Abstract
Context:
The transgenic human islet amyloid polypeptide (HIP) rat model of type 2 diabetes mellitus (T2DM) parallels the functional and structural changes in human islets with T2DM.
Objective:
The transmission electron microscope (TEM) was utilized to observe the ultrastructural changes in islet microcirculation.
Methods:
Pancreatic tissue from male Sprague Dawley rats (2, 4, 8, 14 months) were used as controls (SDC) and compared to the 2-, 4-, 8- and 14-month-old HIP rat models.
Results:
The 2-month-old HIP model demonstrated no islet or microcirculation remodeling changes when compared to the SDC models. The 4-month-old HIP model demonstrated significant pericapillary amyloid deposition and diminution of pericyte foot processes as compared to the SDC models. The 8-month-old model demonstrated extensive islet amyloid deposition associated with pericyte and β-cell apoptosis when compared with SDC. The 14-month-old HIP model demonstrated a marked reduction of β-cells and intra-islet capillaries with near complete replacement of islets by amyloidoses. Increased cellularity in the region of the islet exocrine interface was noted in the 4- to 14-month-old HIP models as compared to SDC. In contrast to intra-islet capillary rarefaction there was noticeable angiogenesis in the islet exocrine interface. Pericytes seemed to be closely associated with collagenosis, intra-islet adipogenesis and angiogenesis in the islet exocrine interface.
Conclusion:
The above novel findings regarding the microcirculation and pericytes could assist researchers and clinicians in a better morphological understanding of T2DM and lead to new strategies for prevention and treatment of T2DM.
Keywords: amylin, angiogenesis, apoptosis, beta cell, islet amyloid, islet fibrosis, exocrine pancreas
Introduction
Type 2 diabetes mellitus (T2DM) has emerged as a pandemic and predictions are that this trend will continue in the future (1-4). Importantly, this pandemic extends beyond the typical middle aged and older aged patient population and now involves our adolescent youth. This alarming trend will place these young patients at risk for more serious complications of end-organ involvement due to a prolonged exposure to the multiple metabolic toxicities associated with these conditions (5). Recently, it has been suggested that the islet itself may be an end-organ in T2DM (isletopathy) and further, that the islet may contain an anatomically important region in the peri-islet area termed the islet exocrine interface (IEI) (6, 7).
T2DM results from pancreatic islet β-cell failure or loss due to apoptosis superimposed on insulin resistance (5-10). The human islet amyloid polypeptide (HIP) rat model of T2DM was created by transfecting the Sprague Dawley control (SDC) rat with the human amylin gene in 2004. The role of the 37 amino acid polypeptide amylin or human amylin derived islet amyloid polypeptide (hIAPP) in the pathogenesis of isletopathy has emerged over the past two decades, and the light microscopic structural abnormalities characterizing this isletopathy have been well described (11). Our understanding of the importance of islet amyloid in the pathogenesis of human T2DM has recently increased due to the availability of animal models of T2DM characterized by having amylin derived islet amyloid (8-16). The HIP model is known to spontaneously develop impaired glucose tolerance at 5 months and overt T2DM between the ages of 6 and 10 months of age while consuming a normal rat chow diet (11-13). Recently, the ultrastructural changes of islet amyloid deposition in the 4-, 8- and 14-month-old HIP model have been described (17).
Transmission electron microscopy (TEM) examination of the islets in this animal model revealed considerable cellular activity and widening at the peri-islet–IEI (6, 7). With progressive deposition of islet amyloid this IEI area was characterized by large numbers of capillaries contemporaneous with intra-islet capillary rarefaction due to islet wounding of the vulnerable islet from progressive deposition of amyloid. Therefore, the aim of the current investigation was to evaluate the ultrastructural changes of the microcirculation remodeling with special emphasis on the pluripotent - plastic pericyte (6, 7) in the islet of the HIP rat model of T2DM (Table 1).
Table 1.
Four Stages of Islet Microcirculation Re-modeling in the HIP Rat Model of Type 2 Diabetes Mellitusa
| I | Quiescent stage: 2-month HIP model |
| No obvious microcirculation remodeling as compared to the SDC model. | |
| Loss of desmosomes and adherens junctions associated with widening of the islet exocrine interface. | |
| II | Islet wounding stage: 4-month HIP model |
| Pericapillary islet amyloid deposition, islet amyloid deposition between the pericyte and endothelial cell of the microcirculation, strongest signal for α-SMA antibody positive staining of pericytes and pericyte hyperplasia and/or migration to the islet exocrine interface. | |
| III | Pericyte and β-cell apoptosis stage: 8-month HIP model |
| Pericyte and β-cell apoptosis, progressive migration and or hyperplasia of pericytes | |
| in the islet exocrine interface. Endocrine islet cell invasion of the exocrine extracellular matrix. | |
| IV | Cellular - Pericyte differentiation stage: 14-month HIP model |
| Adipogenesis, collagenosis and angiogenesis | |
| The islet microcirculation paradox: consisting of intra-islet capillary rarefaction occurring contemporaneously with islet exocrine interface angiogenesis. | |
| Each of the above seems to be strongly associated with the pericyte. |
HIP, human islet amyloid polypeptide; T2DM, type 2 diabetes mellitus.
Materials and Methods
Animal Tissue Samples for Transmission Electron Microscopy (TEM)
Following harvesting, the tail sections of pancreatic tissue in 2-, 4-, 8- and 14-month-old male SDC and male HIP rat models were thinly sliced and placed immediately in standard TEM fixative. Standard TEM tissue preparation, fixation and staining were employed to study these tissues with TEM as previously described (17, 18). Briefly, following secondary fixation, specimens were placed on a rocker overnight, embedded and polymerized at 60°C for 24 hours. 85 nanometer (nm) thin sections were then stained with 5% uranyl acetate and Sato's Triple lead stain for viewing by a Jeol 1200-EX TEM.
Light Microscopic Histological Preparation and Staining
All pancreatic tissues were harvested from the tail region of the pancreas and immediately fixed in 3% paraformaldehyde, infiltrated and embedded in paraffin.
Alpha-Smooth Muscle Actin (α-SMA)
α-SMA antibody staining was utilized to specifically identify islet pericytes–myofibroblast-like precursor cells in tissue as described (19, 20).
Tissue blocks were washed in PBS, suspended in 6.8% sucrose in PBS (pH 7.4), dehydrated in 100% acetone (60 min at 4°C), and embedded in Historesin Plus (3 h at 4°C; no. 7022 2224–861; Leica, Deerfield, IL); or 1 mm3 cubes were washed in PBS, dehydrated in cold ethanol at −4 to −20°C, embedded in Unicryl Resin (British BioCell, Cardiff, UK), and polymerized by ultraviolet light (48 h at −10 to 20°C). 2 μm Historesin Plus sections were stored at 4°C until use; 90-nm Unicryl sections were picked up onto formvar-coated nickel grids and stored at room temperature until use.
