Abstract
Stimulation of neurons in the lateral/dorsolateral periaqueductal grey (l/dlPAG) produces increases in heart rate (HR) and mean arterial pressure (MAP) that are, according to traditional views, mediated through projections to medullary autonomic centres and independent of forebrain mechanisms. Recent studies in rats suggest that neurons in the l/dlPAG are downstream effectors responsible for responses evoked from the dorsomedial hypothalamus (DMH) from which similar cardiovascular changes and increase in core body temperature (Tco) can be elicited. We hypothesized that, instead, autonomic effects evoked from the l/dlPAG depend on neuronal activity in the DMH. Thus, we examined the effect of microinjection of the neuronal inhibitor muscimol into the DMH on increases in HR, MAP and Tco produced by microinjection of N-methyl-d-aspartate (NMDA) into the l/dlPAG in conscious rats. Microinjection of muscimol alone modestly decreased baseline HR and MAP but failed to alter Tco. Microinjection of NMDA into the l/dlPAG caused marked increases in all three variables, and these were virtually abolished by prior injection of muscimol into the DMH. Similar microinjection of glutamate receptor antagonists into the DMH also suppressed increases in HR and abolished increases in Tco evoked from the PAG. In contrast, microinjection of muscimol into the hypothalamic paraventricular nucleus failed to reduce changes evoked from the PAG and actually enhanced the increase in Tco. Thus, our data suggest that increases in HR, MAP and Tco evoked from the l/dlPAG require neuronal activity in the DMH, challenging traditional views of the place of the PAG in central autonomic neural circuitry.
The periaqueductal grey (PAG) is a mesencephalic region thought to be involved in the integration of cardiovascular changes associated with emotional behaviours (Carrive, 1993; Bandler & Shipley, 1994). While activation of neurons in the ventrolateral column of the PAG in conscious animals results in decreases in MAP and HR coupled with immobility responses (Carrive, 1993; Bandler & Shipley, 1994; Bandler et al. 2000), activation of neurons in the lateral/dorsolateral column of the PAG (l/dlPAG) results in increases in mean arterial pressure (MAP) and heart rate (HR) that are accompanied by a flight/fight response (Carrive, 1993; Bandler & Shipley, 1994; Bandler et al. 2000; da Silva et al. 2006). Activation of this region in anaesthetized rats results in sympathetically mediated increases in temperature in interscapular brown adipose tissue (IBAT; Chen et al. 2002). Increases in MAP and HR as well as other components of defensive reactions could be evoked from PAG columns in decerebrate cats (Carrive et al. 1987, 1989; Carrive & Bandler, 1991a,b; DePaulis et al. 1992). Thus, the long-held and currently accepted view is that increases in MAP and HR evoked from these regions are mediated through efferent projections from neurons in the PAG to brainstem autonomic centres (Lovick, 1993; Bandler et al. 2000; Morrison, 2001; McNaughton & Corr, 2004), and the PAG has been traditionally seen as a lower centre for defensive reactions that is influenced by descending afferents from the hypothalamus (see Bandler & Keay, 1996; Bernard & Bandler 1998; Kerman, 2008).
Activation of neurons in the dorsomedial hypothalamus (DMH) by microinjection of bicuculline methiodide (BMI), a GABAA receptor antagonist, or excitatory amino acids results in a pattern of cardiovascular and behavioural changes that resembles the responses observed after activation of l/dlPAG (Soltis & DiMicco, 1991a,b; da Silva et al. 2003; da Silva et al. 2006; de Menezes et al. 2006). Stimulation of the DMH also increases IBAT temperature in anaesthetized rats and core body temperature in both conscious and anaesthetized rats (Morrison et al. 1999; Zaretskaia et al. 2002). Recently, microinjection of either muscimol, a GABAA receptor agonist, or MK-801, an NMDA ionotropic glutamate receptor antagonist, into the l/dlPAG was shown to reduce the increases in MAP, HR, body temperature and locomotor activity evoked by disinhibition of neurons in the DMH (da Silva et al. 2003, 2006; de Menezes et al. 2006). Based on the long-standing views about the relationship between these regions discussed above, and the fact that neurons in the DMH are known to send projections to the l/dlPAG (ter Horst & Luiten, 1986; Thompson et al. 1996), the PAG was proposed to be a downstream component of descending pathways through which the activation of the DMH produces these physiological and behavioural responses (da Silva et al. 2003, 2006; de Menezes et al. 2006).
Because microinjection of muscimol into the DMH has been shown to block the increases in HR and MAP seen in air jet stress (Stotz-Potter et al. 1996a,b), our recent finding that these increases were also reduced by microinjection of muscimol into the l/dlPAG (de Menezes et al. 2008) seemed to provide further evidence that neurons in the l/dlPAG constitute downstream effectors for cardiovascular changes evoked from the DMH. Surprisingly, however, in the same study, microinjection of muscimol into the l/dlPAG also reduced the increases in plasma adrenocorticotropic hormone (ACTH) evoked by air jet stress. Increases in plasma ACTH seen in this paradigm represent activation of the hypothalamic-pituitary-adrenal axis, a hallmark of the response to stress, and have been proposed to be mediated in large part through a direct projection from neurons in the DMH to the hypothalamic paraventricular nucleus (PVN; for review, see DiMicco et al. 2002). On the other hand, neurons in the l/dlPAG have been reported not to project to the PVN (Cameron et al. 1995).
One possible explanation for all of the findings above is that neurons in the l/dlPAG represent an important afferent source of excitatory drive to neurons in the DMH that are ultimately responsible for these effects. The cardiovascular responses produced by blockade of GABAA receptors in the DMH depend on activity at local ionotropic glutamate receptors (Soltis & DiMicco, 1991b), but to date the origin of this tonic activity remains unknown. Neurons in the l/dlPAG are known to project to neurons in the region of the DMH (Shaikh et al. 1987; Cameron et al. 1995; Siegel et al. 1997), and Vianna and Brandao recently suggested that ascending projections from subregions of the PAG to forebrain regions that included the DMH may play a role in defensive reactions (Vianna & Brandao, 2003). In this regard, chemical or electrical stimulation of the l/dlPAG increase the expression of c-fos, a marker for neuronal activation (Dragunow et al. 1987; Dragunow & Robertson, 1987a,b; Hunt et al. 1987; Shaikh et al. 1987; Dragunow & Faull, 1989; Siegel et al. 1997), in the DMH (Sandner et al. 1992; de Oliveira et al. 2000). Thus, during stress, afferents from neurons in the l/dlPAG, perhaps along with those from other regions, may act to excite neurons in the DMH which would in turn activate (1) CRH-containing neurons in the PVN to stimulate the secretion of ACTH, and (2) autonomic centres in the brainstem to increase HR and MAP. Therefore we hypothesized that the increase in HR, MAP and body temperature evoked by excitation of neurons in the l/dlPAG depend on activation of neurons in the DMH.
In this study, we tested this hypothesis by examining the effect of acute inhibition of the DMH by microinjection of muscimol on responses evoked by the microinjection of the excitatory amino acid N-methyl-d-aspartate (NMDA) into l/dlPAG. We also evaluated the role of hypothalamic ionotropic glutamate receptors in the responses evoked from the l/dlPAG by employing microinjections of 2-amino-5-phosphonopentanoate (AP5) and 2,3-dihydroxy-6-nitro-7- sulfamoyl-benzo[f] quinoxaline-2,3-dione (NBQX), antagonists of NMDA and AMPA subtypes, respectively, into the DMH. To establish the anatomical specificity of the effect of muscimol microinjected into the DMH, we also tested the effect of identical microinjections into the PVN in parallel experiments.
