Abstract
Although it has been suggested that individuals with an Autism spectrum disorder (ASD) process faces less holistically than typically developing controls, there are few direct investigations of this hypothesis. This question was addressed before using the composite paradigm (Teunisse & de Gelder, 2003). The results had revealed that adolescents with ASDs were less sensitive than controls to the misalignment of face parts and it was concluded their face processing was less holistic. However, because of shortcomings of the design, it was not possible to distinguish whether individuals with Autism processed both aligned and misaligned composites in a part-based fashion, or both in a holistic fashion. We compared adolescents with ASDs to controls matched on sex, age and IQ on a more complete version of the composite paradigm. The results indicate that individuals with ASDs, like controls, experience interference from facial features that they are told to ignore. However, while such interference is released for controls if parts of face composites are misaligned, individuals with ASDs show comparable interference from irrelevant parts regardless of alignment. Two different interpretations are discussed, both compatible with the idea that perceptual and or attentional abnormalities in ASDs result in a diminished level of expertise for faces.
Introduction
Individuals with Autism Spectrum Disorders (ASD) suffer from multifaceted impairments including deficits in language and communication skills, repetitive behaviors and restricted interests and difficulties with social interactions. Both fMRI and ERP studies suggest abnormal processing of faces in the brain of people with ASDs relative to typically developing controls (Grice et al., 2001; Pierce et al., 2001; Schultz et al., 2000; Webb & Aggarwal, 1981). Behavioral evidence also suggests that faces are processed by individuals with ASDs using an abnormal strategy (Klin et al., 2002; Langdell, 1978; Rutherford et al., 2007). Yet studies on this issue paint a somewhat confusing picture from which it is difficult to extract the exact nature of the behavioral deficit.
One proposal has been that individuals with ASDs process faces in a more part-based fashion than typically developing controls. The idea that faces are perceived more holistically than other objects is a theme that pervades the literature on typical adult face processing (Diamond & Carey, 1986; Farah et al., 1998; Tanaka & Farah, 1993). There are several definitions of holistic processing in the literature but they commonly refer to the facilitatory effect that the context of a whole upright face has on judgments about its parts and their relations (Farah, Wilson, Drain & Tanaka, 1998). Holistic processing was not directly measured in ASD until a study by Joseph and Tanaka (2003). Using a task called the whole-part paradigm (Tanaka & Farah, 1993), these authors reported that children with ASDs processed faces holistically only when recognition depended on the mouth. That is, controls always recognized face parts better in the context of the whole faces in which the parts had been studied (a whole-part advantage) and also recognized whole faces better upright than inverted (an inversion effect). In contrast, children with Autism showed both whole-part and inversion effects when recognition depended on the mouth, whereas these effects were not obtained when recognition depended on the eyes, in which case their performance was very poor. These findings are generally consistent with prior studies that found a reduced, non-existent, or even reversed face inversion effect (Hobson et al., 1988; Langdell, 1978; Tantam et al., 1989) as well as abnormally high attention to the mouth area relative to the eyes in individuals with ASDs (Klin et al., 2002). A recent study measuring the ability to detect small displacements of the eyes and mouth found that while a subgroup of young adults with ASDs (those with lower verbal IQ) showed poor performance with eyes, no deficit or advantage was observed in ASD in the detection of mouth changes (Rutherford, Clements & Sekuler, 2007). Another study argued for a non-specific configural deficit in ASD and reported a correlation between performance on configural tasks with non-face objects and performance on face tasks (Behrmann et al., 2006). However, that study did not assess configural or holistic processing with faces.
At the same time, other studies have failed to replicate some of these effects. One study suggested that once floor effects are controlled for, a normal face inversion effect is obtained in individuals with ASDs (Teunisse & de Gelder, 2003, see also Lahaie et al., 2006). Another study (Lopez et al., 2004) measured the whole-part advantage under two different conditions: without cueing the participants as to the part relevant for recognition (as was done originally by Joseph and Tanaka, 2003) or cueing them to attend to the relevant part. While cueing had little effect on controls, it affected the performance of adolescents with ASDs: uncued trials led to abnormal performance (no whole advantage) while cued trials led to a normal whole-part advantage. Besides suggesting a role for attentional mechanisms in face processing deficits in ASDs, Lopez and colleagues also failed to replicate Joseph and Tanaka’s finding that mouth processing is normal in ASD when parts are not cued. Whether participants need to distribute their attention to all face parts to solve a given task may be an important factor in interpreting the results of other studies, but that factor alone may not explain all the results.
One problem concerning the partly discrepant results of Joseph and Tanaka (2003) and Lopez et al. (2004) resides in the use of the whole-part paradigm. First, it has been suggested that this task does not measure an effect unique to face perception, despite what was originally reported by Tanaka and Farah (1993). For instance, a whole-part advantage for non-face objects has been observed (Gauthier & Tarr, 1997, 2002; Tanaka et al., 1996). Second, in expertise training paradigms where other hallmarks of configural and holistic processing can be obtained for non-face objects following extensive practice at individuating objects, the whole-part advantage is comparable in novice and expert participants even when other measures of holistic processing increase with perceptual expertise (Gauthier & Tarr, 1997, 2002; Gauthier et al., 1998). Moreover, Leder & Carbon (2005) have shown that the whole-part advantage depends on participants initially studying whole faces: when parts are studied instead, part recognition is superior to whole recognition, consistent with the general predictions of the encoding specificity principle (Tulving & Thomson, 1973). As a whole, this suggests that the whole-part advantage may be at best a problematic index of holistic processing and may greatly depend on domain-general principles of memory.
