Abstract
The neuropilin (Nrp)1 receptor is essential for both nervous and vascular system development. Nrp1 is unusually versatile, because it transmits both chemoattractive and repulsive signals in response to vascular endothelial growth factor (VEGF)-A and class 3 semaphorins, respectively. Both Nrp1 and VEGF receptor 2 undergo ligand-dependent endocytosis. We sought to establish the endocytic pathway of Nrp1 and to determine whether uptake is required for its signaling. Whereas Nrp1 underwent clathrin-dependent endocytosis in response to VEGFA165 treatment, semaphorin 3C (sema3C) induced lipid raft–dependent endocytosis. The myosin VI PDZ (postsynaptic density 95, Disk large, Zona occludens-1) adaptor protein synectin was essential for Nrp1 trafficking. Sema3C failed to inhibit migration of synectin−/− endothelial cells, mirroring the lower migratory response of these cells to VEGFA165. These results show that the endocytic pathway of Nrp1 is determined by its ligand and that the trafficking of Nrp1 is essential for its signaling.
Keywords: neuropilin-1, VEGFR2, endocytosis, lipid rafts
Both vascular endothelial growth factor receptor (VEGFR)2 and neuropilin (Nrp)1 are known to undergo endocytosis in response to ligand binding. VEGFR2 internalization was reported to be clathrin-dependent1,2 and to require receptor autophosphorylation.1,3 It was recently shown that internalized VEGFR2 remains active.2 Moreover, endocytosis and recycling of the drosophila VEGFR ortholog was required for cell migration in response to guidance cues.4
Several studies observed Nrp1 internalization in response to semaphorin (sema)3A5,6 and VEGFA165,7 although the endocytic pathway has not been positively identified. Indirect evidence suggested that Nrp1 underwent clathrin-dependent endocytosis,5 but other studies observed Nrp1 in lipid rafts8,9 and an increase in its presence in rafts upon sema3A treatment.8 Cholesterol depletion impaired cell response to sema3A, suggesting that the association of Nrp1 with lipid rafts was required for Nrp1 signaling.
The only cytoplasmic protein known to bind to the intracellular domain of Nrp1 is synectin,10 also known as GIPC,11 and neuropilin-interacting protein (NIP).10 Synectin is a PDZ (postsynaptic density 95, Disk large, Zona occludens-1) adaptor protein that couples uncoated endocytic vesicles to the molecular motor myosin VI and is required for the trafficking of endocytosed membrane receptors.12 The interaction of Nrp1 with synectin is required for developmental angiogenesis in the zebrafish embryo and for EC migration.13 To date, no report has linked Nrp1 function to its internalization.
We sought to identify the endocytic pathway of Nrp1 in response to semaphorin and to VEGFA165 and to track Nrp1 and VEGFR2 trafficking. We took advantage of the synectin−/− mouse model to determine whether synectin is required for Nrp1 trafficking. We then asked whether the internalization of Nrp1 is involved in its cellular function as a VEGF and semaphorin receptor.
Materials and Methods
Antibodies and Reagents
Antibodies to Nrp1 (R&D Systems); VEGFR2 (Fitzgerald Industries International); α-tubulin, β-actin (Sigma); flotillin, EEA1, clathrin heavy chain, alkaline phosphatase (AP)1α, caveolin (Cav)1 (BD Transduction Labs); myc (Cell Signaling); green fluorescent protein (GFP), donkey anti-goat IgG–Alexa-568, donkey anti-rabbit IgG–Alexa-488, chicken anti-rat IgG–Alexa-488, and phalloidin–Alexa-647 (Invitrogen) were used according to the instructions of the manufacturer. Goat IgG was from Jackson Immunologicals, methyl β-cyclodextrin was from Sigma, and VEGF-A165 was from R&D Systems. The single domain antibody to plexin D1 was described before14 and was detected by anti-VSV (Abcam) conjugated to Qdot-565 (Invitrogen).
DNA Constructs and Transfection Method
A carboxyl-terminal fragment of AP180 was previously subcloned into the pCMV-myc plasmid,15 and an EPS15 mutant lacking EH domains 2 and 3 was previously subcloned into pEGFP-C2 vector (Clontech).16 These mutant proteins exerted a dominant-negative (DN) effect on clathrin-dependent endocytosis by competing with their endogenous counterparts for either clathrin or the clathrin adaptor protein AP2, respectively. Both were transfected into human umbilical cord ECs (HUVECs) (purchased from Lonza) using Fugene-6 (Roche) according to the instructions of the manufacturer. We used HUVECs for these experiments because their transfection efficiency (60% to 70%) was significantly higher than that of mouse primary ECs. The transfection efficiency of the latter ECs (<15%) was too low to ensure observation of the potential effects of expressing DN AP180 and EPS15 on Nrp1 uptake.
Recombinant sema3C Preparation and Binding Assay
Either chick sema3C subcloned into the APTag4 plasmid or AP was expressed and collected as described.17 The conditioned medium was concentrated by centrifugation through 30-kDa cutoff filters (Centricon, Millipore). The concentration of the expressed AP or of the AP-sema3C fusion protein was calculated by measuring AP activity (AP Assay Reagent A, GenHunter) according to the instructions of the manufacturer. Binding of AP-sema3C to ECs after 10 minutes of incubation at 37°C was measured in the same manner using cell lysate. AP was used as an experimental control for AP-sema3C.
Primary EC Preparation
Mouse aorta ECs were isolated as described.18 Cells were used up to passage 5 and grown in DMEM and 20% FBS.
Optical Microscopy
ECs were grown on fibronectin-coated glass-bottom 35-mm plates (Microwell, MatTek). Cells were fixed before confocal microscopy in 4% paraformaldehyde and permeabilized in 0.1% Triton X-100, as needed, and then mounted with Prolong Gold (Invitrogen). Images were acquired by laser-scanning confocal microscopy (Zeiss LSM 510 Meta, Thornwood, NY) with a ×63 objective. To minimize potential bleed-through, fluorescence channels were excited and acquired sequentially in each image.
The Nrp1 and VEGFR2 fluorescence signals were imaged on focal sections that traversed the cytoplasm. These signals persisted after the removal of surface-bound antibodies by low pH wash and colocalized with the endocytic marker EEA1. For these reasons, it can be safely assumed that Nrp1 and VEGFR2 clusters observed after the 0 time point represent endocytic vesicles.
Time-lapse imaging of live ECs grown in Microwell plates was done on an inverted microscope (Olympus IX71) equipped with a plexiglass-enclosed stage kept at 37°C and 5% CO2. Images were acquired by cooled software-controlled (QED, Media Cybernetics) charge-coupled device camera (SensiCam, Cooke Corp) using a filter and a ×60 objective (Olympus).
