Abstract
Background
Longitudinal neuroimaging investigations of antidepressant treatment offer the opportunity to identify potential baseline biomarkers associated with poor outcome.
Methods
To explore the neural correlates of nonresponse to cognitive behavioural therapy (CBT) or venlafaxine (VEN), we compared pretreatment (18)F-fluoro-2-deoxy-d-glucose positron emission tomography scans of participants with major depressive disorder responding to either 16 weeks of CBT (n = 7) or VEN treatment (n = 9) with treatment nonresponders (n = 8).
Results
Nonresponders to CBT or VEN, in contrast to responders, exhibited pretreatment hypermetabolism at the interface of the pregenual and subgenual cingulate cortices.
Limitations
Limitations of our study include the small sample sizes and the absence of both arterial sampling to determine absolute glucose metabolism and high-resolution structural magnetic resonance imaging coregistration for region-of-interest analyses.
Conclusion
Our current findings are consistent with those reported in previous studies of relative hyperactivity in the ventral anterior cingulate cortex in treatment-resistant populations.
Abstract
Contexte
Les études de neuro-imagerie longitudinales pendant le traitement antidépresseur offrent la possibilité de repérer certains biomarqueurs de base susceptibles d’être associés à une évolution moins favorable.
Méthodes
Pour explorer les corrélats neuraux propres au phénomène de résistance à la thérapie cognitivo-comportementale (TCC) ou au traitement par venlafaxine (VEN), nous avons comparé les tomographies par émission de positrons au (18)F-fluoro-2-désoxy-d-glucose préthérapeutiques de participants atteints de troubles dépressifs majeurs qui ont répondu soit à 16 semaines de TCC (n = 7), soit à un traitement par VEN (n = 9), à celles de participants n’ayant pas répondu au traitement (n = 8).
Résultats
Comparativement aux participants ayant répondu au traitement, ceux qui n’ont répondu ni à la TCC ni à la VEN présentaient en prétraitement un hypermétabolisme au niveau de l’interface du cortex cingulaire ventro-antérieur du genou du corps calleux.
Limites
Les limites de notre étude sont notamment la petite taille des échantillons et l’absence à la fois de prélèvements artériels pour déterminer le métabolisme absolu du glucose et d’enregistrements simultanés d’imagerie par résonance magnétique structurelle de haute résolution à des fins d’analyse des régions concernées.
Conclusion
Nos résultats actuels concordent avec ceux d’études antérieures sur l’hyperactivité relative du cortex cingulaire ventro-antérieur dans le traitement des populations réfractaires au traitement.
Introduction
The 2 most established acute treatment modalities for major depressive disorder (MDD) are pharmacotherapy and evidence-based psychotherapy, particularly cognitive behavioural therapy (CBT). Both have roughly comparable outcomes.1,2 Nevertheless, up to 50% of patients fail to achieve an adequate response, and even fewer achieve remission following an acute treatment trial.3 Despite advances in neurosciences, cognitive sciences and psychopharmacology, there is no current algorithm to guide optimal treatment selection for individual patients.4,5
Response prediction based on clinical parameters, including symptom clusters or depressive subtype, has yielded disappointing results.6 Early neurobiological predictors, including neuroendocrine markers7,8 and electrophysiological recordings,9,10 have not had a substantial impact on treatment selection, although 2 rapidly advancing techniques that may offer superior predictive value are pharmacogenetics11,12 and functional neuroimaging.13,14
Neuroimaging investigations employing (18)F-fluoro-2-deoxy-d-glucose positron emission tomography and electroencephalography suggest that baseline metabolism in the pregenual cingulate (Brodmann area [BA] 24) and subgenual cingulate (BA 24/25) cortices may predict response to various antidepressant interventions including pharmacotherapy,15–19 sleep deprivation20 and cingulotomy.21 In 2 of 4 pharmacotherapy investigations, lower pretreatment metabolic activity in the anterior cingulate cortex (ACC) predicted favourable response, whereas higher activity in the pregenual ACC predicted response in the other 2.17,18 To date, there have been fewer investigations of metabolic changes following psychological interventions,22–25 and these have not distinguished between treatment responders and nonresponders.
