Abstract
Anatomical studies indicate that synaptic inputs from many cortical and subcortical structures converge on neurons of the ventral tegmental area (VTA). Although in vitro electrophysiological studies have examined synaptic inputs to dopamine (DA) and non-DA neurons in the VTA, they have largely relied upon local electrical stimulation to activate these synapses. This provides little information regarding the distinct properties of synapses originating from different brain areas. Using whole-cell recordings in parasagittal rat brain slices that preserved subcortical axons from the pedunculopontine nucleus (PPN) to the VTA, we compared these synapses with those activated by intra-VTA stimulation. PPN-evoked currents demonstrated longer latencies than intra-VTA-evoked currents, and both VTA and PPN responses were mediated by GABAA and AMPA receptors. However, unlike VTA-evoked currents, PPN currents were exclusively mediated by glutamate in 25–40% of the VTA neurons. Consistent with a cholinergic projection from the PPN to the VTA, nicotinic acetylcholine receptors (nAChR) were activated by endogenous acetylcholine released during PPN, but not VTA, stimulation. This was seen as a reduction of PPN-evoked, and not VTA-evoked, synaptic currents by the α7-nAChR antagonist methyllycaconitine (MLA) and the agonist nicotine. The β2-nAChR subunit antagonist dihydro-β-erythroidine had no effect on VTA- or PPN-evoked synaptic currents. The effects of MLA on PPN-evoked currents were unchanged by the GABAA receptor blocker picrotoxin, indicating that α7-nAChRs presynaptically modulated glutamate and not GABA release. These differences in physiological and pharmacological properties demonstrate that ascending PPN and presumed descending inputs to VTA utilize distinct mechanisms to differentially modulate neuronal activity and encode cortical and subcortical information.
A wide range of experimental work implicates the ventral tegmental area (VTA) as a core component of the mammalian brain reward circuitry (Wise, 2005). The VTA is thought to receive and integrate information from a large number of brain areas to assess the presence and strength of environmental rewards, and to marshal appropriate behavioural responses based on this information (White, 1996; Schultz, 1998; Hyland et al. 2002). This function of the VTA and associated brain reward nuclei is essential for motivated reward seeking behaviour, and hence the survival of the organism. Although there is substantial evidence demonstrating heterogeneity of cellular phenotypes in the VTA (Cameron et al. 1997; Margolis et al. 2006; Yamaguchi et al. 2007; Luo et al. 2008), it is widely accepted that DA neurons located in this midbrain nucleus are critical to the normal processing of reward-relevant information, and are involved in aberrant processes such as addiction and mental illness in humans (Wise, 2005). The patterns and rates at which VTA DA neurons discharge determine the content of information regarding environmental reward salience. Thus, the normally irregular pacemaker-like firing of these neurons can shift to brief bursts of high-frequency firing in response to environmental stimuli that are novel or predict reward (Schultz, 1998; Hyland et al. 2002). This burst or phasic firing pattern is associated with a large increase in DA release in VTA target areas such as the nucleus accumbens and prefrontal cortex (Gonon, 1988; Suaud-Chagny et al. 1992; Garris et al. 1994), and is hypothesized to signal stimulus relevance and aid in the selection of stimulus-relevant behavioural responses (Schultz, 1998). The firing patterns of VTA DA neurons are thought to be highly dependent upon excitatory afferents (Overton & Clark, 1997; Kitai et al. 1999), and evidence exists to suggest that activation of glutamatergic or cholinergic afferents can cause a shift from pacemaker to burst firing mode (Kitai et al. 1999; Georges & Aston-Jones, 2002; Floresco et al. 2003).
Inputs to the VTA arise from numerous descending and ascending afferents (Yeomans et al. 1993; Forster et al. 2002; Forster & Blaha, 2003; Geisler & Zahm, 2005; Geisler et al. 2007; Nelson et al. 2007), and it has recently been demonstrated that rather than receiving heavy inputs from a few distinct nuclei, the VTA receives glutamatergic input from an extensive number of brain regions (Geisler et al. 2007). Despite the important role that synaptic inputs to the VTA play in regulating the function of DA and non-DA neurons, little is understood of the biophysical and pharmacological properties of the extensive and distinct excitatory inputs to this brain region. One brain area that provides substantial afferent input to the VTA is the PPN. The PPN is a subcortical brainstem structure that provides ascending excitatory and inhibitory synaptic input to neurons in the VTA (Sugimoto & Hattori, 1984; Oakman et al. 1995; Geisler & Zahm, 2005). Activation of the PPN increases VTA DA neuron activity and increases extracellular DA levels in the nucleus accumbens (Klitenick & Kalivas, 1994; Floresco et al. 2003), suggesting that the PPN in part regulates the reward and motivational functions of the VTA. In support of this hypothesis, lesions of the PPN block learning of associations between primary rewards and neutral stimuli (Inglis et al. 2000), and inactivation of the PPN can reduce DA neuron responses to conditioned cues in rats (Pan & Hyland, 2005). Lesions of the PPN also block conditioned-place preference to abused drugs, including opiates and psychostimulants (Bechara & van der Kooy, 1989; Olmstead & Franklin, 1993), and decrease the reward value of self-administered heroin (Olmstead et al. 1998). Collectively, these studies indicate that PPN input to the VTA may represent an important link in the brain reward circuitry that can exercise control over the timing and strength of DA neuron activation, perhaps by integrating sensory and limbic input to influence goal-directed behaviour (Olmstead et al. 1998; Pan & Hyland, 2005).
The present study represents a systematic examination of the properties of the PPN synaptic inputs to VTA neurons in a novel brain slice preparation. The properties of this PPN-elicited input to the VTA are also directly compared to those activated via local electrical stimulation, an approach that is often used to study synaptic inputs in VTA neurons in vitro. We report distinct physiological and pharmacological properties for these synapses and demonstrate the potential utility of this model system to study the influence of abused drugs on distinct afferent pathways converging on the same VTA DA neurons.
Methods
Ethical approval
All protocols were conducted under National Institutes of Health Guidelines using the Guide for the Care and Use of Laboratory Animals, which is based upon the United States Animal Welfare Act. The protocols were approved by the Institutional Animal Care and Use Committee (National Institute on Drug Abuse, Intramural Research Program, Baltimore, MD), which is accredited by the International Association for Assessment and Accreditation of Laboratory Animal Care.
Brain slice preparation
Parasagittal brain slices containing the pedunculopontine tegmental nucleus (PPN) and the VTA were prepared from juvenile (14- to 19-day-old) Sprague–Dawley rats (Charles River Laboratories, Raleigh, NC, USA). An animal was decapitated using a guillotine, and the brain removed from the cranium and immediately transferred to a beaker containing oxygenated (95% O2–5% CO2), ice-cold (2–4°C), artificial cerebral spinal fluid (aCSF) of the following composition (mm): NaCl, 126; KCl, 3; MgCl2, 1.5; CaCl2, 2.4; NaH2PO4,1.2; glucose, 11; NaHCO3, 26; ascorbic acid, 0.4; d-2-amino-5-phosphonopentanoate (AP-5), 0.04. The brains were then blocked for slicing as follows (for diagrams depicting the dissection and blocking procedure, see the supplemental file entitled ‘Preparation of parasagittal brain slices containing intact synaptic projections from the pedunculopontine nucleus (PPN) to the ventral tegmental area (VTA)’); with the whole brain placed on its ventral surface, a cut was made transverse to the brain's longitudinal axis posterior to the inferior colliculus, thereby removing the cerebellar lobes, caudal brainstem and cervical spinal cord. According to the coordinates of Paxinos & Watson (1982), the main body of the VTA resides lateral 0.9 mm from midline, whereas the PPN is located 1.9 mm lateral. Therefore, with the brain on its ventral surface, a second cut was made through the right cerebral hemisphere on a bias following that angle, 1 mm lateral from the PPN location. Thus, the caudal portion of this cut began on the lateral side of the right inferior colliculus (through the lateral entorhinal cortex) and was angled rostrally towards the midline. A third cut was made sagittally, approximately 4 mm lateral to the mid-line, through the left hemisphere, using the lateral edge of the left inferior colliculus as a guide. This cut removed the temporal lobe and formed a flat surface. The brain was then flipped onto its remaining right surface, and the tissue dorsal and rostral to the stria medullaris was removed, including the hippocampus, striatum and frontal cortex. The remaining block of tissue containing the thalamus, the colliculi and brainstem (see Fig. 1A) was glued onto the cutting stage of a vibrating tissue slicer (Leica, VT1000) and submersed in ice-cold, oxygenated aCSF. The brain was positioned with the right hemisphere down and the thalamus facing the cutting blade. Two slices (280 μm) containing both the VTA and PPN were obtained from each rat using this procedure (Fig. 1A). The slices were transferred to an oxygenated holding chamber (31°C) and allowed to recover for at least 1 h before recording.
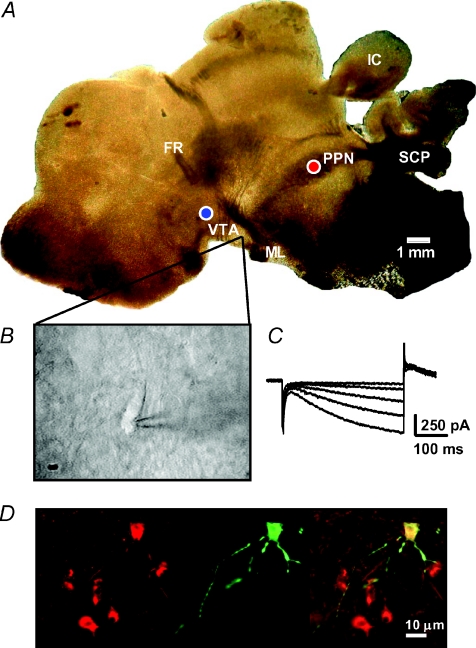
Figure 1. Identification of neurons in PPN-VTA brain slices.