Uptake Assays
ECs grown in Microwell plates were incubated with antibody to Nrp1 (10 µg/mL), VEGFR2 (10 µg/mL), or both, diluted in DMEM plus 0.5% FBS for 15 minutes on ice, then washed with the same medium, and incubated with donkey anti-goat Alexa568 or Alexa488, respectively. After washing again with same ice-cold medium, sema3C or VEGF-A165 in 37°C medium at the indicated concentrations was added to all samples, except that corresponding to the 0-minute time point. The latter plate was left on ice, whereas the others were transferred immediately to 37°C, 5% CO2 incubator, then removed at the indicated times, placed on ice, and washed with ice-cold PBS. Antibody remaining on the cell surface was removed from all plates (except for the 0-minute plate) by a 30-second wash with ice-cold PBS at pH 2.5. Trafficking and recycling were quantified by counting in how many cells out of 100 in each of 3 experiments Nrp1 punctae reached the perinuclear region or the plasma membrane by 30 minutes after the start of uptake. The effect of DN AP180 or EPS15 expression on Nrp1 uptake in HUVEC was quantified by KS400 Image Analysis Software (Zeiss), which calculated the ratio of the perinuclear fluorescence signal to the signal in the surrounding cytoplasm. The average postuptake intensity of control enhanced (e)GFP-transfected ECs was used as a threshold for classifying AP180- and EPS15-expressing ECs as “positive” or “negative” in terms of uptake. Data were obtained from 2 to 4 independent experiments, with a minimum of 30 cells per experiment.
Measurement of Colocalization
Localization between 2 fluorophores was calculated as the percentage of pixels in an image in which the intensity of both wavelengths was at least 100, in a scale of 0 to 255. The extent of colocalization was calculated from confocal images by CoolLocalizer software (Cytolight, Stockholm, Sweden) based on Pearson’s correlation coefficient.19
Cell Fractionation by Sucrose Gradient
Each sample consisted of 2 confluent 15-cm plates. ECs were scraped off on ice in 25 mmol/L Tris-HCl pH 7.4, 150 mmol/L NaCl, 20 mmol/L EDTA, (TNE buffer) supplemented with a protease inhibitor cocktail (Complete, Roche), pelleted by brief centrifugation, and resuspended in 0.5 mL of TNE containing 1% CHAPS. Cells were sheared by 10 passages through a 26-gauge syringe needle, incubated for 30 minutes on ice, and mixed with 0.5 mL of 90% sucrose in TNE. The ensuing 45% sucrose sample was placed at the bottom of a centrifuge tube (Ultra Clear, Beckman) overlaid with 2.5 mL of 30% sucrose and 1 mL of 0.5% sucrose in TNE using a peristaltic pump (Densi-Flow, Labconco) and spun for 14 hours at 4°C in a Ti-55 rotor (Beckman) at 38 000 rpm (LM-8 Ultracentrifuge, Beckman). Fractions of 1 mL were removed from each tube by the same peristaltic pump, of which 45 µL were resolve by SDS-PAGE (10% Bis-Tris Criterion, Bio-Rad) and transferred to poly(vinylidene difluoride) membrane (Pierce).
Chemotaxis Assays
Chemotaxis assays were performed and quantified as described.20 Because epidermal growth factor (EGF) elicited the best response of synectin−/− ECs in migration assays,18 we used it as chemoattractant in combination with sema3C. Both sema3C and EGF were placed in the bottom well.
Fluorescence-Assisted Cell Sorting
Mouse primary ECs labeled with anti-Nrp1 and donkey anti-goat IgG–Alex647 were sorted as described.20
Statistical Analysis
Statistical significance was calculated by Student’s double-tailed t test. The order of probabilities listed in the figure legends corresponds to the left to right order of asterisks in each figure.
Results
Nrp1 Underwent Endocytosis via Lipid Rafts in Response to sema3C
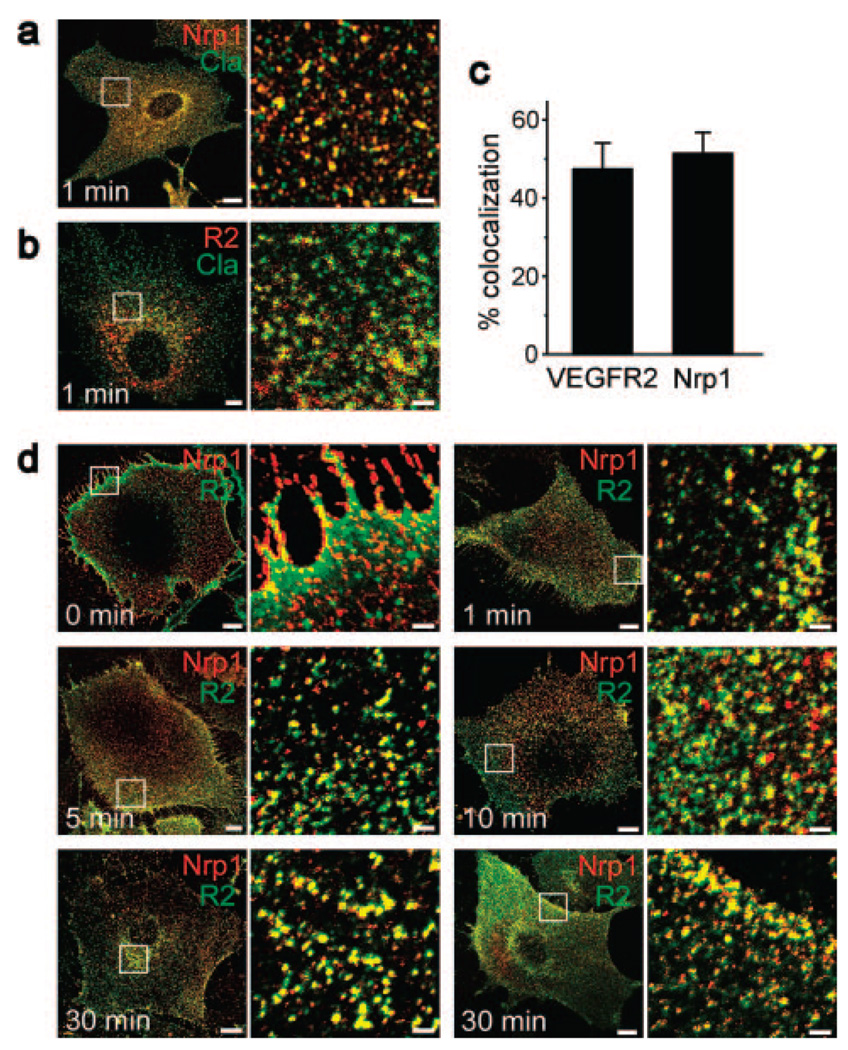
Because of the conflicting evidence on Nrp1 endocytosis,5,8,9 we first sought to identify the endocytic pathway of Nrp1 in response to sema3. Of the several members of the sema3 family, we chose to treat ECs with semaphorin 3C (sema3C) because it was shown to be a Nrp1 ligand involved in the morphogenesis of the mammalian vascular system.21,22 In view of previous results,5 we used markers of clathrin-dependent endocytosis. None of these markers (the clathrin heavy chain [Figure 1a and 1c] and the adaptor protein 1α [data not shown]) colocalized with Nrp1 during sema3C-induced uptake. We also considered the possibility of Cavdependent endocytosis because there is evidence for the presence of Nrp1 in Cav1-containing cell fractions.23 Cavdependent endocytosis was also ruled out, however, because Cav1 did not colocalize with Nrp1 at any time point between 0 to 30 minutes after the start of uptake (Figure 1a and 1c). A strikingly different outcome emerged when we used the lipid raft marker cholera toxin β subunit (CTβ). In quiescent cells, Nrp1 was located primarily along cell edges and colocalized with CTβ only by ≈5% (Figure 1b). By 5 minutes after the start of uptake, close to 20% of the Nrp1 population colocalized with CTβ (Figure 1c and 1d). The general pattern of Nrp1 translocation was radial, from the cell periphery toward the perinuclear region, which was reached by 10 minutes (Figure 1b). By 30 minutes, a large fraction of cellular Nrp1 was perinuclear, reaching, on average, a 23.5% colocalization with CTβ (Fig. 1b, c, and d). At the same time, part of the Nrp1 population recycled to the plasma membrane in as much as 73% of the sampled ECs (Figure 1b and 1e). The extent of Nrp1-CTβ colocalization, as well all other colocalizations described below, are likely to have been underestimated given the high threshold intensity used for qualifying pixels as colocalized (see Materials and Methods). We concurred with a previous study24 in showing that antibody binding did not induce endocytosis of Nrp1 (Figure I in the online data supplement), and we ascertained that anti-Nrp1 did not interfere with sema3C binding to Nrp1 (supplemental Figure II).
