Summary
The hypothesis that α1D-adrenoceptors may mediate the pro-hypertensive actions of angiotensin II (Ang II) was tested in isolated aorta (α1D-adrenoceptor bearing tissue) of the aryl hydrocarbon receptor null mouse (AhR−/−), which shows increased levels of Ang II, cardiac hypertrophy and hypertension.
The effect of captopril (an angiotensin converting enzyme inhibitor) on both blood pressure and aortic α1D-adrenoceptor expression and function in mice were determined.
Basal blood pressure was higher in AhR−/− mice, while captopril therapy decreased it to wild-type (WT) values.
Aortas of adult WT and AhR−/− mice were stimulated by phenylephrine or noradrenaline to induce contraction; the maximal effect was higher in AhR−/− mice, without a significant change in pEC50.
PA2 values for the selective α1D-adrenoceptor antagonist BMY 7378 (8-[2-[4-(2-methoxyphenyl)-1-piperazynil]ethyl]-8-azaspiro [4.5]decane-7,9-dione) were 9.19 and 8.94 for WT and AhR−/−, respectively; while Schild slopes were not different from 1.
PCR experiments showed c. 77% increase in AhR−/− α1D-adrenoceptors cDNA compared with WT mice; while western blot analysis demonstrated c. 88% increase in α1D-adrenoceptor protein in AhR−/− mice.
Captopril therapy decreased α1D-adrenoceptor-induced contraction and protein in AhR−/− mice to WT levels.
These data support the hypothesis that under conditions where Ang II is elevated, vascular α1D-adrenoceptors are increased, and further suggest that both Ang II and vascular α1D-adrenoceptors could be related in the onset of hypertension.
Keywords: angiotensin II, α1D-adrenoceptors, aryl hydrocarbon receptor null mouse, hypertension, captopril
Introduction
Angiotensin II (Ang II) plays an important role in regulating systemic arterial pressure through its synthesis by the renin–angiotensin system (RAS). Ang II is a potent vasoconstrictor that activates Ang II type 1 (AT1) receptors on vascular smooth muscle and affects vascular and cardiac remodelling cardiac contractility and heart rate through increased sympathetic nervous system tone by promoting noradrenaline release (Weir & Dzau, 1999). Several physiological parameters regulated by the RAS, such as plasma renin activity, plasma angiotensinogen concentration (Ruiz et al., 1990; Zicha & Kune, 1999) and kidney renin release (Henrich & Levi, 1991; Zicha & Kune, 1999) are known to be elevated in young spontaneously hypertensive rats (SHR), suggesting that these components might contribute to the pathogenesis of genetic hypertension. Recent evidence suggests that the AT1 receptor signalling pathway mediates both the physiological and pathogenic pleiotropic actions of Ang II (reviewed in Hunyady & Catt, 2006).
On the other hand, a decade ago it was demonstrated that Ang II induces α1-adrenoceptor expression, mainly the α1D-subtype, in isolated rat aorta smooth muscle cells (VSMC) (Hu et al.,1995). Activation of the α1D-adrenoceptor subtype increases protein synthesis in VSMC (Xin et al., 1997). These data suggest that Ang II may facilitate aorta smooth muscle hypersensitivity and hypertrophy through α1D-adrenoceptors expression.
Increasing evidence shows that vascular α1D-adrenoceptors are functionally related with the genesis and/or maintenance of hypertension, they seem to be present prior to the establishment of hypertension, and it has been suggested that an increase in the population of constitutively active α1D-adrenoceptor could be involved in the pathology of elevated sympathetic tone found in SHR (Villalobos-Molina & Ibarra, 1996, 1999; Villalobos-Molina et al., 1999; Guimaraes & Moura, 2001; Gisbert et al., 2002; García-Sáinz & Villalobos-Molina, 2004). We have found that inhibiting Ang II production with captopril decreased both the expression and function of α1D-adrenoceptors in the aorta of pre-hypertensive SHR rats (Godínez-Hernández et al., 2006). Taken together, these data suggest that the RAS and α1D-adrenoceptors might interact with each other at the onset of hypertension. Thus, we hypothesize that under conditions of elevated levels of Ang II, vascular α1D-adrenoceptors will also be increased, and may mediate some of the pro-hypertensive actions of the hormone (Villalobos-Molina & Ibarra, 2005; Godínez-Hernández et al., 2006). One condition where elevated levels of AngII are observed is the aryl hydrocarbon receptor null (AhR−/−) mouse, which exhibits cardiac hypertrophy, increased levels of endothelin-1 and high arterial blood pressure (Lund et al., 2003). However, so far no explanation exists as to why the absence of the aryl hydrocarbon receptor leads to the pathology observed in these mice. As it is known that mouse aorta express α1D-adrenoceptors that mediate contraction (Yamamoto & Koike, 2001; Lázaro-Suárez et al., 2005), we chose this model to test our hypothesis.