A, photomicrograph showing a 280 μm thick parasagittal brain slice preparation containing the PPN and VTA as it appears in the brain slice recording chamber. The placement of stimulation and recording electrodes in the VTA (blue circle) and the PPN (red circle) was done using the superior cerebellar peduncle (SCP), and the fasciculus retroflexus (FR) as landmarks. Also aiding in the orientation were the medial lemniscus (ML) and inferior colliculus (IC). Note that in the brain slice the overlying cerebral cortical layers were removed during dissection (see supplemental file describing the blocking and slicing procedure). B, video microscopic image of a VTA neuron selected for recording. Neurons were visualized using a gradient contrast microscope and infrared illumination. C, inward currents in response to a series of negative voltage steps. Only neurons exhibiting large Ih, such as that shown in C, were included in this study. D, photomicrograph of a VTA neuron filled with biocytin (image at center) and counterstained for the dopamine transporter (image at left). At right is shown the superimposed images.
One brain slice was transferred to a heated recording chamber (31–33°C) and superfused with aCSF (2 ml min−1) that was identical to that used for slice preparation except for the absence of AP-5 and ascorbic acid. Visualization of VTA neurons was performed with an upright microscope (Zeiss Axioscope, Germany), modified to provide a gradient-contrast image utilizing infrared illumination. Recording electrodes (3–5 MΩ) contained (in mm): KCH3SO4, 115; KCl, 20; MgCl2,1; Hepes, 10; EGTA, 1; ATP, 2; GTP, 0.3; phosphocreatine, 10, pH 7.2 using KOH (osmolarity = 270–274 mosmol l−1). Whole-cell voltage clamp recordings were performed using an Axopatch 200B (Axon Instruments, Union City, CA, USA). Voltage steps and stimulation protocols were delivered using the Strathclyde electrophysiology software package (WCP, courtesy of Dr John Dempster, Strathclyde University, Glasgow, UK; http://spider.science.strath.ac.uk/sipbs/software_ses.htm) and an A/D board (ITC-18, Instrutech Corp., Bellmore, NY, USA) residing in a personal computer.
Neurons were first identified visually, and then selected on the basis of a large (>200 pA) hyperpolarization-activated inward current (Ih), as originally described by Johnson & North (1992b). Although more recent evidence demonstrates that the presence of Ih is not an unequivocal marker of VTA DA neurons (Margolis et al. 2006), additional studies show that approximately 90% of tyrosine hydroxylase (TH) positive DA neurons in the VTA demonstrate large Ih, and only 13% of the Ih positive cells did not express TH (Cameron et al. 1997). Therefore, the absolute number of non-DAergic cells demonstrating large Ih in the VTA is likely to be low (Grace & Onn, 1989; Johnson & North, 1992b; Steffensen et al. 1998). To address this, dopamine transporter (DAT) immunohistochemistry was performed in a small number of biocytin-filled VTA neurons to confirm the co-localization of large Ih magnitude with DAT immunohistochemistry (Fig. 1D). To measure Ih, neurons were voltage clamped at −60 mV and voltage steps from −70 to −110 mV were used.
Synaptic currents were evoked using two bipolar tungsten electrodes (FHC, Bowdoinham, ME, USA) with a tip separation of 300 μm, placed both in the posterior PPN and anterior to the VTA (Fig. 1A). Synaptic currents were evoked using single pulses, alternating between the intra-VTA and PPN stimulators (0.2 ms duration pulses), with at least 45 s between each alternating stimulus, and 90 seconds between stimuli applied to the same pathway. This relatively long stimulus interval was necessary because pilot studies determined that electrical stimulation of the PPN at intervals less that 90 s resulted in synaptic currents that rapidly decreased in amplitude.
The antagonist atropine sulfate (1 μm) was included in the aCSF in all experiments to block muscarinic receptors located on PPN and VTA neurons (Forster & Blaha, 2000; Miller & Blaha, 2005). This was done because in pilot experiments atropine was found to increase the probability of neurotransmitter release, as shown by a significant decrease in failures of synaptic transmission (synaptic failures: control = 26.8 ± 4.4%, atropine = 6.4 ± 3%, P < 0.01, paired t test, n= 9 neurons) following electrical stimulation of the PPN, and an increase in the proportion of neurons exhibiting PPN-evoked responses. This was likely to be due to the removal of inhibitory muscarinic influences on PPN neurons following the local release of endogenous ACh during electrical stimulation of this nucleus (Leonard & Llinas, 1994).
In experiments utilizing strontium (Sr2+), asynchronous evoked synaptic currents were collected to the hard drive of a personal computer, and analysed off-line using the MiniAnalysis software package (v 6.0, Synapstosoft, Inc., Leonia, NJ, USA). After automated software detection of asynchronous synaptic currents, each event was examined to eliminate detection errors and to ensure stable background noise levels.
The co-localization of biocytin and the DAT was performed in brain slices containing a single biocytin filled VTA neuron from which a recording was made. These slices were fixed in 4% paraformaldehyde overnight and then transferred to a phosphate buffered solution (PBS) containing 18% sucrose. The slices were then re-sectioned on a cryostat (20 μm), collected on slides, and washed in PBS (2 × 5 min). Sections were then incubated in a blocking buffer composed of PBS containing 4% bovine serum albumin (BSA) and 0.3% Triton X-100 for 1 h at room temperature, then transferred for 24 h to a solution (at 4oC) containing the DAT antibody (Millipore, Billerica, MA, USA), diluted 1 : 100 in blocking buffer. Sections were then washed twice in PBS, and incubated in Alexa Fluor 568-conjugated goat antibody to rat (Invitrogen, Carlsbad, CA, USA) for 2 h at room temperature. To detect biocytin, sections were washed twice in PBS and incubated for 1 h at room temperature in a streptavidin, Alexa Fluor 488 conjugate (Invitrogen), diluted 1 : 800 in blocking buffer.
Data in text and figures are given as means ±s.e.m. Statistical analysis was performed using either Student's two-tailed t test or a one-way ANOVA for multiple comparisons, using GraphPad Prism v 5.01 (GraphPad Software Inc., San Diego, CA, USA). Nicotine, dihydro-β-erythroidine (DHβE), atropine, picrotoxin and Sr2+ were purchased from Sigma-Aldrich (St Louis, MO, USA). Methyllycaconitine (MLA), 6,7-dinitroquinoxaline-2,3-dione (DNQX), 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione (NBQX), bicuculline methiodide (BIC) and AP-5 were supplied from Tocris (Ellisville, MO, USA). All drugs were applied through bath superfusion using calibrated syringe pumps (Razel Scientific Instruments, Stamford, CT, USA).
Results
Parasagittal brain slices that contained both the VTA and PPN were identified using neuroanatomical landmarks visible using a low-power (4×) compound microscope objective. These landmarks included the fasciculus retroflexus (FR) and the superior cerebellar peduncle (SCP), which formed a triangle with the VTA (Fig. 1A). Typically, only two brain slices containing these features could be obtained from each rat brain. The tips of a bipolar stimulating electrode were placed near the rostro-ventral border of the superior cerebellar peduncle and the caudal PPN, to permit activation of PPN afferents during whole-cell voltage clamp recordings from VTA neurons (Fig. 1A). A stimulation electrode of identical construction was placed within the rostro-dorsal VTA, rostral to the fasciculus retroflexus in the same slice (Fig. 1A). This arrangement permitted alternating stimulation of proximal VTA afferents and PPN afferents to the same neurons. Once landmarks were identified and stimulation electrodes placed, neurons were identified within the VTA using a water-immersion objective (total magnification 600×) under infrared illumination. Neurons were selected and whole-cell recording pipettes were positioned using real-time video microscopy (Fig. 1B). Once whole cell access was established, only neurons demonstrating large inward currents (Ih) upon hyperpolarization of the neuronal membrane (Fig. 1C, Methods) were recorded and included in the analyses. Initial experiments indicated there was a much higher probability of observing synaptic currents during PPN stimulation in those neurons located in the posterior VTA. This represents the area of the VTA that is nearest the PPN in these brain slices (Fig. 1A). Although the majority (> 80%) of cells in this study were located in this area, there were several neurons located midway between the anterior and posterior portions of the VTA that also responded to mild single-pulse electrical stimulation of the PPN. In contrast, electrical stimulation of the PPN never resulted in discernable synaptic currents in neurons located in the anterior portion of the VTA.
Physiological characteristics of synaptic inputs to VTA neurons
Electrical stimulation within the VTA near (∼100–200 μm) neurons voltage-clamped at −60 mV elicited inward synaptic currents that varied as a function of stimulus intensity (Fig. 2E). Similarly, electrical stimulation of the PPN elicited inward synaptic currents in VTA neurons. However, the PPN-evoked currents were observed less frequently than those following intra-VTA stimulation, occurring in approximately 50% of the recorded neurons. This may reflect a lower degree of innervation of VTA cells by PPN afferents (Omelchenko & Sesack, 2005), or the loss of some of the more obliquely projecting PPN axons, with respect to the recorded cell, following the slicing procedure.
