Abstract
Background: This study explored the efficacy and tolerability of sunitinib, an inhibitor of tyrosine kinase receptors, in men with castration-resistant prostate cancer (CRPC).
Methods: Men with no prior chemotherapy (group A) and men with docetaxel (Taxotere)-resistant prostate cancer (group B) were treated with sunitinib. The primary end point was confirmed 50% prostate-specific antigen (PSA) decline. Secondary end points included objective response rate and safety. Serum-soluble biomarkers were measured.
Results: Seventeen men were enrolled in each group. One confirmed PSA response was observed in each group, and an additional eight men and seven men had stable PSA at week 12 in groups A and B, respectively. Improvements in imaging were observed in the absence of post-treatment PSA declines. Common adverse effects included fatigue, nausea, diarrhea, myelosuppression and transaminase elevation. Significant changes following sunitinib treatment were observed in serum-soluble biomarkers including soluble vascular endothelial growth factor receptor-2, platelet-derived growth factor aa, placental growth factor and leptin.
Conclusions: Sunitinib monotherapy resulted in few confirmed 50% post-treatment declines in PSA in men with CRPC. Serum markers of angiogenesis confirmed on-target effects of sunitinib. Assessments of radiographic disease status were often discordant with changes in PSA, indicating that alternate end points are important in future trials.
Keywords: angiogenesis, castration, docetaxel, PSA, resistant
introduction
Metastatic prostate cancer develops in a subset of men diagnosed with prostate cancer and is estimated to cause 27 000 deaths per year in the United States [1]. In men with advanced prostate cancer, androgen deprivation therapy is the mainstay of treatment, but ultimately many will develop castration-resistant prostate cancer (CRPC) and may develop metastatic disease. In men with metastatic CRPC, docetaxel-based chemotherapy improves overall survival (OS) compared with mitoxantrone plus steroids [2, 3]. The median survival in men treated with docetaxel-based therapy is 18 months. In men with metastatic, docetaxel-resistant prostate cancer, median survival is <1 year. There is therefore an urgent need for novel treatments in men with CRPC.
Preclinical and clinical studies indicate that prostate cancer growth is dependent on angiogenesis [4–6]. In men with prostate cancer, serum vascular endothelial growth factor (VEGF) levels are elevated and higher levels are associated with greater mortality [7–9]. Another potentially important pathway in prostate cancer growth is the platelet-derived growth factor (PDGF) pathway. In xenograft models of prostate cancer, inhibition of platelet-derived growth factor receptor (PDGFR) with imatinib reduces disease burden [10]. Both PDGF ligand and receptor are overexpressed in human prostate cancer, and high-level overexpression of PDGFR has been demonstrated in bone metastases from CRPC [11–13].
Sunitinib is a small-molecule inhibitor of vascular endothelial growth factor receptor (VEGFR), PDGFR and other receptor tyrosine kinases and is approved for the treatment of advanced kidney cancer and imatinib-resistant gastrointestinal stromal cancer. Dual inhibition of the VEGF and PDGF pathways may be an important therapeutic strategy in advanced prostate cancer and may be accomplished with a multitargeted inhibitor such as sunitinib. We therefore assessed the effects of sunitinib in men with CRPC.
methods
study design
We conducted an open-label phase II trial of sunitinib in men with CRPC. Two cohorts of men were studied: those who received no prior chemotherapy (group A) and those with docetaxel (Taxotere: Sanofi-Aventis, Bridgewater, NJ)-resistant disease (group B). The primary end point of the trial was prostate-specific antigen (PSA) response rate, defined as confirmed ≥50% decline in PSA from baseline [14], and secondary end points included objective response rate, safety and tolerability and changes in serum biomarkers. All men were treated with sunitinib in 6-week cycles consisting of 50 mg daily for 4 weeks followed by 2 weeks off. Dose reductions were allowed to 37.5 or 25 mg. Treatment continued until intolerance to therapy or disease progression, defined as presence of new metastasis or PSA increase of ≥25% from nadir. Subjects were permitted to withdraw consent at any time.
study population
Eligible men had histologically confirmed adenocarcinoma of the prostate and evidence of progressive CRPC. Progression was defined as rising PSA in two consecutive measurements at least 1 week apart, with a minimum increment of at least 2 ng/ml above the nadir. All men had ongoing chemical castration during the study. Concurrent treatment with bisphosphonates was allowed. Previous treatment with radiation or radionuclide therapy must have ended 6 weeks before entry. Adequate renal, hepatic and bone marrow function was required. Exclusion criteria included a second active malignancy, active cardiac disease, cerebrovascular accident or pulmonary embolism within 6 months, human immunodeficiency virus infection, grade 3 hemorrhage within 4 weeks, ongoing cardiac dysrhythmia of grade ≥2 or QTc prolongation >450 ms.