Platelet Derived Growth Factor Receptor Beta (PDGFR-β) Antibody Staining
PDGFR-β antibody has a strong specificity for pericytes and standard fluorescent immunohistochemistry was utilized to identify the presence of pericytes and their islet location in order to supplement the findings of TEM observations.
Briefly, 5 μm paraffin sections of 2, 4, 8, and 14 months of HIP pancreas were dewaxed, rehydrated, and antigens were retrieved in citrate buffer at 95°C for 25 minutes. Non-specific binding sites were blocked with 5% bovine serum albumin and 5% normal donkey serum. Sections were then incubated with 1:100 of rabbit polyclonal PDGFR-β antibody in 10 fold diluted blocker. After 24 h, the sections were washed and incubated with 1:300 secondary antibody, Alexa flour-donkey anti rabbit 594, for 4 h. The slides were washed again and incubated with 1:2000 DAPI for 10 minutes. After washing, the slides were mounted with Mowiol and checked under a multi-photon scanning laser confocal microscope (Carl Zeiss, Thornwood, NY). The images were captured by using imaging software (Carl Zeiss, Thornwood, NY).
Ethics
The generation, housing, diet, metabolic collection, sacrifice techniques and the utilization and treatment of animals have been previously described in detail (11-13). The University of California Los Angeles Institutional Animal Care and Use Committee approved all housing, surgical and experimental procedures (11, 12).
Results
Blood Glucose Values of the Study Animals
Whole blood fasting glucose levels were <100, 70, 123, 187 and 213 mg/ml in the SDC (2–14 month), 2-, 4-, 8- and 14-month HIP rat models respectively that were imaged in this TEM study. Detailed weights, metabolic parameters, statistical analyses and light microscopic morphological changes have been previously described in detail regarding this model at ages 2, 5, 10 and 14 months of age (11-13). Weight and insulin levels were unavailable for the HIP rat models imaged in this paper; however, we do know from previous studies that weights increased to approximately 500 grams at age 5 months and remained constant to age 18 months. HIP rat models are known to become insulin deficient between the ages of 10–18 months compared to the SDC models (11, 12). The extent of islet amyloid by percentage increased progressively to reach a plateau by age 10 months in the HIP model of T2DM (11). Islet amyloid was not present in any of the SDC models because they do not have an amyloidogenic amylin and thus have no islet amyloid deposition for comparison.
Unblinded Ultrastructural Evaluation by TEM at 2, 4, 8 and 14 Months
2-Month-Old HIP Model Microcirculation: (Quiescent Stage I)
The 2-month-old HIP model was specifically evaluated in this study to be certain there were no early changes in the pericyte–endothelial interactions of the microcirculation. Indeed, the 2-month-old HIP model demonstrated no identifiable islet microcirculation abnormalities by light or TEM compared to its SDC counterparts (Fig. 1-3). Additionally, β-cells were prevalent with abundant insulin secretory granule(s) (ISG) and there was a normal interstitium between intra-islet capillaries and β-cells (50–300 nm) of the 2-month-old HIP model; similar to the SDC models (Fig. 3A).
Figure 1.
It takes two: normal pericyte and endothelial morphology in the islet. This image is representative of the normal morphology and the close interaction between pericyte(s) (Pc) and endothelial cell(s) (EC) of the islet microcirculation in the Sprague Dawley control (SDC) rat models. Note how the cytoplasmic foot process(es) (fp) circumferentially surround and envelop the EC as if embracing it. There is an intimate physical relationship between the Pc and EC allowing for crosstalk communication and physical support. In focal areas the fp share their basement membranes via peg-sockets (arrowheads) and adherens junctions (arrow) allowing for direct crosstalk communication via tight, gap (connexins 37, 40 and 43) and N-cadherins junctions respectively. Magnification ×15,000. Bar = 500 nm.
Figure 3.
Comparison of normal 2-month-old and 4-month pericapillary regions in the islet amyloid HIP model: Islet wounding stage II. Panel A is a representative image of the normal microcirculation morphology found in the 2-month-old human islet amyloid polypeptide (HIP) model. Note the similarities of the pericyte (Pc) and foot process(es) (fp) relationships to the endothelial cell found in 2- to 4-month-old Sprague Dawley in Figure 1. Compare the normal relationships in this panel and Figure 1 to the morphological alterations resulting from deposition of islet amyloid in Panels B, C and D. Magnification ×4,000. Bar = 2 μm. Triangular Insert (upper left) depicts the close physical relationship between the pericyte fp and the endothelial cytoplasm (Ec). Note only the presence of loose areolar matrix between the fp and the Ec (X). Magnification ×30,000. Bar = 200 nm. Panel B demonstrates diffuse pericapillary islet amyloid (*) deposition with a thickness of 2–4 μm. Note the significant loss of pericyte (Pc) foot processes as compared to the normal Pc and endothelial cell in Panel A and Figure 1. Additionally, it is important to note that the small black electron dense dots (insulin secretory granule(s), ISG) only come to the edge of the islet amyloid and do not demonstrate normal trafficking or docking of ISG as in Panel A. This image demonstrates the structural diffusion barrier defect associated with pericapillary islet amyloid deposition. β = beta cell, EC = endothelial cell, ISG = insulin secretory granule. Magnification ×3,000. Bar = 2 μm. Panel C depicts islet amyloid (*) deposition between and surrounding a pericyte foot process (Pc-fp) and endothelial cell cytoplasm (Ec). Magnification ×30,000. Bar = 200 nm. Panel D displays pericapillary islet amyloid (*) and early collagenosis (X). Frequent co-expression of islet amyloid and banded-fibrillar collagen was noted in the intra-islet and islet exocrine interface in 4-, 8- and 14-month-old models. Magnification ×6,000. Bar = 1 μm. Insert (d) depicts a high magnification of the banded-fibrillar collagen (X) in Panel D. Magnification ×60,000. Bar = 100 nm.
In contrast, the peri-islet–islet exocrine interface (IEI) was observed to widen in the 2-month-old HIP model as compared to the SDC 2-month-old model (Fig. 2A, B). This widening of the IEI was associated with the loss of adherens junctions and desmosomes, which normally keep the endocrine and exocrine pancreas in close anatomical proximity in the IEI (Fig. 2A Insert a, a′). This early widening of the IEI may allow for later remodeling fibrosis and islet amyloid deposition. Additionally, IEI pericytes seemed to be associated with the collagenosis necessary for matrix maintenance of the loose areolar matrix of the IEI and some pericytes were noted to be actively synthesizing collagen at this early age (Fig. 2C, D).
Figure 2.