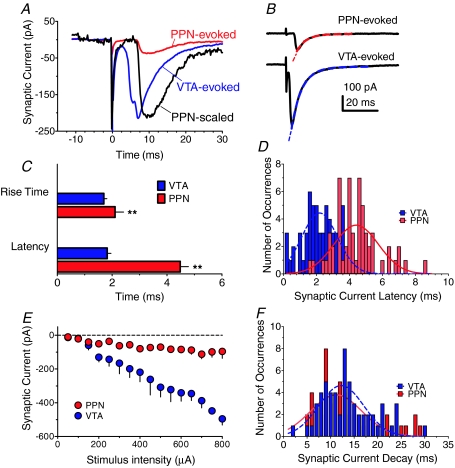
Figure 2. Distinct physiological properties of PPN- and intra-VTA-evoked synaptic currents in VTA neurons.
A, representative examples of mean synaptic currents evoked by PPN (red) or intra-VTA (blue) single-pulse stimulation in the same VTA neuron. Note the longer latency of the PPN-evoked current, evident in the scaled PPN response (black). B, single-exponential decay time constants (τ) for PPN- and VTA-evoked synaptic currents in the same VTA neuron. The τ values were 5.4 ms and 5.9 ms for the PPN- (red line) and VTA-evoked (blue line) responses, respectively. C, mean 10–90% rise times and synaptic latencies for currents evoked by PPN and intra-VTA stimulation in the same neurons (n= 58 cells). Both the rise times and latencies were significantly slower for the PPN-evoked responses (P < 0.01, ANOVA). D, histograms and Gaussian curve fits showing the distribution of latencies for PPN and intra-VTA evoked synaptic currents measured in the same VTA neurons (n= 58). Bin width = 0.2 ms. E, input–output relationship for PPN- and intra-VTA-evoked synaptic currents in the same VTA neurons across a range of stimulus intensities (n= 6 neurons). F, histograms and Gaussian curve fits showing the distribution of decay times (90–10% of peak amplitude) for PPN and intra-VTA-evoked synaptic currents in the same VTA neurons (n= 81).
To determine whether electrical stimulation applied to the PPN, or within the VTA, activated distinct synaptic inputs to the VTA neurons, we compared the properties of synaptic currents activated by alternating single-pulse stimulation of these sites in the same VTA neurons. The synaptic currents evoked by PPN stimulation differed from the VTA-evoked responses in several ways. First, PPN-evoked responses demonstrated a slower latency to rise (stimulus to 10% of peak synaptic current), compared to the intra-VTA elicited currents (PPN: 4.83 ± 0.15 ms, n= 81; intra-VTA: 1.8 ± 0.07 ms, n= 139; P < 0.0001, ANOVA, Fig. 2A–D). Secondly, we observed that the time required for the synaptic response to rise from 10–90% of its maximal amplitude (10–90% rise time) was significantly slower for the PPN-elicited synaptic current (2.11 ± 0.13 ms, n= 81), as compared to the intra-VTA synaptic response (1.7 ± 0.6 ms, n= 139; P < 0.001, ANOVA, Fig. 2C). The PPN responses also demonstrated smaller amplitudes for a given intensity of stimulation. Thus, responses from the PPN pathway reached an asymptotic level of approximately 100 pA, at 200 μA of stimulation intensity, whereas the synaptic currents evoked by intra-VTA stimulation reached a 5-fold larger level in the same neurons, over the same range of stimulus intensities (Fig. 2E). This maximum level was also reached more gradually via VTA stimulation than that for the PPN-evoked response (Fig. 2E), perhaps indicating that only a small number of neurons with similar activation thresholds were depolarized by the PPN stimulus. Whereas the amplitude of the PPN-evoked synaptic currents varied as a function of stimulation intensity, the mean latencies of these responses did not. Thus, the mean latency of the PPN-evoked response ranged from 5.72 ± 1.4 ms at the 100 μA stimulus intensity to 5.4 ± 0.4 ms at the 750 μA stimulus level (n= 6). This lack of stimulus-dependent change in synaptic latency implies that the PPN inputs to VTA neurons were mono- rather than polysynaptic.
In contrast to the differences in synaptic current properties described above, those evoked via PPN and intra-VTA stimulation exhibited similar decay kinetics. Thus, in a sample of 12 VTA neurons in which both intra-VTA and PPN-evoked synaptic currents were recorded, single exponential decay time constants (τ) of 5.6 ± 0.27 and 5.1 ± 0.4 ms were observed, respectively (Fig. 2B). Furthermore, in a larger sample of neurons (n= 81) in which 90–10% of peak decay times, rather than time constants, were measured for PPN- and VTA-evoked synaptic currents, no significant differences were observed (Fig. 2F).
The smaller maximal synaptic response resulting from PPN stimulation may indicate that fewer afferents were activated by PPN stimulation, compared to local VTA stimulation. Alternatively, it was possible that the quantal properties of these synapses differed such that the VTA pathway was more efficacious than the PPN pathway. Therefore, to distinguish these possibilities we compared the properties of quantal synaptic currents at each of these pathways, following their activation in the presence of the divalent cation Sr2+.
Strontium competes with calcium at neurotransmitter release sites in axon terminals, but is less efficient in supporting coordinated release of neurotransmitter. This has the effect of reducing the peak synaptic response, desynchronizing the release process in the stimulus-activated axons (Xu-Friedman & Regehr, 2000), and permitting the analysis of individual asynchronous quantal events over hundreds of milliseconds following the activation of axon terminals by electrical stimulation (Dodge et al. 1969; Xu-Friedman & Regehr, 2000). Bath perfusion with Sr2+ (4 mm) caused a decrement in the size of the initial synchronous inward synaptic current, followed by the appearance of asynchronous inward currents (Fig. 3A). The mean amplitudes of the asynchronous quantal events resulting from stimulation of the PPN (18.87 ± 0.75 pA, n= 159 events, n= 6 neurons) did not differ significantly from those observed following VTA stimulation (20.30 ± 0.52 pA, n= 275, n= 10 neurons; Fig. 3B, Fig. 3Ca, P > 0.05) in the same neurons. However, similar to the data obtained from the synchronous multi-quantal synaptic currents described above, the 10–90% rise times of the synaptic currents evoked by PPN stimulation in Sr2+ (1.49 ± 0.03 ms, n= 159) were significantly slower than those observed following intra-VTA stimulation (0.99 ± 0.02 ms, n= 275; Fig. 3Cd, P < 0.0001, ANOVA). In addition, there was a small, but statistically significant, difference in the time constant for the decay of the asynchronous currents recorded in Sr2+ (PPN: 3.41 ± 0.18 ms, n= 159; intra-VTA: 2.52 ± 0.11 ms, n= 275; Fig. 3Ba, 3Bb and 3Cb, P < 0.0001). Also consistent with this observation, the area of the asynchronous synaptic events was significantly larger following PPN stimulation (61.33 ± 4.03 pA ms, n= 159), compared to intra-VTA stimulation (44.97 ± 2.15 pA ms, n= 275; Fig. 3Cc, P < 0.0001). Data from pharmacological experiments demonstrated that both the PPN- and intra-VTA-evoked synchronous synaptic currents were mediated almost entirely by the release of the neurotransmitters glutamate and GABA (below). Therefore, we also examined the properties of PPN- and VTA-evoked asynchronous synaptic currents in the presence of Sr2+ after blockade of GABAA receptors with picrotoxin (100 μm). These asynchronous glutamatergic synaptic currents exhibited properties that were similar to the asynchronous currents observed in the absence of picrotoxin (Fig. 3). Thus, the mean amplitude, and amplitude distribution of these quantal EPSCs following VTA and PPN stimulation were similar (Fig. 3B and 3Ca). However, significant differences in rise and decay times between the PPN- and VTA-evoked currents were no longer observed during GABAA receptor blockade (Fig. 3Cb and 3Cd). Thus, it appears that in the absence of picrotoxin the asynchronous quantal events are composed of a GABAergic population exhibiting a more rapid rise following VTA stimulation, and a slower decay following PPN stimulation. This may reflect differences in the molecular structure of GABAA receptors which are activated by these distinct afferent inputs. Taken together, the data obtained from the Sr2+ experiments demonstrate that the quantal events activated by stimulating the PPN displayed distinct temporal characteristics compared to those observed following intra-VTA stimulation. However, the unitary quantal amplitudes of the synaptic currents activated by these sites of stimulation did not differ. This provides further evidence that distinct rather than overlapping sets of synapses were activated through these two sites of stimulation, and that differences in quantal amplitude cannot explain the differences in the input–output relationship (Fig. 2E) for these distinct VTA afferents.
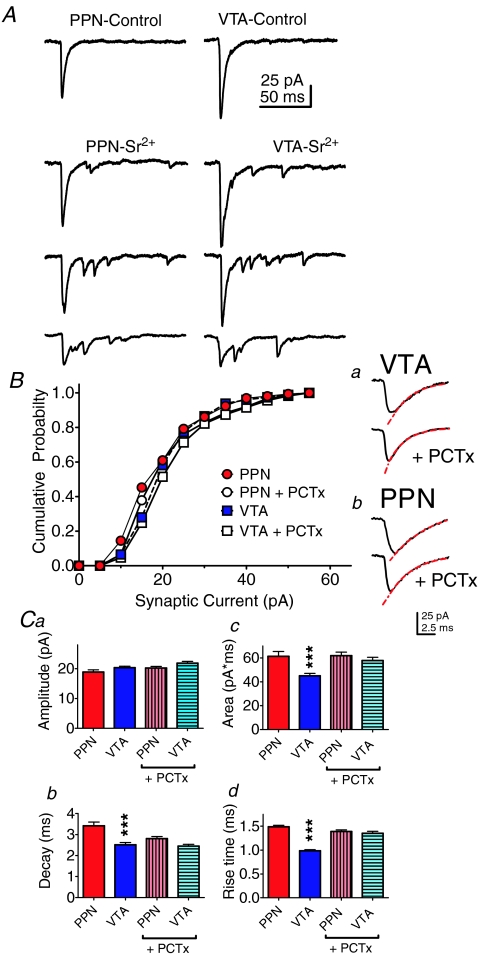
Figure 3. Quantal properties of PPN and intra-VTA evoked currents in the same VTA neurons.