We have previously reported on the differential effects of venlafaxine (VEN) and CBT in altering brain glucose metabolism following a 16-week randomized controlled trial to treat MDD.26 However, there was no assessment of baseline scans as potential predictors of response or nonresponse. The purpose of the present analysis is to examine baseline metabolism in the same population as a predictor of anti-depressant nonresponse to CBT and VEN in this clinical population. We hypothesized that baseline metabolism in either the pregenual or subgenual cingulate cortices would have predictive value.
Methods
We recruited patients aged 20–50 years at the Centre for Addiction and Mental Health at the University of Toronto, Toronto, Ont. Participants were required to meet the DSM-IV criteria for MDD in the context of a current major depressive episode, as assessed by the Structured Clinical Interview for DSM-IV, patient version (SCID-IP),27 and score 20 or greater on the Hamilton Rating Scale for Depression, 17-item version (HAMD-17).28 Antidepressant medication-free status for at least 2 weeks (4 weeks for fluoxetine) preceding the study and good physical health with no evidence of neurologic or other unstable medical conditions were additional inclusion criteria. Other Axis I diagnoses, including concurrent anxiety disorders and substance abuse or dependence within the 6 months preceding the study, evidence of active suicidal ideation, pregnancy and previous failure to respond to an adequate trial of CBT or VEN were exclusion criteria. All participants provided written informed consent. The Research Ethics Board of the Centre for Addiction and Mental Health approved our study.
We randomly assigned participants to receive either VEN (75–225 mg/d) or CBT for 16 weeks. We assessed the severity of depressive symptoms using the HAMD-17. We defined response to treatment as a minimum reduction of 50% in HAMD-17 scores from baseline to end point.29
We obtained positron emission tomography measurements of regional cerebral glucose metabolism within 1 week before treatment initiation and within 1 week of the last treatment visit. For each scan, we injected a 5-mCi (185-Mbq) dose of (18)F-fluoro-2-deoxy-d-glucose intravenously, with image acquisition beginning after 40 minutes (PC 2048b; GEMS-Scanditronix). We acquired all scans at a consistent time, between 9 am and noon, with participants in a supine, awake and resting state with eyes closed and ears uncovered. We asked participants to refrain from food, coffee or alcohol intake for a minimum of 8 hours before each scan session.
We gave participants no explicit cognitive instructions, but we asked them to avoid ruminating on any one topic during the (18)F-fluoro-2-deoxy-d-glucose uptake period. A member of the research staff monitored wakefulness every 10 minutes. We acquired emission data during a 35-minute period (about 1 million counts per slice; 10-cm field of view). We used a customized, thermoplastic face mask to minimize head movement. We corrected raw images (15 parallel slices; 6.5-mm centre-to-centre interslice distance) for attenuation, and we reconstructed and smoothed them to a final in-plane resolution of 7.0 mm at full width at half maximum.
Statistical analysis
We perfomed all statistical analyses using SPM99 statistical software (Wellcome Department of Cognitive Neurology, London, England) and Matlab (version 7.4; Mathworks Inc.). We normalized all scans to the Montreal Neurological Institute ICBM 152 stereotactic template within SPM99. We normalized the scans for differences in whole-brain global metabolism by setting the mean voxel value of each image to 1.0, and we smoothed them, using a Gaussian kernel, to a final in-plane resolution of 12 mm at full width at half maximum. We did not calculate absolute glucose metabolic rates.