Methods
All experiments were performed on male Sprague–Dawley rats (Harlan, Indianapolis, IN, USA) weighing 280–320 g at the time of the first surgery. Animals were housed individually and maintained in a 12 h light–12 dark cycle. Free access to water and food was allowed. All procedures conformed to the regulations set forth by NIH and were approved by the Institutional Animal Care and Use Committee of the Indiana University School of Medicine.
In 36 rats a Dataquest telemetric system (Data Sciences) was employed for measurement of HR, MAP and Tco. Animals were anaesthetized with ketamine and xylazine (80 mg kg−1 ketamine and 11.5 mg kg−1 xylazine, i.p.) and the flexible catheter of telemetric probe (C-50-PXT, Data Sciences, MN, USA) was implanted into the aorta through the right femoral artery. The transmitter body was placed in the abdominal cavity and sutured to the abdominal wall.
Six days after transmitter implantation, rats were re-anaesthetized (80 mg kg−1 ketamine and 11.5 mg kg−1 xylazine, i.p.) and guide cannulae (26 G, Plastics One, Roanoke, VA, USA) were implanted for microinjection of drugs into the l/dlPAG and into the DMH as described previously (da Silva et al. 2003, 2006; de Menezes et al. 2006). Briefly, rats were placed in a stereotaxic apparatus with the incisor bar positioned at 3.5 mm below the level of the interaural line. The guide cannulae were positioned according to the coordinates of the Paxinos and Watson atlas (Paxinos, 2007) using bregma as a reference point; coordinates for the l/dlPAG: 7.8 mm posterior, 0.7 mm lateral, 4.8 mm ventral; for the DMH: 3.2 mm posterior, 0.5 mm lateral and 7.5 mm ventral; for PVN: 1.9 mm posterior, 0.4 mm lateral and 6.8 mm ventral. The guide cannulae were then secured by two screws, Vetbond glue, and dental acrylic. Dummy cannulae were inserted into the guides, and animals were returned to their home cages for recovery. Rats were allowed at least 6 days for recovery before beginning one of the following experimental protocols.
Experiments were performed as follows in a room in which the temperature was maintained at 24–25°C. On the day of the experiment, animals were positioned in their home cages on telemetry receiver plates 2 h prior to the beginning of the protocol. The experiment commenced only after stabilization of physiological parameters (HR, MAP, Tco and locomotor activity) for at least 30 min. Microinjections were performed with a microinjector (33 gauge, 1 mm longer than the guide cannula; Plastics One, Roanoke, VA, USA) connected to a 10 μl Hamilton syringe with Teflon tubing (ID 0.12 mm; OD 0.65 mm; Bioanalytic Systems, West Lafayette, IN, USA). The syringe was mounted in an infusion pump (Harvard Apparatus, Holliston, MA, USA) that was used to deliver 100 nl of injectate over 30 s. The microinjection was considered successful if, immediately after removal of the microinjector, flow appeared within 3 s after the pump was reactivated, indicating that the injector was not clogged.
The first series of experiments examined the effect of microinjection of NMDA (6 pmol/100 nl) into the caudal l/dlPAG (n= 5) on HR, Tco and MAP. Each rat was subjected to two different trials 2 days apart and in random order in which either saline vehicle (100 nl) or NMDA (Sigma; 6 pmol) was microinjected into the l/dlPAG. This dose of NMDA was chosen based on the study of da Silva and colleagues, which demonstrated that microinjection of this dose in the l/dlPAG produces reliable increases in HR and MAP in conscious rats (da Silva et al. 2006).
The second series of experiments was designed to examine the effect of microinjection of muscimol (100 pmol/100 nl) into the ipsilateral DMH on the baseline physiological parameters (n= 6). Each rat was subjected to two different trials 2 days apart and in random order in which either saline vehicle (100 nl) or muscimol was injected into the DMH followed by injection of vehicle (100 nl) into the l/dlPAG. This dose of muscimol was chosen based on earlier studies demonstrating that microinjection of a similar dose (80 pmol) in the DMH effectively reduced the physiological responses evoked by air jet stress (Stotz-Potter et al. 1996a,b).
The third series of experiments examined the effect of microinjection of muscimol (100 pmol/100 nl) into the ipsilateral DMH (n= 8) on the responses evoked by microinjection of NMDA into the PAG. Each rat was subjected to two different trials 2 days apart and in random order in which either saline vehicle (100 nl) or muscimol was injected into the DMH followed by injection of NMDA (6 pmol) into the l/dlPAG.
The fourth series of experiments examined the effect of microinjecting a combination of ionotropic glutamate receptor antagonists (AP5, 200 pmol/100 nl, and NBQX, 100 pmol/100 nl) (n= 7) into the ipsilateral DMH on the responses evoked by microinjection of NMDA into the l/dlPAG. Each rat was subjected to two different trials 2 days apart and in random order in which either saline vehicle (100 nl) or the combination of the glutamate receptor antagonists (AP5, 200 pmol/100 nl; NBQX, 100 pmol/100 nl; both from Sigma) was injected into DMH followed by injection of NMDA (6 pmol) into the l/dlPAG. The dose of AP5 was selected because microinjection of 100 pmol of AP5 into the DMH was shown previously to produce a selective blockade of local NMDA receptors and to reduce the increases in HR and MAP seen during air jet stress (Soltis & DiMicco, 1992). Microinjection of this same dose of NBQX into the DMH was demonstrated in a similar microinjection study to reduce the increases in HR and MAP produced by microinjection of BMI into the amygdala (Soltis et al. 1998).
As an anatomical control for the microinjection of muscimol into the DMH, we examined the effect of identical microinjections into the ipsilateral PVN on the responses evoked by injection of NMDA into l/dlPAG in a parallel set of experiments (n= 6). Each rat was subjected to two different trials 2 days apart and in random order in which either saline vehicle (100 nl) or muscimol was injected into the PVN followed by injection of NMDA (Sigma; 6 pmol) into the l/dlPAG. We also examine the effect of microinjection of muscimol into the PVN on baseline physiological parameters in a study where either saline vehicle (100 nl) or muscimol (100 nmol) was microinjected into the PVN in two different trials 2 days apart and in random order (n= 4).
In all microinjection experiments, the injectate was mixed with fluorescent microspheres (FluoSpheres, 0.04 μm, 5% solids) to mark the site of injection. The solution of microspheres represented 10% of the total drug solution, so that the microspheres themselves constituted 0.5% of the volume of the final injectate. At the completion of experiments, rats were deeply anaesthetized with pentobarbital (80 mg Kg−1, i.p.) and subjected to transcardial perfusion with 60 ml ice-cold saline followed by 120 ml 4% buffered paraformaldehyde in 0.1 m phosphate-buffered saline. The brain was then removed, stored in 4% buffered paraformaldehyde overnight, and then transferred to a 30% sucrose solution until saturation. Coronal sections (40 μm) at the level of the l/dlPAG, DMH, and PVN were cut on a cryostat, mounted on slides with Vectashield HardSet Mounting Medium (Vector laboratories, Burlingame, CA, USA), and coverslipped. Slides were examined using a Leica DM LB microscope with a fluorescence digital camera (Diagnostics Instruments Inc., Starling Heights, MI, USA). Sites of injections were approximated using the atlas of Paxinos and Watson as a reference (Paxinos & Watson, 2007).
Telemetric data for HR, MAP and Tco values were recorded continuously. For all statistical analyses and for representation in figures, averages over 4 min intervals beginning with the last interval prior to the first microinjection (at t= 5 min) and extending through the entire observation period (i.e. to t= 60 min as shown in all figures) were employed. Maximal changes (as means ± standard error of the mean) were calculated using 4 min averages. For calculation of mean maximal changes in HR and MAP, the maximal change occurring within 20 min after microinjection was used. For calculation of mean maximal changes in temperature, the maximal change over the entire 60 min observation period was employed. Where the duration of a response is given, this corresponds to the time from the onset of the increase to the time at which the magnitude of the change had fallen to 60% of the peak. All data were analysed by Student's t test for paired data, again with critical P value at P < 0.05.