A, synaptic currents following PPN or intra-VTA stimulation before and during application of Sr2+, in the absence of picrotoxin. Baseline responses were collected for 10 min before strontium (4 mm) was applied. The example waveforms in Sr2+ were collected at 2 min intervals after beginning its application. B, mean cumulative amplitude distributions of asynchronous quantal events, evoked via intra-VTA (n= 275 events) or PPN stimulation (n= 159 events), after 25 min perfusion with Sr2+, in the presence (n= 10 neurons) or absence (n= 6 neurons) of picrotoxin (PCTx, 100 μm). Note that PCTx did not change the amplitude distributions of the asynchronous synaptic events. a, averaged asynchronous synaptic currents evoked via VTA stimulation in the absence and presence of PCTx (100 μm). b, averaged asynchronous synaptic currents evoked via PPN stimulation in the absence and presence of PCTx (100 μm). In a and b, the red dashed line shows a single exponential decay curve fitted to the mean traces. These time constants were 2.95 and 2.68 ms, respectively, for VTA-evoked (a), and 6.44 and 3.44 ms, respectively, for PPN-evoked (b) currents. C, mean properties of asynchronous quantal synaptic currents evoked by PPN and intra-VTA stimulation after at least 25 min perfusion of Sr2+, in the presence (n= 10 neurons) and absence (n= 6 neurons) of PCTx (100 μm). Both the decay times (b), and the rise times (d) for the quantal currents were significantly slower for the PPN-evoked currents in the absence of PCTx, and the area of these currents was also significantly greater (c, P < 0.001, ANOVA). However, PPN- and intra-VTA-evoked mean quantal amplitudes (a) were not significantly different (P > 0.05, ANOVA).
Pharmacology of distinct synaptic inputs to the VTA
Intra-VTA stimulus-activated synaptic currents
Previous studies have demonstrated that VTA neurons receive strong inputs from both glutamatergic and GABAergic axons (Johnson & North, 1992a). Therefore, we examined the contribution of these inputs to the intra-VTA elicited synaptic currents. The AMPA/kainate receptor antagonists DNQX (10 μm) and NBQX (10 μm; data not shown) reduced the intra-VTA-evoked inward synaptic current by approximately 50% (Fig. 4, n= 15 cells). Furthermore, nearly all of the synaptic current remaining after AMPA/kainate receptor antagonism was eliminated by the GABAA receptor antagonist bicuculline (10 μm; Fig. 4), or by the GABAA chloride channel blocker picrotoxin (100 μm, not shown). This indicated that the inward synaptic currents elicited via single-pulse electrical stimulation within the VTA were largely mediated by the activation of AMPA/kainate and GABAA ionotropic receptors.
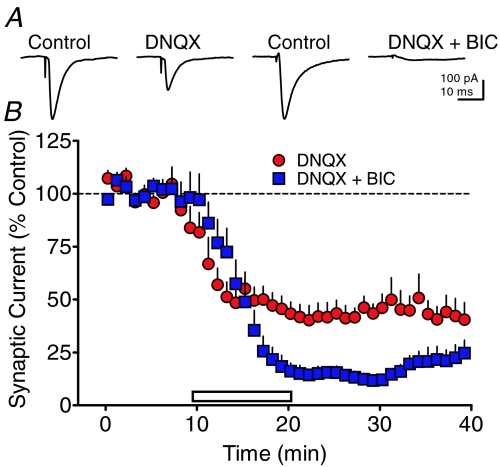
Figure 4. Effects of AMPA receptor and GABAA receptor antagonists on synaptic currents evoked by intra-VTA stimulation recorded in VTA neurons.
A, representative signal averages obtained during baseline periods (Control), or during the indicated maximal drug effect in 2 different VTA neurons. The AMPA antagonist DNQX (10 μm) caused an approximate 50% reduction in synaptic currents evoked by intra-VTA stimulation, whereas the combined application of DNQX and the GABAA receptor antagonist bicuculline (BIC, 10 μm) nearly eliminated these synaptic currents. B, mean time course of effects of DNQX alone and in combination with BIC on intra-VTA-evoked synaptic currents.
PPN stimulus-activated synaptic currents
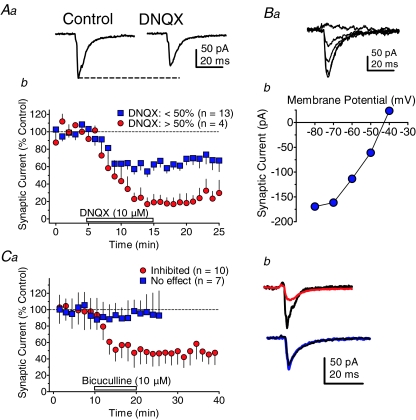
Synaptic currents activated by PPN stimulation were recorded simultaneously in a population of the same cells in which intra-VTA evoked responses were measured. The synaptic currents evoked by PPN stimulation were also sensitive to blockade by DNQX (10 μm, Fig. 5Aa and b). However, whereas the majority (n= 13/17 neurons) of PPN-evoked synaptic currents were inhibited by approximately 40% using DNQX, there was another population of cells in which DNQX caused nearly complete blockade of the synaptic currents (n= 4/17 neurons, Fig. 5Ab). This suggested that at least a portion (23.5%) of the synaptic inputs to the VTA triggered by PPN stimulation were purely glutamatergic. To identify the neurotransmitter mediating the remaining current that was not blocked by DNQX in the majority of cells, GABAA receptors were blocked with bicuculline after the application of DNQX. Bicuculline (10 μm) was found to nearly eliminate the synaptic current remaining after DNQX application in these PPN-evoked responses (not shown). In addition, the current-voltage relationships of the DNQX-insensitive synaptic currents showed reversal near the Nernst predicted equilibrium potential for Cl− under the present conditions (Fig. 5Ba and b). The effect of bicuculline alone on PPN-evoked inward synaptic currents was also examined in another group of cells. Bicuculline caused a large reduction of the PPN-evoked synaptic currents in 10 of 17 (58.8%) of the VTA neurons (Fig. 5Ca and b), but had no effect in the remaining seven neurons (41.2%, Fig. 5Ca and b). These experiments, together with the DNQX experiments described above, suggest that PPN stimulation activated synaptic currents in VTA neurons that were exclusively mediated by ionotropic glutamate receptors in approximately 24–41% of the posterior VTA neurons, and mediated by both ionotropic glutamate and GABAA receptors in the remaining 59–76% of these cells. However, whereas pure glutamatergic synaptic inputs to the VTA neurons were sometimes observed following PPN stimulation, pure GABAergic IPSCs were never observed, because in no instance did bicuculline or picrotoxin completely eliminate the synaptic currents (Fig. 5C). These data also indicated that the synaptic currents evoked by intra-VTA and PPN stimulation were almost entirely mediated by GABA and/or glutamate, without a major contribution by another neurotransmitter under the present recording conditions.
Figure 5. Identification of neurotransmitter receptors mediating PPN-evoked synaptic currents in VTA neurons.
Aa, PPN-evoked synaptic current that was representative of the majority of neurons in displaying partial sensitivity to a saturating concentration of the AMPA receptor antagonist DNQX (10 μm). b, mean time course illustrating 2 populations of PPN-evoked synaptic currents in VTA neurons; a majority inhibited less than 50% by DNQX (blue squares), and a minority inhibited greater than 50% (red circles). Ba, representative PPN-evoked synaptic currents remaining following DNQX application in a VTA neuron. The currents were evoked while the VTA neuron membrane potential was stepped from −40 mV to −80 mV (b). These synaptic currents remaining after DNQX application reversed near the Nernst-predicted reversal potential for Cl− (predicted: −46 mV, actual: −43 mV). The nAChR antagonists MLA (50 nm) and DHβE (1 μm), and the muscarinic cholinergic antagonist, atropine (1 μm) were also present in the aCSF during these experiments. Ca, mean time course of the effects of the GABAA antagonist bicuculline (10 μm) on PPN-evoked synaptic currents recorded in VTA neurons. Two populations of PPN-evoked synaptic currents were observed: those completely unresponsive to bicuculline (No effect, blue squares), and those inhibited by approximately 50% by bicuculline (Inhibited, red circles). b, representative signal averages of PPN-evoked synaptic currents that were sensitive (top, red line), and insensitive to bicuculline (bottom, blue line). Control traces are shown in black.
GABAergic and glutamatergic PPN-evoked synaptic currents exhibit similar latencies
The data shown in Fig. 2 indicated that the latencies of the synaptic currents evoked by PPN stimulation were significantly longer than those evoked by intra-VTA stimulation. Since, as demonstrated above, the PPN-evoked synaptic currents consisted of glutamatergic and GABAergic components, it was important to determine whether one or both of these components of the synaptic response elicited by PPN stimulation demonstrated similarly long latencies. Therefore, we determined whether a shift in the latencies of the pharmacologically isolated PPN-evoked currents occurred following the blockade of either AMPA receptors with DNQX, or GABAA receptors with bicuculline. In those cases where application of DNQX (10 μm) did not completely block the PPN-evoked synaptic current there was no significant change in the synaptic current latency (control latency = 4.52 ± 0.24 ms, DNQX latency = 4.53 ± 0.27 ms, n= 6 neurons, P > .05, paired t-test). Similarly, in another group of neurons in which PPN-evoked responses were recorded, bicuculline (10 μm) did not significantly change the latencies of these synaptic currents (control latency = 3.87 ± 0.42 ms, bicuculline latency = 4.04 ± 0.40 ms, n= 8 neurons, P > 0.05, paired t-test). These data, together with those shown in Fig. 2, indicate that the synaptic currents originating from the activation of both glutamatergic and GABAergic projections from PPN to VTA exhibited similarly long latencies.