Men in group A were not permitted to have received prior cytotoxic chemotherapy for prostate cancer. Men in group B had radiographic evidence of metastatic prostate cancer and must have received one prior docetaxel-based chemotherapeutic regimen. No other prior chemotherapy was permitted. Evidence of disease progression was required during or within 60 days following docetaxel chemotherapy.
The study was approved by the Harvard Cancer Center Institutional Review Board and all subjects provided written informed consent.
study assessments
Serum PSA was measured at baseline and on day 1 of each cycle. Physical examination was carried out on day 1 of each cycle, and ECOG performance status and vital signs were assessed on days 1 and 28 of each cycle. Adverse events were reviewed on days 1, 15 and 28 of each cycle. Routine laboratory studies were measured at baseline, days 1 and 28 of each cycle and day 15 of cycle 1. Serum for biomarker analysis was collected at baseline and on days 1 and 28 of cycles 1 and 2.
Radiographic assessments were done at baseline, every 12 weeks and at study end or subject withdrawal, and responses were assessed using RECIST. Landmark analysis was carried out at week 12 to conform to initial reassessment guidelines as recommended by the Prostate Cancer Clinical Trials Working Group [15].
Subjects were followed for survival status every 12 weeks after treatment discontinuation.
statistical methods
A Simon two-stage design was employed for each group separately, to differentiate a hypothesized 25% response rate from a null rate of 5%, with a 10% probability of rejecting a promising treatment (β) and a 5% probability of accepting an uninteresting treatment (α). The primary end point was confirmed 50% decline in PSA, according to PSA Working Group criteria [14]. Seventeen men per group were enrolled in the first stage, and a minimum of two responses were required to open the second stage to a total accrual of 30 men in each group. The treatment would be considered promising if four or more men in a group achieved the primary end point. The probability of early termination if the null hypothesis were truly correct was 83%.
Changes in serum bone markers and angiogenic markers from day 1 to 28 were assessed using the Wilcoxon signed rank tests. Changes were compared between men who exhibited some PSA decline versus those who had PSA progression at week 12 using Wilcoxon rank sum tests.
biomarker studies
Determination of PSA, alkaline phosphatase and other routine laboratories were measured in the clinical laboratories of the participating hospitals. Levels of soluble vascular endothelial growth factor receptor-2 (sVEGFR2), leptin and placental growth factor (PLGF) (R&D Systems, Minneapolis, MN) and bone-specific alkaline phosphatase (BSAP) and N-telopeptide (NTx) (Quidel, San Diego, CA) were measured using immunoassay-based kits with colorimetric detection. Serum PDGFaa was measured by multiplex assay, using the Upstate Beadlyte human growth factor 2-plex assay (Millipore, Billerica, MA).
results
Thirty-six subjects were enrolled from March to December 2006. Two of the subjects did not receive study therapy and were not assessable for efficacy or safety. The baseline characteristics of the remaining 34 men are shown in Table 1. Group A consisted of men with CRPC who had not received systemic chemotherapy. Group B consisted of men with metastatic, docetaxel-resistant prostate cancer. Six of 17 men in group A and 11 of 17 men in group B were treated concurrently with zoledronic acid.
Table 1.
Baseline patient characteristics
| Group A (no prior chemotherapy) | Group B (docetaxel resistant) | |
| Number | 17 | 17 |
| Age (years) | ||
| Median | 71 | 65 |
| Range | 52–80 | 45–84 |
| ECOG performance status | ||
| 0 | 12 | 7 |
| 1 | 5 | 9 |
| 2 | – | 1 |
| Sites of disease | ||
| Bone metastasis | 12 | 15 |
| PSA-only disease | 1 | 0 |
| PSA (ng/ml) | ||
| Median | 51 | 44 |
| Range | 7–602 | 8–752 |
| Alkaline phosphatase (U/l) | ||
| Median | 99 | 126 |
| Range | 46–991 | 69–495 |
| Hemoglobin (g/dl) | ||
| Median | 13.2 | 12.5 |
| Range | 10.7–14.9 | 8.3–14.1 |
| Prior hormone therapies | ||
| 1–3 | 11 | 12 |
| 4–6 | 6 | 4 |
| Prior cycles of chemotherapy | ||
| Median | 0 | 8 |
| Range | 3–14 | |
| Prior radiation therapy | 8 | 10 |
| Bisphosphonate use | 6 | 11 |
ECOG, Eastern Cooperative Oncology Group; PSA, prostate-specific antigen.