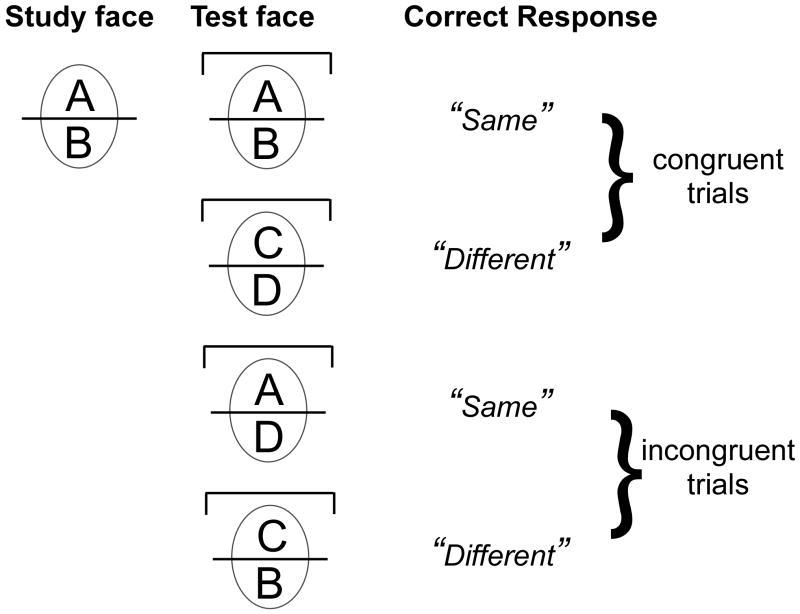
In contrast, the composite paradigm is a different task that offers a better measure of the holistic processing strategy that is specific to faces (Young et al., 1987). In the matching version of the composite paradigm, face halves are combined to create new composite faces, and participants are asked to selectively attend to only the top or bottom half of a study face, which they are asked to match to the equivalent part in a test composite. Specifically, in the design used in this work, congruent trials refer to trials where the relevant (attended) and irrelevant parts are both the same, or both different, between study and test. In contrast, on incongruent trials, the relationship between the study and test irrelevant is opposite to the relationship for the relevant part. For instance, if the relevant parts are identical, then the irrelevant parts are different from each other (see Figure 1). In this task, holistic processing is characterized as a failure of selective attention measured by a congruency effect: performance that is better on congruent than incongruent trials reveals that participants have difficulty ignoring the irrelevant part (Cheung et al., in press; Farah et al., 1998; Richler et al., 2008; in press-b). Typically developing adults process upright faces holistically according to this measure (Boutet et al., 2002; Cheung et al., in press; Gauthier et al., 2003; Goffaux & Rossion, 2006; Hole, 1994; Richler et al., 2008; in press-b; Young et al., 1987). Holistic processing is reduced when the two parts of the faces are inverted (Hole, 1994) or misaligned (Cheung et al., in press; Richler et al., 2008; Young et al., 1987). This alignment effect suggests that when the meaningful configuration of the face is disrupted, so is holistic processing (see also Goffaux & Rossion, 2006; Hole, 1994; Hole, George & Dunsmore, 1999; Le Grand, Mondloch, Maurer & Brent, 2004; Michel, Rossion, Han, Chung, & Caldara, 2006; Robbins & McKone, 2007; Young, Hellawell & Hay, 1987). Note that the alignment effect is a relatively crude measure of the sensitivity of the perception of upright faces to configural information. In other tasks, configural processing translates into exquisite sensitivity to much smaller disruptions of the metric relations between parts (e.g., Le Grand, Mondloch, Maurer & Brent, 2004; Tanaka & Sengco, 1997).
Figure 1.
Definition of congruency in a composite matching task. Trials were the top are relevant are illustrated as an example (bracket on test face illustrate a cue indicating the top is relevant). Congruent trials are those where the test and study faces match in both parts, or differ in both parts. Incongruent trials are those where the relevant part of the test face is the same as in the study face, but the irrelevant parts do not match, or the relevant part of the test face differs from the study face, but the irrelevant parts are identical.
In summary, the composite paradigm allows the measurement of failures of selective attention to parts that result from a holistic processing strategy, and the specificity of this holistic strategy to the normal face configuration. In a typical adult population, both phenomena (holistic processing and its specificity to the normal face configuration) are more important for faces than for other objects (Farah et al., 1998; Gauthier et al., 2003; Richler et al., in press-a) and both increase with expertise (Gauthier et al., 2003). In addition, holistic processing as measured in this task correlates with activity in the FFA (Gauthier & Tarr, 2002), an area that is hypoactive during face perception in ASD (Schultz et al., 2000).
Only one study to date has tested individuals with ASDs using a composite task (Teunisse & de Gelder, 2003). Adolescents with a clinical diagnostic of Autism (mean age 19.5) were compared to typically developing children (age 9 and 10) and to typical undergraduate controls. In addition to differing in chronological age, the groups were not compared on IQ, although only high-functioning individuals with Autism were included. Participants were presented with a three quarter front view target composite face for 1 s (for undergraduate subjects) or 3 s (for the two younger groups), and after a 3 s delay, two probes were shown. Both probes used an identical (and novel) bottom half, but only one probe had the same top half as the studied face. Participants made speeded matching judgments on the top half. In different blocks, the probes were shown upright/aligned, upright/misaligned, inverted/aligned and inverted/misaligned. The undergraduate controls showed the expected pattern of results: better performance for misaligned than aligned trials, but only in the upright orientation. In contrast, the Autism group showed no effect of alignment on either upright or inverted trials. However, typical children also failed to show the expected adult pattern.
On the basis of these results, Teunisse and de Gelder concluded that adolescents with Autism are less susceptible to contextual information in a task where facial features need to be matched. Several aspects of this study limit its interpretation and the current study was designed to address them. First, there was no control group matched in either chronological age or IQ to the participants with Autism. Second, the study only assessed judgments for the top half of faces, so it is not possible to assess differences between mouths and eyes. Third, the inspection time for the adolescents with Autism was three times longer than for the adult group, making any comparison difficult. Fourth, no direct group comparison was performed, to ascertain whether the individuals with Autism really performed differently from the two control groups. Fifth, Autism diagnoses were not confirmed with gold standard research procedures, such as the Autism Diagnostic Interview (ADI, Lord et al., 1994) or the Autism Diagnostic Observation Schedule (Lord et al., 1999). Sixth, the irrelevant part in both probe faces was always different from that in the target face. Although this is similar to the original design used by Young et al. (1987), other recent studies have introduced a new version of the composite task (Boutet et al., 2002; Farah et al., 1998; Gauthier et al., 2003; Richler et al., in press-a). This variant has been called the “complete composite paradigm” because it includes the conditions where the irrelevant parts of the study and test faces are identical, in addition to the standard conditions where these irrelevant parts differ from each other (Cheung et al., in press; Richler et al., submitted; Richler et al., in press-a). It has been argued that analysis of sensitivity in the complete composite design can measure holistic effects without a confounding contribution of the considerable response biases that are often observed in this task (Cheung et al., in press; Richler et al., in press-a; Wenger & Ingvalson, 2002).
To address all of these limitations and revisit the question of whether people with ASD process faces less holistically, we used the complete composite paradigm in adolescents with ASDs and age- and IQ-matched controls.
Method
Participants
Twenty-four adolescent males with an ASD participated in the experiment as well as 17 typically developing adolescent male controls. Five of the participants (3 in the ASD group, 2 in the control group) were later excluded on the basis of chance performance in the experiment. The resulting two groups did not differ in chronological age (ASD: mean 12.84, SD 3.92; Controls: mean 12.01, SD 1.97; t34=.84, p= .41), or in full-scale IQ (ASD: mean 93.14, SD 20.0; Controls: mean 101.6, SD 15.1; t34=1.48, p= .16). Healthy controls were recruited from the local community and were screened for a history of current or lifetime psychiatric and neurological disorder using in house parent report questionnaires. None of the control participants had ever been diagnosed or suspected of having a DSM IV Axis 1 disorder. None had a first or second degree relative with an ASD, each reported good peer relationships, and none displayed any signs of social disability during informal interviews by licensed clinical psychologists experienced in the diagnosis of pervasive developmental disorders.