PPN-evoked glutamatergic synaptic currents are modulated by endogenous ACh acting at α7-nicotinic acetylcholine receptors
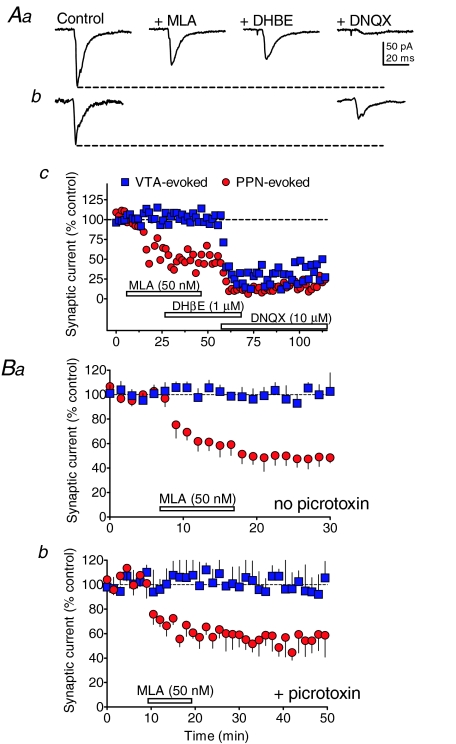
Previous studies have suggested that activation of nAChRs can excite VTA neurons (Calabresi et al. 1989; Pidoplichko et al. 1997, 2004; Mansvelder et al. 2002, 2003), and the LDT/PPN is thought to provide the primary cholinergic input to the ventral midbrain (Oakman et al. 1995; Omelchenko & Sesack, 2005). Therefore, we examined the role that these receptors might play in regulating the synaptic inputs to VTA neurons activated via either intra-VTA or PPN electrical stimulation. A single representative experiment examining the involvement of nicotinic ACh receptors in the synaptic responses evoked from both stimulus sites in the same VTA cell is shown in Fig. 6A. Neither the α4β2-nicotinic receptor antagonist DHβE (1 μm, n= 14), nor the α7-nicotinic receptor antagonist MLA (50 nm, n= 21) altered synaptic currents evoked by stimulation within the VTA in this or any of the cells tested (Figs 6 and 7B). In contrast, MLA (50 nm) reduced the PPN-evoked synaptic currents by approximately 50% in the representative cell shown in Fig. 6A, and in 9 of 17 cells that were recorded (i.e. 52.9%, Fig. 7B). However, as with the intra-VTA-evoked synaptic currents, the β2-containing nAChR antagonist DHβE did not significantly alter the PPN-evoked currents (Fig. 6Ac, Fig. 7B). This suggests that approximately 50% of the PPN-evoked synaptic currents were dependent upon functional α7-nAChRs, whereas β2-nAChRs did not appear to modulate these currents.
Figure 6. Effects of nAChR antagonists on PPN- and intra-VTA-evoked synaptic currents in VTA neurons.
Aa, PPN-evoked synaptic currents collected during the indicated pharmacological treatment from the same neuron represented in c. b, intra-VTA-evoked synaptic currents collected during the indicated pharmacological treatment (labels in a) in the same neuron as shown in c. c, time course of drug effects (indicated by the horizontal bars) on PPN- (red circles) and intra-VTA-evoked (blue squares) synaptic currents in the same VTA neuron. Note that the α7-nAChR antagonist MLA reduced the PPN-evoked response, but did not affect the VTA-evoked response. DHβE did not affect either synaptic response, and DNQX reduced both the PPN- and VTA-evoked responses in this cell. B, mean time courses of the effect of the α7-nAChR antagonist on PPN- and VTA-evoked and synaptic currents in the absence (a) and presence (b) of the GABAA blocker picrotoxin (100 μm). Note that MLA had no effect on the VTA-evoked responses, and that its inhibitory effect on PPN-evoked currents was unaffected by picrotoxin. This indicates that the effects of MLA were likely on glutamate, and not GABA release during PPN stimulation.
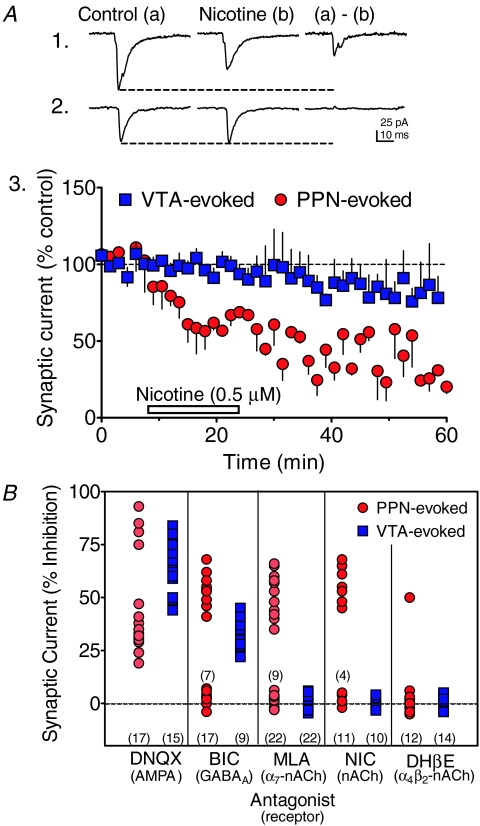
Figure 7. Effects of nicotine and nAChR antagonists on PPN- and VTA-evoked synaptic currents.
A, effects of nicotine on PPN- (1) and VTA-evoked (2) synaptic currents in VTA neurons. Averaged waveforms elicited by alternating PPN and intra-VTA stimulation in the same VTA neuron were collected during the indicated drug application periods. Also shown is the current inhibited by nicotine that was obtained by digital subtraction of the traces (a–b). 3, time course of the effects of nicotine on VTA- and PPN-evoked synaptic currents in VTA neurons. Note the reduction in PPN-evoked currents (red circles), and the absence of nicotine effects on the intra-VTA-evoked currents (blue squares). B, percentage inhibition of PPN- (red circles) and VTA-evoked (blue squares) synaptic currents by all pharmacological manipulations in all of the VTA neurons included in the study. The total number of cells exposed to each pharmacological agent is shown along the abscissa, and subtotals for some of the markers are shown above those groups of points. Drug concentrations: DNQX (10 μm), BIC (10 μm), MLA (5–50 nm), nicotine (NIC, 500 nm), DHβE (1 μm).
Since the experiments shown in Fig. 5 demonstrated that the PPN-activated synaptic currents were mediated either exclusively by AMPA receptors, or by AMPA and GABAA receptors in the remaining cells, we sought to determine whether α7-nAChRs modulated either GABA or glutamate release targeting these postsynaptic receptors. Therefore, MLA effects on synaptic responses evoked by PPN and VTA stimulation were examined in the presence and absence of the GABAA channel blocker picrotoxin (100 μm) in two separate groups of VTA neurons. Exposure of the slices to picrotoxin prior to MLA application did not alter the mean level of inhibition of the PPN-evoked synaptic currents by the α7-nAChR antagonist (Fig. 6Ba versus Fig. 6Bb), Suggesting that α7-nAChRs influenced glutamatergic, rather than GABAergic synaptic inputs to the VTA neurons.
To further examine the contribution of α7-nAChRs to the PPN- and intra-VTA-evoked synaptic currents we tested the effects of the agonist nicotine on these responses. Nicotine (500 nm) reduced the amplitudes of the PPN-evoked synaptic currents in 7 of 11 neurons (i.e. 63.6%; no effect in 4 cells, Fig. 7A), but had no effect on the synaptic currents evoked by intra-VTA stimulation in the same neurons (Fig. 7A2). This provides further evidence for the involvement of nAChRs in the PPN pathway, and for the independence of these inputs in this brain slice preparation. For comparison, the effects of all antagonists used in the characterization of the PPN- and intra-VTA-evoked synaptic inputs for all VTA neurons are shown in Fig. 7B.
Discussion
The present study demonstrates that synaptic currents elicited by electrical stimulation of the PPN differ in several respects from those activated by stimulation within the VTA. This site of stimulation was chosen for comparison with the PPN site because it is often used to elicit glutamatergic synaptic currents in VTA DA neurons in vitro (Bonci & Malenka, 1999; Overton et al. 1999; Jones et al. 2000; Saal et al. 2003; Bellone & Luscher, 2005). Furthermore, this stimulation may result in activation of descending glutamatergic afferents to the VTA arising from prefrontal cortical sites that are thought to be crucial to drug addiction (Bonci & Malenka, 1999; Jones et al. 2000). However, the extent to which intra-VTA stimulation activates glutamatergic PFC afferents to the VTA to the exclusion of other inputs in the brain slice preparation is unknown (Bonci & Malenka, 1999).