post-treatment PSA changes
The primary end point of the trial was confirmed ≥50% decline in PSA from baseline. Only one subject in group A and one subject in group B exhibited confirmed 50% decline in PSA. Since we did not observe at least two responses in either group, enrollment did not continue to the second stage of accrual.
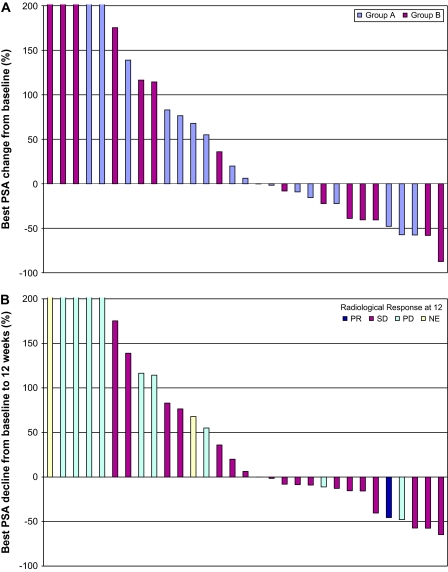
The best PSA response (maximum decline in PSA without requirement of confirmation) for men in both groups is shown in Figure 1A. Of the 34 assessable men in the study, 14 (41%) had some degree of PSA decline and 8 of 34 (24%) had a decline of ≥30%. One subject in group A exhibited >50% decline at 12 weeks, and another eight men had stable PSA at 12 weeks (Table 2). Similarly, in group B, there was one >50% decline and another seven men had stable PSA at 12 weeks. The median duration of treatment was two cycles, with a range from 1 to 15 cycles.
Figure 1.
(A) Best PSA response. Each bar reflects the best percent change in PSA from baseline in an individual subject. The bars are colored according to patient cohort. The y-axis is truncated at 200%; the maximum value was +537%. Three men were not assessable for PSA changes. (B) PSA and radiological response at 12 weeks. Each bar reflects change in PSA from baseline to 12 weeks. The bars are colored according to radiological response at 12 weeks. The y-axis is truncated at 200%; the maximum value is +537%. Three patients were not assessable for PSA changes. PSA, prostate-specific antigen; PR, partial response; SD, stable disease; PD, progressive disease; NE, not assessable.
Table 2.
Response at 12 weeks
| Group A (no prior chemotherapy) | Group B (docetaxel resistant) | |
| No. of men | 17 | 17 |
| PSA | ||
| Response | 1 | 1 |
| Stable | 8 | 7 |
| Progression | 7 | 7 |
| Not evaluable | 1 | 2 |
| Radiological | ||
| Partial response | 0 | 1 |
| Stable | 10 | 8 |
| Progression | 5 | 5 |
| Not evaluable | 1 | 3 |
| Not applicable | 1 | 0 |
PSA: response, confirmed ≥50% decline from baseline; progression, ≥25% increase from baseline or nadir; stable, neither response nor progression. Radiological: partial response, confirmed ≥30% decline from baseline; progression, ≥20% increase from baseline or new metastasis; stable, neither response nor progression.
PSA, prostate-specific antigen.
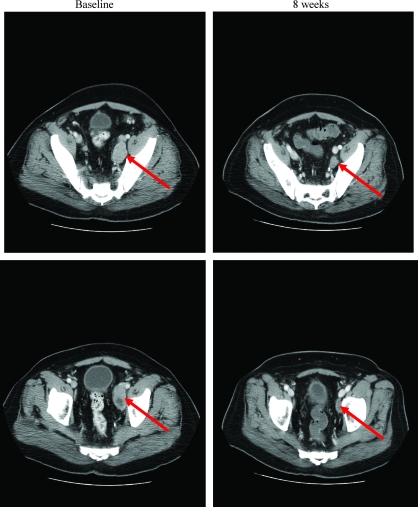
An analysis at 12 weeks demonstrated that changes in PSA did not accurately reflect radiographic changes (Figure 1B). Notably, some men with RECIST-defined stable disease (SD) who had improvement by bone scan or abdominal computed tomography scan nevertheless demonstrated continued rises in their serum PSA values. Figure 2 demonstrates radiographic improvement in lymphadenopathy at 8 weeks, in a subject from group A who did not manifest any PSA decline. The subject in group B with a RECIST-defined partial response had a 46% decline in PSA at 12 weeks and did not have a confirmed 50% PSA decline.