Results
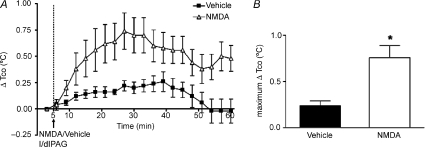
Baseline HR, Tco and MAP were not significantly different between the vehicle control and treatment in any of the protocols (Fig. 1–5). In five rats, microinjection of NMDA in the l/dlPAG produced increases in HR and MAP (mean maximum change = 70 ± 3 bpm and 20 ± 2 mmHg after NMDA vs. +23 ± 7 bpm and +8 ± 1 mmHg after vehicle, P < 0.05 by paired t test) as has been reported previously. These changes were accompanied by increases in Tco that were somewhat delayed (maximum change =+0.7 ± 0.1°C at t= 22 ± 2 min after NMDA vs +0.2 ± 0.05°C at t= 25 ± 4 min after vehicle, P < 0.05 by paired t test) and persisted for the duration of the observation period (Fig. 1). Microinjection of NMDA into l/dlPAG also produced transient increases in locomotor activity (data not shown).
Figure 1. Effect of injection of NMDA or saline into l/dlPAG on Tco.
A, mean changes from baseline for core body temperature (Tco) over time (min) after microinjection of vehicle (100 nl, black squares) or NMDA (6 pmol/100 nl, grey triangles) into l/dlPAG at t= 5 min (n= 5). Baseline Tco: saline, 37.4 ± 0.1°C; muscimol, 37.5 ± 0.1°C. B, maximum delta changes in Tco after microinjection of vehicle or NMDA. *Significant difference between response to microinjection of NMDA versus saline into l/dlPAG by paired t test, P < 0.05.
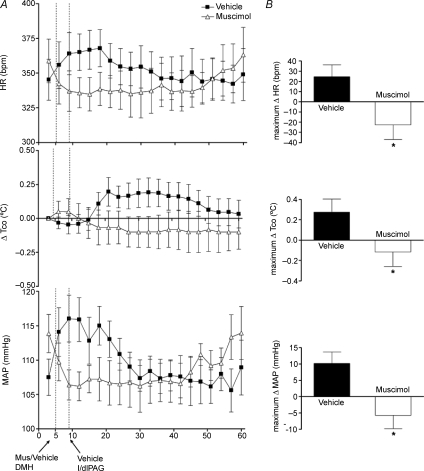
The effect of microinjecting muscimol 100 pmol/100 nl into the DMH on baseline parameters was assessed in experiments in which only vehicle was injected into the l/dlPAG (n= 5). Injection of saline into the DMH under these conditions was followed by modest but significant increases from baseline HR (maximum change =+24 ± 12 bpm at t= 15 ± 1 min), Tco (maximum change =+0.3 ± 0.1°C at t= 26 ± 3 min), and MAP (maximum change =+10 ± 3 mmHg at t= 14 ± 1 min). In contrast, after microinjection of muscimol, modest decreases from baseline HR (maximum change =−22 ± 14 bpm at t= 17 ± 1 min, duration of 19 ± 6 min), Tco (maximum change =−0.1 ± 0.1°C at t= 37 ± 3 min, duration of 49 ± 2 min) and MAP (maximum change =−5 ± 4 mmHg at t= 16 ± 1 min, duration of 34 ± 4 min) were apparent. Thus, microinjection of muscimol produced effects on HR, Tco and MAP that differed significantly from those of vehicle (P < 0.05 by paired t test; Fig. 2).
Figure 2. Effect of injection of muscimol or saline into DMH on HR, Tco, and MAP after microinjection of saline into l/dlPAG (n= 6).
A, mean values for heart rate (HR) and mean arterial pressure (MAP) and mean changes from baseline for core body temperature (Tco) over time (min) after microinjection of vehicle (100 nl, grey triangles) or muscimol (100 pmol/100 nl, black squares) into DMH at t= 5 min followed 5 min later by a microinjection of saline into l/dlPAG. Baseline Tco: saline, 37.6 ± 0.2°C; muscimol, 37.3 ± 0.2°C. B, maximum changes in HR, Tco and MAP after microinjection of vehicle/muscimol into DMH followed by microinjection of saline into l/dlPAG. *Significant difference between response to microinjection of muscimol versus saline into DMH by paired t test, P < 0.05.
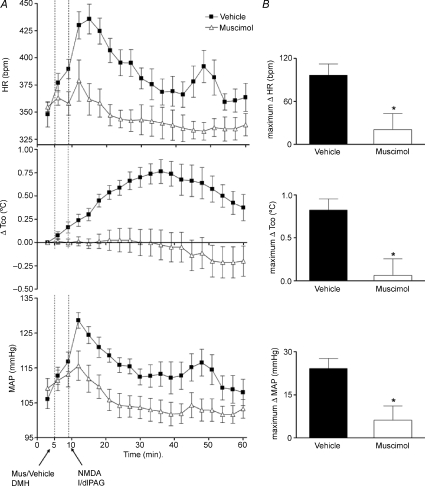
The effect of microinjection of muscimol into the DMH on the response to activation of the l/dlPAG with NMDA was assessed in eight rats. After injection of saline into the DMH, microinjection of NMDA into the l/dlPAG evoked marked increases in HR (mean maximum change =+97 ± 16 bpm at t= 14 ± 1 min), MAP (maximum change =+24 ± 3 mmHg at t= 13 ± 1 min), and Tco (maximum change =+0.8 ± 0.1°C at t= 32 ± 3 min). After microinjection of muscimol into the DMH, significant reductions in the responses to subsequent injection of NMDA into the l/dlPAG were apparent for all three parameters (P < 0.05 by paired t test; Fig. 3). Thus, injection of NMDA into the PAG produced only a slight transient increases in HR (maximum change =+21 ± 22 bpm at t= 15 ± 1 min), MAP (mean maximum change =+7 ± 4 mmHg at t= 15 ± 1 min) and Tco (maximum change =+0.06 ± 0.2°C at t= 35 ± 5 min; Fig. 3). Prior microinjection of muscimol into the DMH also significantly reduced the mean duration of NMDA-induced increases in HR (11 ± 1 min after vehicle versus 6 ± 1 min after muscimol), MAP (9 ± 1 min after vehicle versus 5 ± 1 min after muscimol), and Tco (40 ± 2 min after vehicle versus 33 ± 2 min after muscimol). The increases in locomotor activity observed after microinjection of NMDA into l/dlPAG were not significantly affected by the injection of muscimol into the DMH (data not shown).
Figure 3. Effect of prior microinjection of muscimol into DMH on cardiovascular and thermal response evoked by microinjection of NMDA into l/dlPAG (n= 8).
A, mean values for heart rate (HR) and mean arterial pressure (MAP) and mean changes from baseline for core body temperature (Tco) over time (min) after microinjection of vehicle (100 nl, grey triangles) or muscimol (1300 pmol/100 nl, black squares) into DMH at t= 5 min followed 5 min later by a microinjection of NMDA into l/dlPAG. Baseline Tco: saline, 37.3 ± 0.1°C; muscimolm 37.5 ± 0.1°C. B, maximum changes in HR, Tco and MAP after microinjection of vehicle/muscimol into DMH followed by microinjection of NMDA into l/dlPAG. *Significant difference between response to microinjection of NMDA into l/dlPAG after microinjection of muscimol versus after saline into DMH by paired t test, P < 0.05 m.