Peri-islet – Islet exocrine interface widening and collagen synthesis and extrusion in the 2-month-old HIP model. Panel A demonstrates the normal peri-islet – islet exocrine interface (IEI) (200–300 nm) (arrows) between the endocrine and exocrine pancreas in the 2-month-old Sprague Dawley rat model. Z = zymogen granule(s). Magnification ×1,500. Bar = 1 μm. Exploded Inserts (a) and (a′) represent desmosomes and adherens junctions respectively found in the SDC model at 2 months of age (original magnification ×1,000) and have no precise scale bar or magnification. Panel B depicts widening of the IEI in the 2-month-old HIP model (2,500 nm) and represents a 10 fold increase in width of the IEI. This image demonstrates the loose areolar matrix found in the 2-month-old HIP and further depicts the matrix continuity between the IEI and endoacinar matrix, which allows for cellular trafficking and paracrine communication between the endocrine and exocrine pancreas. Z = zymogen granule. Magnification ×1,500. Bar = 1 μm. Panel C displays an IEI pericyte (Pc) adjacent to a capillary identified in Insert (c) actively synthesizing banded-fibrillar collagen fibrils seen in higher magnification in Insert (c′). This collagen synthesis is felt to represent maintenance of the loose areolar matrix of the IEI in this 2-month-old model. Magnification ×25,000. Bar = 200 nm. Insert (c) magnification ×6,000. Bar = 1 μm. Insert (c′) magnification ×120,000. Bar = 50 nm. Panel D depicts the extrusion of a single collagen fibril and demonstrates the actively synthetic Pc cell with its prominent rough endoplasmic reticulum (rER). Magnification ×40,000. Bar = 200 nm.
4-Month HIP Model (Islet Wounding Stage II)
Islets of the 4-month-old HIP rat model demonstrated substantial pericapillary islet amyloid (hIAPP) deposition as previously reported (Fig. 3B) (17). There was a noticeable loss of pericyte foot processes enveloping the capillary endothelial cells (Fig. 3B), and islet amyloid was present between endothelial cells and pericyte foot processes (Fig. 3C). Additionally, areas of collagenosis (very early ordered banded-fibrillar collagen) were interspersed with inter-β-cell amyloid deposition, both of which were associated with the pericytes (Fig. 3D). The pericapillary islet amyloid resulted in a structural diffusion barrier and interfered with the normal trafficking and docking of ISG of the β-cell and intra-islet capillaries (Fig. 3B, 4A, B). In addition to the loss of pericyte foot processes there was a change in the morphology of the cell, in that, pericytes became more cuboidal in shape (Fig. 4A) as compared to the elongated morphology in the SDC and 2-month-old HIP models (Fig. 1, 3A). Importantly, there was noted to be pericyte loss in many intra-islet capillaries (Fig. 4B), which could contribute to the capillary rarefaction as the animal model aged.
Figure 4.
4-month-old HIP model: islet wounding stage II. Panel A demonstrates cellular remodeling of the pericyte (Pc) within the center of the islet. Note the more cuboidal shape of the Pc (vs. elongated shape) in addition to the loss of Pc foot processes as compared to Figure 1 and Figure 2 (Panel A). Note that the capillary lumen (CL) is surrounded by islet amyloid (*) and that the black electron dense dots - insulin secretory granule(s) (ISG) (arrows) stop abruptly at the edge of the islet amyloid (*) due to the structural barrier created by human amylin derived islet amyloid polypeptide deposition. RBC = red blood cell. β = beta cell. EC = endothelial cell. Magnification ×5,000. Bar = 1 μm. Panel B depicts an elongated central islet capillary devoid of Pc coverage and representative of Pc loss. Again, note the pericapillary islet amyloid deposition (*) and the structural barrier to the ISG. Magnification ×3,000. Bar = 2 μm. Panel C represents the immunohistochemistry light microscopy (IHC-LM) staining with the intra-islet rust colored staining of alpha smooth muscle actin (α-SMA) antibody (arrows), which is specific for the pericyte actin component indicating a strong signal in the 4-month-old HIP model. It is interesting that the strongest signal for Pc staining occurred at this stage of islet wounding as compared to the 2-, 8- and 14-month-old HIP models. Bar = 200 μm. Insert (c) demonstrates the negative intra-islet staining for α-SMA antibody in the Sprague Dawley 4-month control model. Bar = 200 μm. Panel D depicts the specific immunohistochemistry confocal microscopy (IHC-CfM) positive staining of the pericytes with platelet derived growth factor receptor beta (PDGFR-β) antibody (red color) utilizing confocal microscopy. The blue color depicts the nuclei with 49,6-diamidino-2-phenylindole (DAPI) blue co-staining. Dashed white outline equals the confines of the islet. Note the increased peripheral islet positive staining with PDGFR-β antibody (red). Bar = 50 μm. Insert (d) depicts the PDGFR-β antibody staining in the Sprague Dawley control 4-month model and note that there is more intra-islet staining of the pericytes than in the 4-month-old HIP model. The PDGFR-β antibody staining in the 2-, 8- and 14-month-old models are featured in Figure 12. Bar = 50 μm.
Special Staining with Alpha-Smooth Muscle Actin (α-SMA) and Platelet Derived Growth Factor Receptor-Beta (PDGFR-β) Antibody Staining in the 4-Month-Old HIP Model
The 4-month-old HIP model demonstrated the strongest staining signals to α-SMA antibody (Fig. 4C) when compared to the 2-, 8- and 14-month-old models (results not shown). These observations suggest that the signal for α-SMA identification–activation by antibody staining may precede the migration and/or hyperplasic changes found in the IEI of the 8- and 14-month-old HIP model. In addition to staining pericytes within the peripheral islet and interface regions, the α-SMA antibody stained vascular smooth muscle cells and ductal tissue of the exocrine portion of the pancreas. However, there was no other stromal cell staining noted throughout the pancreas (not shown). This pattern of α-SMA pancreatic staining was also recently noted in the Ren2 model of hypertension and insulin resistance (7).
Specific pericyte staining with PDGFR-β antibody at this stage demonstrated a tendency for pericyte migration toward the periphery of the islet into the IEI regions (Fig. 4D).
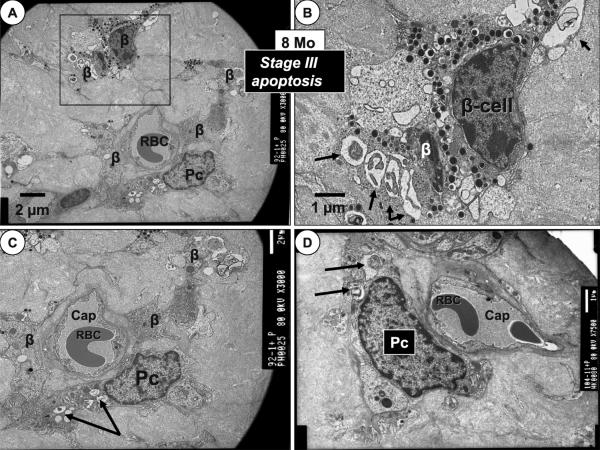
8-Month-Old HIP Model (Pericyte and β-Cell Apoptosis Stage III)
The 8-month-old HIP rat model demonstrated progressive islet hIAPP deposition as compared to the 4-month-old model and intra-islet capillaries were scarce. In addition to known β-cell apoptosis (17) there was novel evidence of pericyte apoptosis (Fig. 7C, D). When found, intra-islet pericytes no longer had their foot processes enveloping the capillary endothelial cells and it appeared as if there was pericyte foot process attenuation and/or loss. The pericyte nucleus and cell body lost it elliptical, elongated shape and assumed a more cuboidal type of morphology in all of the capillaries identified at 8 months (Fig. 5B, 8). There was also noted an adaptive remodeling response to the progressive hIAPP replacement remodeling and the peripheral islet cells and matrix at the IEI appeared to physically invade the surrounding exocrine interstitium (Fig. 9).
