Abstract
Previous work has shown that small depolarizing pulses produce a beat to beat alternation in the amplitude of the systolic Ca2+ transient in ventricular myocytes. The aim of the present work was to investigate the role of changes of SR Ca2+ content and L-type Ca2+ current in this alternans. As the amplitude of the depolarizing pulse was increased from 10 to 30 mV the magnitude of alternans decreased. Confocal linescan studies showed that this was accompanied by an increase in the number of sites from which Ca2+ waves propagated. A sudden decrease in the depolarisation amplitude resulted in three classes of behaviour: (1) a gradual decrease in Ca2+ transient amplitude before alternans developed accompanied by a loss of SR Ca2+, (2) a gradual increase in Ca2+ transient amplitude before alternans accompanied by a gain of SR Ca2+, and (3) immediate development of alternans with no change of SR content. We conclude that alternans develops if the combination of decreased opening of L-type channels and change of SR Ca2+ content results in spatially fragmented release from the SR as long as there is sufficient Ca2+ in the SR to sustain wave propagation. Potentiation of the opening of the ryanodine receptor (RyR) by low concentrations of caffeine (100 μm) abolished alternans for a few pulses but the alternans then redeveloped once SR Ca2+ content fell to the new threshold for wave propagation. Finally we show evidence that inhibiting L-type Ca2+ current with 200 μm Cd2+ produces alternans by means of a similar fragmentation of the Ca2+ release profile and propagation of mini-waves of Ca2+ release.
In the healthy heart, under normal circumstances, the force of contraction and the underlying systolic Ca2+ transient are constant from beat to beat. However, under some conditions, in particular heart failure, this regulation breaks down. There can then follow pulsus alternans where the amplitude of contraction alternates from beat to beat (see Eisner et al. 2006; Weiss et al. 2006; Myles et al. 2008 for recent reviews).
In previous work we have shown that alternans of the Ca2+ transient amplitude can be produced experimentally by manoeuvres that either directly interfere with the opening of the RyR (acidosis or the local anaesthetic tetracaine) (Díaz et al. 2002) or indirectly reduce RyR opening by decreasing the triggering L-type Ca2+ current (e.g. decreasing the amplitude of the depolarizing pulse) (Díaz et al. 2004). The Ca2+ alternans was accompanied by an alternation of the Ca2+ content of the SR. In brief the alternans arose because the initial phase of Ca2+ release from the RyR was confined to a small number of RyRs. If the SR Ca2+ content was below a certain level then Ca2+ release remained confined to these RyRs and a small systolic Ca2+ transient was observed. However, when SR content was above a threshold level, waves of Ca2+ release propagated away from the sites of initial release (mini waves) resulting in a large Ca2+ transient. Such a steep dependence of Ca2+ release on SR Ca2+ content has been suggested previously to produce instability of Ca2+ release (Adler et al. 1985; Eisner et al. 2000; Tao et al. 2008).
Our previous work did not provide any information about the factors that determine whether or not alternans occurs. The aim of the work in the present paper was, therefore, to investigate the effects of factors such as membrane potential, SR Ca2+ content and RyR properties on the occurrence of alternans e.g. the number of release sites involved at different alternans ratios. The results show that altering the amplitude of the depolarising step under voltage clamp conditions can alter the systolic Ca2+ alternans ratio produced. In addition, changes to the ratio of alternans involve changes to the number of release sites that initiate waves of propagating Ca2+-induced Ca2+ release. We also show that systolic Ca2+ alternans brought about by inhibiting the L-type Ca2+ current and, therefore, stimulated Ca2+ release also involves fragmentation of the Ca2+ release profile and propagation of mini-waves of Ca2+ release.
Methods
Myocytes were isolated from rat ventricular muscle using a collagenase and protease technique as previously described (Eisner et al. 1989). Rats were killed by stunning and cervical dislocation. Care and use of animals were in accordance with the UK Animals (Scientific Procedures) Act 1986. Cells were voltage-clamped with the perforated-patch technique using the switch clamp mode of the Axoclamp 2A voltage-clamp amplifier (Axon Instruments, Union City, CA, USA). Pipettes (<5 MΩ) were filled with the following solution (mmol l−1): KCH3O3S, 125; KCl, 12; NaCl, 10; Hepes, 10; MgCl2, 5; EGTA, 0.1; titrated to pH 7.2 with KOH; and a final concentration of amphotericin B of 240 μg ml−1. The bathing solution was as follows (mmol l−1): NaCl, 135; KCl, 4; Hepes, 10; glucose, 11; MgCl2, 1; CaCl2, 5; titrated to pH 7.4 with NaOH. All solutions contained 5 mm 4-aminopyridine and 0.1 mm BaCl2 to ensure that tail currents represented only Na+/Ca2+ exchange (NCX) current.