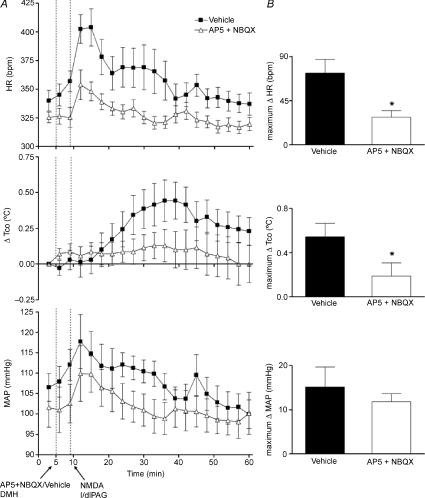
The suppression of cardiovascular and thermogenic responses evoked from the l/dlPAG by microinjection of muscimol into the DMH suggested that activity of neurons in the DMH was largely if not wholly responsible for these changes. To test the idea that ionotropic glutamate receptors in the DMH play a role in this activity, we examined the effect of blockade of these receptors in experiments paralleling those employing muscimol above. Microinjection of a combination of AP5 and NBQX, antagonists of NMDA and non-NMDA subtypes of ionotropic glutamate receptors, respectively, significantly and markedly reduced the increases in HR (maximum change =+28 ± 6 bpm at t= 15 ± 1 min) and Tco (maximum change =+0.2 ± 0.1°C at t= 36 ± 2 min) evoked from the l/dlPAG compared to responses seen after similar injection of vehicle (+72 ± 14 bpm at t= 14 ± 1 min and +0.6 ± 0.1°C at t= 34 ± 3 min, respectively, n= 7, P < 0.05 by paired t test; Fig. 4). Although a clear trend toward reduction of the NMDA-induced increase in MAP was apparent, this effect failed to reach statistical significance. In contrast to the effect of pretreatment with muscimol, prior microinjection of the glutamate receptor antagonists into the DMH failed to reduce significantly the duration of the responses for any parameter (HR: 11 ± 3 min after vehicle, 9 ± 3 min after AP5 + NBQX; MAP: 9 ± 2 min after vehicle, 6 ± 1 min after AP5 + NBQX; Tco: 39 ± 4 min after vehicle, 43 ± 5 min after AP5 + NBQX).
Figure 4. Effect of prior microinjection of a combination of glutamate receptor antagonists (AP5 and NBQX) into the DMH on cardiovascular and thermal response evoked by microinjection of NMDA into l/dlPAG (n= 7).
A, mean values for heart rate (HR) and mean arterial pressure (MAP) and mean changes from baseline for core body temperature (Tco) over time (min) after microinjection of vehicle (100 nl, grey triangles) or AP5 200 pmol/100 nl + NBQX 100 pnol/100 nl (black squares) into DMH at t= 5 min followed 5 min later by a microinjection of NMDA into l/dlPAG. Baseline Tco: saline, 37.4 ± 0.1°C; AP5 + NBQX, 37.2 ± 0.2°C. B, maximum changes in HR, Tco and MAP after microinjection of vehicle/AP5+NBQX into DMH followed by microinjection of NMDA into l/dlPAG. *Significant difference between response to microinjection of NMDA into l/dlPAG after microinjection of glutamate receptor antagonists versus after saline into DMH by paired t test, P < 0.05.
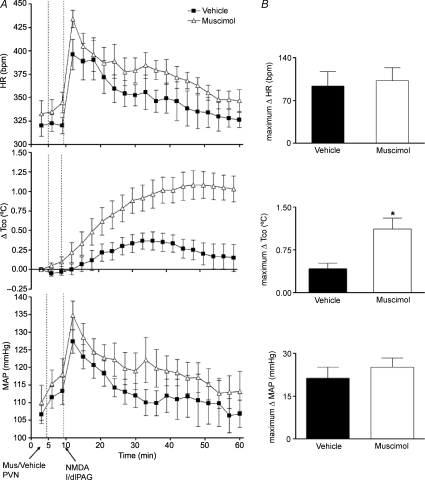
In order to establish the anatomical specificity of the effect of muscimol in the DMH, we examined the response to microinjection of NMDA into the l/dlPAG after microinjection of the same dose of muscimol into the nearby PVN (n= 6). In marked contrast to the reductions seen in the DMH, muscimol in the PVN failed to significantly affect the magnitude or duration of increases in HR and MAP produced by subsequent injection of NMDA into the l/dlPAG (Fig. 5). Surprisingly, both the magnitude and the duration of the increase in Tco produced by stimulation of l/dlPAG were significantly greater after microinjection of muscimol into the PVN (maximum change =+1.1 ± 0.2°C at t= 46 ± 1 min, duration of 51 ± 1 min) when compared with the response to NMDA after microinjection of vehicle (maximum change =+0.4 ± 0.1°C at t= 29 ± 3 min, duration of 36 ± 5 min, P < 0.05 by paired t test; Fig. 5). To examine the basis for this unexpected finding, we assessed the effect of identical microinjection of muscimol into the PVN alone in four additional rats and found it to be significantly different from the response to vehicle for all three parameters (P < 0.05 by paired t test; Fig. 6). Thus, while microinjection of vehicle into the PVN had no effect on any parameter, microinjection of muscimol evoked significant increases in HR (maximum change =+78 ± 20 bpm at t= 23 ± 4 min, duration of 33 ± 7 min) and Tco (maximum change =+0.9 ± 0.3°C at t= 55 ± 5 min, duration of 55 ± 1 min). The time course and magnitude of the effect on Tco were equivalent to the difference seen in the response to activation of the l/dlPAG after injection of muscimol into the PVN.
Figure 5. Effect of microinjection of muscimol into PVN on cardiovascular and thermal response evoked by microinjection of NMDA into l/dlPAG (n= 6).
A, mean values for heart rate (HR) and mean arterial pressure (MAP) and mean changes from baseline for core body temperature (Tco) over time (min) after microinjection of vehicle (100 nl, grey triangles) or muscimol (100 pmol/100 nl, black squares) into PVN at t= 5 min followed 5 min later by a microinjection of NMDA into l/dlPAG. Baseline Tco: saline, 37.3 ± 0.1°C; muscimol, 37.4 ± 0.2°C. B, maximum changes in HR, Tco and MAP after microinjection of vehicle/muscimol into PVN followed by microinjection of NMDA into l/dlPAG. *Significant difference between response to microinjection of NMDA into l/dlPAG after microinjection of muscimol versus after saline into PVN by paired t test, P < 0.05.
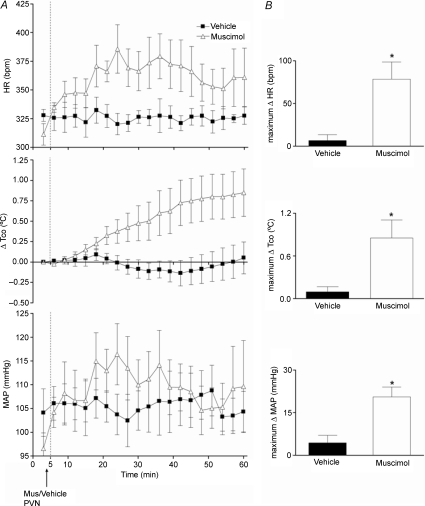
Figure 6. Effect of injection of muscimol or saline into PVN on baseline HR, MAP and Tco (n= 4).
A, mean values for heart rate (HR) and mean arterial pressure (MAP) and mean changes from baseline for core body temperature (Tco) over time (min) after microinjection of vehicle (100 nl, black squares) or muscimol (100 pmol/100 nl, grey triangles) into l/dlPAG at t= 5 min. Baseline Tco: saline, 37.8 ± 0.3°C; muscimol, 37.5 ± 0.2°C. B, maximum changes in Tco after microinjection of vehicle or muscimol into PVN. *Significant difference between response to microinjection of muscimol versus saline into PVN by paired t test, P < 0.05.