Figure 2.
Radiographic changes from baseline to 8 weeks in a subject in group A.
safety
The most common symptomatic adverse effects ascribed to sunitinib were fatigue, nausea, anorexia, taste disturbance, vomiting, diarrhea and skin rash (Table 3). Most adverse effects were grade 1 or 2 and did not necessitate dose modification. Laboratory abnormalities included frequent cytopenias (leukopenia in 68% of men, anemia in 56% of men and thrombocytopenia in 56% of men), as well as transaminitis in 53% of men. Grade 3/4 adverse events requiring dose modification included hypertension, diarrhea, fatigue and single instances of rectal bleeding, sensory neuropathy, seizure, headache and pulmonary embolus (Table 4). Grade 3 leukopenia and neutropenia were seen in five and six men, respectively, and grade 3/4 anemia and thrombocytopenia were observed as well. Overall, the incidence of myelosuppression appeared to be slightly greater than has been described in other malignancies.
Table 3.
Most common adverse events
| Toxicity | Maximum grade |
|||
| 1 | 2 | 3 | 4 | |
| Adverse events | ||||
| Fatigue | 7 | 19 | 3 | – |
| Hand–foot/skin rash | 7 | 6 | – | – |
| Anorexia | 13 | 5 | – | – |
| Diarrhea | 8 | 6 | 4 | – |
| Nausea | 16 | 9 | – | – |
| Taste disturbance | 10 | 7 | – | – |
| Constipation | 7 | 3 | – | – |
| Vomiting | 9 | 6 | – | – |
| Laboratory abnormalities | ||||
| Hemoglobin | 12 | 4 | 2 | 1 |
| Leukocytes | 12 | 5 | 6 | – |
| Neutrophils | 8 | 6 | 5 | – |
| Platelets | 12 | 3 | 3 | 1 |
| Alkaline phosphatase | 10 | 6 | 5 | – |
| ALT elevation | 11 | – | 1 | – |
| AST elevation | 15 | 3 | 1 | – |
| Hyperglycemia | 17 | 1 | 1 | – |
ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Table 4.
Grade 3/4 adverse events
| Toxicity | Maximum grade |
|
| 3 | 4 | |
| Laboratory abnormalities | ||
| Hemoglobin | 2 | 1 |
| Leukocytes | 6 | – |
| Neutrophils | 5 | – |
| Platelets | 3 | 1 |
| ALT/AST elevation | 1 | – |
| Creatinine | 1 | – |
| Hyponatremia | – | 1 |
| Other adverse events | ||
| Hypertension | 5 | – |
| Left ventricular systolic dysfunction | 1 | – |
| Fatigue | 3 | – |
| Diarrhea without prior colostomy | 4 | – |
| Lower GI, hemorrhage NOS | 1 | – |
| Neuropathy-sensory | 1 | – |
| Seizure | 1 | – |
| Headache | 1 | – |
| Thrombosis/embolism | 1 | – |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; GI, gastrointestinal; NOS, not otherwise specified.
biochemical markers of bone turnover
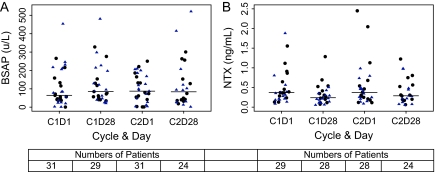
Serum biochemical markers of bone formation (BSAP) and bone resorption (NTx) were measured at the beginning and end of cycles 1 and 2 of sunitinib therapy. BSAP increased during cycle 1 of treatment from a median level of 64 U/l on day 1 to 85 U/l on day 28 and remained stable during cycle 2 (median of 87–84 U/l from days 1 to 28; Figure 3A). In contrast, serum NTx decreased during cycles 1 and 2, from a median level of 0.37 to 0.24 ng/ml in cycle 1 and 0.37 to 0.29 ng/ml in cycle 2 (Figure 3B). There appeared to be no difference in BSAP or NTx effects between men treated or not treated concurrently with zoledronic acid.
Figure 3.