Individuals with an ASD were diagnosed using standardized research diagnostic procedures involving (1) careful interviewing of a primary care taker on early development using the Autism Diagnostic Interview (ADI; Lord et al., 1994); (2) a careful clinical interview of the patient using the Autism Diagnostic Observation Schedule (ADOS; Lord et al., 1999); and (3) a consensus clinical diagnostic process among 2 or more experienced clinicians, weighing all of the available evidence. These are the gold standard diagnostic procedures endorsed by the NIH Collaborative Programs for Excellence in Autism (CPEA) network. Of the 21 individuals with an ASD who successfully completed the paradigm, 11 were diagnosed with Autistic disorder, 7 with Asperger syndrome and 3 with Pervasive Developmental Disorder, Not Otherwise Specified. The mean on the ADOS social algorithm for the individuals with Autism was 11.1 (SD=2.38), for Asperger’s syndrome was 6.67 (SD = 3.08) and for PDD-NOS was 5.33 (SD= 4.04). The ADI social totals for each group respectively were 22.11 (SD=6.29), 18.6 (SD=7.06) and 16.67 (7.02)
All participants were assessed with a battery of clinical and neuropsychological measures as part of a larger project, and provided informed consent. The IQ scores were obtained with the Wechsler Intelligence Scales (WISC III, Wechsler, 1992) or WAIS III (Wechsler, 1997). None of the ASD participants had a known genetic disorder such as fragile × disorder. All were in good health and had normal or corrected 20-20 vision. The face perception tasks were completed in a single session; other testing was completed on a different day.
Procedure and materials
The stimuli were created from twelve digital images of male faces without hair, beard or other salient diagnostic features (from the face database provided by the Max-Planck Institute for Biological Cybernetics in Tuebingen, Germany). Each face was approximately 200 × 160 pixels in size and transformed to grayscale. The top and bottom halves of each face were saved as separate images and reorganized to create 24 composites. Parts were paired systematically so that each top or bottom appeared in two composites. A misaligned version was created for each composite by moving the bottom part towards the right approximately 70 pixels (so that the edge of the bottom half fell on the centre of the top half). A 3 pixel thick black line was positioned at the seam between the two halves of each stimulus (or in the same position for isolated parts). This is done to make it completely clear where the top half starts and the bottom half ends, if anything facilitating selective attention to the cued parts. Thus, any holistic processing we measure cannot be attributed to participants being unclear about the regions of the face to which they were supposed to attend. A 256 × 256 pixel nonsense texture mask was also created.
The experiment was conducted on Mac OS9 computers using RSVP software (Williams & Tarr, no date). Participants were tested in a quiet room, free from distractions, on a MacIntosh laptop with a 15” monitor. Participants were positioned at a comfortable viewing distance, not strictly controlled but approximately 100 cm from the screen. After administering the instructions and guiding the participants through the practice trials, the experimenter remained in the testing room to monitor performance, encourage on-task compliance and to handle any other issues that might arise. There were 240 experimental trials and their order was randomized for each participant, with the constraint that they were blocked by cued part: 4 blocks of 30 trials each for the top or bottom cued conditions were alternated, with the order counterbalanced across subjects. Each block began with a prompt “Are the TOPS the same? (ignore the bottom)” or “Are the BOTTOMS the same? (ignore the top).” The isolated trials (48) showed each of the 24 isolated parts twice, once on a same trial, once on a different trial. Note that isolated parts were shown on the screen in their natural position in the original face, rather than centered on the screen. The remaining trials (192) consisted of a combination of 12 stimuli × 2 cued parts (top, bottom) × 2 configurations (aligned, misaligned) × 2 levels of congruency (congruent, incongruent) × 2 correct response (same, different). Eight practice trials, randomly selected for each participant among all conditions, were not analyzed.
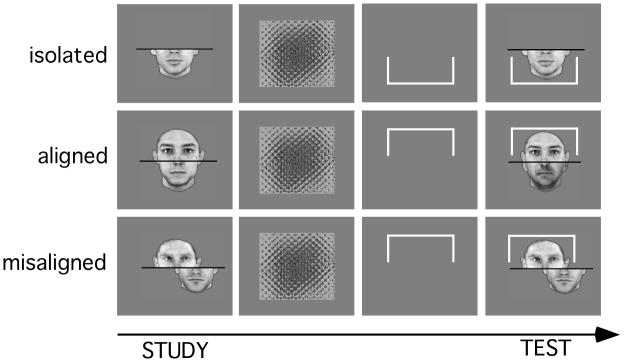
Each trial began with the message ‘press the space bar’, followed by a study face which was shown for 700 ms, a flashing mask (4 identical masks shown each for 120 ms, alternating with 50 ms blanks for a total of 630 ms), a rectangular bracket cueing top or bottom judgments, shown for 800 ms, which remained on the screen when the test face appeared (Figure 2). The cue appeared before the test face and remained present thereafter to ensure that participants were very clear on which part to attend and which to ignore: any holistic processing we measure is unlikely to be due to participants forgetting which part was relevant on any trial. In addition, trials were blocked by cued part, so that participants did not have to switch their attention constantly. Participants indicated by button press whether the cued part was the same at study and test. The test stimulus stayed on the screen until the participant responded using the ‘1’ = same and ‘2’ = different keys on the numerical keypad, or for 5000 ms. Participants were instructed to respond as rapidly as possible once they had made up their mind. Reaction times were measured from the appearance of the test stimulus and trials timed out after 5000 ms. A 500 ms break followed each response, before the start of the next trial. No feedback was given regarding performance. For consistency with previously published composite paradigms, fixation was not controlled. Participants were given frequent breaks. Following each break, participants were reminded again of the instructions and told which of the top or bottom they were then going to respond to for the following block of trials.
Figure 2.
Examples of trials used in the Experiment. Faces were always shown in the same format (isolated, aligned or misaligned) at study and test. The top or the bottom part was cued on each trial (the isolated example shows a bottom cue, the other examples show top cues). In the aligned and misaligned conditions, the irrelevant part could be either congruent or incongruent with the correct response for the cued part (the aligned example shows an incongruent trial, the misaligned example shows a congruent trial).