Material and methods
Animals
Adult (4 months-old) AhR−/− (targeted mutation of aryl hydrocarbon receptor gene) and wild-type C57BL6N/129sv (WT) male mice, were maintained in a pathogen-free environment with a controlled temperature (22 ± 2 °C) at 40–60% humidity. The mice had 12/12 light/dark cycles and were given free access to food and water. Animal housing, care and all procedures were conducted in accordance with the Mexican Regulations of Animal Care and Use (NOM-062-ZOO-1999, Ministry of Agriculture, México), and with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the U.S. National Institutes of Health. All protocols were approved by the institutional committee for the use of animals.
Blood pressure and captopril therapy
Wild-type and AhR−/− mice were used for the measurement of blood pressure, with a tail-cuff device (Letica, Panlab, Barcelona, Spain), as follows: a mouse was restrained in a plastic container (size match), a sensor and a ring containing the inflatable latex were placed in the tail; while the mouse was kept warm in the same device. The animals were trained to be inside the container with the cuff placed in the tail and the latex ring was inflated–deflated (this was done several times in a week). Then mice (3–6 animals) were tested for blood pressure measurement (three stable readings were recorded for each mouse) and the mean was used to construct a time-point.
Aryl hydrocarbon receptor null mice were treated with captopril (10 mg−1kg−1day−1, in the drinking water) over 2 weeks, while blood pressure was measured. After this therapy, animals were killed and the aorta was harvested.
Contraction in isolated aorta
Animals were killed and the thoracic aorta was excised and cleaned from surrounding connective tissue. Isolated arteries were cut into rings (c.5 mm in length) and the endothelium removed by gently rubbing the intimal surface with a metal device, as previously described (Lázaro-Suárez et al., 2005). In brief, aortic rings were placed in tissue chambers filled with 10 ml Krebs–Henseleit solution, maintained at 37 °C, pH 7.4 and bubbled with 95% O2 containing 5% CO2. Arterial rings were hooked to the bottom of the chamber and to a Grass FT03 force displacement transducer (Astro-Med, Inc., West Warwick, RI, USA), connected to a MP100 data-acquisition system (Biopac Systems Inc., Santa Barbara, CA, USA), to record the isometric tension developed by aortic rings. An optimal tension of 1.5 g was applied to mouse aorta then phenylephrine (Sigma-Aldrich, St Louis, MO, USA) or nor-adrenaline (supplemented with 2% ascorbic acid) concentration–response curves were constructed. All curves were constructed in the presence of rauwolscine (1 × 10−7 m) and propranolol (1 × 10−7 m), to antagonize α2- and β-adrenoceptors, respectively. The tissue was challenged with phenylephrine or noradrenaline and washed every 30 min for 2 h. Then, reproducible cumulative concentration–response curves to agonists (1 × 10−9 to 3 × 10−5 m) were obtained for each artery. To avoid fatigue of the preparation, a recovery period of 60 min was allowed between agonist curves.
In some experiments, arteries were incubated with BMY 7378 (8-[2-[4-(2-methoxyphenyl)-1-piperazynil]ethyl]-8-azaspiro [4.5]decane-7,9-dione), a highly selective α1D-adrenoceptor antagonist, for 30 min before and during phenylephrine exposure. Schild analysis was used to obtain pA2 and slope (m) values (Arunlakshana & Schild, 1959). Solutions were prepared daily.
Reverse transcription-polymerase chain reaction
Total RNA of mice aorta artery was purified by the Trizol reagent method (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s protocol. First strand cDNA was synthesized using reverse transcriptase, 1 µg of DNase-treated RNA, 1 mm dNTPs, RNase inhibitor, oligo(dT) and random hexamers as primers in a total volume of 50 µl at 42 °C. The cDNA synthesized was used as template in polymerase chain reaction. The relative abundance of α1D-adrenergic receptor was estimated by normalization with 18S-ribosomal RNA, PCR amplified. For α1D-adrenoceptor amplification, the sequence of primers was: forward, 5′-TGGTATCTGTGGGACCGCTACTAGG-3′, and reverse 5′-TACACGCGGCAGTACATGACCACG-3′. PCR conditions were carried out for 27 cycles each 94 °C for 40 s, 62 °C for 40 s and 72 °C for 30 s, followed by 5 min at 72 °C, expected amplified sequence is a 158-bp fragment. For 18S-ribosomal RNA forward sequence was 5′-GGGAGCCTGAGAAACGGC-3′ and the reverse sequence was 5′-GGGTCGGGAGTGGGTAATTT-3′ the amplicon length is 67 bp; the PCR amplification was conducted for 10 cycles under identical conditions. PCR amplicons were visualized in 2% agarose gels, with SYBR green-1 stain in a FLA-5000 image analyzer (Fujifilm Medical Systems, Stamford, CT, USA). Densitometric analysis was obtained with multi gauge 3.0 software (Fujifilm).
