Abstract
Hormones such as glucagon are secreted by Ca2+-induced exocytosis of large dense-core vesicles, but the mechanisms involved have only been partially elucidated. Studies of pancreatic β-cells secreting insulin revealed that synaptotagmin-7 alone is not sufficient to mediate Ca2+-dependent insulin granule exocytosis, and studies of chromaffin cells secreting neuropeptides and catecholamines showed that synaptotagmin-1 and -7 collaborate as Ca2+ sensors for exocytosis, and that both are equally involved. As no other peptide secretion was analysed, it remains unclear whether synaptotagmins generally act as Ca2+ sensors in large dense-core vesicle exocytosis in endocrine cells, and if so, whether synaptotagmin-7 always functions with a partner in that role. In particular, far less is known about the mechanisms underlying Ca2+-triggered glucagon release from α-cells than insulin secretion from β-cells, even though insulin and glucagon together regulate blood glucose levels. To address these issues, we analysed the role of synaptotagmins in Ca2+-triggered glucagon exocytosis. Surprisingly, we find that deletion of a single synaptotagmin isoform, synaptotagmin-7, nearly abolished Ca2+-triggered glucagon secretion. Moreover, single-cell capacitance measurements confirmed that pancreatic α-cells lacking synaptotagmin-7 exhibited little Ca2+-induced exocytosis, whereas all other physiological and morphological parameters of the α-cells were normal. Our data thus identify synaptotagmin-7 as a principal Ca2+ sensor for glucagon secretion, and support the notion that synaptotagmins perform a universal but selective function as individually acting Ca2+ sensors in neurotransmitter, neuropeptide, and hormone secretion.
Glucagon secretion is stimulated when blood glucose levels are low to restore normal glucose levels by activating glucose production and glucose release in the liver. Like neurotransmitter release by synaptic vesicle exocytosis, secretion of peptide hormones, such as glucagon, is triggered by Ca2+ (Gerber & Südhof, 2002; Gromada et al. 2007; MacDonald et al. 2007; Rorsman et al. 2008). However, little is known about the molecular mechanisms regulating glucagon secretion in particular, or Ca2+-dependent hormone secretion beyond insulin and catecholamine release in general.
Synaptotagmins constitute a family of at least 15 proteins primarily expressed in neurons and endocrine cells. Eight of the 15 synaptotagmins bind Ca2+ (synaptotagmin-1, -2, -3, -5, -6, -7, -9 and -10), and exhibit diverse Ca2+-binding properties (Südhof, 2002). Of these eight Ca2+-binding synaptotagmins, only synaptotagmin-1, -2 and -9 function as Ca2+ sensors for fast synchronous neurotransmitter release (Geppert et al. 1994; Pang et al. 2006; Xu et al. 2007). The differential distribution and distinct properties of the three neuronal Ca2+ sensors support the emerging notion that synaptotagmins serve as individually acting Ca2+ sensors in neurotransmitter release (Geppert et al. 1994; Pang et al. 2006; Sun et al. 2007; Xu et al. 2007). However, it is not clear whether the synaptotagmin-calcium sensor paradigm is generally applicable to exocytosis of large dense-core vesicles (LDCVs), including the hormone-containing secretory granules of endocrine cells. So far, Ca2+-induced exocytosis of two types of LDCVs – of catecholamine- and neuropeptide-containing granules in chromaffin cells, and of insulin-containing granules in pancreatic β-cells – was shown to be mediated by synaptotagmins (Gustavsson et al. 2008; Schonn et al. 2008). Surprisingly, in both cell types, exocytosis was mediated by more than one synaptotagmin isoforms that appeared to cooperate as Ca2+ sensors. Whilst supporting the notion that synaptotagmins generally function as Ca2+ sensors in exocytosis, these findings also raise the possibility that LDCV exocytosis, in contrast to synaptic exocytosis, may always involve the concerted action of two synaptotagmin isoforms, a possibility that is also supported by previous studies using endocrine cell lines (Gao et al. 2000; Xiong et al. 2006; Gauthier et al. 2008).
In the present study, we tested the synaptotagmin- calcium sensor theory in peptide hormone secretion, and investigated whether synaptotagmin-7 could function as an individually acting Ca2+ sensor in LDCV exocytosis. Using biochemical assays and electrophysiology measurements, we demonstrate that synaptotagmin-7 mediates nearly all Ca2+-triggered glucagon secretion in response to physiological stimuli, and thus identify synaptotagmin-7 as the principal synaptotagmin isoform required for glucagon secretion. The present study further validates the synaptotagmin- calcium sensor paradigm, and extends it beyond the established neuronal Ca2+ sensors for synaptic vesicle exocytosis, i.e. synaptotagmin-1, -2 and -9, to include synaptotagmin-7 as an individually acting high affinity Ca2+ sensor for LDCV exocytosis.
Methods
Animal welfare
All experiments involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of A*STAR (Agency for Science, Technology and Research).
Synaptotagmin-7 KO mice
The synaptotagmin-7 KO mice were generated as previously described (Maximov et al. 2008). All mice used in this study were bred and housed in our animal care facilities. For islet isolation, immunostaining and electron microscopy, mice were killed by cervical dislocation. In total, 16 synaptotagmin-7 KO and 16 control mice were used.