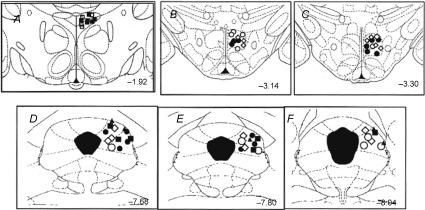
Post-mortem histology confirmed that injection sites in all microinjection experiments for which data were analysed were located in the l/dlPAG, the DMH or the PVN (Fig. 7). Injection sites in the l/dlPAG extended from 7.68 to 8.04 mm caudal to bregma, while injection sites in the DMH extended from 3.14 to 3.30 mm caudal to bregma and in the PVN were located at 1.92 mm caudal to bregma (Fig. 7). Figure 8 illustrates examples of typical injection sites in the PVN, DMH and l/dlPAG.
Figure 7. Sites of injection in the PAG, DMH and PVN in all experiments.
Schematic coronal sections of the rat brain adapted from the atlas of Paxinos and Watson (Paxinos and Watson, 2007) illustrating approximate sites of injections into the PVN and DMH (top) and PAG (bottom) for all experiments for which data are reported. Numbers indicate distance from bregma in millimetres. Injection sites in both regions within the same series of experiments are indicated by matching symbols. Grey diamonds, injection of NMDA (6 pmol/100 nl) or saline (100 nl) into l/dlPAG (n= 5; see Fig. 1); open circles, injection of vehicle into the l/dlPAG after injection of muscimol or vehicle into the DMH (n= 6; see Fig. 2); filled circles, injection of NMDA into PAG after injection of muscimol (100 pmol/100 nl) or saline into DMH (n= 8, see Fig. 3); open diamonds, injection of NMDA into l/dlPAG after microinjection of a combination of glutamate receptor antagonists (AP5, 200 pmol/100 nl, and NBQX, 100 pmol/100 nl) or vehicle into DMH (n= 7; see Fig. 4); filled squares, injection of NMDA into PAG after injection of muscimol or saline into PVN (n= 6, see Fig. 5); open squares, injection of muscimol or vehicle into PVN (n= 4; see Fig. 6).
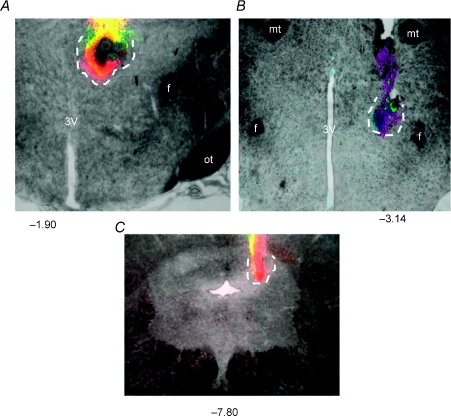
Figure 8. Photomicrographs depicting examples of sites of injection in the PVN (A), the DMH (B) and the l/dlPAG (C).
All drugs were mixed with fluorescent microspheres to mark the site. Approximate extent of spread of beads at injection sites is indicated by dotted line. Numbers represent distance from bregma in millimeters. 3V, third ventricle; f, fornix; mt, mammillothalamic tract; ot, optic tract.
Discussion
Our findings demonstrate that chemical stimulation of neurons in the l/dlPAG results in increases in HR, MAP and, for the first time, Tco in conscious rats, and that these changes depend on neuronal activity in the DMH. Microinjection of NMDA (6 pmol) into the caudal l/dlPAG produced increases in HR, MAP and locomotor activity similar to those observed in previous studies (Carrive, 1993; da Silva et al. 2006), as well as increases in Tco, and all these increases were abolished or markedly reduced by microinjection of muscimol into the DMH. The mechanism responsible for this increase in body temperature probably involves the activation of interscapular brown adipose tissue (IBAT). Microinjection of the excitatory amino acid d,l-homocysteic acid into the lateral/ventrolateral region of the caudal PAG was reported to produce increases in IBAT temperature in anaesthetized rats, although little change in Tco was detected because anaesthesia necessitated the extrinsic support of body temperature (Chen et al. 2002). Disinhibition of neurons in the DMH increases body temperature at least in part through activation of IBAT (Zaretskaia et al. 2002; Cao et al. 2004). Thus, one possible interpretation of our data is that the l/dlPAG represents a source of excitatory projections to neurons in the DMH that have previously been implicated in sympathetically mediated increases in HR, MAP and Tco (see DiMicco et al. 2002).
This notion contrasts with the view recently proposed by da Silva et al. (2003) regarding how neurons in the DMH and the l/dlPAG interact in the generation of cardiovascular responses seen in experimental stress. Disinhibition of neurons in the DMH by local microinjection of the GABAA receptor antagonist bicuculline methiodide (BMI) produces autonomic, neuroendocrine and behavioural changes in conscious rats resembling those seen in experimental stress (DiMicco & Abshire, 1987; Shekhar & DiMicco, 1987; Bailey & DiMicco, 2001), and microinjection of muscimol into the DMH suppresses the increases in HR, MAP and plasma ACTH evoked in air jet stress (Stotz-Potter et al. 1996a,b). Microinjection of either muscimol or ionotropic glutamate receptor antagonists into the l/dlPAG reduced the increases in HR and MAP (da Silva et al. 2003, 2006) as well as the increases in Tco (de Menezes et al. 2006) evoked by microinjection of BMI into the DMH. Together, these previous findings suggested that sympathoexcitatory neurons in the DMH responsible for stress-induced increases in HR and MAP as well as increases in Tco produce these effects at least in part through descending projections to the l/dlPAG (da Silva et al. 2003, d2006; de Menezes et al. 2006). If so, then inhibition of neurons in the l/dlPAG would be expected to reduce increases in HR, MAP and perhaps Tco seen in experimental stress. We recently tested this hypothesis and found that microinjection of muscimol into the l/dlPAG reduced the increases in MAP and HR seen in this paradigm (de Menezes et al. 2008). Thus, previous reports seemed to support the view that neurons in the l/dlPAG represent downstream mediators for sympathoexcitatory responses evoked from the DMH, and that this pathway plays a role in the increases in MAP and HR seen in experimental stress.
However, synthesis of the present results with the previous findings of da Silva and colleagues requires an alternative explanation for the relationship between the DMH and the PAG in this context. One such possibility is that key neurons relevant to these responses in each region may provide critical facilitation required for responses evoked by activation of corresponding neurons in the other region. Consequently, loss of this facilitation from either the DMH or the l/dlPAG would suppress similar responses evoked by disinhibition or excitation of the other. Thus, the DMH and the PAG may communicate bidirectionally as a functionally unified network with specific neurons in each region contributing to different components of the response to experimental stress (see Vianna & Brandao, 2003). A similar hypothesis with regard to behavioural components of the defence reaction has been proposed for communication between the PAG and the amygdala, based upon the finding that microinjection of muscimol into the amygdala suppressed behavioural responses to electrical stimulation of the dorsal PAG in rats (Martinez et al. 2006). Thus, inhibition of activity in the DMH or the PAG would be sufficient to prevent cardiovascular responses generated from either region. A second and closely related possibility is that this critical facilitation from neurons in the DMH and in the PAG converges on common medullary targets relevant to the cardiovascular and thermogenic responses evoked from either region. Neurons in both the DMH and PAG project to the raphe pallidus and the rostral ventrolateral medulla, two brainstem regions that have been implicated in the increase in blood pressure, heart rate and body temperature evoked from either site (Hudson & Lumb, 1996; Farkas et al. 1998; Fontes et al. 2001; Samuels et al. 2002; Horiuchi et al. 2004). Once again, loss of either source of background facilitation may effectively gate responses evoked from the other.