Under voltage clamp cells were stimulated at 0.5 Hz by 100 ms depolarisations from the holding potential (−40 mV) to the test voltage. To calculate changes of SR Ca2+ content, we first calculated the integrals of the L-type Ca2+ current and NCX tail currents and subtracted one from the other. The resulting differences were then summed. These currents represent influx and efflux of Ca2+ from the cell. We found that the Ca2+ efflux was generally greater than measured influx, presumably due to a background influx resulting from the elevated external Ca2+ (Díaz et al. 2004). Any imbalance of these values of influx and efflux we represent as loss or gain of Ca2+ by the cell expressed as μmoles per litre cell volume.
[Ca2+]i was measured after loading myocytes with the acetoxymethyl ester of fluo-3 (5 μm) for 5 min. Epi-fluorescence measurements were made by exciting fluorescence at 488 nm and collecting at wavelengths longer than 500 nm. Confocal Ca2+ measurements were made using a Bio-Rad 1024 confocal microscope in line scan mode. Fluorescence signals were calibrated (Trafford et al. 1999) using the following equation:
The maximum fluorescence (Fmax) was obtained by damaging the cell at the end of the experiment by lowering the pipette. For the purposes of these experiments Kd was assumed to be 400 nm. Confocal experiments have not been calibrated in terms of absolute Ca2+ concentration due to the amount of bleaching over the period of the experiments.
Identification of release sites
This was carried out by three individuals for only those cells with clear examples of 1, 2, 3 or 4 release sites. One of the individuals is not an author on the paper and is therefore impartial. Those that had the agreement of all three judges were used in the study.
All experiments were carried out at room temperature (20°C). All statistics are quoted as means ± s.e.m.
Results
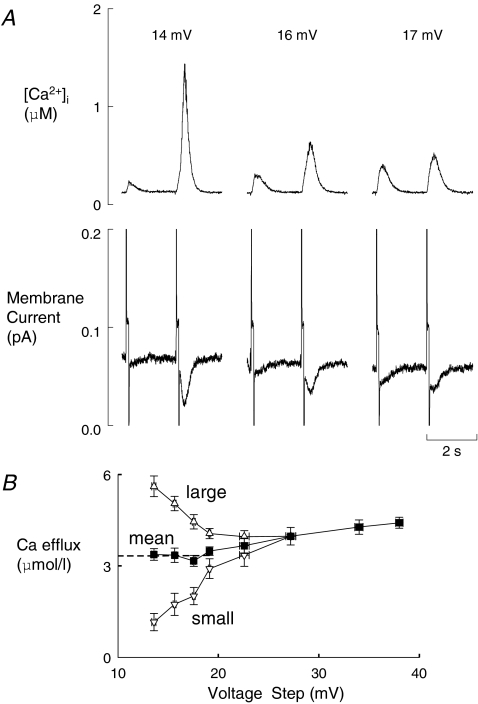
We have previously shown that decreasing the amplitude of the depolarizing pulse results in alternans of the calcium transient. Here we investigate the effects of varying the amplitude of the small, depolarizing pulse. An example is shown in Fig. 1. On the left of Fig. 1A, the depolarisation is 14 mV in amplitude from a holding potential of −40 mV and there is very obvious alternans. A convenient index of the degree of alternans is provided by the ratio of the amplitude of the large and small responses (the ‘alternans ratio’), which is large for this pulse size. As the depolarisation is increased to 16 mV the alternans ratio is decreased. Finally at a depolarisation of 17 mV the systolic Ca2+ transients are almost equal in amplitude, i.e. alternans has disappeared. The membrane currents associated with these changes in alternans ratio are shown in the lower panel of Fig. 1A. The small amplitude of the pulses makes it difficult to resolve the L-type Ca2+ current but Ca2+ efflux on sodium calcium exchange (NCX) can be seen from the tail current on repolarisation. The amplitude of this current parallels that of the underlying Ca2+ transient. Specifically, when the alternans ratio is large there is a large difference of NCX tail current between the pair of current records shown; this difference decreases as the depolarisation increases, in line with the changes of systolic Ca2+ transient. Thus the alternation of Ca2+ efflux from the cell varies with the size of the pulse. The full relationship between depolarisation pulse amplitude and Ca2+ efflux is shown in Fig. 1B. Here the open symbols show Ca2+ efflux for the large and small Ca2+ transient during alternans. As expected, these show that for small depolarisations there is a large difference in Ca2+ efflux activated by each of the transients. This difference declines as the difference in transient amplitude reduces with increasing depolarisation amplitude. The filled symbols show Ca2+ efflux averaged over the cycle of two Ca2+ transients required to describe alternans. These values are much less sensitive to pulse potential and gradually increase as the depolarisation pulse increases in amplitude. Extrapolating this line of mean efflux gives an intercept with the y axis at a value of roughly 3.3 μmol l−1. This suggests that, even in the absence of an applied voltage step, Ca2+ efflux occurs implying that there is a background influx of this magnitude. This is probably not surprising given that the cell is bathed in 5 mm external Ca2+.