Figure 1.
Nrp1 colocalized with CTβ but not with clathrin or Cav during sema3C-induced endocytosis. a, Mouse ECs fixed at the indicated time points during uptake in response to sema3C (250 ng/mL) were labeled with anti-Nrp1 (red) and with anti-clathrin heavy chain (Cla) (green) or with anti-Cav1 (Cav) (green). The white square in this and subsequent figures delineates the magnified subfield on the right. b, Mouse ECs fixed at the indicated time points during uptake in response to sema3C (250 ng/mL), labeled with anti-Nrp1 (red) and cholera toxin β (CTβ) (green). Scale bars: 10 µm (2 µm in the magnified subfields). c, Extent of colocalization between Nrp1 and clathrin at 1 minute, Cav1 at 30 minutes, and CTβ at 30 minutes after start of uptake (n=4–5; ±SD; P=0.0002 and P=0.00002). d, Quantification of the time course of colocalization between Nrp1 and CTβ in ECs treated with sema3C (250 ng/mL, open squares) or VEGF-A165 (50 ng/mL, filled squares) (n=3; mean±SD; P=0.0008, P=0.0005, P=0.0002). e, The fraction of ECs in which Nrp1 recycled to the plasma membrane in ECs treated with sema3C (250 ng/mL) (see the 2 lower right images in b) or with VEGF-A165 (50 ng/mL) (see the 2 lower right images in Figure 4d) (n=3; mean±SD; P=0.004).
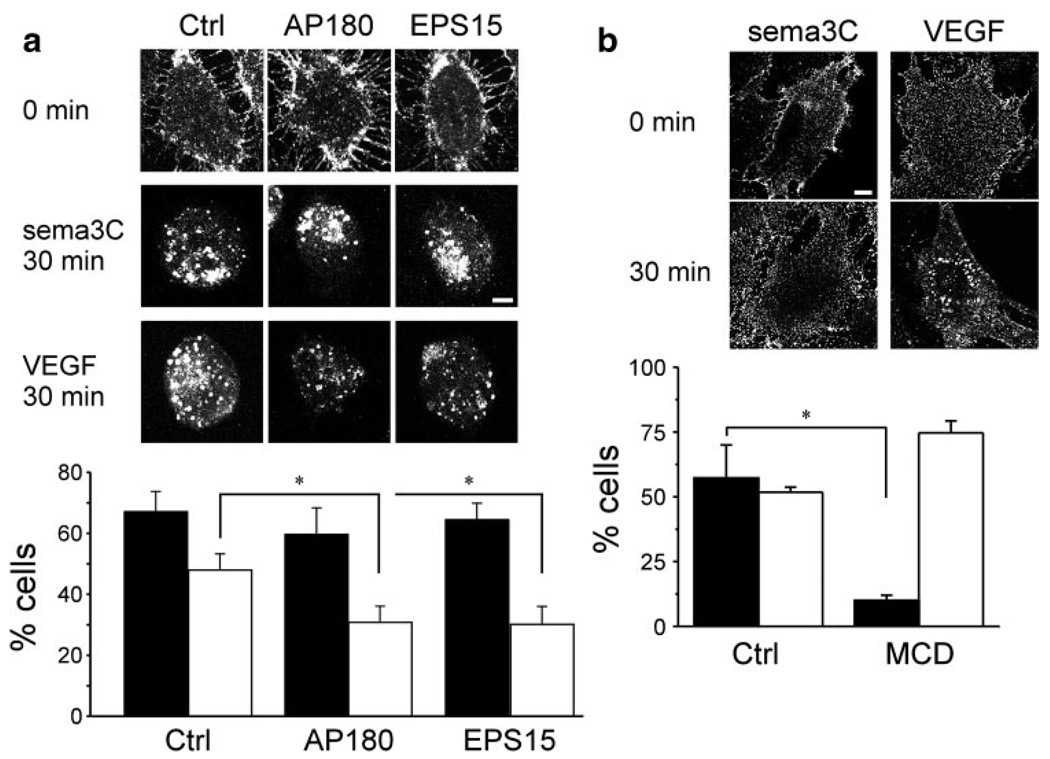
Expression of the DN mutants of the clathrin adaptor proteins AP18015 or EPS1516 (supplemental Figure III) did not inhibit sema3C-induced endocytosis of Nrp1 (Figure 2a) in HUVECs. These mutants are specific to clathrin-dependent endocytosis and, unlike the dynamin-2 K44A DN mutant, do not affect Cav-dependent endocytosis. We were not able to use the latter because of the artifactual effects of its expression on EC cell cycle and VEGFR2 expression level.25 Endocytosis was blocked, however, by the cholesterol-depleting compound methyl β-cyclodextrin (Figure 2b). These results agree with the immunofluorescence observations in suggesting that Nrp1 underwent lipid raft, but not clathrin-dependent, endocytosis in response to sema3C.
Figure 2.
Effect of DN constructs and pharmacological treatment on Nrp1 uptake. a, Representative images (scale bar=5 µm) of sema3C- or VEGF-A165–treated HUVECs transfected with eGFP (Ctrl) or with the DN mutants of AP180 and EPS15. The histogram shows the fraction of either sema3C (black bars) or VEGF-A165–treated (white bars) ECs in which Nrp1 reached the perinuclear region in 30 minutes (n=3; mean±SD; P=0.01). b, Representative images of sema3C- or VEGFA165–treated mouse ECs (scale bar=10 µm) preincubated with methyl β-cyclodextrin (MCD) (10 mmol/L). MCD was applied 30 minutes before sema3C-or VEGF-A165–induced uptake. The histogram shows the fraction of either sema3C (black bars) or VEGF-A165–treated (white bars) ECs preincubated with carrier (Ctrl) (PBS) or with MCD in which Nrp1 reached the perinuclear region in 30 minutes (n=3; mean±SD; P=0.003).