Results
Whole face conditions
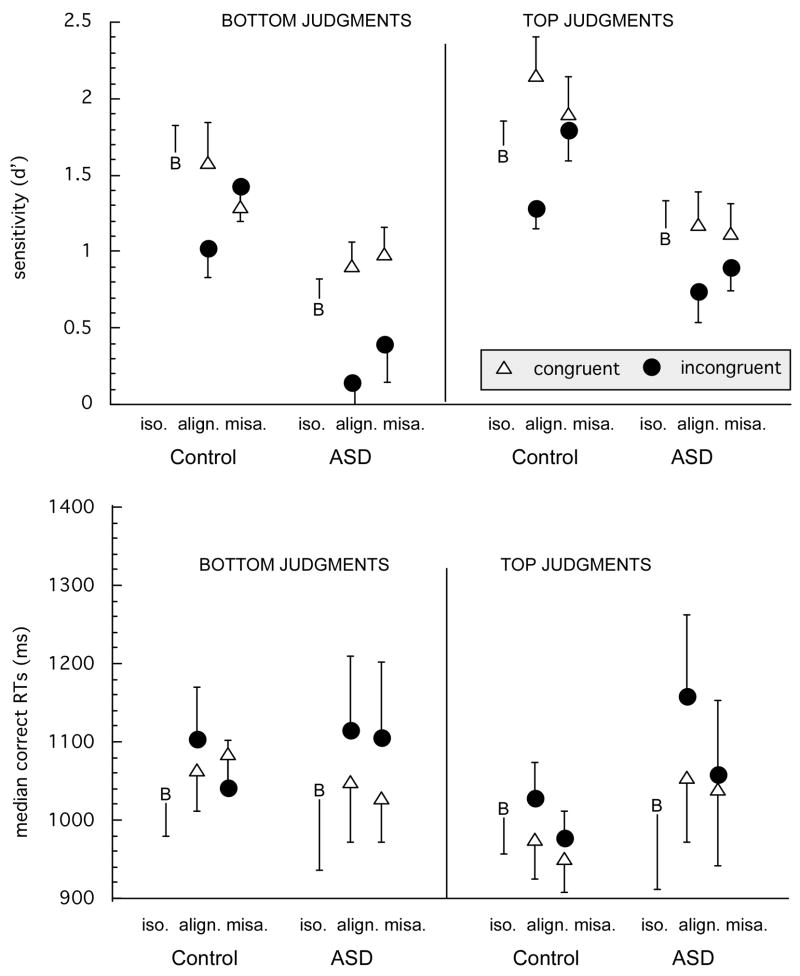
First, to assess holistic and configural processing we analyzed discriminability (d-prime) results (shown in Figure 3) in the whole face conditions (aligned and misaligned trials) in a 2 (Part) × 2 (Alignment) × 2 (Congruency) × 2 (Group) mixed-design ANOVA with group as the only between-subjects factor. Remember that holistic processing is defined as a congruency effect (better performance on congruent vs. incongruent trials; see Figure 1). Sensitivity to configuration in this task is measured by the alignment effect: the typical pattern in this paradigm is for congruency effects to be reduced as the face parts are misaligned, producing an interaction between congruency and alignment. Our analyzes revealed main effects of Group, F(1, 34) = 12.26, p =.001, cued Part F(1, 34) = 20.96, p < .0001 and Congruency F(1, 34) = 26.76, p <.0001. That is, controls performed better than individuals with ASDs, top judgments were better than bottom judgments and performance was better on congruent than incongruent trials. As expected, there was also an interaction between Congruency and Alignment, F(1, 34) = 11.30, p =.002.
Figure 3.
Mean sensitivity (TOP) and median response times for correct responses (BOTTOM) in the different conditions as a function of group, part, configuration and congruency. B denotes baseline trials in the isolated part condition, which are neither congruent nor incongruent. Error bars show the standard error of the mean in each condition.
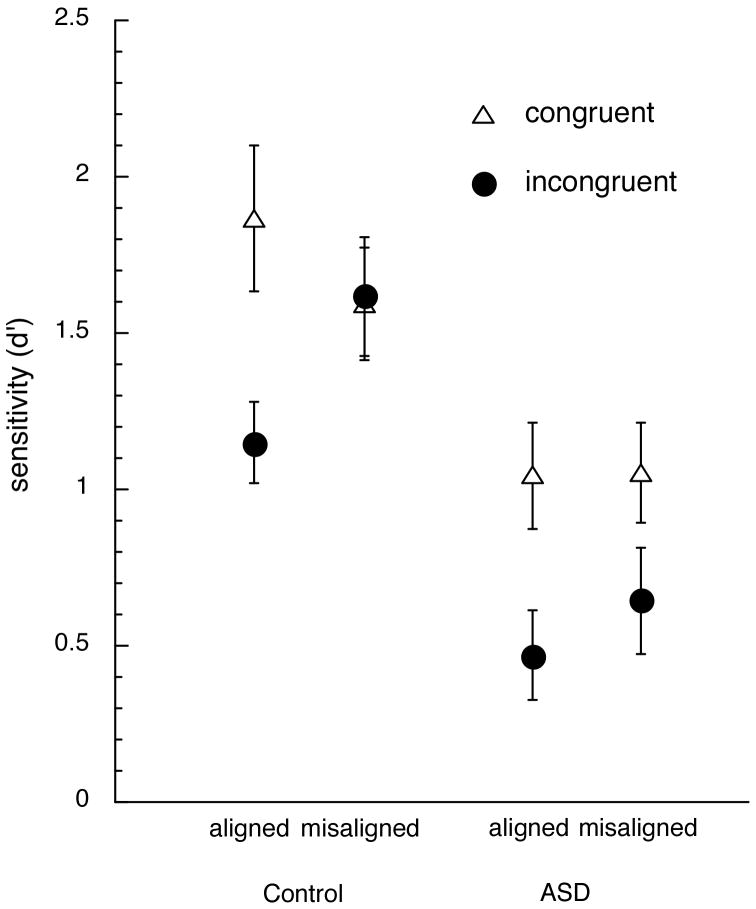
Of particular interest for our hypothesis, we found a significant three-way interaction between Group, Congruency and Alignment, F(1, 34) = 3.64, p =.04, illustrated in Figure 4. This interaction was further explored in separate ANOVAs for each group. The control group showed the expected interaction between congruency and configuration, F(1, 14) = 16.82, p =.001, with a larger congruency effect for aligned than misaligned trials. In contrast, and despite the added power of a larger group, this interaction was not significant in the Autism group (F < 1), who only showed a main effect of congruency F(1, 20) = 16.67, p =.0006).
Figure 4.
Illustration of the significant 3-way interaction between Group, Alignment and Congruency in sensitivity. Error bars show the standard error of the mean in each condition.
In sum, controls processed faces holistically, with the typical congruency effect with aligned faces, and they were also sensitive to configuration, as revealed by a reduction of the congruency effect when parts were misaligned. While individuals with ASDs also showed a congruency effect, it was observed equally for aligned and misaligned faces. Thus, the ASD group showed no sensitivity to configuration. As can been appreciated in Figure 3, the congruency effect was relatively reduced for the ASD group matching the tops of faces. This was suggested by a trend for an interaction between Group, Part and Congruency, F(1, 34) = 2.93, p = .10. This effect was further investigated with an ANOVA limited to the aligned trials, which carry the congruency effect in control participants. However, the 3-way interaction still did not reach significance, F(1, 34) = 2.93, p = .12, and therefore the best summary of the group differences we observe in sensitivity remains the Group × Congruency × Alignment interaction illustrated in Figure 4.