Insulin-induced hypoglycaemia and glucagon sensitivity test
For insulin-induced hypoglycaemia, mice were fasted for 2 h with free access to water before they were weighed and injected i.p. with human insulin (Actrapid) at 1 U (kg body weight)−1. Blood samples of ∼35 μl were collected from tail vein for determination of blood glucose and plasma glucagon levels before (resting), and at 20 and 40 min after the injection. For the glucagon sensitivity test, mice were fasted for 6 h with free access to water before i.p. injection of 0.2 mg kg−1 human glucagon (Sigma). Blood glucose levels were measured before and at 5, 10, 20 and 40 min after glucagon injection. Blood glucose was determined by using the Accu-Chek Advantage glucometer (Roche), and plasma glucagon by using the Mouse Endocrine Panel Lincoplex kit (Millipore) according to the manufacturer's instructions.
Islet isolation, perifusion and glucagon measurements
Islets were isolated and cultured as previously described (Gustavsson et al. 2008). For estimation of glucagon content, islets were lysed by sonication in 200 μl of passive lysis buffer (Promega) after incubation in modified Krebs–Ringer–Hanseleit (KRH) medium (containing, mm: 130 NaCl, 4.7 KCl, 1.2 KH2PO4, 1.2 MgSO4, and 2.56 CaCl2, 1 mg ml−1 BSA, 20 mm Hepes, pH 7.4) supplemented with 10 mm glucose for 2 h at 37 °C. For perifusion experiments, similar-sized islets from a single mouse (10 islets per tube) were first washed with 10 mm glucose-containing KRH medium, placed in a 50 μl flow chamber and further perifused for 40 min at 37 °C. They were then stimulated with 1 mm glucose-containing KRH medium for 30 min at a flow rate of 0.6 ml min−1. Fractions of the perfusate were collected every 2 min, starting from 6 min prior to stimulation. Glucagon concentration was measured using a glucagon radioimmunoassay (RIA) (Millipore).
Immunostaining and electron microscopy
Immunostaining and EM were performed essentially as previously described (Gustavsson et al. 2008). Briefly, 20 μm cryo-sections of synaptotagmin-7 KO and control mouse pancreata were mounted on slides and probed with antibodies against glucagon and synaptotagmin-7 (S757, Synaptic Systems, Göttingen, Germany), and then with Alexa488 and Alexa546 conjugated secondary antibodies (Invitrogen). Slides were visualized by confocal microscopy (Leica). For EM viewing, ∼90 nm sections were stained with 2% uranyl acetate (Analar, UK) for 5 min and examined using a JEOL JEM-1220 electron microscope (JEOL Asia Pte Ltd, Japan). To analyse granule distribution, we grouped glucagon granules into four groups based on the distance from granule membrane to plasma membrane: 0–100 nm, 100–300 nm, 300–500 nm and 500–700 nm. We then counted the number of granules within each group and plotted the percentage of each group as a bar graph.
Electrophysiology measurements
Membrane currents and capacitance were recorded from α-cells in intact isolated islets using the standard whole-cell patch-clamp technique (Gopel et al. 2000, 2004; MacDonald et al. 2007). Exocytosis was elicited by a 500 ms depolarizing pulse from −70 to 0 mV. Single α-cells were identified by a discernable Na+ current and TEA-resistant K+ currents and/or by their electrical activity in the absence of glucose or low glucose that could be suppressed by the addition of glucose (20 mmol l−1). Pipette resistance ranged between 3 and 6 MΩ when pipettes were filled with intracellular solution containing (in mm): 125 potassium glutamate, 10 KCl, 10 NaCl, 1 MgCl2, 5 Hepes, 0.05 EGTA, 0.1 cAMP and 4 MgATP, pH 7.1. Extracellular solution contained (in mm) 118 NaCl, 20 TEA-Cl (tetraethylammonium chloride), 5.6 KCl, 2.6 CaCl2, 1.2 MgCl2, 5 Hepes, pH 7.4. Cells were stimulated at low frequency (< 0.05 Hz) to allow full recovery of exocytotic capacity between pulses. Measurements were performed using EPC9 patch clamp amplifier and Pulse software (HEKA Elektronik, Lambrecht/Pfalz, Germany). Currents were compensated for capacitive transients and linear leak using a –P/4 protocol. Exocytosis was detected as changes in cell membrane capacitance (Cm), which was estimated by the Lindau–Neher technique implementing the ‘Sine+DC’ feature of the lock-in module (Lindau & Neher, 1988). The amplitude of the sine wave was 30 mV and the frequency was set at 1 kHz. All Cm measurements were performed at 28°C.
Statistical analysis
Data are presented as means ±s.e.m. Comparisons of data from synaptotagmin-7 KO and control mice were made using Student's two-tailed t test for independent data. The significance limit was set at P < 0.05.
Results
Synaptotagmin-7 is expressed in glucagon-secreting cells
We first determined whether synaptotagmin-7 was expressed in pancreatic α-cells by immunohistochemistry and confocal microscopy. Immunoreactivity to synaptotagmin-7 was evident in wild-type mouse pancreatic sections, but was absent in synaptotagmin-7 KO sections (Fig. 1). When viewed at higher magnification, the synaptotagmin-7 signal in wild-type α-cells co-localized with that of glucagon, demonstrating that synaptotagmin-7 was expressed in glucagon-secreting α-cells (Fig. 1).
Figure 1. Synaptotagmin-7 expression in glucagon-secreting cells.
Twenty micrometre pancreatic sections were stained with antibodies against synaptotagmin-7 (S757, Synaptic Systems) and glucagon, followed by fluorescence-conjugated secondary antibodies. Representative images of such stained sections, taken on a Leica TCS2 confocal microscope, are shown. Synaptotagmin-7 (Syt 7, red) was expressed in glucagon-positive cells and shown to have a high degree of overlap with glucagon signals (green). Arrows indicate selected overlapping signals of synaptotagmin-7 and glucagon. For comparison, no apparent synaptotagmin-7 signal was detected in islet sections from synaptotagmin-7 KO (Syt7−/−) mouse. Scale bars: 40, 20 and 5 μm for top, middle and bottom rows, respectively.