VEGFR2 Colocalized With Nrp1 in Quiescent and in sema3C-Treated ECs
VEGFR2 was reported to associate with Nrp1 upon VEGF-A165 treatment, but their relative localization in sema3C-treated cells is unknown.24 We found that more than 60% of the cell surface VEGFR2 population colocalized with Nrp1 in quiescent ECs (Figure 3a and 3b). The 2 receptors remained coclustered to a similar extent as in quiescent cells after the application of sema3C and translocated together toward the perinuclear region (Figure 3a and 3b).
Figure 3.
Nrp1 and VEGFR2 colocalized during uptake in response to sema3C. a, Mouse ECs fixed at the indicated time points during uptake in response to sema3C (250 ng/mL), labeled with antiNrp1 (red) and anti VEGFR2 (R2) (green). Scale bars: 10 µm (2 µm in the magnified subfields). b, Quantification of the time course of colocalization of Nrp1 with VEGFR2 in ECs treated with sema3C (250 ng/mL, open squares) or VEGF (50 ng/mL, filled squares) (n=3; mean±SD; P=0.003, P=0.03).
Nrp1 and VEGFR2 Underwent Clathrin-Dependent Endocytosis in Response to VEGF-A165
In ECs treated with VEGF-A165, VEGFR2 colocalized with clathrin between 1 to 2 minutes after the start of endocytosis (Figure 4a and 4c). In agreement with previous findings,2 this observation indicates that VEGF-A165 induced clathrin-dependent endocytosis of VEGFR2. Concordantly, VEGFR2 did not colocalize with Cav1 between 0 to 30 minutes of uptake (data not shown). Nrp1 colocalized with clathrin to the same extent as VEGFR2 (Figure 4b and 4c), as would be expected given the colocalization of the 2 receptors in quiescent ECs (Figure 3a). Similar to the observation made in sema3C-treated ECs, the majority of the Nrp1 and VEGFR2 populations remained coclustered up to 30 minutes after the start of uptake (Figure 4d and Figure 3b). At 10 and 30 minutes, the level of Nrp1-VEGFR2 colocalization in VEGF-A165–treated ECs was lower by a small but significant extent (Figure 3b) than in sema3C-treated ECs. Although the reason for this difference is unclear, it may indicate a divergence in the trafficking routes of Nrp1 and VEGFR2 in VEGF-A165–treated ECs. At 30 minutes, part of the colocalized Nrp1-VEGFR2 was perinuclear, whereas another part recycled to the plasma membrane in 50% of the sampled ECs (Figure 4d and Figure 1e). Generation of artifactual effects by the antibody to VEGFR2 had been ruled out in a previous study.2 Unlike sema3C-induced uptake, expression of the DN AP180 or DN EPS15 reduced significantly the extent of VEGF-A165–induced uptake of Nrp1 (Figure 2a). These results are consistent with the clathrin dependence of Nrp1 uptake in response to VEGF-A165.
Figure 4.
Nrp1 and VEGFR2 colocalized with clathrin and with each other during uptake in response to VEGF-A165. a and b, Mouse ECs fixed 1 minute after the start of uptake in response to VEGF-A165 (50 ng/mL), labeled with anti-Nrp1 (a) or anti-VEGFR2 (R2) (b) (both red) and with anti-clathrin (Cla) (green). Scale bars: 10 µm (2 µm in the magnified subfields). c, Extent of colocalization of Nrp1 or VEGFR2 with clathrin in VEGF-A165–treated ECs (n=3; mean±SD). d, Mouse ECs fixed at the indicated time points during uptake in response to VEGF-A165 (50 ng/mL), labeled with anti-Nrp1 (red) and anti-VEGFR2 (R2) (green). Scale bars: 10 µm (2 µm in the magnified subfields). The time course of Nrp1-VEGFR2 colocalization is shown in Figure 3b.
Unlike VEGF-A165, VEGF-A121 does not does bridge between Nrp1 and VEGFR2, although it binds both receptors.26 For this reason, we examined its effect on Nrp1 and VEGFR2 trafficking. The 2 receptors remained colocalized in VEGF-A121–treated ECs (data not shown) in a manner that was not significantly different from VEGF-A165–treated cells. This is not surprising because Nrp1 and VEGFR2 were colocalized in sema3C-treated ECs (Figure 3b), although this ligand is not known to bind VEGFR2.
Unlike sema3C-treated ECs, Nrp1 did not colocalize with CTβ in cells treated with VEGF-A165 (supplemental Figure IV and Figure 1d). To verify the differences in Nrp1 colocalization with CTβ between sema3C- and VEGF-A165–treated ECs, we resolved by sucrose gradients cell lysates corresponding to each of the 2 conditions, as well as lysate of quiescent ECs. The presence of Nrp1 in the lipid rafts of sema3C-treated ECs was more than 3 times higher than in quiescent ECs and more than twice higher than in VEGF A165–treated cells (Figure 5a), confirming that sema3C treatment recruited Nrp1 to lipid rafts.
Figure 5.
Nrp1 presence in lipid rafts is increased upon sema3C treatment. a, Immunoblots of Nrp1 and the lipid raft marker flotillin in fractions (numbered on top) from sucrose gradients of untreated (Ctrl), sema3C-treated (250 ng/mL), and VEGF-A165–treated (50 ng/mL) ECs. Non-lipid raft fractions containing the rest of the Nrp1 population are not shown. The relative amount of Nrp1 in lipid rafts under each condition was quantified by summing up the densitometric values of the Nrp1 bands in each of the three fractions, and, separately, the densitometric values of the flotillin bands in each of the same fractions. The ratios between the first and second sums for each condition are shown in the histogram. b, Coimmunoprecipitation of Nrp1 and VEGFR2 in ECs treated with either sema3C (250 ng/mL) or VEGF-A165 (50 ng/mL). The top immunoblot shows the level of Nrp1 coimmunoprecipitated by VEGFR2 from ECs lysed at the indicated times after the start of uptake, and the lower immunoblot shows the level of β-actin in lysates of the same samples as a loading control (2.5% of immunoprecipitation input, vol/vol).
Nrp1 Associated With VEGFR2 in Response to Both sema3C and VEGF-A165
The colocalization of Nrp1 with VEGFR2 in unstimulated ECs, as well as in sema3C- and VEGF-A165–stimulated cells (Figure 3a and Figure 4d), does not necessarily imply that Nrp1 and VEGFR2 are bound to each other, either directly or indirectly. In agreement with previous studies,24 we found that Nrp1 and VEGFR2 did not coimmunoprecipitate in the absence of VEGF-A165. Stimulation by VEGF-A165 or by sema3C induced association between Nrp1 and VEGFR2 by 10 minutes, but the extent of association was much higher in VEGF-A165–stimulated than in sema3C-stimulated ECs (Figure 5b). By 30 minutes of sema3C or VEGF-A165 treatment, Nrp1 and VEGFR2 associated at similar levels.