Performance in the ASD group was very poor in some conditions. In particular, there is a potential floor effect in two conditions where the ASD group’s performance was not significantly different from 0, for incongruent bottom trials with both aligned and misaligned configurations (see Table 1). While this floor effect makes it difficult to describe the exact pattern of performance for bottom trials in the ASD group, even if these incongruent trials were not at floor and performance could get worse in these two cells of the design, we would still be left with an abnormal pattern of performance because of the significant congruency effect in the misaligned condition. In other words, we must be cautious in interpreting the absence of a difference in magnitude of the congruency effect between the two configurations, but the presence of a congruency effect in the misaligned condition is robust and qualitatively different from the Control pattern. To further test whether the group difference could be attributed to the overall poor performance in the ASD group, we performed two additional analyses on the ASD data alone. First, we calculated the correlation between overall performance in the whole face conditions and the magnitude of the congruency effect in the critical misaligned condition. Contrary to the idea that participants in the ASD group who perform the best are the most likely to perform like controls, the correlation was non-significant and even in the opposite direction (r = .32, p=.16). Second, we calculated the correlation between overall performance in the whole face conditions and the difference in the congruency effect between the aligned and misaligned condition (i.e. the magnitude of the interaction between congruency and alignment). The concern was that the higher the overall performance, the larger this difference would be. There was, however, no clear relationship between the interaction and performance (r = .08, p=.73). In sum, there is no evidence that the absence of an interaction between congruency and alignment obtained in the ASD group is attributable to poor performance.
Table 1.
| Hits (/12) | False Alarms (/12) | D-prime | C | ||
|---|---|---|---|---|---|
| CONTROL | |||||
| BOTTOM TRIALS | |||||
| isolated | 9.53 | 2.90 | 1.61 | -0.09 | |
| aligned congruent | 9.93 | 3.33 | 1.58 | -0.16 | |
| aligned incongruent | 8.53 | 4.07 | 1.02 | -0.09 | |
| misaligned congruent | 9.33 | 3.53 | 1.28 | -0.13 | |
| misaligned incongruent | 9.33 | 3.40 | 1.44 | -0.09 | |
| TOP TRIALS | |||||
| isolated | 9.6 | 2.60 | 1.64 | -0.05 | |
| aligned congruent | 10.47 | 1.90 | 2.15 | -0.04 | |
| aligned incongruent | 8.6 | 3.00 | 1.28 | 0.05 | |
| misaligned congruent | 10.13 | 2.47 | 1.90 | -0.14 | |
| misaligned incongruent | 10.5 | 3.29 | 1.82 | -0.29 | |
| AUTISM | |||||
| BOTTOM TRIALS | |||||
| isolated | 8.67 | 5.61 | 0.65 | -0.29 | |
| aligned congruent | 8.95 | 4.90 | 0.92 | -0.22 | |
| aligned incongruent | 7.09 | 6.33 | 0.16* | -0.18 | |
| misaligned congruent | 9.23 | 4.95 | 0.99 | -0.28 | |
| misaligned incongruent | 7.95 | 6.19 | 0.40* | -0.26 | |
| TOP TRIALS | |||||
| isolated | 8.9 | 4.48 | 1.11 | -0.24 | |
| aligned congruent | 8.52 | 3.52 | 1.18 | -0.04 | |
| aligned incongruent | 7.57 | 4.29 | 0.78 | -0.02 | |
| misaligned congruent | 9 | 4.33 | 1.12 | -0.18 | |
| misaligned incongruent | 8.48 | 4.67 | 0.90 | -0.17 | |
mean d-prime values not significantly different from 0
However, because of the high error rate, the pattern of correct response times may be unreliable and we report it here only for the sake of completeness. We submitted median response times for correct responses (shown in Figure 3) to the same omnibus 2 × 2 × 2 × 2 ANOVA as we had done for d-prime. Responses faster than 200 ms or slower than 4000 ms were excluded, removing only 2% of the trials. There were significant main effects of Part, F(1, 34) = 4.32, p =.05 and Congruency, F(1, 34) = 5.56, p =.02. Participants were faster for top cued trials and for congruent trials. There was a significant interaction between Congruency and Alignment, F(1, 34) = 6.03, p =.02. Post hoc tests (Scheffé’s, alpha = .05) revealed a congruency effect for aligned but not misaligned trials. In addition, we found a significant Part × Group interaction, F(1, 34) = 5.09, p =.03. Post hoc tests (Scheffé’s, alpha = .05) revealed that the two groups were not different on the bottom part, but the ASD group was slower than the control group on top judgments. However, this pattern is difficult to interpret because participants in the ASD group were mainly guessing on incongruent bottom judgment trials. We conducted a different ANOVA with Part and Alignment as within-subject factors and Group as a between-subject factor, but using only the congruent trials for which performance was above floor in all conditions. The Part × Group interaction was still observed, F(1, 34) = 5.28, p =.03, suggesting a relative disadvantage in speed for top judgments in the ASD group.
Isolated parts conditions
One use for isolated trials in this task is as a baseline to assess whether congruency effects are due to facilitation from congruent parts or to interference from incongruent parts. Here, none of the whole face conditions was statistically different than the isolated parts baseline, so the congruency effects are best conceived as a combination of both type of influences. However, analyzing the isolated trials on their own, comparing across groups, can also be informative.
Although the analyses on whole face composite trials showed no group difference in overall performance for top and bottom parts in sensitivity (both groups performed better with tops), response times revealed that individuals with ASDs were relatively slower when matching top parts. It is unclear whether this is because they found the top parts more difficult to discriminate or whether they looked longer at them for some other reason. Fortunately, because our design includes both whole face trials and isolated parts, we can test whether there is a performance deficit for the top parts that are shown in isolation. That is, if individuals with ASDs really have more difficulty discriminating tops, this should also hold when the tops are shown in isolation and when the two parts do not compete for attention. As can be appreciated in Figure 3 (focusing on the isolated baseline trials), controls performed equally well on top and bottom trials t(14) = .15, p = .88. In contrast, adolescents with ASDs performed better on top than on bottom trials, t(20) = 2.81, p = .01. However, a 2 × 2 ANOVA failed to show a significant interaction between Group and Part, F(1, 34) = 2.35, p =.13, but there clearly was a main effect of Group F(1, 34) = 7.87, p =.008. Analyses on response times showed no significant differences between conditions.
Therefore, contrary to results when parts were presented together, when face parts were shown in isolation there was no indication of a Part × Group interaction in speed (F=.006). This result allows us to reject the hypothesis that the ASD group has more difficulty discriminating the top half of our face stimuli relative to control subjects. The deficit in speed for top judgments when the parts are presented together requires a different explanation. For instance, given all parts of a face, participants in the ASD group may choose to favor the mouth region.