Figure 7.
Islet cell invasion of the interlobular exocrine pancreas in the 8-month-old model. Stage III. Panel A demonstrates a view of the islet exocrine interface (IEI)—endocrine pancreas—islet cells (IC) invading the surrounding exocrine endoacinar extracellular matrix (ECM) as islet amyloid is being progressively deposited within the islets of the 8-month-old model. Magnification ×2,000. Bar = 2 μm. Panels B–D depict the extent of this islet cell IC invasion at higher magnification into the surrounding endoacinar ECM. These images represent the positive outward cellular remodeling of the endocrine pancreas and its islet cells. Note the large zymogen granules (Z) within the acinar cells of the exocrine pancreas. Magnification ×4,000. Bar = 2 μm.
Figure 5.
The islet exocrine interface in the 4-month-old HIP model. Stage II. Panel A depicts the islet exocrine interface (IEI) (arrows). Z = zymogen granules, IC = islet cell. Magnification ×10,000. Bar = 500 nm. Insert (a) depicts the boxed-in area at higher magnification. Magnification ×75,000. Bar = 100 nm. Panel B portrays an IEI capillary with the co-expression of pericapillary islet amyloid (*) and fibrosis (X). Insert (b) is a higher magnification (×125,000 and Bar = 50 nm) of the fibrillar-banded collagen (X) of pericapillary IEI fibrosis co-expression with human amylin derived islet amyloid (hIAPP). Magnification ×7.5K. Bar = 1 μm. Insert (b) depicts a higher magnification of the banded fibrillar collagen in the boxed-in area. Bar = 50 nm. Panel C depicts significant pericapillary islet amyloid (*) surrounding a single capillary with a red blood cell (RBC) within its lumen in the IEI. Magnification ×7,500. Bar = 1 μm. Note that the IEI islet amyloid extends into the exocrine pancreas (arrow). This IEI islet amyloid deposition and deposition of collagen (early fibrosis) could interfere with the proper communication between the islet and the exocrine pancreas matrix communication. Insert (c) is a higher magnification of the islet amyloid (*). Magnification ×120,000. Bar = 50 nm. Panel D demonstrates pericyte (Pc) hypercellularity, which was commonly found in the IEI in the 4-month HIP model. This hypercellularity (arrows) adjacent to the left side of a large capillary or efferent venule could be due to local hyperplasia and/or migration of Pc from the exocrine pancreas into the IEI similar to what has been found in the Ren2 model of hypertension, oxidative stress and insulin resistance (7). There is co-existence of pericapillary islet amyloid (*) and early fibrosis (boxed-in area) around the large capillary as depicted in the previous Panel B. This image is a low power view and the boxed-in area is seen at higher magnification in Panel B, Insert (b). Magnification ×2,000. Bar = 2 μm.
Figure 8.
Pericyte differentiation stage IV: suggesting pericyte-adipocyte differentiation in the 14-month-old HIP model. Panel A may demonstrate the presence of the pericyte undergoing differentiation into an adipocyte. Note the presence of intracellular lipid droplets (Ld). Initially, it was felt that these cells were resident adipocytes but later it was determined that many of these lipid droplets were found to be within the pericyte (Pc) foot processes and further gave the appearance of nourishing the β-cell as represented in Panels B and C. Note the two capillaries (cap) encircled by dashed circles and that they are 3–8 μm distanced from β-cells (β) by islet amyloid (*). Magnification ×2,000. Bar = 2 μm. Insert (a) demonstrates intra-islet banded fibrillar collagen indicating early intra-islet fibrosis from the boxed-in area. Magnification ×60,000. Bar = 100 nm. Panel B demonstrates a pericyte (Pc) and its foot process (fp) and foot plate adjacent to a regional β-cell (boxed-in area). Insert (b) depicts an exploded view of this foot plate (arrow) and in even higher magnification in Panel C. Magnification ×3,000. Bar = 2 μm. Panel C portrays the foot plate of the Pc foot process with its lipid droplets (Ld) and one Ld interacting and seeming to dock with the basement membrane (X) of the β-cell with its insulin secretory granules (IGS) (arrows). These images certainly suggest the differentiation of Pc into adipocytes and further, that if the Pc are not differentiating into adipocytes that they are certainly acting as a ‘carrier cell’ to deliver lipids to the starving β-cells as a result of the human amylin derived islet amyloid replacement remodeling separating them from islet capillaries. Magnification ×30,000. Bar = 200 nm.
Figure 9.
Pericyte foot process abundance in the islet exocrine interface of the 14-month-old model. Stage IV angiogenesis. Panel A demonstrates a small (2.5 × 4 μm lumen) thin walled anuclear capillary tube with an adjacent pericyte(s) (Pc) with its multiple pericyte foot process (fp) separated from the capillary by islet amyloid (*) within the islet exocrine interface (IEI). Magnification ×6,000. Bar = 1 μm. Panel B depicts an abundance of thin pericyte foot processes (fp) within a bed of islet amyloid (*) in the IEI. Note the thin walled small capillary (4 × 6 μm lumen) containing a single red blood cell (RBC) with small atrophic islet cells (IC) nearby with sparse to absent secretory granules and vacuole formation (#). Magnification ×2,500. Bar = 2 μm. Panel C portrays a capillary lumen (CL) with foot processes (fp) embedded within cross section of fibrotic pericapillary collagen (X). Note the unusual morphology of the pericyte fp. ER = endoplasmic reticulum. Magnification ×20,000. Bar = 200 nm. Panel D demonstrates two capillaries (CL) and three islet cells (IC) within the IEI with the remaining cellularity consisting of Pc and fp. Magnification ×1,500. Bar = 5 μm.
14-Month HIP Model (Pericyte Differentiation Stage IV) with Near Total Islet Replacement with hIAPP
Intra-islet capillaries and functioning β-cells were both extremely difficult to locate in the 14-month-old HIP model due to extensive hIAPP deposition with near total islet replacement. When found buried in a sea of islet amyloid–hIAPP, the β-cells were always accompanied by cells with intracellular lipid droplets and these were assumed initially to be from resident adipocytes (17); however, careful evaluation revealed that the lipid droplet formation occurred within pericyte foot processes suggesting differentiation of microvascular pericytes into adipocytes or lipid carrying cells (Fig. 8).