Previous data are consistent with the notion that projections from neurons in the l/dlPAG represent a critical source of excitation to neurons in the DMH whose activation is ultimately responsible for physiological changes seen in experimental stress. Microinjection of muscimol into the DMH in a manner identical to that employed here blocks the increases in HR and BP seen in air jet stress in rats (Stotz-Potter et al. 1996a,b). Evidence for this effect was evident in the present study, where the small increases in HR and MAP associated with microinjection of vehicle, likely to reflect the mild stress of the microinjection procedure, were absent in rats where muscimol was microinjected into the DMH (see Fig. 2). Increases in HR and MAP resulting from microinjection of BMI into the DMH or elicited by air jet stress are markedly reduced or abolished by local microinjection of ionotropic glutamate receptor antagonists (Soltis & DiMicco, 1991b, 1992), suggesting that the excitatory response to either relies on activity at glutamate receptors in the DMH. We also found in the present study that microinjection of drugs that block ionotropic glutamate receptors in the DMH suppresses the increases in HR and Tco evoked by the activation of neurons in the l/dlPAG. Blockade of glutamate receptors in the DMH also suppresses increases in HR and MAP evoked by the microinjection of BMI in the amygdala (Soltis et al. 1998). Thus, the l/dlPAG may represent one of several regions that provide glutamatergic excitation to neurons in the DMH whose activation results in an array of physiological changes seen in stress. The results of functional anatomical studies also fit with the idea that the l/dlPAG represents a source of ascending excitatory drive to neurons in the DMH. Electrical or chemical stimulation of the same region of the PAG increases the number of neurons expressing c-Fos, a marker for neuronal activation, in the DMH (Sandner et al. 1992; de Oliveira et al. 2000; Borelli et al. 2005). De Oliveira and colleagues reported that unilateral microinjection a nitric oxide donor (SIN-1) into l/dlPAG results in increases in expression of c-Fos in the DMH that were restricted to the side ipsilateral to the treatment, suggesting that these increases were not a consequence of the generalized behavioural arousal that was also produced (de Oliveira et al. 2000).
As discussed above, we have shown previously that inhibition of neurons in the l/dlPAG reduced not only the increases in HR, MAP and Tco evoked by air jet stress but also the accompanying increases in plasma levels of ACTH (de Menezes et al. 2008). Although plasma levels of ACTH were not assessed in the present study, the hypotheses presented above would suggest that an increase plasma ACTH accompanied the other physiological changes evoked from the PAG, and that these, too, may be mediated indirectly through activation of the DMH. The PVN, the location of neurons whose activation is responsible for stress-induced release of ACTH, receives modest direct innervation from neurons in the vlPAG (Floyd et al. 1996) but not from the l/dlPAG (Cameron et al. 1995). However, neurons in the l/dlPAG project to neurons in the region of the DMH (Shaikh et al. 1987; Cameron et al. 1995; Siegel et al. 1997), where (1) neurons that innervate the PVN are activated in stress (Cullinan et al. 1996), (2) disinhibition or activation of neurons increases plasma ACTH (Keim & Shekhar, 1996; Bailey & DiMicco, 2001), and (3) inhibition of neuronal activity suppresses air jet stress-induced increases in plasma ACTH and fos expression in the PVN (Stotz-Potter et al. 1996a,b; Morin et al. 2001). Thus, the suppression of stress-induced increases in plasma ACTH by microinjection of muscimol into the l/dlPAG reported previously may have reflected the loss of facilitation of neurons in the DMH originating in the former region.
In order to establish anatomical specificity, we also assessed the effect of microinjection of muscimol into the PVN on the response evoked from the PAG. In contrast to its effect in the DMH, microinjection of muscimol into the PVN failed to reduce the increases in HR and MAP evoked by microinjection of NMDA into the PAG. Unexpectedly, the increases in Tco evoked by activation of the PAG were significantly greater after microinjection of muscimol into the PVN. To examine the basis for this difference, we microinjected muscimol into the PVN without any other treatment, and found that this alone increased Tco, HR and MAP. Furthermore, the time course and magnitude of the effect on Tco were comparable to the difference seen in the response to activation of the l/dlPAG after microinjection of muscimol or saline into the PVN. One possible explanation for this effect is that neurons in the PVN are inhibitory to sympathetic thermoregulatory mechanisms as a recent preliminary report suggests (Morrison, 2006) and that the increases in both parameters seen after microinjection of muscimol into the PVN represent loss of inhibitory tone mediated through these neurons in conscious rats. Another possibility is that diffusion or spread of muscimol injected into the PVN was sufficient to reach the medial preoptic area where direct microinjection of this agent is known to produce such changes (Zaretsky et al. 2006). Nevertheless, the results clearly establish the anatomical specificity of the suppression of PAG-induced increases in HR, MAP and Tco by microinjection of muscimol into the DMH.
Our data provide evidence that the physiological responses produced by activation of neurons in the l/dlPAG depend on neuronal activity in the DMH in rats. This conclusion contradicts the traditional belief, derived from earlier studies in cats (Carrive et al. 1987, 1989), that cardiovascular reactions evoked from the PAG are independent of the participation of forebrain structures. We have also shown that ionotropic glutamate receptors in the region of the DMH are important for the cardiac and thermal responses evoked by stimulation of PAG. Activation of key neurons in the DMH appears to signal the physiological changes seen in stress, and the activity of these neurons is controlled by the balance of tone at inhibitory GABAA receptors and excitatory ionotropic glutamate receptors. Thus, during stress, tonic inhibition of these neurons may be withdrawn permitting the emergence of glutamate-mediated excitation and the resulting sympathetic and neuroendocrine effects. Our results suggest that the l/dlPAG is likely to be at least one source of this tonic excitatory drive to the relevant neurons in the DMH.
Acknowledgments
This work was supported by NIH grant NS19883-19 and by Conselho Nacional de Desenvolvimento Científico e Tecnológico/FAPEMIG. This investigation was conducted in a facility constructed with support from Research Facilities Improvement Program Grant number C06 RR015481-01 from the National Centre for Research Resources, National Institutes of Health.