Bone markers over time for cohorts combined: (A) bone-specific alkaline phosphatase (BSAP) (U/l); (B) N-telopeptide (NTx) (ng/ml). Median values are denoted by horizontal bars. Black circles are patients not on zoledronic acid; blue triangles are patients who were on zoledronic acid. C1D1 levels were not statistically different according to zoledronic use (BSAP, P = 0.72; NTx, P = 0.17).
serum angiogenic biomarkers
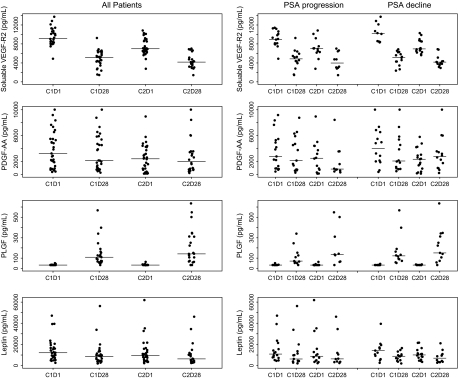
Sunitinib acts primarily through blockade of the VEGFRs and PDGFRs. We assessed target inhibition in our subjects by serial measurement of serum biomarkers. Figure 4 shows the effect of sunitinib therapy on sVEGFR2, PDGFaa, PLGF and leptin. Treatment with sunitinib had a highly reproducible effect on lowering sVEGFR2 levels from day 1 to day 28 of treatment, from a median of 9168 to 5119 pg/ml during cycle 1 and from 6990 to 4174 pg/ml during cycle 2 (P < 0.0001). Levels of PDGFaa also declined during cycle 1 (P = 0.012) and remained at reduced levels during cycle 2. There were statistically significant declines in leptin levels from day 1 to day 28 of each cycle as well, from a median of 12123 to 8596 pg/ml during cycle 1 and from 9598 to 6271 pg/ml during cycle 2 (P < 0.0001 and P = 0.05, respectively). There was no significant correlation between reductions in sVEGFR2, PDGFaa or leptin and changes in PSA.
Figure 4.
Serum angiogenic markers over time for both cohorts combined and according to prostate-specific antigen (PSA) response at 12 weeks. Serum levels of angiogenic markers were measured at C1D1 (cycle 1 day 1), C1D28, C2D1 and C2D28. Median values are denoted by horizontal bars. Each point represents an individual patient value. Statistically significant changes were observed in soluble vascular endothelial growth factor-2 (sVEGFR2), platelet-derived growth factor aa (PDGFaa), placental growth factor (PLGF) and leptin. Groups A and B were combined in this analysis.
Consistent with blockade of proangiogenic receptors, the serum levels of PLGF increased significantly during cycles 1 and 2, likely reflecting a compensatory attempt by cells to overcome angiogenic blockade. Levels at day 1 of each cycle were below the assay detection limit (<33.18 pg/ml) in most men and rose to a median level of 110 pg/ml after cycle 1 and 143 pg/ml after cycle 2 (each P < 0.0001). No correlation was observed between elevation of PLGF and changes in PSA.
discussion
In this phase II study of men with CRPC, treatment with sunitinib resulted in similar effects in two groups, men with or without prior docetaxel-based chemotherapy. Confirmed PSA declines of ≥50% were rare, but evidence of improvement in radiographic disease was observed in men who did not have confirmed PSA declines. Since the primary end point of the study was not met, the second stage of accrual was not initiated in either group. These results indicate that changes in PSA do not correspond with other measures of benefit and are therefore of uncertain value as a surrogate for the activity of sunitinib therapy in CPRC.
Our data support the contention that benefit may be seen from sunitinib in the absence of confirmed 50% PSA declines. A number of men had evidence of clinical or radiographic improvement, despite rising PSA. In group B, three men had evidence of improvement on bone scan, though with SD by RECIST, in the context of rising PSA values. Though not formally measured, we also observed improvement in symptoms, reflected by decreasing use of opiate analgesics, in a number of men whose PSA levels nevertheless continued to rise. These findings have important implications for future study of antiangiogenic agents in prostate cancer. We believe that in the absence of symptomatic or radiographic progression, men receiving antiangiogenic therapy should not be discontinued from treatment on the basis of PSA increase alone. Further, these results indicate that study outcomes should be evaluated using end points other than PSA response or progression.