Intra-islet collagenosis-fibrosis was observed in the 14-month-old model (Fig. 8A) interspersed with diffuse islet amyloid deposition. Islet exocrine interface collagenosis for matrix maintenance was observed in the 2-month-old models while orderly fibrillar-banded collagen typical of fibrosis was present in the IEI of the 4-, 8- and 14-month-old models and reminiscent of the early fibrosis found in the Ren2 model of angiotensin II induced hypertension and insulin resistance. Indeed, it is currently thought that the islet pericyte can differentiate into an islet pancreatic stellate–myofibroblast-like cell capable of synthesizing fibrillar-banded collagen interspersed with peri-islet–islet exocrine interface amyloid deposition (7). Peri-islet fibrosis is also found to be an important extracellular matrix remodeling change in the Zucker obese model of T2DM and is also a prominent finding in islets of humans with T2DM (6, 22, 23). Current observations support the notion that even in the HIP model of T2DM with diffuse amyloid deposition there is concurrent early fibrosis in the intra-islet and IEI regions of the pancreas.
Importantly, the 14-month-old HIP model demonstrated capillary gain or angiogenesis in the IEI and a better understanding of the potential role of the pericyte in this anatomical region with abundant capillaries is necessary (Fig. 9, 10). Here the capillary tubes were frequently anuclear (Fig. 9A) and pericyte foot processes were readily observed in most fields examined (Fig. 9, 10). Curiously, the foot processes adjacent to the capillaries lost their normal finger-like shapes previously observed in controls and the 2-, 4- and 8-month-old models (Fig. 9C). Also, there was increased cellularity consisting primarily of pericytes with unusually long foot processes in the islet exocrine interface (Fig. 9, 10). Interestingly, the findings of intra-islet capillary rarefaction and the observation of interface angiogenesis have not been previously described in other animal models of T2DM.
Figure 10.
Possible pericyte capillary tube formation and stellate appearance of pericytes in the islet exocrine interface of the 14-month-old HIP model. Stage IV. Panel A demonstrates two pericytes (Pc), the superior one appearing to be undergoing tube formation and the inferior one with a stellate-shaped appearance within the islet exocrine interface (IEI) and observed against a background of islet amyloid (*). Foot processes (fp) are everywhere. IC = islet cell. Magnification ×3,000. Bar = 2 μm. Panel B depicts a thin walled (3 × 5 μm) nucleated capillary containing a single red blood cell (RBC) with surrounding thin fp on a background of islet amyloid (*). Magnification ×7,500. Bar = 1 μm. Panel C portrays a single nucleated (2 × 2.5 μm) thin walled capillary adjacent to a Pc fp and an islet cell (IC) with secretory granules. Note the odd shaped pericyte foot processes (fp). Magnification ×10,000. Bar = 500 nm. Panel D demonstrates a larger nucleated capillary embedded within surrounding islet amyloid (*) and adjacent to a nearby Pc fp. Magnification ×10,000. Bar = 500 nm.
In addition to β-cell apoptosis there were also apoptotic changes found in pericytes and their foot processes. When rare intra-islet capillaries were found the pericyte appeared “ghost-like” and less electron dense and this was accompanied by apoptotic changes in the pericyte cytoplasm and foot processes (Fig. 6C–D, 11).
Figure 6.
8-month-old HIP model: pericyte and β-cell apoptosis stage III. Panel A depicts β-cell apoptosis and one pericyte (Pc) undergoing apoptosis embedded in a sea of islet amyloid in the 8-month-old model. Note the near total replacement of the islet with amylin derived islet amyloid and that the Pc does not have any insulin secretory granules as do most of the apoptotic β-cells. Magnification ×3,000. Bar = 2 μm. Panel B demonstrates a higher magnification of the boxed in β-cells noted in Panel A. Note the classic apoptotic bodies (arrows). Magnification ×6,000. Bar = 1 μm. Panel C portrays an apoptotic Pc adjacent to a capillary (cap) with nuclear chromatin clumping, loss of intracellular organelle structure, apoptotic bodies and vesicle formation (arrows). Magnification ×3,000. Bar = 2 μm. Panel D depicts a different apoptotic Pc with apoptotic bodies (arrows) adjacent to a capillary (cap) with similar changes as in Panel C. Magnification ×7,500. Bar = 1 μm.
Figure 11.
Pericyte and pericyte foot processes apoptosis. This image portrays a peripherally located intra-islet capillary with a “halo effect” around the basement membrane of a single endothelial cell (EC) and a luminal red blood cell (RBC) embedded in a sea of islet amyloid (*). Initially this halo effect was thought to possibly represent basement membrane thickening; however, on detailed examination these changes were found to be due to a denser and compacted deposition of adjacent islet amyloid (*). Superior to the EC nucleus is a “ghost-like” Pc nucleus (arrow) without chromatin material with a shriveled appearance and an unusually very light and confluent electron density staining similar to pericytes found in the retina of patients with diabetic retinopathy. The second very large Pc to the upper right of the capillary lumen (CL) is also undergoing apoptotic changes with nuclear chromatin clumping and an apoptotic body (arrow) with abnormal cytoplasmic organelles. Of note is the presence of novel apoptotic bodies within the foot processes (arrows). Magnification ×7,500. Bar = 1 μm.
Platelet Derived Growth Factor Receptor Beta (PDGFR-β) Antibody Staining
PDGFR-β antibody staining was more diffusely present throughout the intra-islet regions in the 2-month-old HIP model; however, the positive staining for pericytes was moderately increased in the 4-month-old model (Fig. 4D) and markedly increased at the periphery or IEI in the 8- and 14-month model indicating migration and/or hyperplasia in support of the TEM findings (Fig. 12). Since the pericyte is in intimate association with the capillary endothelial cell, these changes are suggestive of intra-islet capillary rarefaction and supportive of islet exocrine interface angiogenesis.
Figure 12.
Immunohistochemistry staining with platelet derived growth factor receptor-beta (PDGFR-β) antibody in the 2-, 8- and 14-month-old HIP model. Panel A (2-month-old model) demonstrates immunohistochemistry (IHC) staining with platelet derived growth factor receptor beta (PDGFR-β) antibody by confocal microscopy (CfM). The red florescent color indicates diffuse positive staining of the intra-islet pericytes and the blue color depicts the nuclei with 4′,6-diamidino-2-phenylindole (DAPI) blue co-staining. Each islet image captured (A–C) were approximately the same size so the intra-islet and the islet exocrine interface could be comparably demonstrated. Bar = 50 μm. Insert demonstrates a representative image staining in the 2-month-old Sprague Dawley control (SDC) model. Bar = 25 μm. Panel B (8-month-old model) demonstrates less intra-islet staining of pericytes and suggests increased staining at the periphery. Note the loss of intra-islet cellularity as compared to the 2-month-old human islet amyloid polypeptide (HIP) model in Panel A and the 8-month-old SDC image in the insert. Bar = 50 μm. Insert demonstrates a representative staining in the 8-month-old SDC model. Bar = 25 μm. Panel C (14-month-old model) depicts increased positive red staining of pericytes at the periphery of the islet and near absent staining within the intra-islet area. Image Panels A–C are very supportive of the observed scarcity of intra-islet capillaries with concurrent angiogenesis as the human islet amyloid polypeptide (HIP) model aged. Additionally, the images in Panel A–C support the findings of intra-islet β-cell and pericyte loss via apoptosis depicted in earlier Figures 6 and 11. Bar = 50 μm. Insert demonstrates a representative image in the 14-month-old SDC model. Bar = 25 μm. The 4-month-old staining with PDGFR-β antibody was presented in Figure 4, Panel D.