Synaptotagmin-7 KO mice exhibit impaired glucagon secretion in vivo
Immunohistochemical and histological analyses showed no pathological signs in the islet structure or α-cell distribution in synaptotagmin-7 KO mice. Considering that both insulin and glucagon secretion are triggered by Ca2+, and that synaptotagmin-7 is involved in regulating β-cell secretion (Gustavsson et al. 2008), we tested whether glucagon secretion was disrupted in mice lacking synaptotagmin-7. In male synaptotagmin-7 KO and wild-type littermate control mice fasted for 20 h (fasting state) or 2 h (resting state), resting glucagon levels were lower in synaptotagmin-7 KO mice than in controls (Fig. 2A), whereas blood glucose levels were unaffected as previously reported (Gustavsson et al. 2008; and data not shown). Overnight fasting led to hypoglycaemia in both control and synaptotagmin-7 KO mice, but glucagon levels were increased only in wild-type mice (Fig. 2A), indicating that glucagon secretion in synaptotagmin-7 KO mice was impaired.
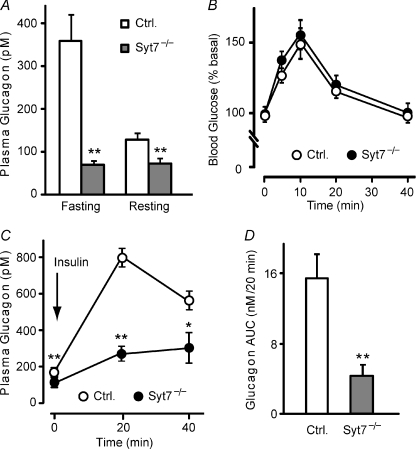
Figure 2. Reduced glucagon level and impaired glucagon secretion, but normal glucagon sensitivity in synaptotagmin-7 KO mice.
A, plasma glucagon levels were measured in synaptotagmin-7 KO and control mice that were fasted for 20 h (Fasting state) or 2 h (Resting state) by using the Lincoplex kit. Synaptotagmin-7 KO mice (grey bar) exhibited lower glucagon levels than control (white bar) in both groups. n= 9 for fasting control, resting control and resting KO, and 12 for fasting KO. **P < 0.01 vs. control. B, blood glucose levels before and at 5, 10, 20 and 40 min after i.p. injection of 0.2 mg kg−1 glucagon in 6 h fasted synaptotagmin-7 KO mice (filled circle) and control (open circle) were measured by using a glucometer. Blood glucose response to glucagon injection was not different between the two groups. n= 10 for each group. C, synaptotagmin-7 KO and control mice were injected with 1 U kg−1 insulin, and their plasma glucagon levels were measured before and at 20 and 40 min after injection. Hypoglycaemia-induced glucagon secretion was greatly reduced in KO mice (filled circle) compared to control (open circle). n= 9 for each group. *P < 0.05; **P < 0.01. D, total stimulated glucagon secretion during insulin-induced hypoglycaemia was calculated as area under curve (AUC) in C after basal secretion subtraction. Hypoglycaemia-stimulated glucagon secretion in vivo was significantly reduced in synaptotagmin-7 KO (grey bar) mice compared to controls (white bar). **P < 0.01.
As we have shown previously, synaptotagmin-7 KO mice exhibit a slower recovery from hypoglycaemia during the insulin tolerance test (Gustavsson et al. 2008; and data not shown). We therefore tested whether KO mice had lower sensitivity to glucagon challenge, which could contribute to the delayed recovery of glucose level from insulin-induced hypoglycaemia. Glucagon induced a similar rise in glucose level in synaptotagmin-7 KO mice and their wild-type controls throughout the tests, indicating that glucagon sensitivity was normal in synaptotagmin-7 KO mice (Fig. 2B). Therefore, the slower recovery from hypoglycaemia in the KO mice was not due to impaired liver response to glucagon, but most likely the result of deficient glucagon secretion.
To measure the severity of the glucagon secretion impairment in synaptotagmin-7 KO mice, we tested glucagon secretion to acute insulin-induced hypoglycaemia in vivo. After intraperitoneal insulin injections, synaptotagmin-7 KO mice exhibited consistently lower blood glucose levels than their littermate controls. Blood glucose levels in synaptotagmin-7 KO mice fell to levels as low as 2 mmol l−1: average blood glucose levels were 2.7 ± 0.2 vs. 3.4 ± 0.2 mmol l−1 for KO and control mice at 60 min after the insulin injection (n= 9 for both genotypes, P < 0.05). This is reminiscent of the results observed in SUR1 KO mice, which was attributed to a defect in glucagon secretion (Shiota et al. 2005). Insulin-induced hypoglycaemia stimulated a <2-fold increase in glucagon secretion in synaptotagmin-7 KO mice at 20 min after the insulin injection, compared with a >4-fold increase in control mice at the same time point (Fig. 2C). Glucagon levels at 40 min after insulin injection were also much higher in control than in synaptotagmin-7 KO mice (Fig. 2C). Total hypoglycaemia-stimulated glucagon secretion, calculated by integrating the area under curve (AUC), was significantly lower in KO than in control: synaptotagmin-7 KO mice secrete less than 30% of their control (Fig. 2D). Collectively, these data demonstrate that synaptotagmin-7 is essential for hypoglycaemia-induced glucagon secretion.