Western blot
Protein homogenates from a pool of three thoracic aorta were obtained by mechanical disruption in a Dounce homogenizer, in RIPA buffer: 10 mM Tris–HCl pH 7.4, 1 mm EDTA, 150 mm NaCl, 0.1% Triton, 0.1% sodium dodecyl sulphate (SDS) and complete mini protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN, USA). The total protein concentration in the extracts was determined with the Folin reagent (Lowry et al., 1951). For electrophoresis, 60 µg of each sample were diluted in one volume of 2× Laemlli buffer, run on 10% SDS-polyacrylamide gel and transferred to polyvinyldenofluoride membranes (Amersham Biosciences, Piscataway, NJ, USA). Membranes were blocked for 1 h at room temperature in 20 mm Tris, pH 7.4, 0.1% Tween 20 (TBS-T) containing 5% non-fat dry milk, rinsed for three times with TBS-T and then incubated overnight at 4 °C with rabbit anti α1D-adrenergic receptor antisera (20, the immunogenic sequence for the utilized antibody is identical for rats and mice), in a 1:2000 dilution. After, membranes were washed with TBS-T, incubated 1 h at room temperature with 1:10000 dilution of horseradish peroxidase-conjugated goat anti-rabbit antibody (Zymed Laboratories Inc., San Francisco, CA, USA) in TBS-T with 5% non-fat milk. After washing, signals were visualized by addition of chemiluminescent substrate (Pierce Biotechnology, Inc., Rockford, IL, USA) and the signal documented by autoradiography. Densitometric analysis of digitalized images was obtained with multi gauge 3.0 software (Fujifilm).
Results
Blood pressure measurements
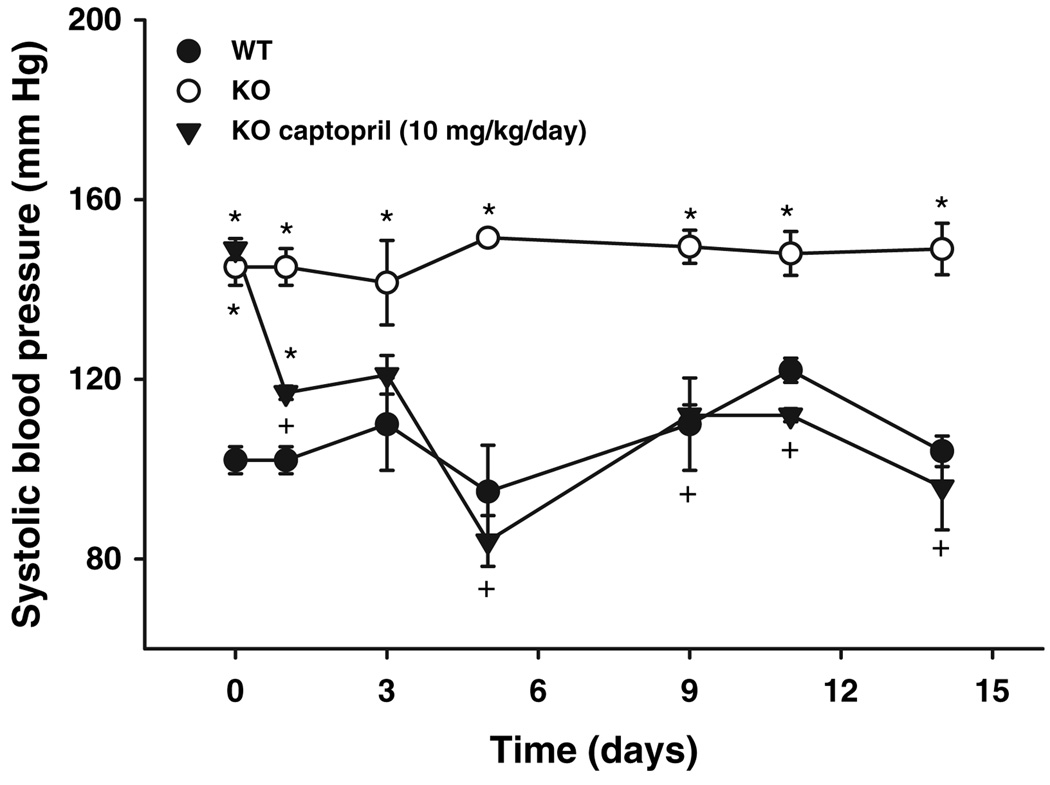
Systolic blood pressure in WT mice was 110 ± 5 mmHg and 150 ± 3 mmHg in AhR−/− mice (Fig. 1). Captopril therapy decreased blood pressure in a time-dependent manner, showing a significant diminution at 24 h of treatment and reaching WT values (Fig. 1).
Figure 1.
Systolic blood pressure in AhR−/− mice. Mice were trained for blood pressure measurements, then subjected to assay conditions: WT (●), AhR−/− (○). In some experiments, AhR−/− mice were treated with captopril (▼). Results represent the mean ± SEM of 3–6 animals. *P < 0.05 AhR−/− vs. WT. +P < 0.05 AhR−/− vs. AhR−/− + captopril.