Figure 1. The effects of varying the amplitude of the depolarizing pulse on Ca2+ alternans.
A, original data. Traces show: [Ca2+]i (top) and membrane current (bottom). Each section shows the response to two depolarizing pulses from a holding potential of −40 mV of the amplitudes (mV) indicated above. B, integral of Ca2+ efflux (obtained by integrating the NCX current on repolarisation) plotted as a function of the pulse size. The open symbols show the integrals accompanying the large and small Ca2+ transients during alternans. The filled symbols show the mean of the large and small responses (n= 4–13 cells). For depolarizing pulses greater than 27 mV no alternans was seen and therefore only the filled symbols are shown. The dashed line shows the fitted line to the last four mean data points (i.e. the filled symbols from 20 mV and below). This has an intercept on the y-axis of 3.3 μmol l−1.
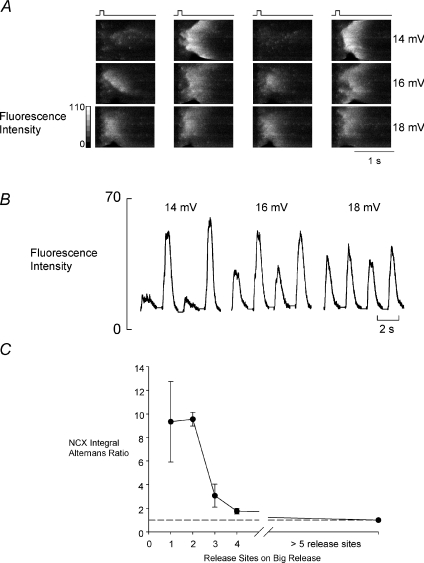
How is the reduction in alternans ratio brought about as depolarisation amplitude increases? The linescans in Fig. 2A show the spatial distribution of Ca2+ release as depolarisation amplitude is changed. Each series of linescan images (left to right) shows four consecutive images. The smallest pulse (14 mV depolarisation, top row) shows the largest alternans ratio. This arises because of complete failure of wave propagation on alternate stimuli, as we have previously reported (Díaz et al. 2004). In this case the large release is the result of initiation of a single propagating wave of Ca-induced Ca2+ release (CICR). However, as the depolarisation amplitude is increased so the number of sites in each image (in both large and small releases) from which propagating Ca2+ release is activated increases. In the bottom series of images (18 mV), although examples of propagating release can still be found, Ca2+ release is practically simultaneous throughout the cell. As a result, alternans are almost completely lacking. The traces in Fig. 2B show the mean values of fluorescence varying with time in each of the linescan series of Fig. 2A. These confirm that the alternans ratio becomes smaller as the depolarisation amplitude increases as in Fig. 1 i.e. the ratio is roughly 3 at 14 mV, 2 at 16 mV and 1 at 18 mV.
Figure 2. The steady state effects of depolarization amplitude on the spatial characteristics of Ca2+ release during alternans.
A, line scans. From left to right are shown the responses to four consecutive depolarizing pulses (applied at the times indicated above). Top to bottom shows the response to depolarizing pulses of the amplitudes shown at the right. The vertical dimension of the linescan is 75 μm. B, average value of fluorescence from the linescans in A. C, graph showing the alternans ratio (measured from the integrated NCX currents as in Fig. 1B in a total of between 2 and 8 cells) as a function of the number of initiating sites observed on the larger responses.