Severely impaired glucagon secretion in synaptotagmin-7 KO mouse islets in vitro
The synaptotagmin-7 deletion may have influenced other organs/cell types besides pancreatic islets, and the α-cell dysfunction may be secondary to a failure of central regulation (Gromada et al. 2007). To exclude this possibility and to pinpoint the defects in glucagon secretion in synaptotagmin-7 KO mice, we measured glucagon secretion in isolated intact islets from KO and control mice, and determined whether the impaired glucagon secretion in vivo persisted in the isolated islet system. In control islets, lowering of the glucose concentration from 10 to 1 mm caused a large increase in secretion, which displayed a steep rise within the first 4 min to reach a steady state, and stayed elevated for the rest of stimulation (Fig. 3A). The response to low glucose stimulation in synaptotagmin-7 KO islets was greatly reduced: glucagon secretion was nearly abolished during the first 10 min, and only slightly elevated afterwards (Fig. 3A). Net glucagon release, calculated as the sum of all the fractions over the entire stimulation period after baseline subtraction, was reduced by almost 80% in synaptotagmin-7 KO islets (Fig. 3B). These results establish that impaired glucagon secretion in synaptotagmin-7 KO mice is not due to a secondary effect of central regulation failure, but rather a defective intrinsic secretion process of glucagon granules. Furthermore, these data reinforce the conclusion that glucagon secretion is principally dependent on synaptotagmin-7.
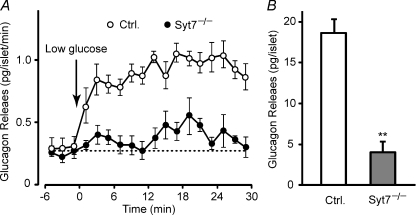
Figure 3. Impaired glucagon secretion in isolated synaptotagmin-7 KO mouse islets.
A, low glucose-induced glucagon secretion from isolated islets was measured in perifusion experiments at a glucose concentration of 10 mm (basal) or 1 mm (stimulatory). Arrow indicates switching from basal to stimulatory perifusion buffer. The perfusate was collected in 2 min intervals, and glucagon levels were determined by using RIA. Synaptotagmin-7 KO islets (Syt7−/−, filled circle) showed impaired glucagon secretion when compared with control (open circle). B, low glucose-stimulated glucagon secretion for the entire stimulation period in the perifusion experiments was lower in isolated islets from synaptotagmin-7 KO (grey bar) than from control (white bar). Glucagon secretion was calculated by integrating the area under each curve in A after baseline subtraction. Data are presented as means ±s.e.m., n= 7 for KO and 9 for control, **P < 0.01.
Glucagon production and packaging, and α-cell ultrastructural characteristics are normal in synaptotagmin-7 KO mouse islets
Impaired glucagon release may result from disrupted exocytosis, but may also be due to impaired glucagon production, packaging and α-cell ultrastructural defects. We tested whether glucagon gene expression was decreased in synaptotagmin-7 KO mice by using qPCR. Glucagon mRNA levels were the same in KO and control mice (Supplemental Fig. 1A), indicating that the synaptotagmin-7 deletion did not affect glucagon production. Total glucagon content was also not different between these two groups of animals (Supplemental Fig. 1B). To check for defects in α-cell ultrastructure associated with glucagon granules, we compared glucagon granule number and distribution in synaptotagmin-7 KO and control islets by EM. Pancreatic α-cells in control and synaptotagmin-7 KO islets had large nuclei with partly condensed chromatin, well-developed Golgi apparatus with zones of granule formation, scattered lamellar rough endoplasmic reticulum, and numerous secretory granules with a large electron-dense core and small white halo (Fig. 4A). Glucagon granules were of similar size and granule density was also similar in synaptotagmin-7 KO and control mice (Fig. 4A and B). Furthermore, we analysed glucagon granule distribution based on distance from the plasma membrane, and found no difference between synaptotagmin-7 KO and control (Fig. 4C). These data indicate that diminished glucagon secretion in synaptotagmin-7 KO mice was not due to insufficient glucagon production, reduced glucagon storage, decreased granule number or defective granule distribution, but was more likely to be the result of impaired exocytosis of glucagon granules.
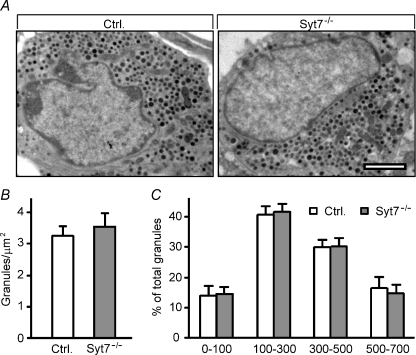
Figure 4. Normal ultrastructure of pancreatic α-cells in synaptotagmin-7 KO mice.
A, pancreatic α-cell ultrastructure was analysed by using transmission EM. One representative image from each genotype is shown. Ultrastructural organizations, including distribution of glucagon secretory granules, were similar in control and KO mouse α-cells. Scale bar = 2 μm. B, glucagon granule number per μm2 was similar in synaptotagmin-7 KO (grey bar) and control (white bar) mouse α-cells. n= 23 α-cells from 3 mice of each genotype. C, glucagon granule distribution was not different between synaptotagmin-7 KO (grey bar) and control (white bar) mouse α-cells. Numbers on the X-axis refer to distance (in nm) between glucagon granule membrane and plasma membrane. Refer to Methods for details. n= 10 α-cells from 3 synaptotagmin-7 KO, and 9 α-cells from 4 control mice.