The finding that participants with ASDs were better at matching top than bottom halves of faces seem to conflict with prior reports of an advantage for processing mouths over eyes (Joseph & Tanaka, 2003; Klin et al., 2002). However, other work suggests that a mouth advantage is not always found. One study found the eyes to be relatively more efficient primes than other parts for individuals with ASDs than for controls (Lahaie et al., 2006). In addition and similar to our findings, Joseph & Tanaka (2003) found less of a difference between performance on eyes and mouths in their Autism group when these parts were recognized in isolation compared to when they were recognized in the context of a whole face.
It may be important to distinguish situations in which participants are presented with several features competing for their attention, which appears to increase attention to the mouth in ASD (Joseph & Tanaka 2003 whole face condition; Klin et al., 2002 but see Lopez et al., 2004 uncued condition) and those situations in which attention is directed to a specific feature or in which isolated features are presented, and where there does not seem to be a mouth advantage (this study, Lopez et al., 2004 cued condition, Joseph & Tanaka isolated part condition). The top advantage in discrimination of isolated parts found here could be partially explained by the top halves being more diagnostic than the bottom halves in our stimuli, something that could drive a group difference if the ASD participants are struggling to find diagnostic features. It is also important to note that the entire face halves were used and provided potentially diagnostic information, so it is impossible to know whether participants focused on the eyes or mouth per se, or if they used other areas such as the forehead and chin.
Correlations with IQ
We considered the possibility that the group differences we observed could be due to IQ differences (despite the fact that the groups were statistically matched in IQ, there is always the possibility that a small difference, undetectable with our sample sizes, may nonetheless have an important effect on performance). Within each group separately, we correlated FSIQ with the three performance measures that represent the most important group differences: the congruency effect in sensitivity with misaligned faces (Cong-Mis), reaction times for top judgments on whole composites (Top-Whole) and sensitivity for top judgments with isolated parts (Top-Iso). In the ASD group, these correlations were not significant (Cong-Mis: p = .24; Top-Whole: p = .11 ; Top-Iso: p = .94) and the same was true of the controls (Cong-Mis: p = .59; Top-Whole: p = .26 ; Top-Iso: p = .24). Similarly, none of the correlations with performance IQ or verbal IQ were significant. Therefore, we find no evidence that IQ is responsible for these group differences.
Discussion
In this study, even after matching the groups for age and IQ, we found that individuals with ASDs were impaired at matching face parts. We also replicated the result previously obtained in the partial design version of the composite paradigm (Teunisse & de Gelder, 2003): while controls show the expected effect of alignment, individuals with ASDs do not. These results were originally interpreted to show that individuals with ASDs process faces less holistically than controls. However, because the prior study used the partial composite design, it was impossible to know whether this was due to their experiencing less interference in the aligned condition (which was assumed) or more interference in the misaligned condition. Our results using the complete composite paradigm reveal that when making a judgment about aligned face parts, adolescents with ASDs, although worse overall, experienced as much interference from the irrelevant parts as controls. Surprisingly, the interaction between group and alignment arises from the fact that, although misalignment reduces holistic processing in controls, it does not reduce holistic processing in the ASD group.
What do these results suggest about the nature of face processing difficulties in individuals with ASDs? For readers more familiar with the literature on ASD than with that on holistic processing of faces, it may be useful to note that psychologists are still debating the nature and processing locus of holistic processing. For instance, while most authors using this paradigm have assumed that the effect reflects perceptual mechanisms, recent work suggests that it may have a more decisional source (Wenger & Ingvalson, 2002, 2003; Richler et al., 2008; in press-b). This is not an uncommon situation: tasks that yield very robust effects in the typical population are often used with atypical populations in the hope that we can “understand the deficit”, but a robust effect is not necessarily one that is fully understood. Therefore, it is expected that both literatures will benefit from future developments in the other field, and for the moment, from tempering their interpretations by acknowledging current limitations.
With such caveats in mind, an important question is whether the normal congruency pattern for aligned faces obtained for children with ASDs can be assumed to be supported by the same mechanisms (whatever they may be) as in controls. Indeed, one straightforward interpretation is that the aligned congruency effect in ASD participants reflects intact holistic processing of upright aligned faces. By this account, children with ASDs might simply perform worse because of a general impairment that may not be face specific (Behrmann et al., 2006; Boucher & Lewis, 1992; Klin et al., 1999; Ozonoff et al., 1990; Teunisse & de Gelder, 2003) but they would be using a normal strategy. This conclusion would be consistent with the suggestion that global face processing is not affected in ASD (Jemel et al., 2006). However, recent work offers strong evidence that the face processing deficits in ASD are not due to a general local processing bias, but reflect a category-specific impairment (Wolf et al., in press).
There is however a different possible interpretation of our results, whereby similar congruency effects for aligned faces in the ASD and control groups may be obtained for different reasons. By this account, a congruency effect that is not modulated by alignment is qualitatively different and stems from abnormal face processing mechanisms. This idea is supported by the results of a recent study using the same composite task, but with composites of artificial novel objects (Greebles) in control individuals who had no prior experience with these objects (Richler et al., in press-a). As might be expected, no holistic processing (no congruency effect) was observed with non-face objects in novice observers, consistent with the idea that the effect is face-specific. But surprisingly, a congruency effect was obtained in one condition, when the study object in each trial was shown in a misaligned configuration. Similar to the congruency effect we observed here in the ASD group, this “contextual” congruency effect was not diminished by misaligning the parts of the test stimulus. It was proposed that having to encode both parts of a misaligned object may encourage an attentional strategy that conflicts with selective attention. Moreover, in a second experiment where only half of the trials showed a misaligned object at study and the other trials used an aligned object at study, a congruency effect was obtained on all trials (even in trials where both objects compared were shown in an aligned configuration). What this suggests is that there are conditions where the context of the experiment can lead novices to process objects holistically. This contextual holistic processing is distinguished from the more automatic holistic processing strategy observed regardless of context with faces by the lack of sensitivity to configuration (i.e. equivalent congruency effects regardless of the alignment of parts at test).
It is possible that the pattern we observe for faces in ASD participants is not unlike that seen when novice observers process objects. Indeed, in the present design, some of the faces were studied in a misaligned configuration. This was originally chosen because for typically developing adults, this does not change the result for faces – face experts may have developed automatic perceptual routines that are less easily influenced by context. But if individuals with ASDs process faces more like novices (Schultz, 2005), then we might expect them to show a contextual congruency effect with faces, because of the presence of misaligned study faces in the experiment. This conjecture could be tested in future work in a modified experiment including only aligned faces at study. This modified design should considerably reduce the face congruency effect in individuals with ASDs, if indeed they can be described as face novices, while it should have little influence in controls.
Importantly, this admittedly speculative account could explain the current disconnect between general theories of perceptual processing in ASDs and empirical results in face perception. For instance, in a review of several empirical studies, Happe and Frith (2006) discuss the Weak Coherence Account of Autism according to which several aspects of perception in Autism are explained by a superiority in local (rather than global) processing. To address the fact that some of the research on face perception in Autism reveals holistic effects, as exemplified by the present work, these authors suggest that it is because faces “may be so special in terms of evolutionary significance and developmental expertise that findings from face studies cannot be generalized to other stimulus classes” (p.13, Happe, 2006). However, if the face holistic findings in ASD were context driven rather than indexing expertise, they could be reconciled with other results that reveal face deficits in ASD participants. The recent findings distinguishing expertise driven and context driven holistic processing (Richler et al., in press-a) suggests that normal holistic processing of faces should only be claimed when there is a modulation of holistic effects by configuration.