Contraction in isolated aorta
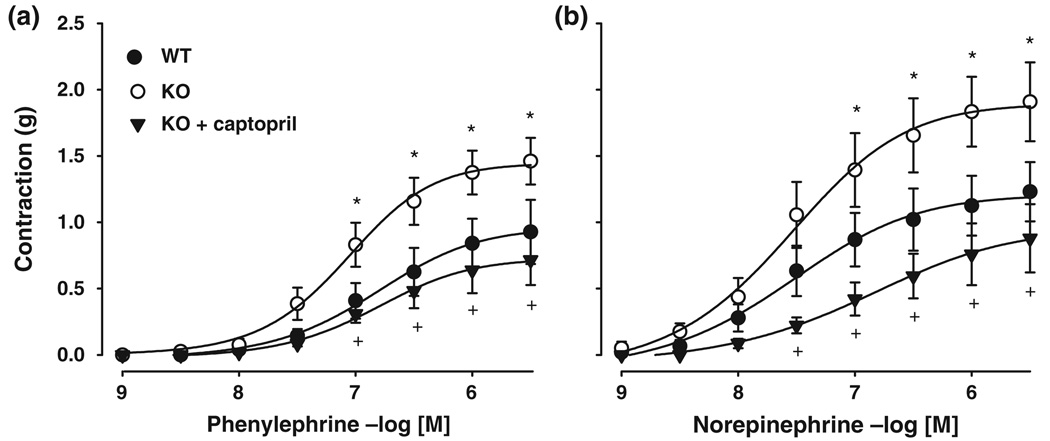
Noradrenaline induced contraction in aorta of both WT and AhR−/− mice in a concentration-dependent manner (Fig. 2). Agonist potency was similar between mice strains (pEC50 of 7.6 ± 0.1 vs. 7.4 ± 0.2 in AhR−/− and WT), but efficacy was different because the AhR−/− aorta showed a maximal effect of 1.9 ± 0.3 vs. 1.2 ± 0.2 g in the WT mouse (P < 0.05). Similar effects were obtained with the agonist phenylephrine (pEC50, 7.1 ± 0.1 vs. 6.8 ± 0.1, Emax, 1.5 ± 0.2 vs. 0.9 ± 0.2 g in AhR−/− vs. WT, respectively, P < 0.05). Captopril treatment reduced the magnitude of contractions in AhR−/− aorta (P < 0.05), such that the values obtained were similar to those WT irrespective of the agonist used (NE pEC50, 7.0 ± 0.1 vs. 6.8 ± 0.1, Emax, 0.9 ± 0.3 vs. 0.9 ± 0.2 g in AhR−/− + captopril vs. WT, respectively and phenylephrine pEC50, 6.9 ± 0.1 vs 6.8 ± 0.1, Emax, 0.8 ± 0.2 vs. 0.9 ± 0.2 g in AhR−/− vs. WT, respectively) (Fig. 2).
Figure 2.
Contractions of aorta taken from AhR−/− (KO, ○) and WT (•) mice exposed to increasing concentrations of phenylephrine (a) or noradrenaline (b). In some experiments, aorta derived from captopril-treated AhR−/− mice were used (▼). Results are reported as mean ± SEM of 3–6 animals. *P < 0.05 AhR−/− vs. WT; +P < 0.05 AhR−/− vs. AhR−/− + captopril.
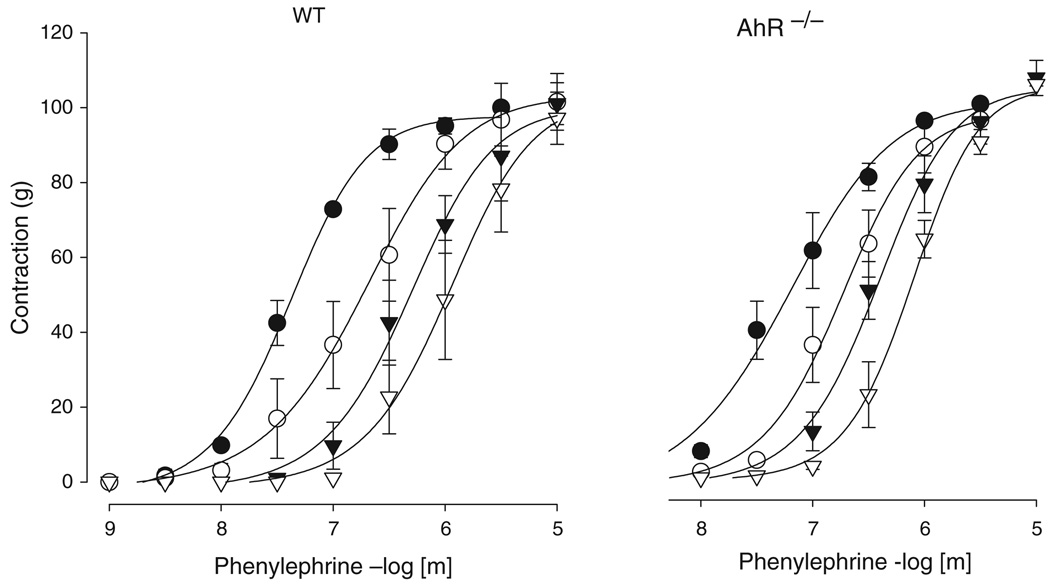
pA2 values for BMY 7378 were 8.94 ± 0.21 vs. 9.19 ± 0.29 for AhR−/− vs. WT, respectively (Fig. 3); whereas Schild slopes were 0.76 (95% confidence limits, −1.13 to −0.4) vs. −0.90 (95% confidence limits, −1.59 to −0.21) for AhR−/− vs. WT, respectively (n = 3 in each group), and not statistically different from 1.