The Patterns of Nrp1 Colocalization With plexin D1 or With VEGFR2 Were Dissimilar
Sema3C binds to both Nrp1 and plexin D1,22 where the latter receptor is thought to initiate the intracellular signaling, in a manner analogous to the role of VEGFR2 in VEGF-A165 signaling. Therefore, we analyzed the localization of Nrp1 and plexin D1 during uptake in response to sema3C. Unlike VEGFR2, plexin D1 was not colocalized with Nrp1 in quiescent ECs (Figure 6a and 6b). By 10 minutes, the colocalization of plexinD1 with Nrp1 reached close to 20% and did not significantly exceed that level by 30 minutes (Figure 6a and 6b). Thus, the colocalization of plexinD1 with Nrp1 was lower and more transient than the VEGFR2-Nrp1 colocalization.
Figure 6.
Nrp1 and plexin D1 colocalized during uptake in response to sema3C. a, Mouse ECs fixed at the indicated time points during uptake in response to sema3C (250 ng/mL), labeled with anti-Nrp1 (red) and anti-plexin D1 (D1) (green). Scale bars: 10 µm (2 µm the magnified subfields). b, Quantification of the time course of the colocalization of Nrp1 with plexin D1 (filled squares) and of Nrp1 with VEGFR2 (open squares, shown also in Figure 3b) in ECs treated with sema3C (250 ng/mL) (n=3; mean±SD; P=1×10−6, P=0.00015, P=0.0005).
Nrp1 Trafficking Was Slower in synectin−/− ECs
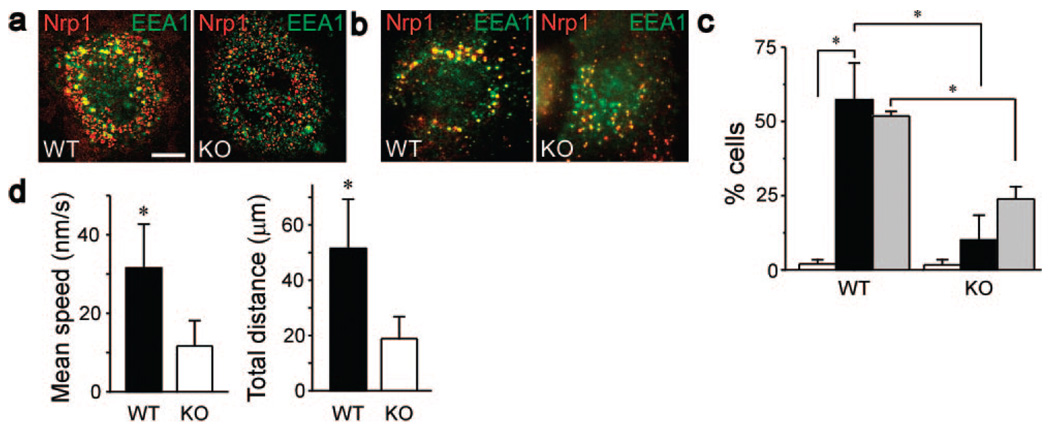
To examine to role of synectin in Nrp1 trafficking, we compared the rate of Nrp1 trafficking in wild-type (WT) and synectin−/− ECs Following the application of sema3C or of VEGF-A165. Both cell types had similar levels of Nrp1 on their plasma membranes (supplemental Figure IV). The extent of Nrp1 colocalization with EEA1 in the perinuclear region was defined as the trafficking end point. The trafficking rate of Nrp1 in synectin−/− ECs in response to either sema3C (Figure 7a) or VEGF-A165 (Figure 7b) was reduced by more than 5- or 2-fold, respectively (Figure 7c), indicating that Nrp1 trafficking was significantly slower in the absence of synectin.
Figure 7.
Nrp1 trafficking in response to either sema3C or VEGF-A165 is faster in WT than in synectin−/− ECs. a and b, WT and synectin−/− (KO) mouse ECs fixed 30 minutes after the start of uptake and stained with anti-Nrp1 (red) and the early endosome marker EEA1 (green) in response to sema3C (250 ng/mL) (a) or VEGF (50 ng/mL) (b). Scale bar: 10 µm. c, Percentage of WT and synectin−/− (KO) ECs in which Nrp1 reached the perinuclear region in 30 minutes in response to AP (white columns), which was used as a control substance, AP-sema3C (250 ng/mL, black columns), or VEGF-A165 (50 ng/mL, gray columns) (n=3; mean±SD; P=0.002, P=0.02, P=0.002). d, Movement parameters of Nrp1 clusters in WT and synectin−/−(KO) mouse ECs, as measured by time-lapse microscopy. Each time interval was 30 seconds, and the total duration was 30 minutes. Nrp1 cluster movement in live cells is visualized in supplemental Movie 1 and supplemental Movie 2 (n=30; mean±SD; P=1×10−11, P=2×10−12).
To further compare Nrp1 trafficking in WT and synectin−/− ECs, we tracked Nrp1 cluster movement in live cells of each type by time-lapse microscopy. Both the rate and pattern of movement were different in the 2 cell types. In WT ECs, Nrp1 clusters moved in the radial direction over relatively large distances, reaching the perinuclear region within 30 minutes (supplemental Movie 1). Nrp1 cluster movement was twice slower and over less than half the distance in synectin−/− ECs (supplemental Movie 2 and Figure 7d), in agreement with the observations made with fixed ECs.
Nrp1 Translocated Along Actin Filaments and Microtubules
Vesicle tracks often follow actin filaments and microtubules because they are driven by either myosin or kinesin molecular motors, respectively. During the first 5 minutes after the application of sema3C, Nrp1 clusters moved in short increments (supplemental Movie 1) and without a uniform orientation. At later times Nrp1 cluster movement occurred in longer increments, mostly in the radial direction toward the nucleus. Nrp1 clusters in Sema3C-treated ECs fixed at 2 and 10 minutes after the start of uptake colocalized with either actin filaments or microtubules, respectively (Figure 8a, and 8b). This suggests that Nrp1-containing vesicles translocated initially along actin filaments, then switched to microtubulebased movement, as reported previously.27 To further test this possibility, we measured the degree of Nrp1 colocalization with actin filaments and with microtubules at 2- and 10-minute time points. At 2 minutes, Nrp1 colocalization with actin filaments was more than 5-fold higher than its colocalization with microtubules, whereas, at 10 minutes, the ratio was reversed: Nrp1 colocalization with tubulin was close to 6-fold higher than with actin filaments (Figure 8c).