Another phase I/II trial in prostate cancer is studying combination therapy with sunitinib, docetaxel and prednisone in men with metastatic CRPC [16]. Preliminary results demonstrated that 9 of 18 men exhibited PSA responses of ≥50% and 5 of 13 manifested partial radiographic responses. Preliminary results of a study similar to the current trial have been presented [17]. Thirty-six men with docetaxel-resistant metastatic prostate cancer were treated with sunitinib and evaluated for progression-free survival (PFS). While investigators found a 12-week PFS of 79%, only three men exhibited PSA decline of ≥50%. These data again indicate that PSA may not be an accurate gage of dynamic response to therapy.
Continuous dosing of sunitinib would likely provide constant VEGFR blockade, though whether this would increase its clinical effectiveness compared with intermittent dosing is unknown. In a phase II trial combining sunitinib with ablative hormone therapy in men with high-risk localized prostate cancer, sunitinib 37.5 mg daily was administered continuously for 3 months before prostatectomy and was well tolerated [18].
In the preliminary report by Periman et al., the most common adverse effects were myelosuppression, fatigue, diarrhea, mucositis and anorexia. Our findings are similar, with the exception that we observed more grade 3/4 hematologic toxicity. This may reflect a more heavily pretreated population, higher incidence of prior radiation therapy or differences in usage of growth factor support. In general, men with advanced prostate cancer may have a higher risk of myelosuppression than patients with other cancers, due to advanced age, prior irradiation and extensive bone and bone marrow involvement.
Numerous preclinical and clinical studies have demonstrated that growth of prostate cancer may be dependent on angiogenesis [4–6]. Serum VEGF levels are elevated in men with prostate cancer, and higher levels may be associated with worse outcome [7–9]. Other angiogenesis inhibitors have been studied in advanced prostate cancer. Bevacizumab, a mAb against VEGF, is currently being tested in a phase III trial in men with metastatic CRPC [19]. Thalidomide and lenalidomide have shown evidence of modest antitumor activity in prostate cancer [20, 21]. Several phase II studies have assessed the activity of sorafenib, an inhibitor of VEGFR, in advanced prostate cancer, with indication of some benefit by clinical but not PSA criteria [22–24]. In one study, a substantial number of men manifested PSA decline after stopping sorafenib, indicating a possible effect on PSA production or secretion [22]. This hypothesis requires validation on a larger scale.
Analysis of biomarkers showed a predictable effect of sunitinib on serum levels of sVEGFR2, PDGFaa and PLGF. In prior studies in renal cell carcinoma, treatment with sunitinib resulted in increased serum levels of VEGF and PLGF and decreased levels of sVEGFR2 and sVEGFR3 [25–27]. Our findings are similar and demonstrate that at the dose and schedule utilized in this study, sunitinib had the expected antiangiogenic impact. The patterns of change in sVEGFR2 and PLGF were characteristic of sunitinib given with the current schedule; levels of sVEGFR2 decreased and PLGF increased during the period of sunitinib administration, indicating effective blockade of the VEGFR, and returned toward baseline during the period off drug, indicating relief of VEGFR blockade.
Leptin has been shown to act on vascular endothelium to promote angiogenesis and increase vascular permeability and may exhibit synergistic effects with VEGF [28, 29]. Elevated levels of leptin have been associated with development of prostate cancer although the significance of variation in levels among men with prostate cancer has not been studied [30]. We found that the serum levels of leptin decreased significantly during the first cycle of sunitinib administration, rose slightly during the period off drug and then decreased significantly again during the second cycle of sunitinib. Changes in sVEGFR2, PDGFaa, PLGF and leptin, however, were not associated with post-treatment changes in PSA. Additional studies are needed to assess the relationship between changes in biomarkers and clinical outcomes including PFS and OS.
The results of our study indicate that sunitinib decreases osteoclast activity. Serum NTx, a biomarker of osteoclast activity, significantly decreased after treatment with sunitinib. Similar decreases in serum NTx were observed in men who were receiving concurrent zoledronic acid and those who were not receiving bisphosphonate therapy. This observation indicates that the mechanism of osteoclast inhibition by sunitinib is distinct from that of zoledronic acid. There was no notable impact of sunitinib on serum BSAP, a biomarker of osteoblast activity.
A chief limitation of our study was the choice of confirmed 50% decline in PSA as the primary end point. Additionally, because of the PSA end point, the study did not proceed to the second stage, limiting sample size. Since changes in PSA did not correspond well with other measures of benefit such as radiographic changes, the study is limited in its ability to define the efficacy of sunitinib in CRPC. The trial was not powered to compare the two groups of men and was not powered to assess PFS or OS.