PPN and VTA evoked currents are distinct
Both physiological and pharmacological evidence obtained in the present study supports the idea that synaptic currents elicited through stimulation of the PPN and VTA were independent. Thus, the synaptic currents evoked by PPN stimulation demonstrated longer latencies (4.83 ± 0.15 ms) than those evoked via intra-VTA stimulation (1.8 ± 0.7 ms), and these latencies were not significantly altered when glutamatergic or GABAergic synaptic currents were pharmacologically isolated. This suggests that both glutamatergic and GABAergic axons projecting to the VTA from the PPN possessed similarly slow synaptic latencies when compared to those evoked via intra-VTA stimulation. The relatively long latencies for the PPN-evoked responses observed in our study agrees with a previous in vivo study of antidromically activated PPN neurons projecting to the substantia nigra pars compacta (SNc) (Scarnati et al. 1987), and with an in vitro study examining PPN-evoked glutamate EPSCs in the SNc (Futami et al. 1995). These longer latencies likely result from the observed small diameter, unmyelinated axons that arise from the PPN neurons, the greater distance of action potential propagation, and the typical slow conduction velocities that these fibres exhibit (0.45–1.25 m s−1) (Scarnati et al. 1987; Takakusaki et al. 1997). It is likely that the synaptic connection between the PPN and the ventral midbrain is monosynaptic, rather than polysynaptic, since the latencies of the PPN-evoked synaptic currents remained constant across a wide range of stimulus intensities in the present study. Furthermore, other studies have shown that the antidromic latencies of PPN neuron activation are comparable to those for orthodromic PPN stimulation of SNc neurons in vivo (Scarnati et al. 1987), and that PPN-activated synaptic potentials in SNc DA neurons follow multiple high frequency stimuli in vitro (Futami et al. 1995).
The synaptic currents evoked via PPN and intra-VTA stimulation also differed in amplitude, with the PPN-evoked responses consistently smaller than the VTA-evoked responses at the same stimulus intensity in the same VTA neurons. However, this did not appear to result from differences in quantal properties between these inputs because asynchronous quantal synaptic currents elicited from these sites during Sr2+ application showed similar mean amplitudes. This suggests that the quantal properties of these synapses were similar and that the divergent size of their multi-quantal evoked currents resulted either from the severing of axons coursing between the PPN and VTA during the slicing procedure, or a smaller number of PPN axons converging on the VTA, as compared to those activated via intra-VTA stimulation (Omelchenko & Sesack, 2005). It is interesting to note that the quantal synaptic currents elicited by PPN stimulation in the presence of Sr2+ in the absence of picrotoxin were also significantly slower to rise than those elicited by VTA stimulation, confirming the results observed with the multi-quantal evoked responses.
PPN and VTA synapses exhibit unique pharmacological profiles
The PPN- and intra-VTA evoked synaptic currents also differed in their sensitivity to pharmacological agents. The currents activated by stimulation of both sites were inhibited by AMPA and GABAA receptor antagonists applied alone, and were largely eliminated by their combined application, suggesting that glutamate and GABA were the predominant neurotransmitters mediating fast synaptic transmission in these pathways. However, whereas the VTA-evoked synaptic currents were never completely eliminated by the AMPA receptor antagonist alone, 24% of the PPN-evoked currents were completely blocked by DNQX. Furthermore, in a different group of VTA neurons, 41% of the PPN-evoked synaptic currents were insensitive to bicuculline. These data indicate that some VTA neurons receive only glutamatergic inputs from the PPN, rather than a mix of GABAergic and glutamatergic inputs. This is consistent with the identification of three major independent projection neurons in the PPN that use GABA or glutamate (Sugimoto & Hattori, 1984; Clements & Grant, 1990; Ford et al. 1995), or ACh, or ACh co-localized with glutamate (Clements et al. 1991; Lavoie & Parent, 1994; Honda & Semba, 1995). However, a more recent study has demonstrated that greater than 95% of all neurons in the PPN and LDT expressing the cholinergic marker choline acetyltransferase (ChAT) did not express markers for GABAergic (glutamic acid decarboxylase mRNA) or glutamatergic (type-2 vesicular glutamate transporter, vGluT2 mRNA) neurons, strongly suggesting the existence of three wholly independent populations of projection neurons in these nuclei (Wang & Morales 2009).
PPN inputs to VTA neurons are selectively modulated by presynaptic nAChRs
Several studies have identified nAChRs in the VTA (Wada et al. 1989; Marks et al. 1992; Clarke, 1993; Klink et al. 2001) that can depolarize midbrain DA neurons when activated (Calabresi et al. 1989; Pidoplichko et al. 1997; Fisher et al. 1998; Zhang et al. 2005). The somatodendritic nAChRs mediating this effect appear to contain β2 subunits (see Pidoplichko et al. 2004 for discussion), since the response to ACh or nicotine is usually blocked by antagonists specific for this subunit. However, α7-nAChRs have also been reported to mediate fast depolarization in a minority of VTA cells (Picciotto et al. 1998; Wooltorton et al. 2003). α7-nAChRs have also been identified on vGluT2-expressing glutamate terminals within the VTA (Jones & Wonnacott, 2004). This glutamate transporter is found at particularly high levels on the glutamatergic axons of brainstem neurons that exhibit a higher probability of release, as compared to vGluT1 expressing glutamate terminals (Fremeau, Jr. et al. 2001; Fremeau, Jr. et al. 2004; Jones & Wonnacott, 2004; Omelchenko & Sesack, 2007). Activation of these presynaptic α7-nAChRs by ACh or nicotine increases the release of glutamate, probably through Ca2+ gating (Grillner & Svensson, 2000; Mansvelder & McGehee, 2000; Pidoplichko et al. 2004).
Since the PPN/LDT is thought to provide the bulk of cholinergic innervation of the midbrain, we examined the sensitivity of synaptic currents evoked via PPN and intra-VTA stimulation to nAChR antagonists and to nicotine. We found that PPN-evoked synaptic currents were reduced by the α7-nAChR antagonist MLA (5–50 nm), and unaffected by the β2-subunit antagonist DHβE (1 μm). In contrast, intra-VTA-evoked currents in the same neurons were completely insensitive to both antagonists. Similarly, nicotine, at a concentration near that thought to be found in the brains of cigarette smokers (0.5 μm) (Pidoplichko et al. 2004), reduced the amplitudes of PPN-evoked synaptic currents without altering those evoked by VTA stimulation, an effect apparently resulting from the desensitization of presynaptic α7-nAChRs. As the magnitude of the effect of MLA on the PPN-evoked currents was unaffected by the GABAA antagonist picrotoxin, we concluded that α7-nAChRs located solely on glutamatergic axon terminals were activated by endogenous ACh that was released by PPN stimulation, and that nAChRs previously identified on GABAergic terminals in the VTA (Mansvelder et al. 2002; Pidoplichko et al. 2004) were not activated by this endogenously released ACh. In addition, the selectivity of the α7-nAChR antagonist MLA for the PPN-, but not intra-VTA-evoked synaptic currents also indicated that the endogenous ACh released by single pulse activation of the PPN was sufficient to activate α7-nAChRs located only on the glutamatergic PPN projection to the VTA, and not those glutamatergic afferents activated by intra-VTA stimulation.
Our findings differ from prior studies that found that spontaneous and intra-VTA-evoked glutamatergic EPSCs were increased by ACh (Grillner & Svensson, 2000; Mansvelder & McGehee, 2000; Pidoplichko et al. 2004), and that nicotine could first increase GABA (via non-α7, presumably β2-containing nAChRs) and glutamate (via α7-nAChRs) release, followed by desensitization of nAChRs by nicotine (Mansvelder et al. 2002; Pidoplichko et al. 2004; Keath et al. 2007). Although several differences exist between the methods used to conduct these studies and our own, the most significant is our use of single-pulse activation of the PPN in a parasaggital brain slice developed specifically to permit the isolation of PPN- and intra-VTA-activated synaptic inputs to VTA neurons. Therefore, the selectivity shown between synaptic currents activated by intra-VTA and PPN stimulation may reflect the spatial segregation of inputs to the same VTA neurons that was preserved in our preparation, and the limited spread of endogenous ACh using single-pulse stimulation of the PPN. In addition, the absence of β2-nAChR antagonist effects on synaptic GABA release, and the absence of a nicotine-induced increase in PPN-evoked synaptic currents observed in our study may have resulted from the complete desensitization of β2-containing nAChRs, and the partial desensitization of α7-nAChRs by relatively large amounts of endogenous ACh released by PPN stimulation. Thus, our inability to observe nicotine-induced increases in PPN-evoked synaptic currents, or effects of DHβE on these responses may reflect the much greater degree of exposure of α7- and β2-containing nAChRs to endogenous ACh in our preparation versus these earlier studies in which the PPN inputs to VTA were not as selectively activated, nor as well-preserved.
Despite the well-documented expression of nAChRs on the somatodendritic membranes of VTA neurons (Calabresi et al. 1989; Pidoplichko et al. 1997; Fisher et al. 1998; Picciotto et al. 1998), we did not observe postsynaptic currents mediated by these receptors during PPN stimulation in any of the neurons in this study. Considering β2- or α7-containing receptors can mediate the direct depolarization of VTA DA neurons by nAChR agonists (Picciotto et al. 1998; Klink et al. 2001; Wooltorton et al. 2003), the reason for the absence of ‘synaptic’ nAChR currents in our study is unknown. However, since these prior studies of somatodendritic nAChR activation were performed using exogenous agonist application, rather than endogenously released ACh as in the present study, the conditions favouring synaptic activation of these receptors are not well understood. An anatomical study has shown that whereas α7-nAChRs were largely located on extrasynaptic axon terminal membranes, and on postsynaptic dendrites in the VTA, they were not found in apposition to vesicular ACh transporters that are associated with cholinergic axon terminals (Jones & Wonnacott, 2004). This lack of axo-axonic and axo-dendritic synaptic cholinergic specialization has been used to support a paracrine model of ACh signalling in the VTA and elsewhere (Jones & Wonnacott, 2004), and it may explain the lack of postsynaptic nAChR activation by endogenous ACh following single-pulse activation of the PPN in the present study. Alternatively, it is possible, for reasons similar to those described above, that prior exposure of these somatodendritic nAChRs to endogenous ACh released by PPN stimulation in our preparation caused desensitization of these receptors.