To conclude, the present work is consistent with the suggestion that individuals with ASDs may lack expertise with faces, which could result both in abnormal holistic processing of faces and hypoactivity of the fusiform face area (Schultz et al., 2000). A review of face processing in Autism (Jemel et al., 2006) has misrepresented the conclusions we have drawn from our prior work: It was stated that because of recent evidence that a boy with Autism who was expert with the Digimon cartoon characters activated the fusiform gyrus for these objects (Grelotti et al., 2005), we abandoned the hypothesis that participants with ASDs may lack face expertise. On the contrary, we believe that this result only strengthens the notion that interest and experience (which is deficient for face perception in ASD participants) can specialize the fusiform gyrus for an object category. This conclusion is not entirely incompatible with the existence of more general configural deficits in ASD (Behrmann et al., 2006), which could be a factor in limiting their perceptual expertise with faces in the first place. Because perceptual expertise appears to rely on hyperspecific representations (that do not generalize across many transformations such as inversion or changes in configuration), a host of different anomalies affecting visual processing could perturb its acquisition. These may include a local processing bias, as has been reported in ASD participants. The effects of a general impairment could lead to more important deficits in the domain of face processing compared to most other domains because the comparison is with typically developing individuals who have acquired exquisite perceptual skills with faces.
Acknowledgments
This research was supported by the James S. McDonnell Foundation (to I.G. and R.T.S), the National Eye Institute and the National Science Foundation (to I.G.) and the Temporal Dynamics of Learning Center (NSF Science of Learning Center SBE-0542013). The authors would like to thank the members of the Perceptual Expertise Network for helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
ISABEL GAUTHIER, Email: isabel.gauthier@vanderbilt.edu.
CHERYL KLAIMAN, Email: cklaiman@chconline.org.
ROBERT T. SCHULTZ, Email: schultzrt@email.chop.edu.
References
- Behrmann M, Avidan G, Leonard GL, Kimchi R, Luna B, Humphreys K, et al. Configural processing in autism and its relationship to face processing. Neuropsychologia. 2006;44(1):110–129. doi: 10.1016/j.neuropsychologia.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Boucher J, Lewis V. Unfamiliar face recognition in relatively able autistic children. Journal of Child Psychology and Psychiatry. 1992;33:843–859. doi: 10.1111/j.1469-7610.1992.tb01960.x. [DOI] [PubMed] [Google Scholar]
- Boutet I, Gentes-Hawn A, Chaudhuri A. The influence of attention on holistic face encoding. Cognition. 2002;84:321–341. doi: 10.1016/s0010-0277(02)00072-0. [DOI] [PubMed] [Google Scholar]
- Cheung OS, Richler JJ, Palmeri TJ, Gauthier I. Revisiting the role of spatial frequencies in the holistic processing of faces. Journal of Experimental Psychology: Human Perception and Performance. doi: 10.1037/a0011752. in press. [DOI] [PubMed] [Google Scholar]
- Diamond R, Carey S. Why faces are and are not special: An effect of expertise. Journal of Experimental Psychology: General. 1986;115(2):107–117. doi: 10.1037//0096-3445.115.2.107. [DOI] [PubMed] [Google Scholar]
- Farah M-J, Wilson K-D, Drain M, Tanaka J-N. What is “Special” About face perception? Psychological Review. 1998;105(3):482–498. doi: 10.1037/0033-295x.105.3.482. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Bukach CM. Should we reject the expertise hypothesis? Cognition. 2007;103(2):322–330. doi: 10.1016/j.cognition.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Curran T, Curby KM, Collins D. Perceptual interference supports a non-modular account of face processing. Nat Neurosci. 2003;6(4):428–432. doi: 10.1038/nn1029. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Tarr MJ. Becoming a “Greeble” Expert: Exploring mechanisms for face recognition. Vision Research. 1997;37(12):1673–1682. doi: 10.1016/s0042-6989(96)00286-6. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Tarr MJ. Unraveling mechanisms for expert object recognition: Bridging brain activity and behavior. Journal of Experimental Psychology: Human Perception and Performance. 2002;28(2):431–446. doi: 10.1037//0096-1523.28.2.431. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Williams P, Tarr MJ, Tanaka J. Training “Greeble” Experts: A framework for studying expert object recognition processes. Vision Research. 1998;38(1516):2401–2428. doi: 10.1016/s0042-6989(97)00442-2. [DOI] [PubMed] [Google Scholar]
- Goffaux V, Rossion B. Faces are “Spatial” -- holistic face perception is supported by low spatial frequencies. Journal of Experimental Psychology: Human Perception and Performance. 2006;32(4):1023–1039. doi: 10.1037/0096-1523.32.4.1023. [DOI] [PubMed] [Google Scholar]
- Grelotti DJ, Klin AJ, Gauthier I, Skudlarski P, Cohen DJ, Gore JC, et al. Fmri activation of the fusiform gyrus and amygdala to cartoon characters but not to faces in a boy with autism. Neuropsychologia. 2005;43(3):373–385. doi: 10.1016/j.neuropsychologia.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Grice S, Spratling M, Karmiloff-Smith A, Halit H, Csibra G, de Haan M, et al. Disordered visual processing and oscillatory brain activity in autism and williams syndrome. Neuroreport. 2001;12(12):2697–2700. doi: 10.1097/00001756-200108280-00021. [DOI] [PubMed] [Google Scholar]
- Hobson RP, Ouston J, Lee A. What’s in a face? The case of autism. Br J Psychol. 1988;79:441–453. doi: 10.1111/j.2044-8295.1988.tb02745.x. [DOI] [PubMed] [Google Scholar]
- Hole GJ. Configurational factors in the perception of unfamiliar faces. Perception. 1994;23(1):65–74. doi: 10.1068/p230065. [DOI] [PubMed] [Google Scholar]
- Hole GJ, George PA, Dunsmore V. Evidence for holistic processing of faces viewed as photographic negatives. Perception. 1999;28:341–359. doi: 10.1068/p2622. [DOI] [PubMed] [Google Scholar]
- Jemel B, Mottron L, Dawson M. Impaired face processing in autism: Fact or artifact? J Autism Dev Disord. 2006:1–16. doi: 10.1007/s10803-005-0050-5. [DOI] [PubMed] [Google Scholar]