References
- Bailey TW, DiMicco JA. Chemical stimulation of the dorsomedial hypothalamus elevates plasma ACTH in conscious rats. Am J Physiol Regul Integr Comp Physiol. 2001;280:R8–15. doi: 10.1152/ajpregu.2001.280.1.R8. [DOI] [PubMed] [Google Scholar]
- Bandler R, Keay KA. Columnar organization in the midbrain periaqueductal gray and the integration of emotional expression. Prog Brain Res. 1996;107:285–300. doi: 10.1016/s0079-6123(08)61871-3. [DOI] [PubMed] [Google Scholar]
- Bandler R, Keay KA, Floyd N, Price J. Central circuits mediating patterned autonomic activity during active vs. passive emotional coping. Brain Res Bull. 2000;53:95–104. doi: 10.1016/s0361-9230(00)00313-0. [DOI] [PubMed] [Google Scholar]
- Bandler R, Shipley MT. Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? Trends Neurosci. 1994;17:379–389. doi: 10.1016/0166-2236(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Bernard JF, Bandler R. Parallel circuits for emotional coping behaviour: new pieces in the puzzle. J Comp Neurol. 1998;401:429–436. [PubMed] [Google Scholar]
- Borelli KG, Ferreira-Netto C, Coimbra NC, Brandao ML. Fos-like immunoreactivity in the brain associated with freezing or escape induced by inhibition of either glutamic acid decarboxylase or GABAA receptors in the dorsal periaqueductal gray. Brain Res. 2005;1051:100–111. doi: 10.1016/j.brainres.2005.05.068. [DOI] [PubMed] [Google Scholar]
- Cameron AA, Khan IA, Westlund KN, Cliffer KD, Willis WD. The efferent projections of the periaqueductal gray in the rat: a Phaseolus vulgaris-leucoagglutinin study. I. Ascending projections. J Comp Neurol. 1995;351:568–584. doi: 10.1002/cne.903510407. [DOI] [PubMed] [Google Scholar]
- Cao WH, Fan W, Morrison SF. Medullary pathways mediating specific sympathetic responses to activation of dorsomedial hypothalamus. Neurosci. 2004;126:229–240. doi: 10.1016/j.neuroscience.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Carrive P. The periaqueductal gray and defensive behavior: functional representation and neuronal organization. Behav Brain Res. 1993;58:27–47. doi: 10.1016/0166-4328(93)90088-8. [DOI] [PubMed] [Google Scholar]
- Carrive P, Bandler R. Control of extracranial and hindlimb blood flow by the midbrain periaqueductal grey of the cat. Exp Brain Res. 1991a;84:599–606. doi: 10.1007/BF00230972. [DOI] [PubMed] [Google Scholar]
- Carrive P, Bandler R. Viscerotopic organization of neurons subserving hypotensive reactions within the midbrain periaqueductal grey: a correlative functional and anatomical study. Brain Res. 1991b;541:206–215. doi: 10.1016/0006-8993(91)91020-2. [DOI] [PubMed] [Google Scholar]
- Carrive P, Bandler R, Dampney RA. Somatic and autonomic integration in the midbrain of the unanesthetized decerebrate cat: a distinctive pattern evoked by excitation of neurones in the subtentorial portion of the midbrain periaqueductal grey. Brain Res. 1989;483:251–258. doi: 10.1016/0006-8993(89)90169-8. [DOI] [PubMed] [Google Scholar]
- Carrive P, Dampney RA, Bandler R. Excitation of neurones in a restricted portion of the midbrain periaqueductal grey elicits both behavioural and cardiovascular components of the defence reaction in the unanaesthetised decerebrate cat. Neurosci Lett. 1987;81:273–278. doi: 10.1016/0304-3940(87)90395-8. [DOI] [PubMed] [Google Scholar]
- Chen XM, Nishi M, Taniguchi A, Nagashima K, Shibata M, Kanosue K. The caudal periaqueductal gray participates in the activation of brown adipose tissue in rats. Neurosci Lett. 2002;331:17–20. doi: 10.1016/s0304-3940(02)00757-7. [DOI] [PubMed] [Google Scholar]
- Cullinan WE, Helmreich DL, Watson SJ. Fos expression in forebrain afferents to the hypothalamic paraventricular nucleus following swim stress. J Comp Neurol. 1996;368:88–99. doi: 10.1002/(SICI)1096-9861(19960422)368:1<88::AID-CNE6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- da Silva LG, de Menezes RC, dos Santos RA, Campagnole-Santos MJ, Fontes MA. Role of periaqueductal gray on the cardiovascular response evoked by disinhibition of the dorsomedial hypothalamus. Brain Res. 2003;984:206–214. doi: 10.1016/s0006-8993(03)03157-3. [DOI] [PubMed] [Google Scholar]
- da Silva LG, Jr, Menezes RC, Villela DC, Fontes MA. Excitatory amino acid receptors in the periaqueductal gray mediate the cardiovascular response evoked by activation of dorsomedial hypothalamic neurons. Neuroscience. 2006;139:1129–1139. doi: 10.1016/j.neuroscience.2005.12.041. [DOI] [PubMed] [Google Scholar]
- de Menezes RC, Zaretsky DV, Fontes MA, DiMicco JA. Microinjection of muscimol into caudal periaqueductal gray lowers body temperature and attenuates increases in temperature and activity evoked from the dorsomedial hypothalamus. Brain Res. 2006;1092:129–137. doi: 10.1016/j.brainres.2006.03.080. [DOI] [PubMed] [Google Scholar]
- de Menezes RC, Zaretsky DV, Sarkar S, Fontes MA, DiMicco JA. Microinjection of muscimol into the periaqueductal gray suppresses cardiovascular and neuroendocrine response to air jet stress in conscious rats. Am J Physiol Regul Integr Comp Physiol. 2008;295:R881–890. doi: 10.1152/ajpregu.00181.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira RW, Del Bel EA, Guimaraes FS. Behavioral and c-fos expression changes induced by nitric oxide donors microinjected into the dorsal periaqueductal gray. Brain Res Bull. 2000;51:457–464. doi: 10.1016/s0361-9230(99)00248-8. [DOI] [PubMed] [Google Scholar]
- Depaulis A, Keay KA, Bandler R. Longitudinal neuronal organization of defensive reactions in the midbrain periaqueductal gray region of the rat. Exp Brain Res. 1992;90:307–318. doi: 10.1007/BF00227243. [DOI] [PubMed] [Google Scholar]
- DiMicco JA, Abshire VM. Evidence for GABAergic inhibition of a hypothalamic sympathoexcitatory mechanism in anesthetized rats. Brain Res. 1987;402:1–10. doi: 10.1016/0006-8993(87)91041-9. [DOI] [PubMed] [Google Scholar]
- DiMicco JA, Samuels BC, Zaretskaia MV, Zaretsky DV. The dorsomedial hypothalamus and the response to stress: part renaissance, part revolution. Pharmacol Biochem Behav. 2002;71:469–480. doi: 10.1016/s0091-3057(01)00689-x. [DOI] [PubMed] [Google Scholar]
- Dragunow M, Faull R. The use of c-fos as a metabolic marker in neuronal pathway tracing. J Neurosci Methods. 1989;29:261–265. doi: 10.1016/0165-0270(89)90150-7. [DOI] [PubMed] [Google Scholar]
- Dragunow M, Peterson MR, Robertson HA. Presence of c-fos-like immunoreactivity in the adult rat brain. Eur J Pharmacol. 1987;135:113–114. doi: 10.1016/0014-2999(87)90767-9. [DOI] [PubMed] [Google Scholar]
- Dragunow M, Robertson HA. Generalized seizures induce c-fos protein(s) in mammalian neurons. Neurosci Lett. 1987a;82:157–161. doi: 10.1016/0304-3940(87)90121-2. [DOI] [PubMed] [Google Scholar]
- Dragunow M, Robertson HA. Kindling stimulation induces c-fos protein(s) in granule cells of the rat dentate gyrus. Nature. 1987b;329:441–442. doi: 10.1038/329441a0. [DOI] [PubMed] [Google Scholar]
- Farkas E, Jansen AS, Loewy AD. Periaqueductal gray matter input to cardiac-related sympathetic premotor neurons. Brain Res. 1998;792:179–192. doi: 10.1016/s0006-8993(98)00029-8. [DOI] [PubMed] [Google Scholar]
- Floyd NS, Keay KA, Arias CM, Sawchenko PE, Bandler R. Projections from the ventrolateral periaqueductal gray to endocrine regulatory subdivisions of the paraventricular nucleus of the hypothalamus in the rat. Neurosci Lett. 1996;220:105–108. doi: 10.1016/s0304-3940(96)13240-7. [DOI] [PubMed] [Google Scholar]
