Abstract
Background
Pericentrin (PCNT) interacts with disruption-in-schizophrenia 1 (DISC1), a known genetic risk factor for schizophrenia, bipolar disorder and major depressive disorder (MDD). We sought to determine whether the PCNT gene is implicated in MDD.
Methods
We performed case–control association analyses in the Japanese population. We analyzed 9 single nucleotide polymorphisms (SNPs) in 173 patients with MDD and 348 healthy controls.
Results
We found a significant allelic association between 3 SNPs (rs3788265, rs2073376 and rs2073380) of the PCNT gene and MDD (p = 0.006, 0.005 and 0.021, respectively). After correction for multiple testing, 2 SNPs (rs3788265 and rs2073376) retained significant allelic associations with MDD. In addition, we found a significant association between the 2 marker haplotypes (r3788265 and rs2073376) and MDD (permutation p = 0.011).
Limitations
Our sample was small and comprised only Japanese participants. In addition, owing to the late onset of MDD, it is possible that the disorder will develop in at least some participants in our control group. Finally, we did not show how SNPs of the PCNT gene alter its function.
Conclusion
Our results suggest that genetic variations in the PCNT gene may play a significant role in the etiology of MDD in the Japanese population.
Abstract
Contexte
La péricentrine interagit avec un facteur reconnu de risque génétique de schizophrénie, de trouble bipolaire et de trouble dépressif majeur, le DISC1 (pour disruption-in-schizophrenia 1). Nous avons voulu vérifier si le gène de la péricentrine est associé au trouble dépressif majeur.
Méthodes
Nous avons effectué des analyses d’association cas–témoins dans la population japonaise. Nous avons analysé 9 polymorphismes de nucléotides simples (PNS) chez 173 patients atteints de trouble dépressif majeur et chez 348 témoins en bonne santé.
Résultats
Nous avons découvert une association allélique significative entre 3 PNS (le rs3788265, le rs2073376 et le rs2073380) du gène de la péricentrine et le trouble dépressif majeur (p = 0,006, 0,005 et 0,021, respectivement). Après correction pour tests d’hypothèses multiples, 2 PNS (le rs3788265 et le rs2073376) maintenaient des liens alléliques significatifs avec le trouble dépressif majeur. De plus, nous avons observé un lien entre les 2 haplotypes marqueurs (le r3788265 et le rs2073376) et le trouble dépressif majeur (permutation p = 0,011).
Limites
Notre échantillon était de petite taille et les participants étaient tous japonais. En outre, compte tenu du déclenchement tardif du trouble dépressif majeur, il est possible que la maladie frappe éventuellement au moins quelques-uns des participants de notre groupe témoin. Finalement, nous n’avons pas démontré de quelle façon les PNS du gène de la péricentrine en modifient la fonction.
Conclusion
Selon nos résultats, les variations génétiques du gène de la péricentrine pourraient jouer un rôle important dans l’étiologie du trouble dépressif majeur chez la population japonaise.
Introduction
The lifetime population prevalence for major depressive disorder (MDD) is 5%–10%. Heritability based on twin studies is 40%–50%, and adoption studies provide some support for a role for genetic factors in MDD.1 The pericentrin (PCNT) gene, also called the kendrin gene, is located at 21q22.3, a region of susceptibility for mood disorders.2, 3
Disrupted-in-schizophrenia 1 (DISC1) is an important genetic risk factor for mental disorders such as schizophrenia,4, 5 bipolar disorder6 and MDD.7 It localizes to the centrosome by binding to PCNT, and PCNT anchors the γ-tubulin complex to the centrosome, providing microtubule nucleation sites. Thus DISC1–PCNT interaction might be involved in the pathophysiology of mental disorders owing to their putative effect on centrosomal function.8
Recently, Anitha and colleagues9 reported that significantly higher expression of PCNT was observed in brain samples of patients with bipolar disorder compared with controls and that the mRNA expression of the PCNT gene in peripheral blood lymphocytes was significantly higher in patients with bipolar disorder and MDD than in controls.