Figure 3.
Effect of α1D-adrenoceptor antagonism in isolated mouse aorta. Isolated aortic rings of WT and AhR−/− mice were incubated with phenyleprhine in the absence and in the presence of increasing concentrations of the selective α1D-adrenoceptor antagonist BMY 7378 (●, control; ○, BMY 3 × 10−9 m; ▼, BMY 1 × 10−8 m; ▽, 3 × 10−8 m). Data are reported as a percentage of the maximum response (mean ± SEM, n = 3).
Determination of α1D-adrenoceptor transcript in aorta of AhR−/− mice
Relative abundance of the α1D-adrenoceptor transcript was explored by reverse transcription of aortic total RNA and PCR amplification with specific primers designed against Adra1d cDNA sequence (NCBI accession number NM_013460).
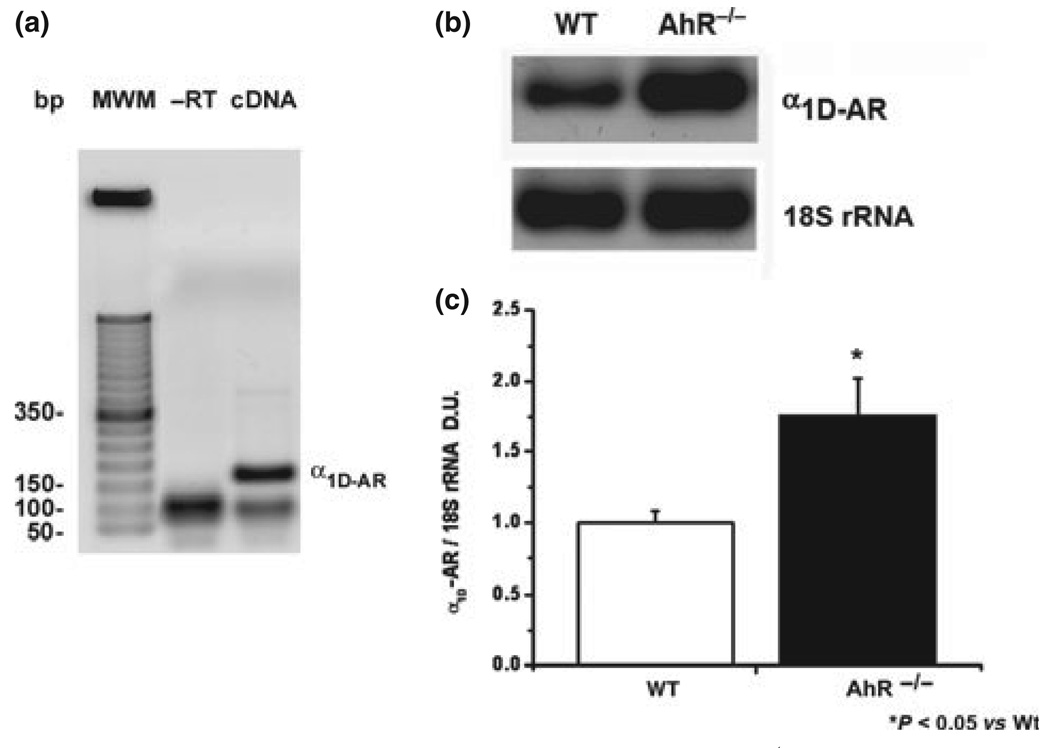
Electrophoretic analysis of the PCR reaction exhibited a single band of the expected size (158 bp) without unspecific or ambiguous bands, reverse transcriptase negative control did not show amplification (Fig. 4a). The lower size band could be an oligomer formed by primers once the reaction cools down. The melting point analysis of this amplicon using SYBR Green showed a single amplicon with a mean temperature of c. 89 °C, while for -RT no amplicon was detected (data not shown). We concluded that the reaction product is specific and no amplification efficiency is affected. Quantity was corrected against 18S ribosomal RNA because it is widely referred in literature and there are no reports of in variation in the quantity of this rRNA or in the ribosome number in the hypertensive state (Fig. 4b).
Figure 4.
Reverse transcription and PCR analysis of α1D-adrenoceptor mRNA in AhR−/− mice. (a) PCR amplification of the receptor was visualized in 2% agarose gels as a band of 158 bp. (b) Representative amplification of α1D-adrenoceptor and 18S rRNA in wild type and mutant mice. (c) Relative abundance was normalized vs. 18S ribosomal RNA amplification. Results are the mean ± SEM of three animals. *P < 0.05 AhR−/− vs. WT.