Figure 8.
a and b, Mouse ECs fixed at the indicated time points during uptake in response to sema3C (250 ng/mL), labeled with anti-Nrp1 (red) and phalloidin–Alexa-647 (Act) (green) (a) or with anti-Nrp1 (red) and anti–α-tubulin (Tub) (green) (b). Scale bars: 10 µm (5 µm in the magnified subfields). Arrows mark Nrp1 punctae along actin filaments (a) or along microtubules (b). c, Extent of colocalization of Nrp1 with actin filaments (black bars) or with microtubules (white bars) at the indicated time points (n=3; mean±SD; P=0.001, P=0.00008). d, Results of chemotaxis assays of WT and synectin−/− (KO) ECs in response to EGF (5 ng/mL) together with AP (black bars) or with sema3C (white bars), both at 250 ng/mL (n=3; mean±SD; P=0.009). AU indicates arbitrary units.
Chemotactic Response of synectin−/− ECs to sema3C Was Impaired
Because it has already been shown that the migratory response of synectin−/− ECs to VEGF-A165 is significantly lower than that of WT ECs,18 it remained to us to compare the chemotactic response of WT and synectin−/− ECs to sema3C. Although sema3C reduced WT EC chemotaxis by more than 40%, there was no significant reduction in the chemotaxis of synectin−/− ECs (Figure 8d), showing that Nrp1 signaling is impaired in synectin−/− ECs.
Discussion
We demonstrated here that the endocytic pathway of Nrp1 depends on its ligand: VEGF-A165 induces clathrin-dependent endocytosis, whereas sema3C induces lipid raft−dependent endocytosis. Nrp1 and VEGFR2 were coclustered in quiescent ECs and remained so after sema3C treatment. It follows, surprisingly, that VEGFR2 is internalized in response to sema3C, although, unlike VEGF-A165, sema3C is not a known VEGFR2 ligand.
The clathrin dependence of VEGFR2 uptake is in agreement with previous observations.1,2 Internalization in response to VEGF required the autophosphorylation of VEGFR2.1 Therefore, it is likely that this tyrosine phosphorylation targeted VEGFR2 to the clathrin endocytic pathway, possibly by providing binding sites for clathrin adaptor proteins.28 We observed a high level of basal colocalization of Nrp1 and VEGFR2, which persisted when ECs were treated by either VEGF-A165 or sema3C. It is possible that on binding VEGF-A165, the tyrosine-phosphorylated VEGFR2 bound Nrp124 and “dragged” it along into the clathrin-dependent endocytic pathway.
Recruitment of receptors to lipid rafts by their ligands has been observed before, including that of Nrp1 in response to sema3A8 and of syndecan-4 in response to fibroblast growth factor-2.29 The recruitment mechanism of Nrp1 to lipid rafts is not known. The recruitment of tyrosine kinase receptors to lipid rafts requires their autophosphorylation.30,31 Tyrosine phosphorylation of VEGFR2 cannot account for its corecruitment with Nrp1 to lipid rafts, however, because it is unlikely that VEGFR2 underwent such phosphorylation upon binding of sema3C to Nrp1. Palmitoylation can also serve as a lipid raft recruitment signal for membrane receptors.32,33 Although Nrp1 has 2 putative palmitoylation sites on cysteines 881 and 883 in its cytoplasmic domain, there is no evidence as yet for their actual palmitoylation. Formation of a lipid shell around the transmembrane domain could also target receptors to lipid rafts,34 as in the case of the platelet-derived growth factor receptor35 and influenza virus hemagglutinin.36 If a lipid shell were present around the transmembrane domain of Nrp1, sema3C binding could cluster the receptor into larger aggregates, producing Nrp1-populated lipid rafts. The sequence of the hydrophobic N-terminal region of the transmembrane domain of hemagglutinin, which determines its lipid affinity, resembles the sequence of the same region in Nrp1.
CTβ, which we used as an endocytic marker, is among several toxins endocytosed via lipid rafts.37 Its endocytosis is frequently also Cav1-dependent and involves the association with Cav-stabilized membrane domains.38 The semaphoring-induced endocytosis of CTβ described here was not Cav1dependent, however. Indeed, CTβ was observed before to undergo lipid raft–dependent endocytosis in cells that do not express Cav, showing that the latter is not essential for this process.37 Shiga toxin, another bacterial protein, also undergoes Cav-independent endocytosis via lipid raft–containing tubular structures.39 Because we did not observe such structures, the endocytic pathway of CTβ, as well as that of Nrp1, differs from that of Shiga toxin.
Previous studies detected VEGFR2 in a Cav1-containing cell fraction and reported that VEGF treatment removed VEGFR2 from that fraction.23 Those results do not necessarily disagree with the absence of VEGFR2-Cav1 colocalization we observed by immunofluorescence, because caveolae and lipid rafts sediment together when cells are fractionated by ultracentrifugation through a gradient of high-molecularweight medium.40 VEGFR2-Cav1 colocalization was previously detected by immunofluorescence in VEGF-treated HUVECs.41 Because we tracked Cav1 together with Nrp1 in sema3C-treated ECs, but did not track Cav1 together with VEGFR2 in VEGF-A165–treated cells, we cannot rule out the possibility of their colocalization under the latter conditions. However, our results are in closer agreement with the observation of Labrecque et al23 than with Ikeda et al41 concerning the effect of VEGF on the association of VEGFR2 and Cav1.
Although Nrp1 and VEGFR2 cocluster in quiescent ECs, our results, as well as those of others,24 show that they are not bound to each other, whether directly or indirectly. Nrp1 and VEGFR2 remained colocalized following stimulation by either VEGF-A165 or sema3C. The close proximity of Nrp1 and VEGFR2 probably facilitates their binding once treated by VEGF-A165, as shown here and in other studies.24 Nrp1-VEGFR2 binding is essential for VEGF signaling,24 but the significance of the lesser extent of Nrp1-VEGFR2 binding in response to sema3C is unclear at this point. Nrp1 coclustered with the semaphorin receptor plexin D1 in sema3C-treated ECs, but its coclustering was more transient and less pronounced that with VEGFR2. These dissimilarities likely reflect differences between the signaling mechanisms of Nrp1 as a VEGFR2 and as a plexin D1 coreceptor.
We found that both sema3C- and VEGF-A165–induced trafficking required synectin. Synectin is a “universal” adaptor between ligands that bind to its PDZ domain, including Nrp1 and myosin VI.12 It follows, therefore, that trafficking in response to both sema3C and VEGF-A165 requires myosin VI–driven translocation along actin filaments. Nrp1 trafficking in synectin−/− ECs was significantly slower than in WT ECs, and the regulation of the migration of synectin−/− EC by sema3C was impaired. This suggest that trafficking is essential for the participation of Nrp1 in both VEGF and semaphorin signaling. Therefore, it is likely that internalization and trafficking facilitate the coupling of Nrp1 and its associated signaling partners (whether VEGFR2 or a plexin receptor) to downstream effectors in signaling pathways regulating cell migration. This conclusion is in agreement with previous studies linking receptor trafficking with directional cell migration4 and studies that attributed a migration-specific role to VEGF signaling through Nrp1.24 Each of the 2 disparate endocytic pathways used by Nrp1 could contribute to its signaling specificity by coupling it to a different set of downstream effectors, depending on which ligand it binds.