Figure 2C shows the relationship, in a total of five cells, between the number of sites where propagating release is initiated on a large release and the alternans ratio (measured here as the ratio of NCX current integrals). This confirms the impression given by Fig. 2A that fewer release sites initiate propagating release when the alternans ratio is large. It should be noted that the maximum value of the alternans ratio in this plot is somewhat higher than in Fig. 1B i.e. ∼9 in Fig. 2Cvs. ∼6 in Fig. 1B. Of course, in Fig. 1B we have no real idea of the number of release sites involved in producing the largest degree of alternans illustrated. In contrast, the values in Fig. 2C have been selected from confocal images to have only a single release site on the confocal line. If there is only one wave on the confocal line, this represents the value for the whole cell (other sites releasing Ca2+ should have time to enter the linescan if present). With more release sites the possibility exists that a single line will not be able to show all release sites. In many cases where it was impossible to define a number of release sites it may be that there were several other sites outside the confocal line contributing to the overall release.
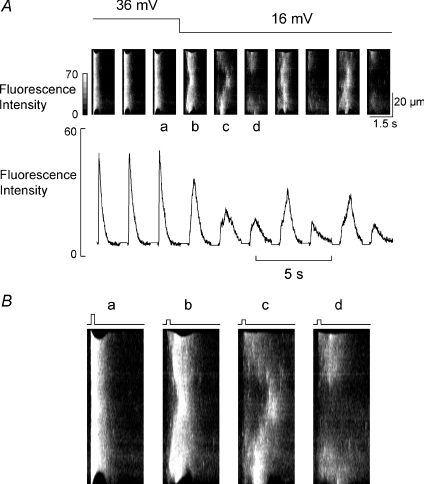
The question now arises: why do more Ca2+ release sites initiate propagating release as depolarisation amplitude increases? There are two potential factors to consider: (1) the number of L-type Ca2+ channels that open and (2) the SR Ca2+ content. We have investigated the relative contribution of these factors by examining the time course of the effects of changing the amplitude of depolarization. Effects due only to changes of the L-type current should occur on the first pulse whereas those due to changes of SR content might be expected to develop following a delay. Figure 3A shows the linescan images of a cell under voltage clamp in which the pulse size is reduced from 36 mV to 16 mV. The 36 mV pulse produces synchronous release throughout the cell. The first pulse to 16 mV results in somewhat less synchronous release. However, the subsequent two responses become more and more fragmented as the Ca2+ transient amplitude falls (the mean fluorescence of each linescan image is shown in the panel below) until alternans begins. This behaviour was seen in a total of five cells imaged in this way. Once again, as we would predict from Fig. 2, the appearance of alternans requires that the profile of Ca2+ release become less synchronous to allow propagation of waves of CICR. In Fig. 3B the images labelled a–d in Fig. 3A are shown enlarged to emphasise the increasing lack of synchrony of release that develops.
Figure 3. Reducing the amplitude of the depolarization does not result in immediate alternans.
A, the amplitude of the depolarizing pulse was decreased from 36 to 16 mV at the time indicated (as indicated by the bars above the linescans). The top record shows original linescans and the lower the mean fluorescence from the linescan. B, enlarged versions of linescans a–d; the depolarisation steps are indicated by the bars above the individual linescans.