Abundance of the receptor in AhR−/− mice was higher (c. 77.5% ± 12%) than in WT mice (Figs. 4b,c). These results strongly suggest that α1D-adrenoceptor is overexpressed in aorta of null mice.
Determination of α1D-adrenoceptor protein in aorta of AhR−/− mice
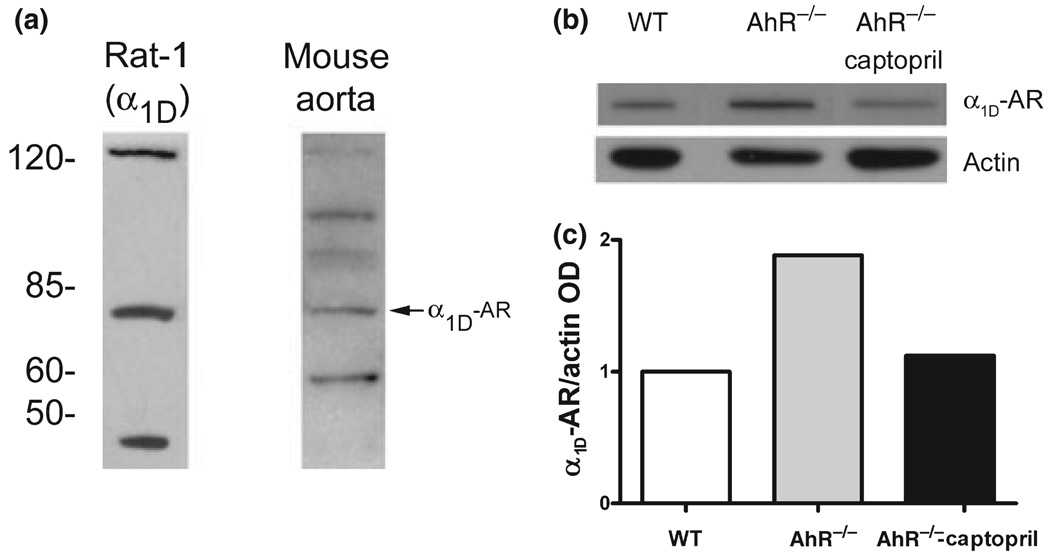
Western blot was conducted with a rabbit policlonal antibody directed against the carboxi termini of rat α1D-adrenoceptor (García-Sáinz et al., 2001). As positive control crude extracts of Rat-1 cells stably expressing rat α1D-adrenoceptor were utilized (kindly donated by Dr J. A. García-Sáinz). The receptor is detectable with an apparent molecular weight of c. 85 kDa.
In mouse aorta homogenates, the specific c. 85 kDa band is present (Fig. 5a). The analysis of the receptor’s relative abundance was carried out in a pool of three aorta from each group, and data corrected relative to actin as a reference protein (Fig. 5b). Aortic homogenates from AhR−/− mice showed c. 88% increase in α1D-adrenoceptors compared with WT (Fig. 5c) while captopril therapy levels to that of WT mice (Fig. 5c).
Figure 5.
Western blot analysis of α1D-adrenoceptor protein in AhR−/− mice. (a) α1D-Adrenoceptor is detected as a band of c. 85 kDa in Rat-1 cells stably expressing the receptor and in mouse aorta. (b) Representative autoradiography of α1D-adrenoceptor and actin in WT, AhR−/− and captopril-treated AhR−/− mice. (c) Relative abundance analysis of α1D-adrenoceptor protein vs. actin in a pool of three mice aorta per group.
Discussion
It is well recognized that the pathogenic actions of Ang II, signalling via AT1 receptors, are involved in diseases such as hypertension. In support of this contention, therapy with angiotensin converting enzyme (ACE) inhibitors and AT1 receptor antagonists have proven to be successful in patients and rats with diabetic nephropathy, heart failure, hypertension and left ventricular hypertrophy (Martínez & Villalobos-Molina, 2003; Ferrario et al., 2004). This suggests that these agents have actions other than just the control of blood pressure. In this regard, it has been reported that the knockout of the AhR gene, involved in regulation of xenobiotic metabolism, generates mice with liver alterations, elevated levels of Ang II, endothelin-1, blood pressure and also develop cardiac hypertrophy (Gonzales & Fernandez-Salguero, 1998; Lund et al., 2003), the latter events decreased towards normal levels after chronic therapy (8–12 weeks) with the ACE inhibitor captopril (Lund et al., 2003). Our results confirm the data reported by Lund et al., in that AhR−/− mice are hypertensive and that captopril therapy decreases blood pressure, but over a shorter time-course (2 weeks). This result means that captopril exerts its antihypertensive action quite rapidly, as expected, and suggests that the results reported by Lund et al. could be observed after several days of therapy. It is known that Ang II induces the expression of α1D-adrenoceptors in rat-derived VSMC (Hu et al., 1995), suggesting that the tissue might become hypersensitive. It is also known that stimulation of α1-adrenoceptors is involved in VSMC hypertrophy (Xin et al., 1997). As both phenomena have been reported to occur in hypertension then, it was important to assess whether aortic arterial diameter in AhR−/− mice was affected by captopril treatment.