In conclusion, this phase II study of sunitinib monotherapy in two groups of men with advanced prostate cancer showed a minimal impact on serum PSA, but nevertheless indicated that sunitinib is well tolerated and may have modest benefit in this patient population. Additional trials of this agent in metastatic prostate cancer will require alternative end points. Based on results from our trial and others, a phase III trial of prednisone in combination with sunitinib or placebo has been launched in men with metastatic prostate cancer following treatment with docetaxel (www.clinicaltrials.gov, NCT00676650). The primary end point of the phase III trial is OS, and results of that study will ultimately determine whether sunitinib has an important role in the therapeutic management of advanced prostate cancer.
funding
Department of Defense Prostate Cancer Research Program Clinical Trial Award number (PC-050010) to MDM; NIH K24 Midcareer Investigator Award (5K24CA121990-02) and Prostate Cancer Foundation to MRS.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 3.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 4.Singh RP, Sharma G, Mallikarjuna GU, et al. In vivo suppression of hormone-refractory prostate cancer growth by inositol hexaphosphate: induction of insulin-like growth factor binding protein-3 and inhibition of vascular endothelial growth factor. Clin Cancer Res. 2004;10:244–250. doi: 10.1158/1078-0432.ccr-1080-3. [DOI] [PubMed] [Google Scholar]
- 5.Takei Y, Kadomatsu K, Yuzawa Y, et al. A small interfering RNA targeting vascular endothelial growth factor as cancer therapeutics. Cancer Res. 2004;64:3365–3370. doi: 10.1158/0008-5472.CAN-03-2682. [DOI] [PubMed] [Google Scholar]
- 6.Fox WD, Higgins B, Maiese KM, et al. Antibody to vascular endothelial growth factor slows growth of an androgen-independent xenograft model of prostate cancer. Clin Cancer Res. 2002;8:3226–3231. [PubMed] [Google Scholar]
- 7.Kohli M, Kaushal V, Spencer HJ, Mehta P. Prospective study of circulating angiogenic markers in prostate-specific antigen (PSA)-stable and PSA-progressive hormone-sensitive advanced prostate cancer. Urology. 2003;61:765–769. doi: 10.1016/s0090-4295(02)02424-x. [DOI] [PubMed] [Google Scholar]
- 8.Shariat SF, Anwuri VA, Lamb DJ, et al. Association of preoperative plasma levels of vascular endothelial growth factor and soluble vascular cell adhesion molecule-1 with lymph node status and biochemical progression after radical prostatectomy. J Clin Oncol. 2004;22:1655–1663. doi: 10.1200/JCO.2004.09.142. [DOI] [PubMed] [Google Scholar]
- 9.George DJ, Regan MM, Oh WK, et al. Radical prostatectomy lowers plasma vascular endothelial growth factor levels in patients with prostate cancer. Urology. 2004;63:327–332. doi: 10.1016/j.urology.2003.09.059. [DOI] [PubMed] [Google Scholar]
- 10.Uehara H, Kim SJ, Karashima T, et al. Effects of blocking platelet-derived growth factor-receptor signaling in a mouse model of experimental prostate cancer bone metastases. J Natl Cancer Inst. 2003;95:458–470. doi: 10.1093/jnci/95.6.458. [DOI] [PubMed] [Google Scholar]
- 11.Fudge K, Bostwick DG, Stearns ME. Platelet-derived growth factor A and B chains and the alpha and beta receptors in prostatic intraepithelial neoplasia. Prostate. 1996;29:282–286. doi: 10.1002/(SICI)1097-0045(199611)29:5<282::AID-PROS2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 12.Fudge K, Wang CY, Stearns ME. Immunohistochemistry analysis of platelet-derived growth factor A and B chains and platelet-derived growth factor alpha and beta receptor expression in benign prostatic hyperplasias and Gleason-graded human prostate adenocarcinomas. Mod Pathol. 1994;7:549–554. [PubMed] [Google Scholar]
- 13.Chott A, Sun Z, Morganstern D, et al. Tyrosine kinases expressed in vivo by human prostate cancer bone marrow metastases and loss of the type 1 insulin-like growth factor receptor. Am J Pathol. 1999;155:1271–1279. doi: 10.1016/S0002-9440(10)65229-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bubley GJ, Carducci M, Dahut W, et al. Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 1999;17:3461–3467. doi: 10.1200/JCO.1999.17.11.3461. [DOI] [PubMed] [Google Scholar]