For the regions of the pregenual and subgenual cingulate cortices defined a priori, we evaluated clusters meeting the “minimum expected cluster size in SPM” (k > 74) and the uncorrected p < 0.01 height threshold for differences in relative regional glucose metabolism between nonresponder and responder groups. We also evaluated supplementary between-group comparisons across the whole brain (CBT nonresponders v. responders and VEN nonresponders v. responders) at an uncorrected p < 0.001 height threshold. We converted the resulting F values to z scores, with brain locations reported as x, y, and z coordinates in Montreal Neurological Institute space with approximate BA identified by mathematical transformation of SPM99 coordinates into Talairach space.26
Results
Thirty-one patients (13 men and 18 women) participated in our study. Of these, we randomly assigned 14 to the VEN group and 17 to the CBT group. After random assignment, 4 patients failed to return to initiate treatment (CBT, n = 3; VEN, n = 1). During the 16-week treatment phase, 1 patient discontinued VEN and 2 discontinued CBT owing to lack of efficacy. Characteristics of the remaining 12 patients in each group are provided in Table 1.
Table 1.
Characteristics of responders and nonresponders to treatment with venlafaxine or cognitive behavioural therapy for major depressive disorder
| Treatment group
|
||||
|---|---|---|---|---|
| Venlafaxine
|
Cognitive behavioural therapy
|
|||
| Characteristic | Responders | Nonresponders | Responders | Nonresponders |
| No. of patients | 9 | 3 | 7 | 5 |
| Age, mean (SD) yr | 40.1 (8.6) | 37.8 (12.0) | 32.7 (11.4) | 26.2 (6.1) |
| Female sex, % | 44.4 | 100.0 | 71.4 | 40.0 |
| HAMD-17 score, mean (SD) | ||||
| Baseline | 20.0 (3.2) | 21.0 (2.9) | 19.6 (3.5) | 22.0 (3.2) |
| End point | 4.1 (1.1) | 13.8 (1.3) | 5.4 (3.8) | 18.4 (3.9) |
HAMD-17 = Hamilton Rating Scale for Depression, 17-item version28; SD = standard deviation.
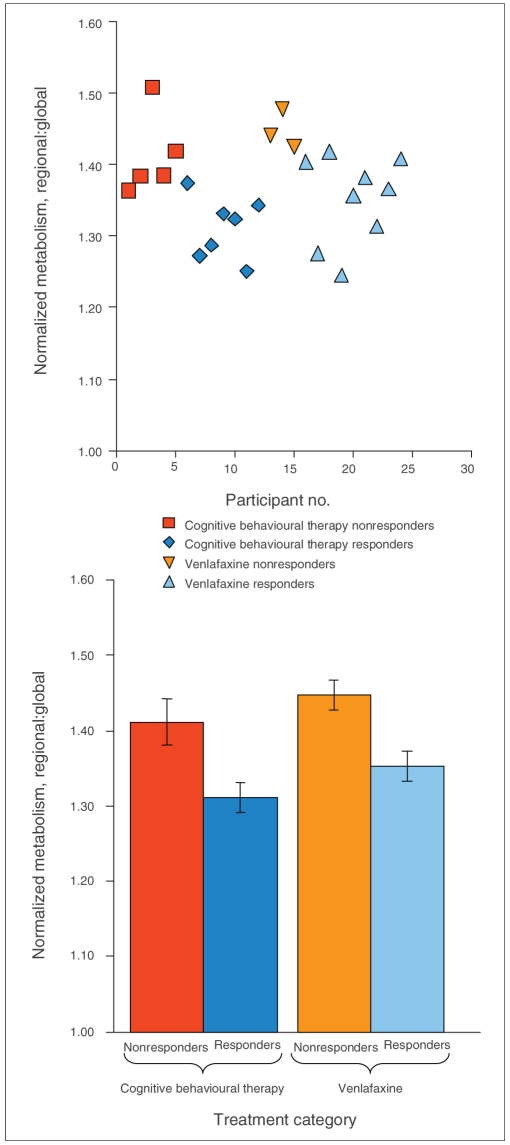
Nine participants treated with VEN and 7 treated with CBT responded to treatment. At baseline, there were no statistically significant differences in age or HAMD-17 scores between eventual responders and nonresponders (Table 1).