Supplementary Material
Acknowledgments
We thank Dr Jonathan Raper (University of Pennsylvania) for sharing the APTag4-sema3C and Dr Richard Pagano (Mayo Clinic) for sharing the AP180 and EPS15 DN constructs. We thank the Englert Cell Analysis Laboratory at Dartmouth for help with confocal microscopy.
Sources of Funding
This work was supported by NIH grants HL62289, 84619, and 53793 (to M.S.); CA78383, HL072178, and HL70567 (to D.M.); and HL67960 (to A.H.). This work was also supported by a generous gift from Bruce and Martha Atwater (to D.M.) and by the Hitchcock Foundation (to A.H.).
Footnotes
Disclosures
None.
References
- 1.Ewan LC, Jopling HM, Jia H, Mittar S, Bagherzadeh A, Howell GJ, Walker JH, Zachary IC, Ponnambalam S. Intrinsic tyrosine kinase activity is required for vascular endothelial growth factor receptor 2 ubiquitination, sorting and degradation in endothelial cells. Traffic. 2006;7:1270–1282. doi: 10.1111/j.1600-0854.2006.00462.x. [DOI] [PubMed] [Google Scholar]
- 2.Lampugnani MG, Orsenigo F, Gagliani MC, Tacchetti C, Dejana E. Vascular endothelial cadherin controls VEGFR-2 internalization and signaling from intracellular compartments. J Cell Biol. 2006;174:593–604. doi: 10.1083/jcb.200602080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dougher M, Terman BI. Autophosphorylation of KDR in the kinase domain is required for maximal VEGF-stimulated kinase activity and receptor internalization. Oncogene. 1999;18:1619–1627. doi: 10.1038/sj.onc.1202478. [DOI] [PubMed] [Google Scholar]
- 4.Jekely G, Sung HH, Luque CM, Rorth P. Regulators of endocytosis maintain localized receptor tyrosine kinase signaling in guided migration. Dev Cell. 2005;9:197–207. doi: 10.1016/j.devcel.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Castellani V, Falk J, Rougon G. Semaphorin3A-induced receptor endocytosis during axon guidance responses is mediated by L1 CAM. Mol Cell Neurosci. 2004;26:89–100. doi: 10.1016/j.mcn.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Piper M, Salih S, Weinl C, Holt CE, Harris WA. Endocytosis-dependent desensitization and protein synthesis-dependent resensitization in retinal growth cone adaptation. Nat Neurosci. 2005;8:179–186. doi: 10.1038/nn1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Narazaki M, Tosato G. Ligand-induced internalization selects use of common receptor neuropilin-1 by VEGF165 and semaphorin3A. Blood. 2006;107:3892–3901. doi: 10.1182/blood-2005-10-4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guirland C, Suzuki S, Kojima M, Lu B, Zheng JQ. Lipid rafts mediate chemotropic guidance of nerve growth cones. Neuron. 2004;42:51–62. doi: 10.1016/s0896-6273(04)00157-6. [DOI] [PubMed] [Google Scholar]
- 9.Moretti S, Procopio A, Lazzarini R, Rippo MR, Testa R, Marra M, Tamagnone L, Catalano A. Semaphorin3A signaling controls Fas (CD95)-mediated apoptosis by promoting Fas translocation into lipid rafts. Blood. 2008;111:2290–2299. doi: 10.1182/blood-2007-06-096529. [DOI] [PubMed] [Google Scholar]
- 10.Cai H, Reed RR. Cloning and characterization of neuropilin-1-interacting protein: a PSD-95/Dlg/ZO-1 domain-containing protein that interacts with the cytoplasmic domain of neuropilin-1. J Neurosci. 1999;19:6519–6527. doi: 10.1523/JNEUROSCI.19-15-06519.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Vries L, Lou X, Zhao G, Zheng B, Farquhar MG. GIPC, a PDZ domain containing protein, interacts specifically with the C terminus of RGS-GAIP. Proc Natl Acad Sci U S A. 1998;95:12340–12345. doi: 10.1073/pnas.95.21.12340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naccache SN, Hasson T, Horowitz A. Binding of internalized receptors to the PDZ domain of GIPC/synectin recruits myosin VI to endocytic vesicles. Proc Natl Acad Sci U S A. 2006;103:12735–12740. doi: 10.1073/pnas.0605317103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang L, Mukhopadhyay D, Xu X. C terminus of RGS-GAIP-interacting protein conveys neuropilin-1-mediated signaling during angiogenesis. FASEB J. 2006;20:1513–1515. doi: 10.1096/fj.05-5504fje. [DOI] [PubMed] [Google Scholar]
- 14.Roodink I, Raats J, van der Zwaag B, Verrijp K, Kusters B, van Bokhoven H, Linkels M, de Waal RM, Leenders WP. Plexin D1 expression is induced on tumor vasculature and tumor cells: a novel target for diagnosis and therapy? Cancer Res. 2005;65:8317–8323. doi: 10.1158/0008-5472.CAN-04-4366. [DOI] [PubMed] [Google Scholar]
- 15.Zhao X, Greener T, Al-Hasani H, Cushman SW, Eisenberg E, Greene LE. Expression of auxilin or AP180 inhibits endocytosis by mislocalizing clathrin: evidence for formation of nascent pits containing AP1 or AP2 but not clathrin. J Cell Sci. 2001;114:353–365. doi: 10.1242/jcs.114.2.353. [DOI] [PubMed] [Google Scholar]
- 16.Benmerah A, Bayrou M, Cerf-Bensussan N, Dautry-Varsat A. Inhibition of clathrin-coated pit assembly by an Eps15 mutant. J Cell Sci. 1999;112:1303–1311. doi: 10.1242/jcs.112.9.1303. [DOI] [PubMed] [Google Scholar]
- 17.Feiner L, Koppel AM, Kobayashi H, Raper JA. Secreted chick semaphorins bind recombinant neuropilin with similar affinities but bind different subsets of neurons in situ. Neuron. 1997;19:539–545. doi: 10.1016/s0896-6273(00)80370-0. [DOI] [PubMed] [Google Scholar]
- 18.Chittenden TW, Claes F, Lanahan AA, Autiero M, Palac RT, Tkachenko EV, Elfenbein A, Ruiz de Almodovar C, Dedkov E, Tomanek R, Li W, Westmore M, Singh JP, Horowitz A, Mulligan-Kehoe MJ, Moodie KL, Zhuang ZW, Carmeliet P, Simons M. Selective regulation of arterial branching morphogenesis by synectin. Dev Cell. 2006;10:783–795. doi: 10.1016/j.devcel.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 19.Manders EM, Stap J, Brakenhoff GJ, van Driel R, Aten JA. Dynamics of three-dimensional replication patterns during the S-phase, analysed by double labelling of DNA and confocal microscopy. J Cell Sci. 1992;103(pt 3):857–862. doi: 10.1242/jcs.103.3.857. [DOI] [PubMed] [Google Scholar]