Taken together, these findings suggest that the PCNT gene may be a candidate gene in MDD. We sought to determine whether the PCNT gene is implicated in MDD in the Japanese population.
Methods
Participants
We recruited biologically unrelated Japanese patients from Tokushima University and the Ehime University Hospital in Japan. At least 2 experienced psychiatrists (S.N., J.I., M.N., S.U., and T.O.) diagnosed MDD according to DSM-IV criteria10 on the basis of extensive clinical interviews and a review of medical records. All patients underwent medical, neurologic, psychological and laboratory evaluation before participating in this study. We excluded patients with organic disorders or with other comorbid psychiatric disturbances (e.g., alcohol or substance abuse). We recruited volunteers for the control group among hospital staff, students and company employees. We included those who were genetically unrelated residents living in Japan with no personal or family history (among first-degree relatives) of mental disorders. For the genetic studies, we obtained genomic DNA samples from the participants. All participants provided written informed consent, and the institutional ethics committees approved our genetic association studies.
Genetic analysis
We performed the genotyping using commercially available TaqMan probes for the PCNT gene with the 7500 Fast Real-Time PCR System, according to the protocol recommended by the manufacturer (Applied Biosystems). We selected 8 tagging single nucleotide polymorphisms (SNPs) using SNP Browser 3.5 (Applied Biosystems; pair-wise r2 > 85%, minor allele frequency > 20%, Japanese population): rs11702684, rs2249057, rs11701058, rs2839226, rs2839231, rs3788265, rs2073376 and rs1010111. We additionally selected rs2073380, because the 8 tagging SNPs did not cover the third block of the PNCT2 gene from HapMap data. We determined haplotype block structure using the Haploview program.11 Blocks were defined according to the criteria set by Gabriel and colleagues.12
Statistical analysis
We compared the allelic and genotypic frequencies of patients and controls using the Fisher exact test. In single-marker analyses, we used the program SNPSpD,13 which is able to reflect the correlation of markers (linkage-disequilibrium) on corrected p values, to control inflation of the type I error rate. We used SNPAlyze 3.2Pro software (Dynacom) to estimate haplotype frequencies, linkage-disequilibrium, permutation p values (10 000 replications) and deviation from Hardy–Weinberg distribution of alleles. We calculated pair-wise linkage-disequilibrium indices (D′ and r2) for the control group. We performed power calculations for our sample size using the G*Power program.14 We set statistical significance at p < 0.05 for all tests.
Results
Participants
We included 173 unrelated Japanese patients with MDD (74 men, mean age 45.1, standard deviation [SD] 12.4 yr; 99 women, mean age 45.0, SD 15.3 yr). The characteristics of patients with MDD are shown in Table 1. We included 348 healthy controls (148 men, mean age 45.1, SD 12.4 yr; 200 women, mean age 44.9, SD 13.0 yr) recruited from hospital staff, students and company employees.
Table 1.
Demographic data and clinical characteristics of patients with major depressive disorder and controls
| Characteristic | MDD | Control |
|---|---|---|
| Age, mean (SD) [range], yr | 45.1 (14.1) [21–79] | 45.0 (12.7) [18–71] |
| Sex, male:female | 74:99 | 148:200 |
| No. of episodes, single:recurrent | 92:81 | — |
| Age at onset, mean (SD), yr | 41.0 (13.7) | — |
| Family history, yes:no | 52:121 | 0:348 |
MDD = major depressive disorder; SD = standard deviation.