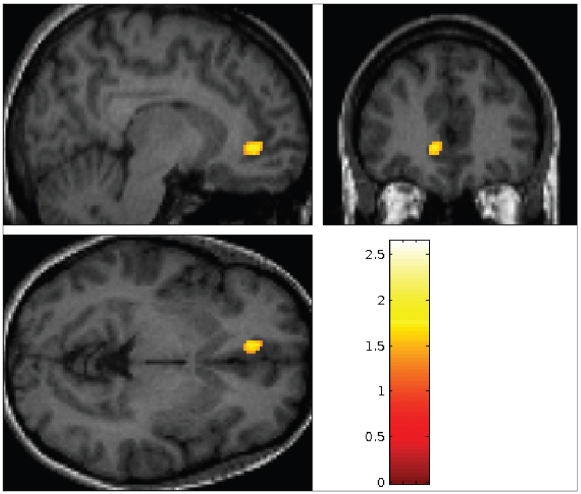
Nonresponders to either treatment modality displayed hypermetabolism at the interface between the pregenual and subgenual cingulate cortices (ventral ACC, BA 24/32), in contrast to responders (Fig. 1, Fig. 2, Table 2) (p < 0.012). We noted no other statistically significant differences in glucose metabolism between the group of pooled responders (n = 16) and nonresponders (n = 8).
Fig. 1.
Ventral anterior cingulate cortex (Montreal Neurological Institute coordinates −10, 40, −04) hypermetabolism in non-responders to either treatment (pooled nonresponders, n = 8 > pooled responders, n = 16, p < 0.01 uncorrected, k > 74).
Fig. 2.
Hypermetabolism in nonresponders to either treatment (p > 0.01 uncorrected, k > 74).
Table 2.
Between-group differences in normalized glucose metabolism
| Coordinates
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Group; brain region | L/R | BA | Direction of change | x | y | z | Voxels in cluster | z score* |
| All nonresponders v. responders | ||||||||
| Ventral anterior cingulate cortex | L | 24/32 | ↑ | −10 | 40 | −4 | 79 | 3.04 |
| CBT nonresponders v. responders | ||||||||
| Dorsal occipital cortex | R | 19 | ↑ | 36 | −88 | 22 | 115 | 3.82 |
| Parahippocampal gyrus | R | 36/37 | ↑ | 20 | −42 | −14 | 67 | 3.54 |
| VEN nonresponders v. responders | ||||||||
| Cerebellum | L | — | ↓ | −28 | −82 | −32 | 60 | 3.58 |
BA = Brodmann area; CBT = cognitive behavioural therapy; L = left; R = right; VEN = venlafaxine.
Scores above 2.34 correspond to p < 0.01 uncorrected; scores above 3.12 correspond to p < 0.001 uncorrected.
Subsequent analyses focused on responder/nonresponder differences within each treatment arm. Unique to the CBT groups, nonresponders (in contrast to responders) also displayed hypermetabolism in the parahippocampal gyrus (BA 36/37) (p < 0.001) and dorsal occipital cortex (BA 19) (p < 0.001) (Table 2). In the VEN group, decreased baseline metabolism in the cerebellum differentiated eventual non-responders from responders (p < 0.001) (Table 2). It should be noted that owing to the small sample size involved, these reported differences must be considered very preliminary, and will not be discussed further, but are included for completeness.
Discussion
Depressed participants who did not respond to treatment displayed relative hyperactivity in a region at the interface between the pregenual and subgenual cingulate cortices, in contrast to responders.