Discussion
The Islet Exocrine Interface (IEI)
The IEI may be considered an important structural and functional region, which contains the neurovascular supply including sympathetic and parasympathetic innervations as well as the afferent and efferent vessels (including the insulo-acinar-portal pathway) (6, 7). This anatomical region has gained increasing importance during the past decade with the advent of islet transplantation and the need for purification of donor islets (24). Also, this region may be important for communication between the endocrine - exocrine pancreatic subdivisions and currently the pancreas may be considered as an integrated organ with two interrelated subdivisions (endocrine-exocrine) in constant communication and functioning synergistically (6, 25, 26).
Interestingly, the cellular and extracellular matrix remodeling of the IEI cannot be interpreted by light microscopy because the magnification is not sufficient to delineate this anatomical region (7). This may be one of the reasons why this specialized anatomical region has been largely overlooked in the past. Imaging studies with TEM has allowed the examination of this specialized anatomical region in greater detail and describe its adaptive–pathological remodeling. As the HIP model aged there seemed to be increased cellular and extracellular matrix activity in this widened and otherwise relatively quiet region. The IEI displayed pericyte hypercellularity in the 4-month-old model (Fig. 5D) and later manifested an increase in capillaries and pericapillary islet amyloid and collagenosis with early deposition of fibrillar-banded collagen (Fig. 3, 5, 8-10). Increased pericytes seemed to be closely associated with the production of new collagen and new capillaries. In some areas of the IEI when there was excessive hIAPP deposition (8- and 14-month models) there seemed to be pericyte foot processes in abundance and some even appeared to be forming capillary tube-like structures (Fig. 10A), which has been described in the retina and malignant tumors (27). Additionally, the evaluation of the IEI allowed for an improved understanding of how islet cells might extend into the adjacent exocrine tissue noted in the 8-month-old model (Fig. 7).
Significant islet amyloid deposition and early fibrosis in this anatomical region occurred concurrently in the HIP model similar to its occurrence in human patients with T2DM (6, 23). Importantly, these novel remodeling changes could potentially interfere with paracrine and endocrine communication with the exocrine pancreas and with the afferent delivery of oxygen and nutrients to the islet and the efferent delivery of islet hormones (insulin) and toxicmetabolic byproducts of islet metabolism. These structural changes could have marked detrimental results on islet function and could contribute to the delay of 1st phase insulin secretion and promote dysfunctional islet exocrine interactions.
Pericyte Endothelial Interaction in the Microcirculation
For a fully competent microvessel to function, pericytes and endothelial cells must function in a synergistic fashion: It takes two (Fig. 1, 3A) (6, 7). The pericyte is an obligatory mural and mesenchymally derived cell serving many functions to the islet microcirculation including vascular development (post-natal angiogenesis), maturation, remodeling and a stabilizing, supportive-protective role to the capillary endothelial cell (Fig. 1, 3A) (28, 29). Vascular smooth muscle cells (VSMC) and pericytes share a close homology and it is thought that one may give rise to the other, but there is also evidence that pericytes may arise from native bone marrow progenitor cells (29, 30). Circumferential pericyte foot processes are in close contact with capillary endothelial cells and communicate by intimately sharing their basement membranes through readily identifiable ultrastructures termed peg-sockets and adherens junctions (Fig. 1) (6, 7, 31). Likewise, pericytes may have elongated longitudinal and thin cytoplasmic foot processes, such as those found in the IEI in this investigation (Fig. 9, 10).
Pericytes are quite vulnerable to multiple metabolic toxicities and oxidative-redox stress states associated with pre-diabetes and overt T2DM (especially glucotoxicity, dyslipidemia, minimally and advanced glycation endproducts (AGE) and their receptors RAGE) (32, 33). For example, pericyte loss in the retinae due to these metabolic abnormalities is commonly observed and may be related to the subsequent development of microaneurysms, acellular capillaries, capillary closure and subsequent capillary loss associated with diabetic retinopathy (33, 34).
There is considerable phenotypic plasticity of pericytes and they are known to be capable of differentiating into pancreatic stellate cells, fibroblasts–myofibroblasts, chondrocytes, osteoblasts, skeletal myocytes, adipocytes, Leydig cells and the pericyte-VSMC axis (7, 35-37). In addition to the pleiotropic differentiating properties of the pericytes, they, like VSMC seem to be capable of activation–migration (7) and frequently undergo apoptosis due to the aforementioned toxicities.
The Pericytes' Proposed Role as an Islet Mesenchymal Stem Cell
A recent publication supports the notion that mesenchymal stem cells (MSC) reside in virtually all post-natal organs including the endocrine and exocrine pancreas (38). These authors point to the possibility that MSC may have a perivascular niche pointing to the pericyte as the possible undifferentiated precursor cell that may be capable of differentiating into adipocytes - lipid carrying cells, pancreatic islet stellate cell–fibroblast–myofibroblasts (7) and angiogenic cells associated with adipogenesis, collagenosis and angiogenesis, respectively.
Pericyte apoptosis was readily identified as this animal model aged (Fig. 6C–D, 11); however, endothelial cell apoptosis was not identified. Therefore, we hypothesize that the islet pericyte may be more susceptible to injurious islet redox stress stimuli than the endothelial cell similar to the retinal pericyte (39). Robust production of injurious islet metabolites associated with T2DM (glucotoxicity, angiotensin II excess, redox–oxidative stress, ROS and islet wounding associated with amyloid deposition) may result in apoptosis; however, less robust production of these islet metabolites might result in pericyte hypertrophy, hyperplasia, increased α-SMA staining and increased matrix metalloproteinase expression. This, in turn, could result in pericyte migration from the intra-islet region or the exocrine pancreas to the IEI while this cell is undergoing differentiation. Further, if the pericyte is destroyed due to apoptosis, its potential as a perivascular MSC could be entirely lost.
Interestingly, it was observed that pericytes might be capable of forming closed capillary tubes (Fig. 10A). This novel finding is of potential pathophysiological importance since islet amyloid, intra-islet and islet exocrine interface collagenosis – fibrosis and intra-islet adipogenesis is observed at autopsy in human T2DM (6, 40). Since the deposition of islet amyloid in this model results in islet wounding it would not be surprising to observe an integral part of the chronic wound healing response: angiogenesis. Thus, we have been able to relate the islet capillary pericyte to islet wounding via a response to injury wound healing mechanism. These observations also suggest that there are remodeling changes associated with early collagenosis-fibrosis, islet adipogenesis and peri-islet–IEI angiogenesis.
A potential weakness of this observational study is that only four snapshots in time (2-, 4-, 8- and 14-month-old images) of HIP model microcirculation remodeling and specific pericyte remodeling were conducted. Despite this limitation, these findings have allowed for a significant gain of information regarding the cellular and extracellular islet remodeling as it relates to islet microcirculation and pericyte remodeling changes.