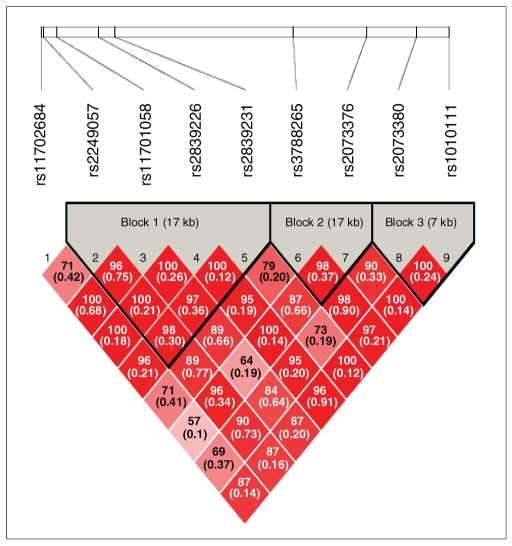
We genotyped 9 SNPs in the PCNT gene (Table 2). Genotypic distributions of these 9 SNPs did not deviate significantly from Hardy–Weinberg equilibrium in the control group. The values of absolute D′ and r2 for the healthy controls are presented in Figure 1. There were 3 linkage-disequilibrium blocks12 in the PCNT gene, with rs2249057, rs11701058, rs2839226 and rs2839231 residing in block 1, rs3788265 and rs2073376 residing in block 2 and rs2073380 and rs1010111 residing in block 3 (Fig. 1). We observed significant differences in allelic frequencies between patients with MDD and controls for 3 SNPs (rs3788265, rs2073376 and rs2073380) in intron29, exon38 and exon45 (p = 0.006, 0.005 and 0.021, respectively). The T allele of rs3788265, the G allele of rs2073376 and the C allele of rs2073380 occurred more frequently in the MDD group than in the control group. After applying the SNPSpD software to correct for multiple testing, 2 SNPs (rs3788265 and rs2073376) retained significant allelic associations with MDD. The control minor allele frequency of rs2073376 in our study was almost the same as that in the study by Anitha and colleagues9 involving Japanese participants (33% in our study v. 31.7% that of Anitha and colleagues9). Next, we performed haplotype analyses. We found a significant association between the 2 marker haplotypes (r3788265 and rs2073376) and MDD (permutation p = 0.011) (Table 3). In power calculations using the G*Power program, our sample size had a post-hoc power of 0.99 to detect an effect size of 0.5 (moderate) at the 0.05 significance level (2-tailed).
Table 2.
Allele frequencies of 9 single nucleotide polymorphisms in the PCNT gene in patients with major depressive disorder and controls
| SNP; group | Allele, no. | p value* | Frequency† | |
|---|---|---|---|---|
| rs11702684 | C | T | ||
| Intron 9 | ||||
| MDD | 195 | 151 | 0.12 | 0.44 |
| Control | 427 | 267 | 0.39 | |
| rs2249057 | C | A | ||
| Exon 10 | ||||
| MDD | 179 | 159 | 0.18 | 0.47 |
| Control | 397 | 295 | 0.43 | |
| rs11701058 | C | T | ||
| Intron 12 | ||||
| MDD | 185 | 161 | 0.10 | 0.54 |
| Control | 333 | 363 | 0.48 | |
| rs2839226 | C | T | ||
| Intron 14 | ||||
| MDD | 78 | 268 | 0.81 | 0.23 |
| Control | 152 | 542 | 0.22 | |
| rs2839231 | A | G | ||
| Intron 15 | ||||
| MDD | 83 | 263 | 0.07 | 0.24 |
| Control | 204 | 488 | 0.29 | |
| rs3788265 | G | T | ||
| Intron 29 | ||||
| MDD | 164 | 182 | 0.006 | 0.53 |
| Control | 393 | 301 | 0.43 | |
| rs2073376 | A | G | ||
| Exon 38 | ||||
| MDD | 85 | 261 | 0.005 | 0.25 |
| Control | 230 | 466 | 0.33 | |
| Rs2073380 | C | A | ||
| Exon 45 | ||||
| MDD | 183 | 163 | 0.021 | 0.53 |
| Control | 313 | 381 | 0.45 | |
| Rs1010111 | A | G | ||
| Intron 47 | ||||
| MDD | 269 | 77 | 0.99 | 0.22 |
| Control | 539 | 155 | 0.22 | |
MDD = major depressive disorder; SNP = single nucleotide polymorphism.
Fisher exact test.
Percentage of minor allele frequency.
Fig. 1.