Pre–post investigations of changes in brain glucose metabolism during antidepressant response to disparate treatment modalities have consistently noted decreases in metabolism in the subgenual cingulate cortex.22,26,30–35 Differences in baseline metabolism within subdivisions of the anterior cingulate gyrus have also been reported as predictors of nonresponse to treatment with selective serotonin reuptake inhibitors and selective norepinephrine reuptake inhibitors. Hyperactivity in the subgenual portions of the ACC was associated with treatment nonresponse,16,36 as was hypoactivity of the pregenual (rostral) ACC.17,18,37
Historically, the primary function ascribed to the ACC is affective processing.38 Investigations of cytoarchitecture, connectivity and function of the ACC have divided the region that encompasses BAs 24, 25, 32 and 33 into 2 subdivisions with distinct functions: a dorsal cognitive division and a rostral–ventral affective division.39–41 Mayberg42 has hypothesized that the pregenual ACC represents an interface between these 2 subdivisions.
Using functional magnetic resonance imaging, hyperactivation of the subgenual cingulate cortex in response to emotional stimuli in participants with MDD (n = 14) was associated with poor response to 16 sessions of CBT.43 These findings raise the possibility that hyperactivity in the subgenual cingulate cortex predicts treatment resistance to both pharmacotherapy and psychotherapy. Indeed, in an evaluation of participants with MDD who had failed to respond to a minimum of 4 different antidepressant treatments, elevated blood flow in the subgenual cingulate cortex was noted in contrast to a healthy control group.34 Hyperactivity in the ventral ACC has also been associated with other medication-resistant depressed populations seeking alternative anti-depressant treatments.20,21
Mood and anxiety disorders are associated with dysfunctional limbic–cortical interactions, with illness remission being conceptualized as appropriate network modulation by various forms of treatment.33 Furthermore, it has been proposed that an initial modulation of subcortical targets may be a necessary first step that facilitates subsequent adaptive changes in network homeostasis.14 In support of this model, an evaluation of more than 100 depressed patients revealed differences in connectivity between the dorsolateral prefrontal (BA 9), subgenual cingulate (BA 25) and orbitofrontal (BA 11) cortices between pharmacotherapy responders and nonresponders. Similarly, limbic–cortical connectivity also differentiated CBT responders from pharmacotherapy responders.44 Our results are consistent with previous glucose investigations evaluating response to antidepressant treatment in patients with MDD in that nonresponders in our study demonstrated abnormalities in limbic–subcortical pathways involving parts of the pregenual (BA 24) and subgenual (BA 25) cortices and the ACC.13,15–17,20,43,45
Limitations
Limitations of our study include the small sample sizes, particularly in the within-treatment comparisons, which limited our ability to detect low-magnitude differences. As such, our within-treatment comparison findings must be regarded as preliminary. Other limitations include the absence of both arterial sampling to determine absolute glucose metabolism and high-resolution structural magnetic resonance imaging coregistration for region-of-interest analyses. Additionally, it is worth noting that the methods employed in our analysis were more likely to identify baseline group differences after treatment than individual differences that could guide treatment selection.
Emergent evidence from cerebral blood flow, glucose metabolism and blood oxygenation studies indicates that alteration in subgenual cingulate cortex activity alone, or in concert with other limbic or cortical targets, may mediate severity of depressive symptoms and resistance to treatment.14,44 Our report of hyperactivity in the pregenual and subgenual cortices of the ACC as a marker of nonresponse to both pharmacotherapy and psychotherapy further corroborates these results and complements extant models that emphasize comparable and distinct neural connectivity mediating the therapeutic effects of CBT and medication.44,45
Acknowledgements
We thank Doug Hussey, BSc; Alvina Ng, BSc; and Alan A. Wilson, PhD, from the PET Centre, University of Toronto. This study was supported by the Canadian Institutes of Health Research (# 86023 H.S.M., S.H.K., Z.V.S.) and Wyeth Pharmaceuticals (S.H.K., H.S.M., Z.V.S.).