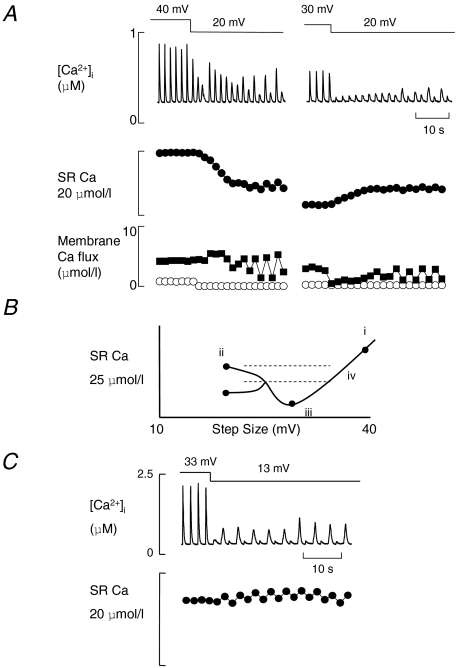
We have also investigated the effect on SR Ca2+ content of a broader range of pulse size in Fig. 4. On the left of Fig. 4A the depolarizing pulse has been changed from large (40 mV) to small (20 mV) amplitude. No alternans was seen with the 40 mV pulse. When the pulse amplitude was decreased to 20 mV the immediate effect was the production of three Ca2+ waves (these interfere with the stimulated Ca2+ transient making Ca2+ release look smaller). Again, as we saw in Fig. 3, there was then a gradual decrease of the amplitude of the Ca2+ transient before alternans began. The decrease of systolic Ca2+ transient is accompanied by a decrease of SR Ca2+ content that we calculate from the balance of Ca2+ fluxes on each pulse (the bottom plots in Fig. 4A; see Methods for the technique used). A similar loss of Ca2+ on reducing the pulse size was measured in a total 6 out of 8 cells. However, very different behaviour is seen in the right panel of Fig. 4A. Here, in the same cell, the depolarizing pulse was reduced from intermediate (30 mV) to small (20 mV) amplitude. The immediate effect of this manoeuvre was to greatly decrease the amplitude of the Ca2+ transient. However, the Ca2+ transient then increased in amplitude until alternans developed. In this case, the calculated SR content change shows an increase (in contrast to the decrease on the left). Using the changes of SR Ca2+ content shown in Fig. 4A we have constructed the graph shown in Fig. 4B. The symbols are the calculated values from Fig. 4A, and the lines have been added for didactic purposes. Here we see that SR Ca2+ is at a minimum at point iii with an intermediate pulse size. Changing the pulse size in either direction from here increases the SR content. However, reducing the pulse size leads to alternans, whereas, increasing the pulse size does not.
Figure 4. Changes of SR Ca2+ content and the onset of alternans on changing pulse amplitude.
A, the effects of reducing the amplitude of the depolarizing pulse from 40 (i.e. from −40 to 0 mV) to 20 mV (left) and from 30 to 20 mV (right). In both panels traces show (from top to bottom): [Ca2+]i; calculated change of SR Ca2+; membrane fluxes (open symbols are Ca2+ influx through the L-type channel and filled symbols the efflux). All data are from the same cell and are typical of 6 out of 8 cells on the left and 6 of 7 cells on the right. B, schematic diagram of the SR Ca2+ content at each pulse size in the cell shown in A. C, changing the depolarizing pulse amplitude from 33 to 13 mV results in the immediate appearance of alternans. (Data from a different cell to that illustrated in A.)
Although the technique used to measure the change of SR Ca2+ content is unable to resolve such small changes, the range in which alternans takes place has been drawn in Fig. 4B to indicate that the SR content changes continuously with the step size. This seems reasonable but we are sure that other factors will also be involved, e.g. the size and spatial distribution of L-type Ca2+ channel openings. Such factors might explain the different levels of alternans seen in the top two panels of Fig. 4A and also may explain how the alternans ratio changes in Fig. 4C following five cycles of alternans with no change in the stimulus regime.
The plot in Fig. 4B also suggests that there is a range of SR Ca2+ content shown by the horizontal dashed lines at which, depending upon the voltage step, Ca2+ release should either alternate from beat-to-beat (region ii) or be uniform in time (region iv). That this occurs can be seen in Fig. 4C (data from another cell); the pulse size is changed from 33 mV to 13 mV and the cell begins to alternate immediately. The calculated change of SR Ca2+ below shows that almost no change of SR Ca2+ content is required for this to take place.
The varied effects of changing pulse size on SR Ca2+ content can be explained as follows. Decreasing the amplitude of the pulse will decrease the size of the calcium current. This has two effects on excitation–contraction coupling (Fabiato, 1985; Trafford et al. 2001): (1) the SR Ca2+ release trigger is reduced and (2) less Ca2+ enters the cell for refilling the SR. The first of these effects tends to increase SR content; however, decreased Ca2+ entry will tend to decrease SR content. Between i and iii in Fig. 4B the loss of the loading of the cell with Ca2+ predominates. When the SR is filling mainly from background influx (see Fig. 1B) and L-type current is already small (i.e. between iii and ii) loss of release trigger predominates and this allows the SR to fill. Thus the overall direction of the effect on SR content depends on the relative strength of these two factors (Trafford et al. 2001) (see Discussion for further consideration).