- Fontes MA, Tagawa T, Polson JW, Cavanagh SJ, Dampney RA. Descending pathways mediating cardiovascular response from dorsomedial hypothalamic nucleus. Am J Physiol Heart Circ Physiol. 2001;280:H2891–2901. doi: 10.1152/ajpheart.2001.280.6.H2891. [DOI] [PubMed] [Google Scholar]
- Hunt SP, Pini A, Evan G. Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature. 1987;328:632–634. doi: 10.1038/328632a0. [DOI] [PubMed] [Google Scholar]
- Horiuchi J, McAllen RM, Allen AM, Killinger S, Fontes MA, Dampney RA. Descending vasomotor pathways from the dorsomedial hypothalamic nucleus: role of medullary raphe and RVLM. Am J Physiol Regul Integr Comp Physiol. 2004;287:R824–832. doi: 10.1152/ajpregu.00221.2004. [DOI] [PubMed] [Google Scholar]
- Hudson PM, Lumb BM. Neurones in the midbrain periaqueductal grey send collateral projections to nucleus raphe magnus and the rostral ventrolateral medulla in the rat. Brain Res. 1996;733:138–141. doi: 10.1016/0006-8993(96)00784-6. [DOI] [PubMed] [Google Scholar]
- Keim SR, Shekhar A. The effects of GABAA receptor blockade in the dorsomedial hypothalamic nucleus on corticotrophin (ACTH) and corticosterone secretion in male rats. Brain Res. 1996;739:46–51. doi: 10.1016/s0006-8993(96)00810-4. [DOI] [PubMed] [Google Scholar]
- Kerman IA. Organization of brain somatomotor-sympathetic circuits. Exp Brain Res. 2008;187:1–16. doi: 10.1007/s00221-008-1337-5. [DOI] [PubMed] [Google Scholar]
- Lovick TA. The periaqueductal gray-rostral medulla connection in the defence reaction: efferent pathways and descending control mechanisms. Behav Brain Res. 1993;58:19–25. doi: 10.1016/0166-4328(93)90087-7. [DOI] [PubMed] [Google Scholar]
- McNaughton N, Corr PJ. A two-dimensional neuropsychology of defense: fear/anxiety and defensive distance. Neurosci Biobehav Rev. 2004;28:285–305. doi: 10.1016/j.neubiorev.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Martinez RC, de Oliveira AR, Brandao ML. Conditioned and unconditioned fear organized in the periaqueductal gray are differentially sensitive to injections of muscimol into amygdaloid nuclei. Neurobiol Learn Mem. 2006;85:58–65. doi: 10.1016/j.nlm.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Morin SM, Stotz-Potter EH, DiMicco JA. Injection of muscimol in dorsomedial hypothalamus and stress-induced Fos expression in paraventricular nucleus. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1276–1284. doi: 10.1152/ajpregu.2001.280.5.R1276. [DOI] [PubMed] [Google Scholar]
- Morrison SF. Differential control of sympathetic outflow. Am J Physiol Regul Integr Comp Physiol. 2001;281:R683–698. doi: 10.1152/ajpregu.2001.281.3.R683. [DOI] [PubMed] [Google Scholar]
- Morrison SF. 2006 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2006. Disinhibition of paraventricular nucleus neurons inhibits evoked increases in brown adipose tissue thermogenesis. Program No. 662.17. [Google Scholar]
- Morrison SF, Sved AF, Passerin AM. GABA-mediated inhibition of raphe pallidus neurons regulates sympathetic outflow to brown adipose tissue. Am J Physiol Regul Integr Comp Physiol. 1999;276:R290–297. doi: 10.1152/ajpregu.1999.276.2.R290. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Amsterdam: Academic Press; 2007. [Google Scholar]
- Samuels BC, Zaretsky DV, DiMicco JA. Tachycardia evoked by disinhibition of the dorsomedial hypothalamus in rats is mediated through medullary raphe. J Physiol. 2002;538:941–946. doi: 10.1113/jphysiol.2001.013302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandner G, Di Scala G, Rocha B, Angst MJ. C-fos immunoreactivity in the brain following unilateral electrical stimulation of the dorsal periaqueductal gray in freely moving rats. Brain Res. 1992;573:276–283. doi: 10.1016/0006-8993(92)90773-3. [DOI] [PubMed] [Google Scholar]
- Shaikh MB, Barrett JA, Siegel A. The pathways mediating affective defense and quiet biting attack behavior from the midbrain central gray of the cat: an autoradiographic study. Brain Res. 1987;437:9–25. doi: 10.1016/0006-8993(87)91522-8. [DOI] [PubMed] [Google Scholar]
- Shekhar A, DiMicco JA. Defense reaction elicited by injection of GABA antagonists and synthesis inhibitors into the posterior hypothalamus in rats. Neuropharmacology. 1987;26:407–417. doi: 10.1016/0028-3908(87)90020-7. [DOI] [PubMed] [Google Scholar]
- Siegel A, Schubert KL, Shaikh MB. Neurotransmitters regulating defensive rage behavior in the cat. Neurosci Biobehav Rev. 1997;21:733–742. doi: 10.1016/s0149-7634(96)00056-5. [DOI] [PubMed] [Google Scholar]
- Soltis RP, Cook JC, Gregg AE, Stratton JM, Flickinger KA. EAA receptors in the dorsomedial hypothalamic area mediate the cardiovascular response to activation of the amygdala. Am J Physiol Regul Integr Comp Physiol. 1998;275:R624–631. doi: 10.1152/ajpregu.1998.275.2.R624. [DOI] [PubMed] [Google Scholar]
- Soltis RP, DiMicco JA. GABAA and excitatory amino acid receptors in dorsomedial hypothalamus and heart rate in rats. Am J Physiol Regul Integr Comp Physiol. 1991a;260:R13–20. doi: 10.1152/ajpregu.1991.260.1.R13. [DOI] [PubMed] [Google Scholar]
- Soltis RP, DiMicco JA. Interaction of hypothalamic GABAA and excitatory amino acid receptors controlling heart rate in rats. Am J Physiol Regul Integr Comp Physiol. 1991b;261:R427–433. doi: 10.1152/ajpregu.1991.261.2.R427. [DOI] [PubMed] [Google Scholar]
- Soltis RP, DiMicco JA. Hypothalamic excitatory amino acid receptors mediate stress-induced tachycardia in rats. Am J Physiol Regul Integr Comp Physiol. 1992;262:R689–697. doi: 10.1152/ajpregu.1992.262.4.R689. [DOI] [PubMed] [Google Scholar]
- Stotz-Potter EH, Morin SM, DiMicco JA. Effect of microinjection of muscimol into the dorsomedial or paraventricular hypothalamic nucleus on air stress-induced neuroendocrine and cardiovascular changes in rats. Brain Res. 1996a;742:219–224. doi: 10.1016/s0006-8993(96)01011-6. [DOI] [PubMed] [Google Scholar]
- Stotz-Potter EH, Willis LR, DiMicco JA. Muscimol acts in dorsomedial but not paraventricular hypothalamic nucleus to suppress cardiovascular effects of stress. J Neurosci. 1996b;16:1173–1179. doi: 10.1523/JNEUROSCI.16-03-01173.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Horst GJ, Luiten PG. The projections of the dorsomedial hypothalamic nucleus in the rat. Brain Res Bull. 1986;16:231–248. doi: 10.1016/0361-9230(86)90038-9. [DOI] [PubMed] [Google Scholar]
- Thompson RH, Canteras NS, Swanson LW. Organization of projections from the dorsomedial nucleus of the hypothalamus: a PHA-L study in the rat. J Comp Neurol. 1996;376:143–173. doi: 10.1002/(SICI)1096-9861(19961202)376:1<143::AID-CNE9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Vianna DM, Brandao ML. Anatomical connections of the periaqueductal gray: specific neural substrates for different kinds of fear. Brazil J Med Biol Res. 2003;36:557–566. doi: 10.1590/s0100-879x2003000500002. [DOI] [PubMed] [Google Scholar]
- Zaretskaia MV, Zaretsky DV, Shekhar A, DiMicco JA. Chemical stimulation of the dorsomedial hypothalamus evokes non-shivering thermogenesis in anesthetized rats. Brain Res. 2002;928:113–125. doi: 10.1016/s0006-8993(01)03369-8. [DOI] [PubMed] [Google Scholar]
- Zaretsky DV, Hunt JL, Zaretskaia MV, DiMicco JA. Microinjection of prostaglandin E2 and muscimol into the preoptic area in conscious rats: comparison of effects on plasma adrenocorticotrophic hormone (ACTH), body temperature, locomotor activity, and cardiovascular function. Neurosci Lett. 2006;397:291–296. doi: 10.1016/j.neulet.2005.12.032. [DOI] [PubMed] [Google Scholar]