Synaptotagmin-7 is required for glucagon secretion downstream of Ca2+ influx
Although the cellular mechanism of glucagon granule exocytosis remains unclear, accumulating data implicate KATP and Ca2+ channels in generating the Ca2+ signal required for glucagon secretion (Gopel et al. 2000; Gromada et al. 2004; Miki & Seino, 2005; Pereverzev et al. 2005; Shiota et al. 2005; Gromada et al. 2007; MacDonald et al. 2007). To test whether the impairment of glucagon secretion was due to defective membrane electrical activities or an insufficient Ca2+ signal, we compared action potentials and Ca2+ channel activity of α-cells in intact islets from synaptotagmin-7 KO and control mice. Pancreatic α-cells can be functionally identified by their characteristic rapidly activating and inactivating TTX-sensitive Na+ current, TEA-resistant K+ currents and spontaneous electrical activities at low glucose concentrations (Gopel et al. 2000). We recorded spontaneous action potentials in the presence of 1 mm glucose in functionally identified control and KO α-cells (Fig. 5A and B), and found no difference in resting membrane potential, action potential frequency, and action potential amplitudes between the two groups of cells (Fig. 5C–E). To investigate Ca2+ channel activity in control and KO α-cells, depolarization-evoked Ca2+ currents were recorded in the presence of 0.1 μg ml−1 TTX, 20 mm TEA, 4 mm 4-AP, and equimolar substitution of Ba2+ for Ca2+ (2.6 mm) in the extracellular buffer. Ca2+ channel activity was indistinguishable between synaptotagmin-7 KO and control α-cells (Fig. 5F and G).
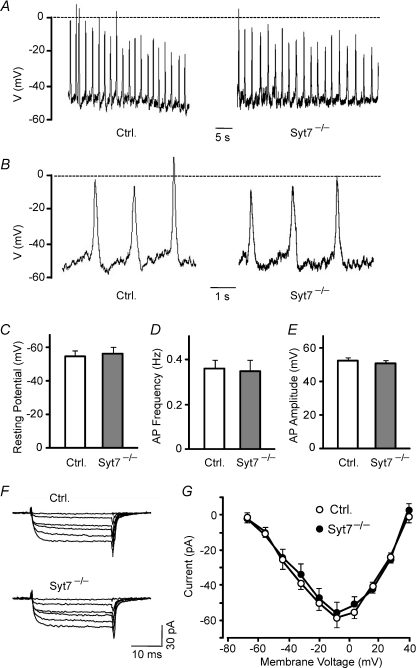
Figure 5. Normal electrical properties and Ca2+ channel activity in synaptotagmin-7 KO α-cells.
A, spontaneous action potentials were recorded in synaptotagmin-7 KO and control α-cells in the presence of 1 mm glucose. B, action potentials from panel A were displayed in expanded horizontal scales to depict spontaneous membrane depolarizations between action potentials. KO α-cells (Syt7−/−, grey bar) exhibited normal resting membrane potential (C), action potential frequency (D) and action potential amplitude (E) compared to control (Ctrl, white bar). F, Ca2+ currents were recorded in synaptotagmin-7 KO and control α-cells in the presence of 0.1 μg ml−1 TTX, 20 mm TEA, 4 mm 4-AP and equimolar substitution of Ba2+ for Ca2+ (2.6 mm). The currents were evoked by depolarizations from −70 mV to −8 mV. Ca2+ currents were indistinguishable between synaptotagmin-7 KO (Syt7−/−) and control (Ctrl) α-cells in the absence of glucose. G, current–voltage relationship of peak Ca2+ current amplitude against membrane depolarisations from −70 mV to +40 mV showed no difference between synaptotagmin-7 KO (filled circle) and control (open circle) α-cells. Data are presented as means ±s.e.m., n= 5 for each group.
We next compared depolarization-evoked exocytosis from synaptotagmin-7 KO and control α-cells at the single cell level using the whole-cell patch-clamp technique (Gopel et al. 2000). The capacitance increase, indicative of exocytosis-induced membrane expansion, was reduced by >70% in synaptotagmin-7 KO α-cells compared to that in control (Fig. 6). The fact that these measurements were conducted in individual α-cells argues against the idea that the impaired glucagon secretion is secondary to defective paracrine regulation (Ishihara et al. 2003), and further reveals that synaptotagmin-7 is essential for glucagon secretion at a step downstream of Ca2+ influx, consistent with the notion that synaptotagmin-7 functions as a Ca2+ sensor regulating glucagon secretion.
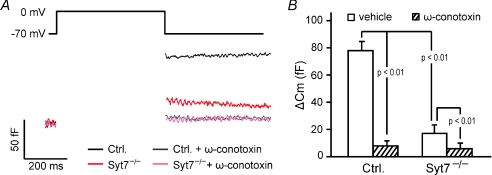
Figure 6. Ca2+ dependence of residual glucagon secretion in synaptotagmin-7 KO α-cells.
A, membrane capacitance was recorded in functionally identified α-cells in isolated synaptotagmin-7 KO and control islets. Capacitance increase elicited by 500 ms depolarizations from −70 to 0 mV in synaptotagmin-7 KO α-cells (red) was lower than in control α-cells (black). The N-type Ca2+ channel blocker ω-conotoxin (1 μm) inhibited membrane capacitance jump, and depolarization-induced capacitance change was not different in synaptotagmin-7 KO (pink) and control (grey) α-cells in the presence of the blocker. B, recordings from similar experiments as represented in A are summarized and presented as means ±s.e.m.n= 12 and 10 for KO (Syt7−/−) without and with ω-conotoxin treatment; and n= 9 and 10 for control (Ctrl) without and with ω-conotoxin treatment, respectively. Statistics (P values) are indicated in the graph. There was no difference in capacitance change between synaptotagmin-7 KO and control in the presence of 1 μmω-conotoxin (hatched bars).