- 15.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.George DJ, Liu G, Wilding G, et al. Sunitinib in combination with docetaxel and prednisone in patients with metastatic hormone-refractory prostate cancer—preliminary results. J Clin Oncol. 2008;26(Suppl):5131a. [Google Scholar]
- 17.Periman P, Sonpavde G, Bernold D, et al. Sunitinib malate for metastatic castration resistant prostate cancer following docetaxel-based chemotherapy: a US oncology phase II trial. J Clin Oncol. 2008;26(Suppl):5157a. doi: 10.1093/annonc/mdp323. [DOI] [PubMed] [Google Scholar]
- 18.Zurita A, Ward J, Araujo JP, et al. Presurgical sunitinib malate and androgen ablation in patients with localized prostate cancer at high risk for recurrence. J Clin Oncol. 2008;26(Suppl):16004a. [Google Scholar]
- 19.Di Lorenzo G, Figg WD, Fossa SD, et al. Combination of bevacizumab and docetaxel in docetaxel-pretreated hormone-refractory prostate cancer: a phase 2 study. Eur Urol. 2008;54:1089–1094. doi: 10.1016/j.eururo.2008.01.082. [DOI] [PubMed] [Google Scholar]
- 20.Aragon-Ching JB, Dahut WL. The role of angiogenesis inhibitors in prostate cancer. Cancer J. 2008;14:20–25. doi: 10.1097/PPO.0b013e318161c014. [DOI] [PubMed] [Google Scholar]
- 21.Zhu D, Corral LG, Fleming YW, Stein B. Immunomodulatory drugs Revlimid((R)) (lenalidomide) and CC-4047 induce apoptosis of both hematological and solid tumor cells through NK cell activation. Cancer Immunol Immunother. 2008;57:1849–1859. doi: 10.1007/s00262-008-0512-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chi KN, Ellard SL, Hotte SJ, et al. A phase II study of sorafenib in patients with chemo-naive castration-resistant prostate cancer. Ann Oncol. 2008;19:746–751. doi: 10.1093/annonc/mdm554. [DOI] [PubMed] [Google Scholar]
- 23.Dahut WL, Scripture C, Posadas E, et al. A phase II clinical trial of sorafenib in androgen-independent prostate cancer. Clin Cancer Res. 2008;14:209–214. doi: 10.1158/1078-0432.CCR-07-1355. [DOI] [PubMed] [Google Scholar]
- 24.Steinbild S, Mross K, Frost A, et al. A clinical phase II study with sorafenib in patients with progressive hormone-refractory prostate cancer: a study of the CESAR Central European Society for Anticancer Drug Research-EWIV. Br J Cancer. 2007;97:1480–1485. doi: 10.1038/sj.bjc.6604064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Motzer RJ, Michaelson MD, Redman BG, et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24:16–24. doi: 10.1200/JCO.2005.02.2574. [DOI] [PubMed] [Google Scholar]
- 26.Deprimo SE, Bello CL, Smeraglia J, et al. Circulating protein biomarkers of pharmacodynamic activity of sunitinib in patients with metastatic renal cell carcinoma: modulation of VEGF and VEGF-related proteins. J Transl Med. 2007;5:32. doi: 10.1186/1479-5876-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rini BI, Michaelson MD, Rosenberg JE, et al. Antitumor activity and biomarker analysis of sunitinib in patients with bevacizumab-refractory metastatic renal cell carcinoma. J Clin Oncol. 2008;26:3743–3748. doi: 10.1200/JCO.2007.15.5416. [DOI] [PubMed] [Google Scholar]
- 28.Cao R, Brakenhielm E, Wahlestedt C, et al. Leptin induces vascular permeability and synergistically stimulates angiogenesis with FGF-2 and VEGF. Proc Natl Acad Sci U S A. 2001;98:6390–6395. doi: 10.1073/pnas.101564798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sierra-Honigmann MR, Nath AK, Murakami C, et al. Biological action of leptin as an angiogenic factor. Science. 1998;281:1683–1686. doi: 10.1126/science.281.5383.1683. [DOI] [PubMed] [Google Scholar]
- 30.Stattin P, Soderberg S, Hallmans G, et al. Leptin is associated with increased prostate cancer risk: a nested case-referent study. J Clin Endocrinol Metab. 2001;86:1341–1345. doi: 10.1210/jcem.86.3.7328. [DOI] [PubMed] [Google Scholar]