- Joseph R, Tanaka J. Holistic and part-based face recognition in children with autism. Journal of Child Psychology and Psychiatry. 2003;44(4):529–542. doi: 10.1111/1469-7610.00142. [DOI] [PubMed] [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Arch Gen Psychiatry. 2002;59(9):809–816. doi: 10.1001/archpsyc.59.9.809. [DOI] [PubMed] [Google Scholar]
- Klin A, Sparrow SS, de Bildt A, Cicchetti DV, Cohen DJ, Volkmar FR. A normed study of face recognition in autism and related disorders. J Autism Dev Disord. 1999;29(6):499–508. doi: 10.1023/a:1022299920240. [DOI] [PubMed] [Google Scholar]
- Lahaie A, Mottron L, Arguin M, Berthiaume C, Jemel B, Saumier D. Face perception in high-functioning autistic adults: Evidence for superior processing of face parts, not for a configural face-processing deficit. Neuropsychology. 2006;20(1):30–41. doi: 10.1037/0894-4105.20.1.30. [DOI] [PubMed] [Google Scholar]
- Langdell T. Recognition of faces: An approach to the study of autism. J Child Psychol Psychiatry. 1978;19:255–268. doi: 10.1111/j.1469-7610.1978.tb00468.x. [DOI] [PubMed] [Google Scholar]
- Leder H, Carbon C. When context hinders! Learn-test compatibility in face recognition. Quarterly Journal of Experimental Psychology A. 2005;58(2):235–250. doi: 10.1080/02724980343000936. [DOI] [PubMed] [Google Scholar]
- Le Grand R, Mondloch CJ, Maurer D, Brent HP. Impairment in holistic spatial frequencies and holistic processing face processing following early visual deprivation. Psychological Science. 2004;15:762–768. doi: 10.1111/j.0956-7976.2004.00753.x. [DOI] [PubMed] [Google Scholar]
- Lopez B, Donnelly N, Hadwin JA, Leekam SR. Face processing in high-functioning adolescents with autism: Evidence for weak central coherence. Visual Cognition. 2004;11(6):673–688. [Google Scholar]
- Lord C, Rutter M, DiLavore PC, R S. Autism diagnostic observation schedule - wps (ados-wps) Los Angeles, CA: Western Psychological Services; 1999. [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Pennington BF, Rogers SJ. Are there emotion perception deficits in young autistic children? J Child Psychol Psychiatry. 1990;31(3):343–361. doi: 10.1111/j.1469-7610.1990.tb01574.x. [DOI] [PubMed] [Google Scholar]
- Pierce K, Muller RA, Ambrose J, Allen G, Courshenes E. Face processing occurs outside the fusiform “face area” In autism: Evidence form functional mri. Brain. 2001;124:2059–2073. doi: 10.1093/brain/124.10.2059. [DOI] [PubMed] [Google Scholar]
- Richler JJ, Bukach CM, Gauthier I. Context influences holistic processing of non-face objects in the composite task. Perception & Psychophysics. doi: 10.3758/APP.71.3.530. in press-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richler JJ, Gauthier I, Tanaka J, Brown D. Why does selective attention to parts fail in face processing? Journal of Experimental Psychology: Learning, Memory and Cognition. doi: 10.1037/a0013080. in press-b. [DOI] [PubMed] [Google Scholar]
- Richler JJ, Gauthier I, Wenger MJ, Palmeri TJ. Holistic processing of faces: Perceptual and decisional components. J Exp Psychol Learn Mem Cogn. 2008;34:328–342. doi: 10.1037/0278-7393.34.2.328. [DOI] [PubMed] [Google Scholar]
- Robbins R, McKone E. No face-like processing for objects-of-expertise in three behavioral tasks. Cognition. 2007;103:34–79. doi: 10.1016/j.cognition.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Rutherford MD, Clements KA, Sekuler AB. Differences in discrimination of eye and mouth displacement in autism spectrum disorders. Vision Research. 2007;47:2099–2110. doi: 10.1016/j.visres.2007.01.029. [DOI] [PubMed] [Google Scholar]
- Schultz RT, Gauthier I, Klin A, Fulbright RK, Anderson AW, Volkmar F, et al. Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and asperger syndrome. Archives of General Psychiatry. 2000;37:331–340. doi: 10.1001/archpsyc.57.4.331. [DOI] [PubMed] [Google Scholar]
- Schultz RT. Developmental deficits in social perception in autism: the role of the amygdala and the fusiform face area. Int J Devl Neuroscience. 2005;23:125–141. doi: 10.1016/j.ijdevneu.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Tanaka JW, Farah MJ. Parts and wholes in face recognition. Quarterly Journal of Experimental Psychology. 1993;46A:225–245. doi: 10.1080/14640749308401045. [DOI] [PubMed] [Google Scholar]
- Tanaka JW, Giles M, Szechter L, Lantz JA, Stone A, Franks L, et al. Measuring parts and wholes recognition of cell, car, and dog experts: A test of the expertise hypothesis. Department of Psychology; 1996. [Google Scholar]
- Tanaka JW, Sengco JA. Features and their configuration in face recognition. Memory & Cognition. 1997;25:583–592. doi: 10.3758/bf03211301. [DOI] [PubMed] [Google Scholar]
- Tantam D, Monaghan L, Nicholson H, Stirling J. Autistic children’s ability to interpret faces: A research note. J Child Psychol Psychiatry. 1989;30:623–630. doi: 10.1111/j.1469-7610.1989.tb00274.x. [DOI] [PubMed] [Google Scholar]
- Teunisse JP, de Gelder B. Face processing in adolescents with autistic disorder: The inversion and composite effects. Brain Cogn. 2003;52(3):285–294. doi: 10.1016/s0278-2626(03)00042-3. [DOI] [PubMed] [Google Scholar]
- Tulving E, Thomson DM. Encoding specificity and retrieval processes in episodic memory. Psychological Review. 1973;80:352–373. [Google Scholar]
- Webb JA, Aggarwal JK. Visually interpreting the motions of objects in space. Computer. 1981;14:40–49. [Google Scholar]
- Wechsler D. Wechsler intelligence scale for children. third edition. San Antonio, TX: 1992. [Google Scholar]
- Wechsler D. Wechsler adult intelligence scale. 3. San Antonio, TX: Psychological Corp; 1997. [Google Scholar]
- Wenger MJ, Ingvalson EM. A decisional component of holistic encoding. J Exp Psychol Learn Mem Cogn. 2002;28(5):872–892. [PubMed] [Google Scholar]
- Williams P, Tarr MJ. Rsvp: Experimental control software for macos [online] no date Retrieved June 22, 1998. [Google Scholar]
- Wolf JM, Tanaka JW, Klaiman C, Cockburn J, Herlihy L, Brown C, South M, McPartland J, Kaiser MD, Phillips R, Schultz RT. Specific impairment of face processing abilities in children with autism spectrum disorder using the Let’s Face It! Skills Battery. Autism Research. doi: 10.1002/aur.56. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AW, Hellawell D, Hay D. Configural information in face perception. Perception. 1987;10:747–759. doi: 10.1068/p160747. [DOI] [PubMed] [Google Scholar]