The present study suggests that the ascending PPN input to the VTA exhibits several properties that differ from those of presumed descending inputs, including slower activation latencies, different pharmacological sensitivities, and the presence of a modulatory nAChR influence on excitatory synaptic transmission only on PPN afferents. Although it is clear that the properties of the PPN pathway are distinct from the ill-defined pathways activated by intra-VTA stimulation typically used to assess DA neuron function in vitro, more work is needed to determine whether these inputs are involved in processing information regarding environmental rewards, and whether these inputs are involved in addiction.
Acknowledgments
This work was supported by The U. S. Department of Health and Human Services, the National Institutes of Health, and the National Institute on Drug Abuse Intramural Research Program. We would like to thank Dr Cristina Backman and YaJun Zhang for performing DAT immunohistochemistry and biocytin reconstruction of VTA neurons.
Supplementary material
Online supplemental material for this paper can be accessed at:
http://jp.physoc.org/cgi/content/full/jphysiol.2008.164194/DC1
References
- Bechara A, Van Der Kooy D. The tegmental pedunculopontine nucleus: a brain-stem output of the limbic system critical for the conditioned place preferences produced by morphine and amphetamine. J Neurosci. 1989;9:3400–3409. doi: 10.1523/JNEUROSCI.09-10-03400.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellone C, Luscher C. mGluRs induce a long-term depression in the ventral tegmental area that involves a switch of the subunit composition of AMPA receptors. Eur J Neurosci. 2005;21:1280–1288. doi: 10.1111/j.1460-9568.2005.03979.x. [DOI] [PubMed] [Google Scholar]
- Bonci A, Malenka RC. Properties and plasticity of excitatory synapses on dopaminergic and GABAergic cells in the ventral tegmental area. J Neurosci. 1999;19:3723–3730. doi: 10.1523/JNEUROSCI.19-10-03723.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Lacey MG, North RA. Nicotinic excitation of rat ventral tegmental neurones in vitro studied by intracellular recording. Br J Pharmacol. 1989;98:135–140. doi: 10.1111/j.1476-5381.1989.tb16873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron DL, Wessendorf MW, Williams JT. A subset of ventral tegmental area neurons is inhibited by dopamine, 5-hydroxytryptamine and opioids. Neuroscience. 1997;77:155–166. doi: 10.1016/s0306-4522(96)00444-7. [DOI] [PubMed] [Google Scholar]
- Clarke PB. Nicotinic receptors in mammalian brain: localization and relation to cholinergic innervation. Prog Brain Res. 1993;98:77–83. doi: 10.1016/s0079-6123(08)62383-3. [DOI] [PubMed] [Google Scholar]
- Clements JR, Grant S. Glutamate-like immunoreactivity in neurons of the laterodorsal tegmental and pedunculopontine nuclei in the rat. Neurosci Lett. 1990;120:70–73. doi: 10.1016/0304-3940(90)90170-e. [DOI] [PubMed] [Google Scholar]
- Clements JR, Toth DD, Highfield DA, Grant SJ. Glutamate-like immunoreactivity is present within cholinergic neurons of the laterodorsal tegmental and pedunculopontine nuclei. Adv Exp Med Biol. 1991;295:127–142. doi: 10.1007/978-1-4757-0145-6_5. [DOI] [PubMed] [Google Scholar]
- Dodge FA, Jr, Miledi R, Rahamimoff R. Strontium and quantal release of transmitter at the neuromuscular junction. J Physiol. 1969;200:267–283. doi: 10.1113/jphysiol.1969.sp008692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JL, Pidoplichko VI, Dani JA. Nicotine modifies the activity of ventral tegmental area dopaminergic neurons and hippocampal GABAergic neurons. J Physiol Paris. 1998;92:209–213. doi: 10.1016/s0928-4257(98)80012-0. [DOI] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6:968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- Ford B, Holmes CJ, Mainville L, Jones BE. GABAergic neurons in the rat pontomesencephalic tegmentum: codistribution with cholinergic and other tegmental neurons projecting to the posterior lateral hypothalamus. J Comp Neurol. 1995;363:177–196. doi: 10.1002/cne.903630203. [DOI] [PubMed] [Google Scholar]
- Forster GL, Blaha CD. Laterodorsal tegmental stimulation elicits dopamine efflux in the rat nucleus accumbens by activation of acetylcholine and glutamate receptors in the ventral tegmental area. Eur J Neurosci. 2000;12:3596–3604. doi: 10.1046/j.1460-9568.2000.00250.x. [DOI] [PubMed] [Google Scholar]
- Forster GL, Yeomans JS, Takeuchi J, Blaha CD. M5 muscarinic receptors are required for prolonged accumbal dopamine release after electrical stimulation of the pons in mice. J Neurosci. 2002;22:RC190. doi: 10.1523/JNEUROSCI.22-01-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster GL, Blaha CD. Pedunculopontine tegmental stimulation evokes striatal dopamine efflux by activation of acetylcholine and glutamate receptors in the midbrain and pons of the rat. Eur J Neurosci. 2003;17:751–762. doi: 10.1046/j.1460-9568.2003.02511.x. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Troyer MD, Pahner I, Nygaard GO, Tran CH, Reimer RJ, Bellocchio EE, Fortin D, Storm-Mathisen J, Edwards RH. The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron. 2001;31:247–260. doi: 10.1016/s0896-6273(01)00344-0. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Voglmaier S, Seal RP, Edwards RH. VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends Neurosci. 2004;27:98–103. doi: 10.1016/j.tins.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Futami T, Takakusaki K, Kitai ST. Glutamatergic and cholinergic inputs from the pedunculopontine tegmental nucleus to dopamine neurons in the substantia nigra pars compacta. Neurosci Res. 1995;21:331–342. doi: 10.1016/0168-0102(94)00869-h. [DOI] [PubMed] [Google Scholar]
- Garris PA, Ciolkowski EL, Pastore P, Wightman RM. Efflux of dopamine from the synaptic cleft in the nucleus accumbens of the rat brain. J Neurosci. 1994;14:6084–6093. doi: 10.1523/JNEUROSCI.14-10-06084.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Derst C, Veh RW, Zahm DS. Glutamatergic afferents of the ventral tegmental area in the rat. J Neurosci. 2007;27:5730–5743. doi: 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Zahm DS. Afferents of the ventral tegmental area in the rat-anatomical substratum for integrative functions. J Comp Neurol. 2005;490:270–294. doi: 10.1002/cne.20668. [DOI] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. Activation of ventral tegmental area cells by the bed nucleus of the stria terminalis: a novel excitatory amino acid input to midbrain dopamine neurons. J Neurosci. 2002;22:5173–5187. doi: 10.1523/JNEUROSCI.22-12-05173.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonon FG. Nonlinear relationship between impulse flow and dopamine released by rat midbrain dopaminergic neurons as studied by in vivo electrochemistry. Neuroscience. 1988;24:19–28. doi: 10.1016/0306-4522(88)90307-7. [DOI] [PubMed] [Google Scholar]
- Grace AA, Onn SP. Morphology and electrophysiological properties of immunocytochemically identified rat dopamine neurons recorded in vitro. J Neurosci. 1989;9:3463–3481. doi: 10.1523/JNEUROSCI.09-10-03463.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner P, Svensson TH. Nicotine-induced excitation of midbrain dopamine neurons in vitro involves ionotropic glutamate receptor activation. Synapse. 2000;38:1–9. doi: 10.1002/1098-2396(200010)38:1<1::AID-SYN1>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Honda T, Semba K. An ultrastructural study of cholinergic and non-cholinergic neurons in the laterodorsal and pedunculopontine tegmental nuclei in the rat. Neuroscience. 1995;68:837–853. doi: 10.1016/0306-4522(95)00177-k. [DOI] [PubMed] [Google Scholar]
- Hyland BI, Reynolds JN, Hay J, Perk CG, Miller R. Firing modes of midbrain dopamine cells in the freely moving rat. Neuroscience. 2002;114:475–492. doi: 10.1016/s0306-4522(02)00267-1. [DOI] [PubMed] [Google Scholar]
- Inglis WL, Olmstead MC, Robbins TW. Pedunculopontine tegmental nucleus lesions impair stimulus–reward learning in autoshaping and conditioned reinforcement paradigms. Behav Neurosci. 2000;114:285–294. doi: 10.1037//0735-7044.114.2.285. [DOI] [PubMed] [Google Scholar]
- Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992a;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, North RA. Two types of neurone in the rat ventral tegmental area and their synaptic inputs. J Physiol. 1992b;450:455–468. doi: 10.1113/jphysiol.1992.sp019136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones IW, Wonnacott S. Precise localization of α-7 nicotinic acetylcholine receptors on glutamatergic axon terminals in the rat ventral tegmental area. J Neurosci. 2004;24:11244–11252. doi: 10.1523/JNEUROSCI.3009-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S, Kornblum JL, Kauer JA. Amphetamine blocks long-term synaptic depression in the ventral tegmental area. J Neurosci. 2000;20:5575–5580. doi: 10.1523/JNEUROSCI.20-15-05575.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keath JR, Iacoviello MP, Barrett LE, Mansvelder HD, McGehee DS. Differential modulation by nicotine of substantia nigra versus ventral tegmental area dopamine neurons. J Neurophysiol. 2007;98:3388–3396. doi: 10.1152/jn.00760.2007. [DOI] [PubMed] [Google Scholar]