Haplotype block structure of the PCNT gene. We determined the structure using the Haploview program.11 We defined blocks according to the criteria of Gabriel and colleagues.12 There were 3 linkage-disequilibrium blocks in the PCNT gene: rs2249057, rs11701058, rs2839226 and rs2839231 reside in block 1, rs3788265 and rs2073376 reside in block 2 and rs2073380 and rs1010111 reside in block 3. Each box represents D′ (r2) values corresponding to each pair-wise single nucleotide polymorphism.
Table 3.
Haplotype analysis among patients with major depressive disorder and controls*
| Haplotype | Overall, % | MDD | Control | χ2 | p value | Permutation p value |
|---|---|---|---|---|---|---|
| GG | 23.4 | 22.8 | 23.7 | 0.091 | 0.76 | 0.78 |
| TG | 46.3 | 52.6 | 43.2 | 8.234 | 0.004 | 0.005 |
| GA | 30.2 | 24.6 | 33 | 7.711 | 0.005 | 0.007 |
| Select locus rs3788265 and rs2073376† | 10.5 | 0.015 | 0.011 |
MDD = major depressive disorder.
Haplotypes were omitted from analysis if the estimated haplotype probabilities were less than 5%.
The 2 marker haplotypes of block 2 (rs3788265 and rs2073376) were associated with MDD (permutation p = 0.011). Replications = 10000.
Discussion
To our knowledge, ours is the first study to examine an association between the PCNT gene and MDD. We observed significant allelic association with rs3788265 and rs2073376 after applying multiple test correction. Furthermore, 2 marker haplotypes of block 2 (rs3788265 and rs2073376) were significantly associated with MDD. In block2, the most common haplotype (TG) was present in 53% of patients with MDD and 43% of controls. Therefore, this haplotype might be a risk factor for MDD. The second most common haplotype (GA) was present in 25% of patients with MDD and 33% of controls, suggesting that this haplotype might be protective against MDD.
Anitha and colleagues9 reported that expression of the PCNT gene in peripheral blood lymphocytes was significantly higher in medication-naive patients with MDD than in healthy controls. A nonsynonymous exonic SNP (rs2073376, Gln2792Arg) that was significantly associated with MDD in our study may contribute to altered expression of this gene in peripheral lymphocytes of MDD.
Hashimoto and colleagues7 reported an association between a genetic variation of DISC1 (Ser704Cys) and MDD and brain morphology in the Japanese population. In our study, we provided the evidence that PCNT, an interacting partner of DISC1, is a candidate gene for MDD. Thus the DISC1–PCNT pathway may play an important role in the pathophysiology of MDD owing to abnormalities in centrosomal function.
Limitations
Our study has several limitations. First, our sample size was relatively small; however, our study had a power of 0.99 to detect an effect size of 0.5 (moderate) at the 0.05 significance level (2-tailed). Larger studies with participants of different races and meta-analyses are needed to confirm these associations. Second, owing to the late onset of MDD, it is possible that the disorder will develop in at least some of the participants in our control group. This potential misclassification in the control group would bias and weaken any association between the PCNT gene and MDD. On the other hand, selected controls who were recruited from employees without a personal or family history of mental disorders may enhance the likelihood of a positive association. Third, we did not reveal how SNPs of the PCNT gene, which were significantly associated with MDD in our study, alter its function. Recently it has been reported that Seckel syndrome, an auto-somal recessive disorder of markedly reduced brain and body size, is associated with mutations in the PCNT gene.15 The mutations have defects in DNA signalling. Further investigations, including intermediate phenotype approaches, are needed to reveal how genetic variations in the PCNT gene are involved in the etiology of MDD.
Conclusion
Our findings indicate that PCNT is a candidate gene for MDD in the Japanese population. Larger studies are needed to confirm these associations.
Acknowledgements
We thank all the volunteers who participated in this study and the physicians who helped us to obtain clinical data and blood samples. We thank Mrs. Akemi Okada and Mrs. Kazue Tugawa for their technical assistance. This work was supported by a Grant-in-Aid for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science and Technology and a Grant-in-Aid for Scientific Research from the 21st Century COE program, Human Nutritional Science on Stress Control, Tokushima, Japan.