Residual Ca2+-dependent component of glucagon secretion in the absence of synaptotagmin-7
The synaptotagmin-7 KO resulted in severely impaired glucagon secretion (down ∼ 80%) to insulin-induced hypoglycaemia in vivo, to low glucose in isolated islets, and to membrane depolarizations in individual α-cells in isolated islets (Figs 2, 3 and 6). However, glucagon secretion was not abolished. We examined whether residual glucagon release was independent of Ca2+, or still required Ca2+. Cytoplasmic Ca2+ in α-cells is mostly controlled by voltage-dependent Ca2+ channels (Gromada et al. 2007). Mouse α-cells are equipped with both N- and L-type Ca2+ channels, and some studies suggest that N-type Ca2+ channels are particularly important for glucagon secretion induced by hypoglycaemia (Gopel et al. 2004; MacDonald et al. 2007). We applied the N-type Ca2+ channel blocker ω-conotoxin, and measured capacitance response in synaptotagmin-7 KO and control α-cells. Consistent with previous reports on the role of N-type Ca2+ channels in glucagon secretion (MacDonald et al. 2007), ω-conotoxin caused a dramatic decrease in depolarization-induced membrane capacitance in control α-cells (Fig. 6). In synaptotagmin-7 KO α-cells, ω-conotoxin inhibited the already-impaired glucagon secretion further (Fig. 6). In the presence of the N-type Ca2+ channel blocker, exocytosis was comparable in synaptotagmin-7 KO and control mice (Fig. 6).
Discussion
Combined genetic and electrophysiological studies have established that in mammalian brain, synaptotagmins-1, -2 and -9 are the only Ca2+ sensors for fast synchronous synaptic vesicle exocytosis, and that these synaptotagmins act individually in this function. These findings prompted the emergence of the synaptotagmin calcium sensor paradigm, i.e. members of the synaptotagmin family generally function as principal Ca2+ sensors in neurotransmitter and peptide hormone secretion (Geppert et al. 1994; Pang et al. 2006; Sun et al. 2007; Xu et al. 2007). In addition to the three neuronal Ca2+ sensors, five other Ca2+-binding synaptotagmins are expressed in mammalian brain and endocrine tissues. These synaptotagmins (synaptotagmin-3, -5, -6, -7 and -10) may function as Ca2+ sensors for other forms of synaptic vesicle exocytosis, for LDCV exocytosis of neuropeptides and peptide hormones, or for completely different forms of Ca2+-dependent exocytosis. Consistent with this notion, synaptotagmin-7 has been shown to contribute to the Ca2+ sensing in insulin and catecholamine exocytosis, but puzzlingly, synaptotagmin-7 was shown in these processes not to act alone but in collaboration with another synaptotagmin (Gustavsson et al. 2008; Schonn et al. 2008). In addition, synaptotagmin-7 has also been proposed to regulate Ca2+-dependent lysosome fusion during wound repair (Martinez et al. 2000; Reddy et al. 2001; Jaiswal et al. 2004), glucose transporter 4 trafficking (Li et al. 2007), and osteoclast and osteoblast secretion (Zhao et al. 2008), although alternative interpretations were offered regarding some of the proposed functions (Shen et al. 2005; Steinhardt, 2005). These studies suggest that synaptotagmin-7 may function as an accessory to a principal Ca2+ sensor, e.g. synaptotagmin-1, or a non-selective regulator for various membrane fusion events.
To investigate whether synaptotagmin-7 has a specific role as an individually acting Ca2+ sensor for hormone/neuropeptide secretion, we examined the function of synaptotagmin-7 in glucagon secretion using synaptotagmin-7 KO mice. Glucagon is the body's principal hyperglycaemic hormone. It is released in response to hypoglycaemia, adrenergic stimulation and stimulation by lipids and amino acids (Rorsman et al. 2008). However, the molecular regulation of glucagon granule exocytosis remains unclear. Moreover, the findings that glucagon secretion becomes dysregulated in diabetes and that hyperglucagonaemia contributes to the hyperglycaemia caused by the insulinopaenia (reviewed in Rorsman et al. 2008) associated with the disease further illustrate the importance of such studies. Here we confirm that glucagon secretion was enhanced 3- to 5-fold by an overnight fast and in response to insulin-induced hypoglycaemia, respectively (Fig. 2A and C), and demonstrate that both responses were nearly abolished in synaptotagmin-7 KO mice. Biochemical measurements of glucagon secretion to low glucose in isolated islets, and single-cell capacitance measurements of glucagon granule exocytosis confirmed the severe impairment of glucagon secretion. Severe impairment of glucagon secretion has previously been reported in several other KO mouse models. For example, mice lacking SUR1 or Kir-6.2 showed nearly abolished hypoglycaemia-induced glucagon response (Miki & Seino, 2005; Shiota et al. 2005). Ablation of Cav2.3/E-type voltage-gated Ca2+ channel caused an impairment of glucagon response and the suppression of glucagon release by high glucose (Pereverzev et al. 2005). The impaired secretion in these studies was likely to be due to defects in Ca2+ signal generation. In the present study, KO α-cells showed normal Ca2+ channel activity, indicating that the defects for diminished glucagon secretion lie downstream of the Ca2+ signal. Our study thus established the requirement of synaptotagmin-7 in glucagon secretion at a step downstream of calcium influx, revealing that synaptotagmin-7 functions as a Ca2+ sensor in glucagon secretion in pancreatic α-cells.