Footnotes
Competing interests: This study was supported by the Canadian Institutes of Health Research and Wyeth Pharmaceuticals. None declared for Mr. Konarski and Drs. Segal, Lau and Bieling. Dr. Kennedy has conducted paid consultancies for Pfizer, Servier and Wyeth; received research support from ANS, AstraZeneca, CIHR, Eli Lilly, GlaxoSmithKline, Lundbeck, NARSAD, OMHF, OPGRS and the Stanley Foundation; and has received speaker fees from ANS, Astra-Zeneca, Biovail, Eli Lilly, Lundbeck, Servier and Wyeth. Dr. McIntyre sits on the advisory boards of AstraZeneca, Bristol-Myers Squibb, France Foundation, GlaxoSmithKline, Janssen-Ortho, Solvay/Wyeth, Eli Lilly, Organon, Lundbeck, Biovail, Pfizer and Shire; he has received speaker fees from Janssen-Ortho, AstraZeneca, Eli Lilly, Lundbeck and Biovail and research support from Eli Lilly. Dr. Mayberg has consulted for Advanced Neuromodulation Systems Inc.
Contributors: Drs. Mayberg, Kennedy and Segal designed the study. Mr. Konarski and Drs. Segal, Lau, Bieling and Mayberg acquired the data. Mr. Konarski and Drs. Kennedy and Mayberg analyzed the data. Mr. Konarski and Drs. Kennedy and McIntyre wrote the paper. Mr. Konarski and Drs. Kennedy, Segal, Lau, Bieling, McIntyre and Mayberg reviewed the article. All authors approved final publication.
References
- 1.DeRubeis RJ, Hollon SD, Amsterdam JD, et al. Cognitive therapy vs medications in the treatment of moderate to severe depression. Arch Gen Psychiatry. 2005;62:409–16. doi: 10.1001/archpsyc.62.4.409. [DOI] [PubMed] [Google Scholar]
- 2.Hollon SD, DeRubeis RJ, Shelton RC, et al. Prevention of relapse following cognitive therapy vs medications in moderate to severe depression. Arch Gen Psychiatry. 2005;62:417–22. doi: 10.1001/archpsyc.62.4.417. [DOI] [PubMed] [Google Scholar]
- 3.Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–17. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 4.Hasler G, Drevets WC, Manji HK, et al. Discovering endophenotypes for major depression. Neuropsychopharmacology. 2004;29:1765–81. doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- 5.Hollon SD, Jarrett RB, Nierenberg AA, et al. Psychotherapy and medication in the treatment of adult and geriatric depression: Which monotherapy or combined treatment? J Clin Psychiatry. 2005;66:455–68. doi: 10.4088/jcp.v66n0408. [DOI] [PubMed] [Google Scholar]
- 6.Joyce PR, Paykel ES. Predictors of drug response in depression. Arch Gen Psychiatry. 1989;46:89–99. doi: 10.1001/archpsyc.1989.01810010091014. [DOI] [PubMed] [Google Scholar]
- 7.Amsterdam JD, Fava M, Maislin G, et al. TRH stimulation test as a predictor of acute and long-term antidepressant response in major depression. J Affect Disord. 1996;38:165–72. doi: 10.1016/0165-0327(96)00010-9. [DOI] [PubMed] [Google Scholar]
- 8.Hollander E, Stein DJ, DeCaria CM, et al. A pilot study of biological predictors of treatment outcome in obsessive-compulsive disorder. Biol Psychiatry. 1993;33:747–9. doi: 10.1016/0006-3223(93)90126-x. [DOI] [PubMed] [Google Scholar]
- 9.Gallinat J, Bottlender R, Juckel G, et al. The loudness dependency of the auditory evoked N1/P2-component as a predictor of the acute SSRI response in depression. Psychopharmacology (Berl) 2000;148:404–11. doi: 10.1007/s002130050070. [DOI] [PubMed] [Google Scholar]
- 10.Knott VJ, Telner JI, Lapierre YD, et al. Quantitative EEG in the prediction of antidepressant response to imipramine. J Affect Disord. 1996;39:175–84. doi: 10.1016/0165-0327(96)00003-1. [DOI] [PubMed] [Google Scholar]