Conclusion
The current observational TEM investigation allowed for an examination of the microcirculation within islets and the IEI of the 2-, 4-, 8- and 14-month-old HIP models suggesting 4 stages of islet microcirculation remodeling (Table 1). Further, this investigation has highlighted the important roles of the IEI and the pluripotent–plastic pericytes involvement in islet microcirculation remodeling. These observations emphasize the impact of both the soluble hIAPP oligomers associated with β-cell apoptosis as well as the impact of the replacement remodeling of the insoluble–fibrillar mature hIAPP with specific attention to the pericyte and critical islet microcirculation. The marked structural remodeling within the islet associated with islet amyloid deposition suggested several pathological processes, including failure of maintaining an intact islet microcirculation involved in the development of T2DM.
The novel HIP rat model appears to be an ideal animal model to better understand both the structural and functional changes that occur in human T2DM and better understand the progressive nature of this chronic and progressive pandemic disease. Additionally, these observations have allowed for a more insightful understanding of islet pericyte structural morphology and cellular remodeling as a result of amyloid deposition. In this regard, it is important to note that pericytes constitute a potential mechanism for lifelong repair and regeneration of injured islet tissues. Thus, the possibility of exploiting their regenerative potential is very attractive and could lead to the future development of novel therapies. Collectively this TEM investigation also suggests that islet amyloid deposition and islet pericyte loss may also play an important role in islet transplant failure.
Acknowledgments
Authors wish to acknowledge the Peter C Butler laboratory of the Larry Hillblom Islet Research Center, David Geffen School of Medicine, University of California, Los Angeles, California, who provided tissue specimens for morphologic study with TEM.
A special thank you is extended to Tatyana Gurlo, Ph.D. who kindly collected and prepared the TEM tissue for mailing. Additionally, the authors would like to acknowledge the Electron Microscopic Core Center at the University of Missouri–Columbia, Missouri for their excellent help and tissue preparation of animal samples for viewing.
This research was supported by NIH (R01 HL73101-01A1), the Veterans Affairs Merit System (0018), and Novartis Pharmaceuticals for James R Sowers, M.D.
References
- 1.Amos AF, McCarty DJ, Zimmet P. The rising global burden of diabetes and its complications: estimates and projections to the year 2010. Diabetes Med. 1997;14:S1–S85. [PubMed] [Google Scholar]
- 2.Zimmet P. The burden of type 2 diabetes: are we doing enough? Diabetes Metab. 2003;29:6S9–6S18. doi: 10.1016/S1262-3636(03)72783-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hayden MR, Stump C, Sowers JR. Organ involvement in cardiometabolic syndrome. J Cardiometab Syndr. 2006;1(1):16–24. doi: 10.1111/j.0197-3118.2006.05454.x. [DOI] [PubMed] [Google Scholar]
- 4.Hayden MR, Sowers JR. Redox imbalance in diabetes. Antioxid Redox Signal. 2007;9(7):865–867. doi: 10.1089/ars.2007.1640. [DOI] [PubMed] [Google Scholar]
- 5.Arslanian SA. Type 2 diabetes mellitus in children: pathophysiology and risk factors. J Pediatr Endocrinol Metab. 2000;13(suppl 6):1385–1394. doi: 10.1515/jpem-2000-s612. [DOI] [PubMed] [Google Scholar]
- 6.Hayden MR, Sowers JR. Isletopathy in type 2 diabetes mellitus: implications of islet RAS, islet fibrosis, islet amyloid, remodeling and oxidative stress. Antioxid Redox Signal. 2007;9(7):891–910. doi: 10.1089/ars.2007.1610. [DOI] [PubMed] [Google Scholar]
- 7.Hayden MR, Karuparthi PR, Habibi J, Wasekar C, Lastra G, Manrique C, Stas S, Sowers JR. Ultrastructural islet study of early fibrosis in the Ren2 rat model of hypertension. Emerging role of the islet pancreatic pericyte-stellate cell. JOP. 2007;8(6):725–738. [PubMed] [Google Scholar]
- 8.Cefalu WT. Animal models of type 2 diabetes: clinical presentation and pathophysiological relevance to the human condition. ILAR J. 2006;47(3):186–198. doi: 10.1093/ilar.47.3.186. [DOI] [PubMed] [Google Scholar]
- 9.Hoppener JW, Oosterwijk C, Nieuwenhuis MG, Posthuma G, Thijssen JH, Vroom TM, Ahren B, Lips CJ. Extensive islet amyloid formation is induced by development of Type II diabetes mellitus and contributes to its progression: pathogenesis of diabetes in a mouse model. Diabetologia. 1999;42(4):427–434. doi: 10.1007/s001250051175. [DOI] [PubMed] [Google Scholar]
- 10.Hull RL, Andrikopoulos S, Verchere CB, Vidal J, Wang F, Cnop M, Prigeon RL, Kahn SE. Increased dietary fat promotes islet amyloid formation and beta-cell secretory dysfunction in a transgenic mouse model of islet amyloid. Diabetes. 2003;52(2):372–379. doi: 10.2337/diabetes.52.2.372. [DOI] [PubMed] [Google Scholar]
- 11.Butler AE, Jang J, Gurlo T, Carty MD, Soeller WC, Butler PC. Diabetes due to a progressive defect in beta-cell mass in rats transgenic for human islet amyloid polypeptide (HIP Rat): a new model for type 2 diabetes. Diabetes. 2004;53(6):1509–1516. doi: 10.2337/diabetes.53.6.1509. [DOI] [PubMed] [Google Scholar]
- 12.Matveyenko AV, Butler PC. β-Cell deficit due to increased apoptosis in the human islet amyloid polypeptide transgenic (HIP) rat recapitulates the metabolic defects present in type 2 diabetes. Diabetes. 2006;55(7):2106–2114. doi: 10.2337/db05-1672. [DOI] [PubMed] [Google Scholar]
- 13.Matveyenko AV, Butler PC. Islet amyloid polypeptide (IAPP) transgenic rodents as models for type 2 diabetes. ILAR J. 2006;47(3):225–233. doi: 10.1093/ilar.47.3.225. [DOI] [PubMed] [Google Scholar]
- 14.Hull RL, Andrikopoulos S, Verchere CB, Vidal J, Wang F, Cnop M, Prigeon RL, Kahn SE. Increased dietary fat promotes islet amyloid formation and beta-cell secretory dysfunction in a transgenic mouse model of islet amyloid. Diabetes. 2003;52(2):372–379. doi: 10.2337/diabetes.52.2.372. [DOI] [PubMed] [Google Scholar]
- 15.Henson MS, O'Brien TD. Feline models of type 2 diabetes mellitus. ILAR J. 2006;47(3):234–242. doi: 10.1093/ilar.47.3.234. [DOI] [PubMed] [Google Scholar]