- 20.Liu M, Horowitz A. A PDZ-binding motif as a critical determinant of Rho guanine exchange factor function and cell phenotype. Mol Biol Cell. 2006;17:1880–1887. doi: 10.1091/mbc.E06-01-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feiner L, Webber AL, Brown CB, Lu MM, Jia L, Feinstein P, Mombaerts P, Epstein JA, Raper JA. Targeted disruption of semaphorin 3C leads to persistent truncus arteriosus and aortic arch interruption. Development. 2001;128:3061–3070. doi: 10.1242/dev.128.16.3061. [DOI] [PubMed] [Google Scholar]
- 22.Gitler AD, Lu MM, Epstein JA. PlexinD1 and semaphorin signaling are required in endothelial cells for cardiovascular development. Dev Cell. 2004;7:107–116. doi: 10.1016/j.devcel.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Labrecque L, Royal I, Surprenant DS, Patterson C, Gingras D, Beliveau R. Regulation of vascular endothelial growth factor receptor-2 activity by caveolin-1 and plasma membrane cholesterol. Mol Biol Cell. 2003;14:334–347. doi: 10.1091/mbc.E02-07-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan Q, Chanthery Y, Liang WC, Stawicki S, Mak J, Rathore N, Tong RK, Kowalski J, Yee SF, Pacheco G, Ross S, Cheng Z, Le Couter J, Plowman G, Peale F, Koch AW, Wu Y, Bagri A, Tessier-Lavigne M, Watts RJ. Blocking neuropilin-1 function has an additive effect with anti-VEGF to inhibit tumor growth. Cancer Cell. 2007;11:53–67. doi: 10.1016/j.ccr.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 25.Bhattacharya R, Kang-Decker N, Hughes DA, Mukherjee P, Shah V, McNiven MA, Mukhopadhyay D. Regulatory role of dynamin-2 in VEGFR-2/KDR-mediated endothelial signaling. FASEB J. 2005;19:1692–1694. doi: 10.1096/fj.05-3889fje. [DOI] [PubMed] [Google Scholar]
- 26.Pan Q, Chathery Y, Wu Y, Rathore N, Tong RK, Peale F, Bagri A, Tessier-Lavigne M, Koch AW, Watts RJ. Neuropilin-1 binds to VEGF121 and regulates endothelial cell migration and sprouting. J Biol Chem. 2007;282:24049–24056. doi: 10.1074/jbc.M703554200. [DOI] [PubMed] [Google Scholar]
- 27.Kuznetsov SA, Langford GM, Weiss DG. Actin-dependent organelle movement in squid axoplasm. Nature. 1992;356:722–725. doi: 10.1038/356722a0. [DOI] [PubMed] [Google Scholar]
- 28.Nesterov A, Kurten RC, Gill GN. Association of epidermal growth factor receptors with coated pit adaptins via a tyrosine phosphorylation-regulated mechanism. J Biol Chem. 1995;270:6320–6327. doi: 10.1074/jbc.270.11.6320. [DOI] [PubMed] [Google Scholar]
- 29.Tkachenko E, Lutgens E, Stan RV, Simons M. Fibroblast growth factor 2 endocytosis in endothelial cells proceed via syndecan-4-dependent activation of Rac1 and a Cdc42-dependent macropinocytic pathway. J Cell Sci. 2004;117:3189–3199. doi: 10.1242/jcs.01190. [DOI] [PubMed] [Google Scholar]
- 30.Paratcha G, Ledda F, Baars L, Coulpier M, Besset V, Anders J, Scott R, Ibanez CF. Released GFRalpha1 potentiates downstream signaling, neuronal survival, and differentiation via a novel mechanism of recruitment of c-Ret to lipid rafts. Neuron. 2001;29:171–184. doi: 10.1016/s0896-6273(01)00188-x. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki S, Numakawa T, Shimazu K, Koshimizu H, Hara T, Hatanaka H, Mei L, Lu B, Kojima M. BDNF-induced recruitment of TrkB receptor into neuronal lipid rafts: roles in synaptic modulation. J Cell Biol. 2004;167:1205–1215. doi: 10.1083/jcb.200404106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fragoso R, Ren D, Zhang X, Su MW, Burakoff SJ, Jin YJ. Lipid raft distribution of CD4 depends on its palmitoylation and association with Lck, and evidence for CD4-induced lipid raft aggregation as an additional mechanism to enhance CD3 signaling. J Immunol. 2003;170:913–921. doi: 10.4049/jimmunol.170.2.913. [DOI] [PubMed] [Google Scholar]
- 33.Chakrabandhu K, Herincs Z, Huault S, Dost B, Peng L, Conchonaud F, Marguet D, He HT, Hueber AO. Palmitoylation is required for efficient Fas cell death signaling. EMBO J. 2007;26:209–220. doi: 10.1038/sj.emboj.7601456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson RG, Jacobson K. A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science. 2002;296:1821–1825. doi: 10.1126/science.1068886. [DOI] [PubMed] [Google Scholar]
- 35.Liu P, Wang P, Michaely P, Zhu M, Anderson RG. Presence of oxidized cholesterol in caveolae uncouples active platelet-derived growth factor receptors from tyrosine kinase substrates. J Biol Chem. 2000;275:31648–31654. doi: 10.1074/jbc.M004599200. [DOI] [PubMed] [Google Scholar]
- 36.Scheiffele P, Roth MG, Simons K. Interaction of influenza virus haem-agglutinin with sphingolipid-cholesterol membrane domains via its transmembrane domain. EMBO J. 1997;16:5501–5508. doi: 10.1093/emboj/16.18.5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parton RG, Richards AA. Lipid rafts and caveolae as portals for endocytosis: new insights and common mechanisms. Traffic. 2003;4:724–738. doi: 10.1034/j.1600-0854.2003.00128.x. [DOI] [PubMed] [Google Scholar]
- 38.Pelkmans L, Burli T, Zerial M, Helenius A. Caveolin-stabilized membrane domains as multifunctional transport and sorting devices in endocytic membrane traffic. Cell. 2004;118:767–780. doi: 10.1016/j.cell.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 39.Romer W, Berland L, Chambon V, Gaus K, Windschiegl B, Tenza D, Aly MR, Fraisier V, Florent JC, Perrais D, Lamaze C, Raposo G, Steinem C, Sens P, Bassereau P, Johannes L. Shiga toxin induces tubular membrane invaginations for its uptake into cells. Nature. 2007;450:670–675. doi: 10.1038/nature05996. [DOI] [PubMed] [Google Scholar]
- 40.Xu W, Yoon SI, Huang P, Wang Y, Chen C, Chong PL, Liu-Chen LY. Localization of the kappa opioid receptor in lipid rafts. J Pharmacol Exp Ther. 2006;317:1295–1306. doi: 10.1124/jpet.105.099507. [DOI] [PubMed] [Google Scholar]
- 41.Ikeda S, Ushio-Fukai M, Zuo L, Tojo T, Dikalov S, Patrushev NA, Alexander RW. Novel role of ARF6 in vascular endothelial growth factor-induced signaling and angiogenesis. Circ Res. 2005;96:467–475. doi: 10.1161/01.RES.0000158286.51045.16. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.