In synaptotagmin-7 KO α-cells, ω-conotoxin inhibited the severely reduced glucagon secretion further to baseline level (Fig. 6), revealing the presence of N-type Ca2+ channel-dependent component in the residual glucagon secretion in synaptotagmin-7 KO mice. This indicates at least two possibilities: (1) synaptotagmin-7 functions as the principal Ca2+ sensor for glucagon secretion, and other Ca2+-sensing protein(s) regulate the minor component of glucagon secretion; (2) synaptotagmin-7 is the sole Ca2+ sensor for glucagon secretion, and other Ca2+-binding protein(s) only partially (∼20%) compensate for the loss of synaptotagmin-7, and perform the Ca2+-sensing role in the absence of synaptotagmin-7. In an initial attempt to distinguish the two scenarios, we tested the presence of Ca2+-binding synaptotagmins in α-cells. We previously showed that mRNA levels of synaptotagmin-7 and -9 were the highest among all Ca2+-binding synaptotagmins in mouse islets (Gustavsson et al. 2008). Synaptotagmin-2 and -3 mRNAs, although at a much lower level than synaptotagmin-7 and -9, could also be detected in mouse islets (Gustavsson et al. 2008). However, immunofluorescence failed to detect synaptotagmin-2 or -3 in α-cells (Supplemental Fig. 2), leaving synaptotagmin-9 as the most likely Ca2+ sensor for the minor component of glucagon secretion. Regarding the identities of Ca2+ sensors and possible compensation for synaptotagmin-7 in glucagon secretion, analyses of synaptotagmin-9 KO and synaptotamgin-7/-9 double KO mice will be invaluable. Although our findings appear to favour the first model, we cannot rule out a compensation and cooperation mechanism in the regulation of Ca2+-dependent glucagon secretion.
In conclusion, our results support the synaptotagmin calcium sensor theory, and further extend it beyond the three established fast synchronous Ca2+ sensors in neurotransmitter release, namely, synaptotagmin-1, -2 and -9, to include synaptotagmin-7 as an individually acting Ca2+ sensor for LDCV exocytosis.
Acknowledgments
We thank Dr Ong Wei-Yi for support and discussion on the electrophysiology analyses, Dr Cai Li for critical reading and comments on the manuscript, and Dr Clement Khaw and the SBIC-Nikon Imaging Center for support on confocal microscopy. This study was supported by intramural funding from A*STAR (Agency for Science, Technology and Research, Singapore) Biomedical Research Council. T.C.S. is a Howard Hughes Medical Institute investigator.
Supplementary material
Online supplemental material for this paper can be accessed at:
http://jp.physoc.org/cgi/content/full/jphysiol.2008.168005v1/DC1
References
- Gao Z, Reavey-Cantwell J, Young RA, Jegier P, Wolf BA. Synaptotagmin III/VII isoforms mediate Ca2+-induced insulin secretion in pancreatic islet β-cells. J Biol Chem. 2000;275:36079–36085. doi: 10.1074/jbc.M004284200. [DOI] [PubMed] [Google Scholar]
- Gauthier BR, Duhamel DL, Iezzi M, Theander S, Saltel F, Fukuda M, Wehrle-Haller B, Wollheim CB. Synaptotagmin VII splice variants α, β, and δ are expressed in pancreatic β-cells and regulate insulin exocytosis. FASEB J. 2008;22:194–206. doi: 10.1096/fj.07-8333com. [DOI] [PubMed] [Google Scholar]
- Geppert M, Goda Y, Hammer RE, Li C, Rosahl TW, Stevens CF, Südhof TC. Synaptotagmin I: a major Ca2+ sensor for transmitter release at a central synapse. Cell. 1994;79:717–727. doi: 10.1016/0092-8674(94)90556-8. [DOI] [PubMed] [Google Scholar]
- Gerber SH, Südhof TC. Molecular determinants of regulated exocytosis. Diabetes. 2002;51(Suppl 1):S3–11. doi: 10.2337/diabetes.51.2007.s3. [DOI] [PubMed] [Google Scholar]
- Gopel S, Zhang Q, Eliasson L, Ma XS, Galvanovskis J, Kanno T, Salehi A, Rorsman P. Capacitance measurements of exocytosis in mouse pancreatic α-, β- and δ-cells within intact islets of Langerhans. J Physiol. 2004;556:711–726. doi: 10.1113/jphysiol.2003.059675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopel SO, Kanno T, Barg S, Weng XG, Gromada J, Rorsman P. Regulation of glucagon release in mouse α-cells by KATP channels and inactivation of TTX-sensitive Na+ channels. J Physiol. 2000;528:509–520. doi: 10.1111/j.1469-7793.2000.00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromada J, Franklin I, Wollheim CB. α-Cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr Rev. 2007;28:84–116. doi: 10.1210/er.2006-0007. [DOI] [PubMed] [Google Scholar]
- Gromada J, Ma X, Hoy M, Bokvist K, Salehi A, Berggren PO, Rorsman P. ATP-sensitive K+ channel-dependent regulation of glucagon release and electrical activity by glucose in wild-type and SUR1−/− mouse α-cells. Diabetes. 2004;53(Suppl 3):S181–189. doi: 10.2337/diabetes.53.suppl_3.s181. [DOI] [PubMed] [Google Scholar]
- Gustavsson N, Lao Y, Maximov A, Chuang JC, Kostromina E, Repa JJ, Li C, Radda GK, Südhof TC, Han W. Impaired insulin secretion and glucose intolerance in synaptotagmin-7 null mutant mice. Proc Natl Acad Sci U S A. 2008;105:3992–3997. doi: 10.1073/pnas.0711700105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara H, Maechler P, Gjinovci A, Herrera PL, Wollheim CB. Islet β-cell secretion determines glucagon release from neighbouring α-cells. Nat Cell Biol. 2003;5:330–335. doi: 10.1038/ncb951. [DOI] [PubMed] [Google Scholar]