- 16.Howard CF., Jr Longitudinal studies on the development of diabetes in individual Macaca nigra. Diabetologia. 1986;29(5):301–306. doi: 10.1007/BF00452067. [DOI] [PubMed] [Google Scholar]
- 17.Hayden MR, Karuparthi PR, Manrique CM, Lastra G, Habibi J, Sowers JR. Longitudinal ultrastructure study of islet amyloid in the HIP rat model of type 2 diabetes mellitus. Exp Biol Med (Maywood) 2007;232(6):772–779. [PubMed] [Google Scholar]
- 18.Hayden MR, Chowdhury NA, Cooper SA, Whaley-Connell A, Habibi J, Witte L, Wiedmeyer C, Manrique CM, Lastra G, Ferrario C, Stump C, Sowers JR. Proximal tubule microvilli remodeling and albuminuria in the Ren2 transgenic rat. Am J Physiol Renal Physiol. 2007;292(2):F861–F867. doi: 10.1152/ajprenal.00252.2006. [DOI] [PubMed] [Google Scholar]
- 19.Rajkumar VS, Howell K, Csiszar K, Denton CP, Black CM, Abraham DJ. Shared expression of phenotypic markers in systemic sclerosis indicates a convergence of pericytes and fibroblasts to a myofibroblast lineage in fibrosis. Arthritis Res Ther. 2005;7(5):R1113–R1123. doi: 10.1186/ar1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skalli O, Pelte MF, Peclet MC, Gabbiani G, Gugliotta P, Bussolati G, Ravazzola M, Orci L. Alpha-smooth muscle actin, a differentiation marker of smooth muscle cells, is present in microfilamentous bundles of pericytes. J Histochem Cytochem. 1989;37:315–321. doi: 10.1177/37.3.2918221. [DOI] [PubMed] [Google Scholar]
- 21.Habibi J, Whaley-Connell A, Qazi MA, Hayden MR, Cooper SA, Tramontano A, et al. Rosuvastatin, a HMG-CoA reductase inhibitor, decreases cardiac oxidative stress and remodeling in Ren2 transgenic rats. Endocrinology. 2007;148(5):2181–2188. doi: 10.1210/en.2006-1355. [DOI] [PubMed] [Google Scholar]
- 22.Tikellis C, Wookey PJ, Candido R, Andrikopoulos S, Thomas MC, Cooper ME. Improved islet morphology after blockade of the reninangiotensin system in the ZDF rat. Diabetes. 2004;53(4):989–997. doi: 10.2337/diabetes.53.4.989. [DOI] [PubMed] [Google Scholar]
- 23.Hayden MR. Islet amyloid and fibrosis in the cardiometabolic syndrome and type 2 diabetes mellitus. J Cardiometab Syndr. 2007;2(1):70–75. doi: 10.1111/j.1559-4564.2007.06159.x. [DOI] [PubMed] [Google Scholar]
- 24.Hughes SJ, Clark A, McShane P, Contractor HH, Gray DW, Johnson PR. Characterization of collagen VI within the islet-exocrine interface of the human pancreas: implications for clinical islet isolation? Transplantation. 2006;81(3):423–426. doi: 10.1097/01.tp.0000197482.91227.df. [DOI] [PubMed] [Google Scholar]
- 25.Bertelli E, Bendayan M. Association between endocrine pancreas and ductal system. More than an epiphenomenon of endocrine differentiation and development? J Histochem Cytochem. 2005;53(9):1071–1086. doi: 10.1369/jhc.5R6640.2005. [DOI] [PubMed] [Google Scholar]
- 26.Leung PS, Carlsson PO. Pancreatic islet renin angiotensin system: its novel roles in islet function and in diabetes mellitus. Pancreas. 2005;30(4):293–298. doi: 10.1097/01.mpa.0000158028.76666.76. [DOI] [PubMed] [Google Scholar]
- 27.Ozerdem U, Stallcup WB. Early contribution of pericytes to angiogenic sprouting and tube formation. Angiogenesis. 2003;6(3):241–249. doi: 10.1023/B:AGEN.0000021401.58039.a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97(6):512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- 29.Rajantie I, Ilmonen M, Alminaite A, Ozerdem U, Alitalo K, Salven P. Adult bone marrow-derived cells recruited during angiogenesis comprise precursors for periendothelial vascular mural cells. Blood. 2004;104(7):2084–2086. doi: 10.1182/blood-2004-01-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ozerdem U, Alitalo K, Salven P, Li A. Contribution of bone marrow-derived pericyte precursor cells to corneal vasculogenesis. Invest Ophthalmol Vis Sci. 2005;46(10):3502–3506. doi: 10.1167/iovs.05-0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shojaee N, Patton WF, Hechtman HB, Shepro D. Myosin translocation in retinal pericytes during free-radical induced apoptosis. J Cell Biochem. 1999;75(1):118–129. [PubMed] [Google Scholar]
- 32.Yamagishi S, Takeuchi M, Matsui T, Nakamura K, Imaizumi T, Inoue H. Angiotensin II augments advanced glycation end product-induced pericyte apoptosis through RAGE overexpression. FEBS Lett. 2005;579(20):4265–4270. doi: 10.1016/j.febslet.2005.06.058. [DOI] [PubMed] [Google Scholar]
- 33.Brignardello E, Beltramo E, Molinatti PA, Aragno M, Gatto V, Tamagno E, Danni O, Porta M, Boccuzzi G. Dehydroepiandrosterone protects bovine retinal capillary pericytes against glucose toxicity. J Endocrinol. 1998;158(1):21–26. doi: 10.1677/joe.0.1580021. [DOI] [PubMed] [Google Scholar]
- 34.Gerhardt H, Betsholtz C. Endothelial-pericyte interactions in angio-genesis. Cell Tissue Res. 2003;14(1):15–23. doi: 10.1007/s00441-003-0745-x. [DOI] [PubMed] [Google Scholar]
- 35.Farrington-Rock C, Crofts NJ, Doherty MJ, Ashton BA, Griffin-Jones C, Canfield AE. Chondrogenic and adipogenic potential of micro-vascular pericytes. Circulation. 2004;110(15):2226–2232. doi: 10.1161/01.CIR.0000144457.55518.E5. [DOI] [PubMed] [Google Scholar]
- 36.Davidoff MS, Middendorff R, Enikolopov G, Riethmacher D, Holstein AF, Müller D. Progenitor cells of the testosterone-producing Leydig cells revealed. J Cell Biol. 2004;167(5):935–944. doi: 10.1083/jcb.200409107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, et al. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007;9(3):255–267. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- 38.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119(Pt 11):2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 39.Hammes HP, Lin J, Renner O, Shani M, Lundqvist A, Betsholtz C, Brownlee M, Deutsch U. Pericytes and the pathogenesis of diabetic retinopathy. Diabetes. 2002;51(10):3107–3112. doi: 10.2337/diabetes.51.10.3107. [DOI] [PubMed] [Google Scholar]
- 40.Zhao HL, Lai FM, Tong PC, Zhong DR, Yang D, Tomlinson B, Chan JC. Prevalence and clinicopathological characteristics of islet amyloid in Chinese patients with type 2 diabetes. Diabetes. 2003;52(11):2759–2766. doi: 10.2337/diabetes.52.11.2759. [DOI] [PubMed] [Google Scholar]