It has been shown that chronic therapy with captopril (50 mg kg−1 day−1 per 10 weeks) abolished the increase in resting tone (IRT) response, a measure of α1D-adrenoceptor constitutive activity, in several arteries taken from either Wistar-Kyoto rats (WKY) or SHR rats. It was suggested that IRT was not observed because SHR rats did not become hypertensive (Gisbert et al., 2002). However, recent findings in aorta of pre-hypertensive SHR exhibited elevated basal levels of α1D-adrenoceptor mRNA and protein as compared with normotensive WKY rats, and that therapy with a lower dose of captopril (3 mg kg−1 day−1) over a shorter time (1 week) decreased both expression (mRNA as well as protein) and function of aorta (α1D-adrenoceptor bearing tissue) from pre-hypertensive rats (Godínez-Hernández et al., 2006). These results indicated that the absence of IRT reported by Gisbert et al. occurred because captopril inhibited Ang II production and as a consequence, prevented the increase in α1D-adrenoceptor population, which are putatively involved in the pathology of the SHR (Villalobos-Molina & Ibarra,1999, 2005; Villalobos-Molina et al.,1999; Gisbert et al., 2002; Godínez-Hernández et al., 2006). Our current results agree with the latter suggestion, i.e. the reduction in Ang II levels by captopril is associated with a decrease in α1D-adrenoceptor expression and function. The fact that AhR−/− mouse shows the described phenotype, would allow the study of Ang II-α1D-adrenoceptor cross-talk at the genomic level, an interesting but as yet unknown interrelationship that would support our hypothesis (Villalobos-Molina & Ibarra, 2005). Furthermore, we found similar results in a model where Ang II is continuously infused into rats, increasing blood pressure and contractile responses in isolated aorta (I. A. Gallardo-Ortíz, P. López-Sánchez and R. Villalobos-Molina, unpublished observations). As a corollary, Tsujimoto and coworkers showed that disruption of the α1D-adrenoceptor gene resulted in hypotensive mice and concluded that these receptors are important for blood pressure control (Tanoue et al., 2002). Taken together, the data suggest that Ang II and α1D-adrenoceptors interact in the genesis and/or maintenance of hypertension.
On the other hand, although the mechanism by which knockout of the AhR causes an increase in vasoactive peptides, high blood pressure and cardiac hypertrophy is unknown, it is reasonable to conclude that it is associated with Ang II-dependent hypertension and vascular reactivity to adrenoceptor ligands. Our data suggests that this augmentation of vascular reactivity may be due to elevated α1D-adrenoceptor expression.
Our data support the hypothesis that under conditions where Ang II is elevated, vascular α1D-adrenoceptor expression is upregulated, and suggests that Ang II and vascular α1D-adrenoceptors could interact at the onset of hypertension.
Acknowledgments
Supported in part by grants 47481 from CONACYT and IN224408 from PAPIIT, DGAPA, U. N. A. M.
References
- Arunlakshana O, Schild HO. Some quantitative uses of drug antagonists. Br. J. Pharmacol. 1959;14:48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario C, Abdelhamed AI, Moore M. Angiotensin II antagonists in hypertension, heart failure, and diabetic nephropathy: focus on losartan. Curr. Med. Res. Opin. 2004;20:279–293. doi: 10.1185/030079903125003017. [DOI] [PubMed] [Google Scholar]
- García-Sáinz JA, Villalobos-Molina R. The elusive α1D-adrenergic receptor: molecular and cellular characteristics and integrative roles. Eur. J. Pharmacol. 2004;500:113–120. doi: 10.1016/j.ejphar.2004.07.016. [DOI] [PubMed] [Google Scholar]
- García-Sáinz JA, Vázquez-Cuevas FG, Romero-Avila MT. Phosphorylation and desensitization of α1D-adrenergic receptors. Biochem. J. 2001;353:603–610. doi: 10.1042/0264-6021:3530603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gisbert R, Ziani K, Miquel R, Noguera MA, Ivorra MD, Anselmi E, Docon P. Pathological role of a constitutively active population of α1D-adrenoceptors in arteries of spontaneously hypertensive rats. Br. J. Pharmacol. 2002;135:206–216. doi: 10.1038/sj.bjp.0704447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godínez-Hernández D, Gallardo-Ortíz IA, López-Sánchez P, Villalobos-Molina R. Captopril therapy decreases the expression and function of α1D-adrenoceptors in aorta of pre-hypertensive rats. Auton. Autacoid. Pharmacol. 2006;26:21–29. doi: 10.1111/j.1474-8673.2005.00358.x. [DOI] [PubMed] [Google Scholar]