- Jaiswal JK, Chakrabarti S, Andrews NW, Simon SM. Synaptotagmin VII restricts fusion pore expansion during lysosomal exocytosis. PLoS Biol. 2004;2:E233. doi: 10.1371/journal.pbio.0020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wang P, Xu J, Gorelick F, Yamazaki H, Andrews N, Desir GV. Regulation of insulin secretion and GLUT4 trafficking by the calcium sensor synaptotagmin VII. Biochem Biophys Res Commun. 2007;362:658–664. doi: 10.1016/j.bbrc.2007.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindau M, Neher E. Patch-clamp techniques for time-resolved capacitance measurements in single cells. Pflugers Arch. 1988;411:137–146. doi: 10.1007/BF00582306. [DOI] [PubMed] [Google Scholar]
- MacDonald PE, De Marinis YZ, Ramracheya R, Salehi A, Ma X, Johnson PR, Cox R, Eliasson L, Rorsman P. A KATP channel-dependent pathway within α cells regulates glucagon release from both rodent and human islets of Langerhans. PLoS Biol. 2007;5:e143. doi: 10.1371/journal.pbio.0050143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez I, Chakrabarti S, Hellevik T, Morehead J, Fowler K, Andrews NW. Synaptotagmin VII regulates Ca2+-dependent exocytosis of lysosomes in fibroblasts. J Cell Biol. 2000;148:1141–1149. doi: 10.1083/jcb.148.6.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maximov A, Lao Y, Li H, Chen X, Rizo J, Sorensen JB, Südhof TC. Genetic analysis of synaptotagmin-7 function in synaptic vesicle exocytosis. Proc Natl Acad Sci U S A. 2008;105:3986–3991. doi: 10.1073/pnas.0712372105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki T, Seino S. Roles of KATP channels as metabolic sensors in acute metabolic changes. J Mol Cell Cardiol. 2005;38:917–925. doi: 10.1016/j.yjmcc.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Pang ZP, Melicoff E, Padgett D, Liu Y, Teich AF, Dickey BF, Lin W, Adachi R, Südhof TC. Synaptotagmin-2 is essential for survival and contributes to Ca2+ triggering of neurotransmitter release in central and neuromuscular synapses. J Neurosci. 2006;26:13493–13504. doi: 10.1523/JNEUROSCI.3519-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereverzev A, Salehi A, Mikhna M, Renstrom E, Hescheler J, Weiergraber M, Smyth N, Schneider T. The ablation of the Cav2.3/E-type voltage-gated Ca2+ channel causes a mild phenotype despite an altered glucose induced glucagon response in isolated islets of Langerhans. Eur J Pharmacol. 2005;511:65–72. doi: 10.1016/j.ejphar.2005.01.044. [DOI] [PubMed] [Google Scholar]
- Reddy A, Caler EV, Andrews NW. Plasma membrane repair is mediated by Ca2+-regulated exocytosis of lysosomes. Cell. 2001;106:157–169. doi: 10.1016/s0092-8674(01)00421-4. [DOI] [PubMed] [Google Scholar]
- Rorsman P, Salehi SA, Abdulkader F, Braun M, MacDonald PE. KATP-channels and glucose-regulated glucagon secretion. Trends Endocrinol Metab. 2008;19:277–284. doi: 10.1016/j.tem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Schonn JS, Maximov A, Lao Y, Südhof TC, Sorensen JB. Synaptotagmin-1 and -7 are functionally overlapping Ca2+ sensors for exocytosis in adrenal chromaffin cells. Proc Natl Acad Sci U S A. 2008;105:3998–4003. doi: 10.1073/pnas.0712373105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen SS, Tucker WC, Chapman ER, Steinhardt RA. Molecular regulation of membrane resealing in 3T3 fibroblasts. J Biol Chem. 2005;280:1652–1660. doi: 10.1074/jbc.M410136200. [DOI] [PubMed] [Google Scholar]
- Shiota C, Rocheleau JV, Shiota M, Piston DW, Magnuson MA. Impaired glucagon secretory responses in mice lacking the type 1 sulfonylurea receptor. Am J Physiol Endocrinol Metab. 2005;289:E570–577. doi: 10.1152/ajpendo.00102.2005. [DOI] [PubMed] [Google Scholar]
- Steinhardt RA. Shaky ground for lysosome-dependent membrane repair. Sci STKE. 2005;2005 doi: 10.1126/stke.3042005lc1. lc1; author reply lc2. [DOI] [PubMed] [Google Scholar]
- Südhof TC. Synaptotagmins: why so many? J Biol Chem. 2002;277:7629–7632. doi: 10.1074/jbc.R100052200. [DOI] [PubMed] [Google Scholar]
- Sun J, Pang ZP, Qin D, Fahim AT, Adachi R, Südhof TC. A dual-Ca2+-sensor model for neurotransmitter release in a central synapse. Nature. 2007;450:676–682. doi: 10.1038/nature06308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X, Zhou KM, Wu ZX, Xu T. Silence of synaptotagmin I in INS-1 cells inhibits fast exocytosis and fast endocytosis. Biochem Biophys Res Commun. 2006;347:76–82. doi: 10.1016/j.bbrc.2006.06.045. [DOI] [PubMed] [Google Scholar]
- Xu J, Mashimo T, Südhof TC. Synaptotagmin-1, -2, and -9: Ca2+ sensors for fast release that specify distinct presynaptic properties in subsets of neurons. Neuron. 2007;54:567–581. doi: 10.1016/j.neuron.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Zhao H, Ito Y, Chappel J, Andrews NW, Teitelbaum SL, Ross FP. Synaptotagmin VII regulates bone remodeling by modulating osteoclast and osteoblast secretion. Dev Cell. 2008;14:914–925. doi: 10.1016/j.devcel.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.