- 11.Eichelbaum M, Ingelman-Sundberg M, Evans WE. Pharmacogenomics and individualized drug therapy. Annu Rev Med. 2006;57:119–37. doi: 10.1146/annurev.med.56.082103.104724. [DOI] [PubMed] [Google Scholar]
- 12.Malhotra AK, Murphy GM, Jr, Kennedy JL. Pharmacogenetics of psychotropic drug response. Am J Psychiatry. 2004;161:780–96. doi: 10.1176/appi.ajp.161.5.780. [DOI] [PubMed] [Google Scholar]
- 13.Evans KC, Dougherty DD, Pollack MH, et al. Using neuroimaging to predict treatment response in mood and anxiety disorders. Ann Clin Psychiatry. 2006;18:33–42. doi: 10.1080/10401230500464661. [DOI] [PubMed] [Google Scholar]
- 14.Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull. 2003;65:193–207. doi: 10.1093/bmb/65.1.193. [DOI] [PubMed] [Google Scholar]
- 15.Brody AL, Saxena S, Mandelkern MA, et al. Brain metabolic changes associated with symptom factor improvement in major depressive disorder. Biol Psychiatry. 2001;50:171–8. doi: 10.1016/s0006-3223(01)01117-9. [DOI] [PubMed] [Google Scholar]
- 16.Little JT, Ketter TA, Kimbrell TA, et al. Venlafaxine or bupropion responders but not nonresponders show baseline prefrontal and paralimbic hypometabolism compared with controls. Psychopharmacol Bull. 1996;32:629–35. [PubMed] [Google Scholar]
- 17.Mayberg HS, Brannan SK, Mahurin RK, et al. Cingulate function in depression: a potential predictor of treatment response. Neuroreport. 1997;8:1057–61. doi: 10.1097/00001756-199703030-00048. [DOI] [PubMed] [Google Scholar]
- 18.Pizzagalli D, Pascual-Marqui RD, Nitschke JB, et al. Anterior cingulate activity as a predictor of degree of treatment response in major depression: evidence from brain electrical tomography analysis. Am J Psychiatry. 2001;158:405–15. doi: 10.1176/appi.ajp.158.3.405. [DOI] [PubMed] [Google Scholar]
- 19.Pizzagalli D, Oakes T, Fox A, et al. Functional but not structural subgenual prefrontal cortex abnormalities in melancholia. Mol Psychiatry. 2004;9:393–405. doi: 10.1038/sj.mp.4001501. [DOI] [PubMed] [Google Scholar]
- 20.Wu J, Buchsbaum MS, Gillin JC, et al. Prediction of antidepressant effects of sleep deprivation by metabolic rates in the ventral anterior cingulate and medial prefrontal cortex. Am J Psychiatry. 1999;156:1149–58. doi: 10.1176/ajp.156.8.1149. [DOI] [PubMed] [Google Scholar]
- 21.Dougherty DD, Weiss AP, Cosgrove GR, et al. Cerebral metabolic correlates as potential predictors of response to anterior cingulotomy for treatment of major depression. J Neurosurg. 2003;99:1010–7. doi: 10.3171/jns.2003.99.6.1010. [DOI] [PubMed] [Google Scholar]
- 22.Brody AL, Saxena S, Stoessel P, et al. Regional brain metabolic changes in patients with major depression treated with either paroxetine or interpersonal therapy: preliminary findings. Arch Gen Psychiatry. 2001;58:631–40. doi: 10.1001/archpsyc.58.7.631. [DOI] [PubMed] [Google Scholar]
- 23.Martin SD, Martin E, Rai SS, et al. Brain blood flow changes in depressed patients treated with interpersonal psychotherapy or venlafaxine hydrochloride: preliminary findings. Arch Gen Psychiatry. 2001;58:641–8. doi: 10.1001/archpsyc.58.7.641. [DOI] [PubMed] [Google Scholar]
- 24.Roffman JL, Marci CD, Glick DM, et al. Neuroimaging and the functional neuroanatomy of psychotherapy. Psychol Med. 2005;35:1385–98. doi: 10.1017/S0033291705005064. [DOI] [PubMed] [Google Scholar]
- 25.Deldin PJ, Chiu P. Cognitive restructuring and EEG in major depression. Biol Psychol. 2005;70:141–51. doi: 10.1016/j.biopsycho.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Kennedy SH, Konarski JZ, Segal ZV, et al. Differences in Brain Glucose Metabolism Between Responders to CBT and Venlafaxine in a 16-Week Randomized Controlled Trial. Am J Psychiatry. 2007;164:778–88. doi: 10.1176/ajp.2007.164.5.778. [DOI] [PubMed] [Google Scholar]