- Gonzales FJ, Fernandez-Salguero P. The aryl hydrocarbon receptor: studies using the AHR-null mice. Drug Metab. Disp. 1998;26:1194–1198. [PubMed] [Google Scholar]
- Guimaraes S, Moura D. Vascular adrenoceptors: an update. Pharmacol. Rev. 2001;53:319–356. [PubMed] [Google Scholar]
- Henrich WL, Levi M. Ontogeny of renal rennin release in spontaneously hypertensive rat and Wistar–Kyoto rat. Am. J. Physiol. 1991;260:F530–F535. doi: 10.1152/ajprenal.1991.260.4.F530. [DOI] [PubMed] [Google Scholar]
- Hu ZW, Shi XY, Okazaki M, Hoffman BB. Angiotensin II induces transcription and expression of alpha-1 adrenergic receptors in vascular smooth muscle cells. Am. J. Physiol. 1995;268:H1006–H1014. doi: 10.1152/ajpheart.1995.268.3.H1006. [DOI] [PubMed] [Google Scholar]
- Hunyady L, Catt KJ. Pleiotropic AT1 receptor signaling pathways mediating physiological and pathogenic actions of angiotensin II. Mol. Endocrinol. 2006;20:953–970. doi: 10.1210/me.2004-0536. [DOI] [PubMed] [Google Scholar]
- Lázaro-Suárez ML, Gómez-Zamudio JH, Gallardo-ortíz IA, Tanoue A, Tsujimoto G, Farias-Rodríguez VM, Villalobos-Molina R. Chloroethylclonidine reveals α1A-adrenoceptors mediate contraction in aorta of α1D-adrenoceptor knockout mice. Auton. Autacoid. Pharmacol. 2005;25:179–183. doi: 10.1111/j.1474-8673.2005.00348.x. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Lund AK, Goens MB, Kanagy NL, Walker MK. Cardiac hypertrophy in aryl hydrocarbon receptor null mice is correlated with elevated angiotensin II, endothelin-1 and mean arterial blood pressure. Toxicol. Appl. Pharmacol. 2003;193:177–187. doi: 10.1016/j.taap.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Martínez AL, Villalobos-Molina R. Early and chronic captopril or losartan therapy reduces infarct size and avoids congestive heart failure after myocardial infarction in rats. Arch. Med. Res. 2003;34:357–361. doi: 10.1016/S0188-4409(03)00076-6. [DOI] [PubMed] [Google Scholar]
- Ruiz P, Basso N, Cannata MA, Taquini AC. The renin–angiotensin system in different stages of spontaneous hypertension in the rat (SHR) Clin. Exp. Hypertens. A. 1990;12:63–81. doi: 10.3109/10641969009074720. [DOI] [PubMed] [Google Scholar]
- Tanoue A, Nasa Y, Koshimizu T, Shinoura H, Oshikawa S, Kawai T, Sunada S, Takeo S, Tsujimoto G. The α1D-adrenergic receptor directly regulates arterial blood pressure via vasoconstriction. J. Clin. Invest. 2002;109:765–775. doi: 10.1172/JCI14001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalobos-Molina R, Ibarra M. α1-Adrenoceptors that mediate contraction in arteries of normotensive and spontaneously hypertensive rats are of the α1D or α1A subtypes. Eur. J. Pharmacol. 1996;298:257–263. doi: 10.1016/0014-2999(95)00781-4. [DOI] [PubMed] [Google Scholar]
- Villalobos-Molina R, Ibarra M. Vascular α1D-adrenoceptors: are they related to hypertension? Arch. Med. Res. 1999;30:347–352. doi: 10.1016/s0188-0128(99)00047-0. [DOI] [PubMed] [Google Scholar]
- Villalobos-Molina R, Ibarra M. Increased expression and function of vascular α1D-adrenoceptors may mediate the pro-hypertensive effects of angiotensin II. Mol. Interv. 2005;5:340–342. doi: 10.1124/mi.5.6.6. [DOI] [PubMed] [Google Scholar]
- Villalobos-Molina R, López-Guerrero JJ, Ibarra M. Functional evidence of α1D-adrenoceptors in the vasculature of young and adult spontaneously hypertensive rats. Br. J. Pharmacol. 1999;126:1534–1536. doi: 10.1038/sj.bjp.0702468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir MR, Dzau VJ. The renin–angiotensin-aldosterone system: a specific target for hypertension management. Am. J. Hypertens. 1999;12:205S–213S. doi: 10.1016/s0895-7061(99)00103-x. [DOI] [PubMed] [Google Scholar]
- Xin X, Yang N, Eckhart AD, Faber JE. α1D-Adrenergic receptors and mitogen-activated protein kinase mediate increased protein synthesis by arterial smooth muscle. Mol. Pharmacol. 1997;51:764–775. doi: 10.1124/mol.51.5.764. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Koike K. Characterization of alpha1-adrenoceptor-mediated contraction in the mouse thoracic aorta. Eur. J. Pharmacol. 2001;424:131–140. doi: 10.1016/s0014-2999(01)01134-7. [DOI] [PubMed] [Google Scholar]
- Zicha J, Kune J. Ontogenetic aspects of hypertension development: analysis in the rat. Physiol. Rev. 1999;79:1227–1282. doi: 10.1152/physrev.1999.79.4.1227. [DOI] [PubMed] [Google Scholar]