Footnotes
Competing interests: None declared.
Contributors: Drs. Numata and Iga designed the study and acquired the data. Drs. Nakataki, Itakura, Ueno and Ohmori also acquired the data, which Drs. Numata, Nakataki, Tayoshi, Tanahashi, Ueno and Ohmori analyzed. Dr. Numata wrote the article, which all authors reviewed and approved for publication.
References
- 1.Levinson DF. The genetics of depression: a review. Biol Psychiatry. 2006;60:84–92. doi: 10.1016/j.biopsych.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 2.Baron M. Manic-depression genes and the new millennium: poised for discovery. Mol Psychiatry. 2002;7:342–58. doi: 10.1038/sj.mp.4000998. [DOI] [PubMed] [Google Scholar]
- 3.Lin PI, McInnis MG, Potash JB, et al. Assessment of the effect of age at onset on linkage to bipolar disorder: evidence on chromosomes 18p and 21q. Am J Hum Genet. 2005;77:545–55. doi: 10.1086/491602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cannon TD, Hennah W, van Erp TG, et al. Association of Drosoph Inf ServC1/TRAX haplotypes with schizophrenia, reduced pre-frontal gray matter, and impaired short- and long-term memory. Arch Gen Psychiatry. 2005;62:1205–13. doi: 10.1001/archpsyc.62.11.1205. [DOI] [PubMed] [Google Scholar]
- 5.Hennah W, Varilo T, Kestilä M, et al. Haplotype transmission analysis provides evidence of association for Drosoph Inf ServC1 to schizophrenia and suggests sex-dependent effects. Hum Mol Genet. 2003;12:3151–9. doi: 10.1093/hmg/ddg341. [DOI] [PubMed] [Google Scholar]
- 6.Thomson PA, Wray NR, Millar JK, et al. Association between the TRAX/Drosoph Inf ServC locus and both bipolar disorder and schizophrenia in the Scottish population. Mol Psychiatry. 2005;10:657–68. doi: 10.1038/sj.mp.4001669. [DOI] [PubMed] [Google Scholar]
- 7.Hashimoto R, Numakawa T, Ohnishi T, et al. Impact of the Drosoph Inf ServC1 Ser704Cys polymorphism on risk for major depression, brain morphology and ERK signaling. Hum Mol Genet. 2006;15:3024–33. doi: 10.1093/hmg/ddl244. [DOI] [PubMed] [Google Scholar]
- 8.Miyoshi K, Asanuma M, Miyazaki I, et al. Drosoph Inf ServC1 localizes to the centrosome by binding to kendrin. Biochem Biophys Res Commun. 2004;317:1195–9. doi: 10.1016/j.bbrc.2004.03.163. [DOI] [PubMed] [Google Scholar]
- 9.Anitha A, Nakamura K, Yamada K, et al. Gene and expression analyses reveal enhanced expression of pericentrin 2 (PCNT2) in bipolar disorder. Biol Psychiatry. 2008;63:678–85. doi: 10.1016/j.biopsych.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 10.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington (DC): The Association; 1994. [Google Scholar]
- 11.Barrett JC, Fey B, Maller J, et al. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–5. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 12.Gabriel SB, Schaffner SF, Nguyen H, et al. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–9. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- 13.Nyholt DR. A simple correction for multiple testing for single-nucleotide polymorphism in linkage disequilibrium with each other. Am J Hum Genet. 2004;74:765–9. doi: 10.1086/383251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erdfelder E, Faul F, Buchner A. GPOWER: a general power analysis program. Behav Res Methods Instrum Comput. 1996;28:1–11. [Google Scholar]
- 15.Griffith E, Walker S, Martin CA, et al. Mutations in pericentrin cause Seckel syndrome with defective ATR-dependent DNA damage signaling. Nat Genet. 2008;40:232–6. doi: 10.1038/ng.2007.80. [DOI] [PMC free article] [PubMed] [Google Scholar]