- 27.First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York (NY): Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 28.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frank E, Prien R, Jarrett R, et al. Conceptualization and rationale for consensus definitions of terms in major depressive disorder. Remission, recovery, relapse, and recurrence. Arch Gen Psychiatry. 1991;48:851–5. doi: 10.1001/archpsyc.1991.01810330075011. [DOI] [PubMed] [Google Scholar]
- 30.Liotti M, Mayberg H, McGinnis S, et al. Unmasking disease-specific cerebral blood flow abnormalities: mood challenge in patients with remitted unipolar depression. Am J Psychiatry. 2002;159:1830–40. doi: 10.1176/appi.ajp.159.11.1830. [DOI] [PubMed] [Google Scholar]
- 31.Mayberg HS, Liotti MS, Brannan S, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675–82. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- 32.Mayberg HS, Brannan SK, Tekell JL, et al. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry. 2000;48:830–43. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- 33.Mayberg HS. Modulating limbic-cortical circuits in depression: targets of antidepressant treatments. Semin Clin Neuropsychiatry. 2002;7:255–68. doi: 10.1053/scnp.2002.35223. [DOI] [PubMed] [Google Scholar]
- 34.Mayberg HS, Lozano A, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–60. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 35.Nobler MS, Oquendo MA, Kegeles LS, et al. Decreased regional brain metabolism after ect. Am J Psychiatry. 2001;158:305–8. doi: 10.1176/appi.ajp.158.2.305. [DOI] [PubMed] [Google Scholar]
- 36.Brody A, Saxena S, Silverman D, et al. Brain metabolic changes in major depressive disorder from pre- to post-treatment with paroxetine. Psychiatry Res. 1999;91:127–39. doi: 10.1016/s0925-4927(99)00034-7. [DOI] [PubMed] [Google Scholar]
- 37.Saxena S, Brody AL, Ho ML, et al. Differential brain metabolic predictors of response to paroxetine in obsessive-compulsive disorder versus major depression. Am J Psychiatry. 2003;160:522–32. doi: 10.1176/appi.ajp.160.3.522. [DOI] [PubMed] [Google Scholar]
- 38.Papez J. A proposed mechanism of emotion. Arch Neurol Psychiatry. 1937;38:725–44. [Google Scholar]
- 39.Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–22. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 40.Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- 41.Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb Cortex. 1992;2:435–43. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
- 42.Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. J Neuropsychiatry Clin Neurosci. 1997;9:471–81. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- 43.Siegle GJ, Carter CS, Thase ME. Use of FMRI to predict recovery from unipolar depression with cognitive behavior therapy. Am J Psychiatry. 2006;163:735–8. doi: 10.1176/ajp.2006.163.4.735. [DOI] [PubMed] [Google Scholar]
- 44.Seminowicz DA, Mayberg HS, McIntosh AR, et al. Limbic-frontal circuitry in major depression: a path modeling metanalysis. Neuroimage. 2004;22:409–18. doi: 10.1016/j.neuroimage.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 45.Goldapple K, Segal Z, Garson C, et al. Modulation of cortical-limbic pathways in major depression: treatment-specific effects of cognitive behavior therapy. Arch Gen Psychiatry. 2004;61:34–41. doi: 10.1001/archpsyc.61.1.34. [DOI] [PubMed] [Google Scholar]