- Kitai ST, Shepard PD, Callaway JC, Scroggs R. Afferent modulation of dopamine neuron firing patterns. Curr Opin Neurobiol. 1999;9:690–697. doi: 10.1016/s0959-4388(99)00040-9. [DOI] [PubMed] [Google Scholar]
- Klink R, de Kerchove d’Exaerde A, Zoli M, Changeux JP. Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J Neurosci. 2001;21:1452–1463. doi: 10.1523/JNEUROSCI.21-05-01452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klitenick MA, Kalivas PW. Behavioral and neurochemical studies of opioid effects in the pedunculopontine nucleus and mediodorsal thalamus. J Pharmacol Exp Ther. 1994;269:437–448. [PubMed] [Google Scholar]
- Lavoie B, Parent A. Pedunculopontine nucleus in the squirrel monkey: distribution of cholinergic and monoaminergic neurons in the mesopontine tegmentum with evidence for the presence of glutamate in cholinergic neurons. J Comp Neurol. 1994;344:190–209. doi: 10.1002/cne.903440203. [DOI] [PubMed] [Google Scholar]
- Leonard CS, Llinas R. Serotonergic and cholinergic inhibition of mesopontine cholinergic neurons controlling REM sleep: an in vitro electrophysiological study. Neuroscience. 1994;59:309–330. doi: 10.1016/0306-4522(94)90599-1. [DOI] [PubMed] [Google Scholar]
- Luo AH, Georges FE, Aston-Jones GS. Novel neurons in ventral tegmental area fire selectively during the active phase of the diurnal cycle. Eur J Neurosci. 2008;27:408–422. doi: 10.1111/j.1460-9568.2007.05985.x. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, Keath JR, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002;33:905–919. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, De Rover M, McGehee DS, Brussaard AB. Cholinergic modulation of dopaminergic reward areas: upstream and downstream targets of nicotine addiction. Eur J Pharmacol. 2003;480:117–123. doi: 10.1016/j.ejphar.2003.08.099. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, McGehee DS. Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron. 2000;27:349–357. doi: 10.1016/s0896-6273(00)00042-8. [DOI] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Hjelmstad GO, Fields HL. The ventral tegmental area revisited: Is there an electrophysiological marker for dopaminergic neurons? J Physiol. 2006;577:907–924. doi: 10.1113/jphysiol.2006.117069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MJ, Pauly JR, Gross SD, Deneris ES, Hermans-Borgmeyer I, Heinemann SF, Collins AC. Nicotine binding and nicotinic receptor subunit RNA after chronic nicotine treatment. J Neurosci. 1992;12:2765–2784. doi: 10.1523/JNEUROSCI.12-07-02765.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AD, Blaha CD. Midbrain muscarinic receptor mechanisms underlying regulation of mesoaccumbens and nigrostriatal dopaminergic transmission in the rat. Eur J Neurosci. 2005;21:1837–1846. doi: 10.1111/j.1460-9568.2005.04017.x. [DOI] [PubMed] [Google Scholar]
- Nelson CL, Wetter JB, Milovanovic M, Wolf ME. The laterodorsal tegmentum contributes to behavioral sensitization to amphetamine. Neuroscience. 2007;146:41–49. doi: 10.1016/j.neuroscience.2007.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakman SA, Faris PL, Kerr PE, Cozzari C, Hartman BK. Distribution of pontomesencephalic cholinergic neurons projecting to substantia nigra differs significantly from those projecting to ventral tegmental area. J Neurosci. 1995;15:5859–5869. doi: 10.1523/JNEUROSCI.15-09-05859.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmstead MC, Franklin KB. Effects of pedunculopontine tegmental nucleus lesions on morphine-induced conditioned place preference and analgesia in the formalin test. Neuroscience. 1993;57:411–418. doi: 10.1016/0306-4522(93)90072-n. [DOI] [PubMed] [Google Scholar]
- Olmstead MC, Munn EM, Franklin KB, Wise RA. Effects of pedunculopontine tegmental nucleus lesions on responding for intravenous heroin under different schedules of reinforcement. J Neurosci. 1998;18:5035–5044. doi: 10.1523/JNEUROSCI.18-13-05035.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. Laterodorsal tegmental projections to identified cell populations in the rat ventral tegmental area. J Comp Neurol. 2005;483:217–235. doi: 10.1002/cne.20417. [DOI] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. Glutamate synaptic inputs to ventral tegmental area neurons in the rat derive primarily from subcortical sources. Neuroscience. 2007;146:1259–1274. doi: 10.1016/j.neuroscience.2007.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton PG, Clark D. Burst firing in midbrain dopaminergic neurons. Brain Res Brain Res Rev. 1997;25:312–334. doi: 10.1016/s0165-0173(97)00039-8. [DOI] [PubMed] [Google Scholar]
- Overton PG, Richards CD, Berry MS, Clark D. Long-term potentiation at excitatory amino acid synapses on midbrain dopamine neurons. Neuroreport. 1999;10:221–226. doi: 10.1097/00001756-199902050-00004. [DOI] [PubMed] [Google Scholar]
- Pan WX, Hyland BI. Pedunculopontine tegmental nucleus controls conditioned responses of midbrain dopamine neurons in behaving rats. J Neurosci. 2005;25:4725–4732. doi: 10.1523/JNEUROSCI.0277-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 2nd. New York: Academic Press Inc.; 1982. [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, Fuxe K, Changeux JP. Acetylcholine receptors containing the β2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Pidoplichko VI, DeBiasi M, Williams JT, Dani JA. Nicotine activates and desensitizes midbrain dopamine neurons. Nature. 1997;390:401–404. doi: 10.1038/37120. [DOI] [PubMed] [Google Scholar]
- Pidoplichko VI, Noguchi J, Areola OO, Liang Y, Peterson J, Zhang T, Dani JA. Nicotinic cholinergic synaptic mechanisms in the ventral tegmental area contribute to nicotine addiction. Learn Mem. 2004;11:60–69. doi: 10.1101/lm.70004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Scarnati E, Proia A, Di LS, Pacitti C. The reciprocal electrophysiological influence between the nucleus tegmenti pedunculopontinus and the substantia nigra in normal and decorticated rats. Brain Res. 1987;423:116–124. doi: 10.1016/0006-8993(87)90831-6. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Steffensen SC, Svingos AL, Pickel VM, Henriksen SJ. Electrophysiological characterization of GABAergic neurons in the ventral tegmental area. J Neurosci. 1998;18:8003–8015. doi: 10.1523/JNEUROSCI.18-19-08003.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suaud-Chagny MF, Chergui K, Chouvet G, Gonon F. Relationship between dopamine release in the rat nucleus accumbens and the discharge activity of dopaminergic neurons during local in vivo application of amino acids in the ventral tegmental area. Neuroscience. 1992;49:63–72. doi: 10.1016/0306-4522(92)90076-e. [DOI] [PubMed] [Google Scholar]
- Sugimoto T, Hattori T. Organization and efferent projections of nucleus tegmenti pedunculopontinus pars compacta with special reference to its cholinergic aspects. Neuroscience. 1984;11:931–946. doi: 10.1016/0306-4522(84)90204-5. [DOI] [PubMed] [Google Scholar]
- Takakusaki K, Shiroyama T, Kitai ST. Two types of cholinergic neurons in the rat tegmental pedunculopontine nucleus: electrophysiological and morphological characterization. Neuroscience. 1997;79:1089–1109. doi: 10.1016/s0306-4522(97)00019-5. [DOI] [PubMed] [Google Scholar]
- Wada E, Wada K, Boulter J, Deneris E, Heinemann S, Patrick J, Swanson LW. Distribution of α2, α3, α4, and β2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. J Comp Neurol. 1989;284:314–335. doi: 10.1002/cne.902840212. [DOI] [PubMed] [Google Scholar]
- Wang H-L, Morales MM. Pedunculopontine and laterodorsal tegmental nuclei contain independent populations of cholinergic, glutamatergic and GABAergic neurons in the rat. Eur J Neurosci. 2009;29:340–358. doi: 10.1111/j.1460-9568.2008.06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White FJ. Synaptic regulation of mesocorticolimbic dopamine neurons. Annu Rev Neurosci. 1996;19:405–436. doi: 10.1146/annurev.ne.19.030196.002201. [DOI] [PubMed] [Google Scholar]
- Wise RA. Forebrain substrates of reward and motivation. J Comp Neurol. 2005;493:115–121. doi: 10.1002/cne.20689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooltorton JRA, Pidoplichko VI, Broide RS, Dani JA. Differential desensitization and distribution of nicotinic acetylcholine receptor subtypes in midbrain dopamine areas. J Neurosci. 2003;23:3176–3185. doi: 10.1523/JNEUROSCI.23-08-03176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu-Friedman MA, Regehr WG. Probing fundamental aspects of synaptic transmission with strontium. J Neurosci. 2000;20:4414–4422. doi: 10.1523/JNEUROSCI.20-12-04414.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Sheen W, Morales M. Glutamatergic neurons are present in the rat ventral tegmental area. Eur J Neurosci. 2007;25:106–118. doi: 10.1111/j.1460-9568.2006.05263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeomans JS, Mathur A, Tampakeras M. Rewarding brain stimulation: role of tegmental cholinergic neurons that activate dopamine neurons. Behav Neurosci. 1993;107:1077–1087. doi: 10.1037//0735-7044.107.6.1077. [DOI] [PubMed] [Google Scholar]
- Zhang L, Liu Y, Chen X. Carbachol induces burst firing of dopamine cells in the ventral tegmental area by promoting calcium entry through L-type channels in the rat. J Physiol. 2005;568:469–481. doi: 10.1113/jphysiol.2005.094722. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.