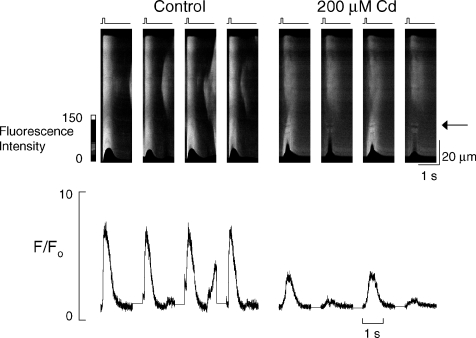
All of the data thus far have been obtained by altering the amplitude of L-type Ca2+ current by changing the voltage pulse amplitude. We have also inhibited current by applying Cd2+ in voltage clamp. Figure 5 shows linescan data from a voltage clamped cell with a 36 mV depolarisation under control conditions and with 200 μm Cd2+. In control conditions the 5 mmCa 2+o is sufficient to produce spontaneous release of Ca2+ from the SR, otherwise release is spatially uniform. When L-type Ca2+ current is inhibited (still using 36 mV depolarising pulses) there is a fragmentation of release and propagation of mini-waves of Ca2+ release. In addition there is clear alternans of systolic Ca2+ in the lower portion of the linescan. These results are representative of all 11 cells in which this experiment was performed.
Figure 5. Inhibition of L-type Ca2+ current with Cd2+ (200 μm) allows the appearance of systolic [Ca2+] alternans.
Linescan data from a voltage clamped cell being stimulated to contract by a depolarising pulse of 36 mV from a holding potential of −40 mV, 4 consecutive stimuli are shown before and after inhibition of L-type Ca2+ current with Cd2+. The traces below show the mean fluorescence of a 20 pixel wide box at the level of the arrow expressed as F/Fo.
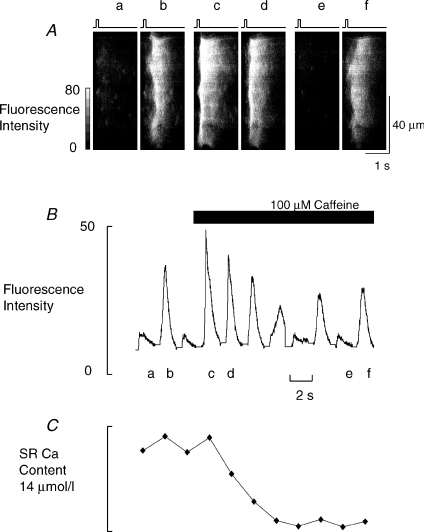
If, as we have suggested, the appearance of alternans is due to the small number of sites at which Ca2+ release is initiated, we should be able to abolish it if we can increase the number of these sites. We have attempted to do this by increasing the sensitivity of release sites to stimulation by applying a low concentration of caffeine. We have set conditions in Fig. 6 to produce alternans (depolarising pulse 17 mV) and then applied 100 μm caffeine (as indicated by the bar). Initially during alternans release is fragmented (a and b) and as predicted caffeine abolishes alternans (c and d) by making release more uniform. However, this effect is only transient, as after 10–15 s alternans returns essentially as before (e and f). Similar effects were seen in all six cells studied. The return of alternans is probably due to the fall of SR Ca2+ shown in Fig. 6C (O’Neill & Eisner, 1990; Trafford et al. 2000). Thus, RyR responsiveness and SR Ca2+ content interact in the production of alternans.
Figure 6. Caffeine transiently abolishes alternans.
A, original linescans. Alternans was produced by 17 mV amplitude depolarizing pulses (shown by the bars above the linescans). The first two linescans (a and b) show alternans. Caffeine (100 μm) was then added. Linescans c and d are the first two responses in caffeine and e and f are the 7th and 8th. B, the average fluorescence from the full sequence of linescans in this experiment. C, the loss of SR Ca2+ calculated from the integrals of NCX tail currents.
Discussion
In previous work we have shown that manoeuvres that decrease the open probability of the RyR (either directly in the case of tetracaine or acidosis) or indirectly, by decreasing the amplitude of the Ca2+ current, can produce beat to beat alternation of the amplitude of the Ca2+ transient (Díaz et al. 2002, 2004). The alternation was suggested to arise because of the steepened dependence of Ca2+ release on the SR Ca2+ content, with large responses depending on the propagation of waves. In the present work we have investigated the voltage dependence of this effect and also the role of SR Ca2+ content. Our results show that the ratio of alternans (the ratio of the amplitudes of large and small systolic Ca2+ transients) declines as the voltage step used to stimulate release increases and that this is associated with increasing uniformity of SR Ca2+ release. The change in alternans ratio is brought about by interaction between the number of wave initiation sites and the SR Ca2+ content and can be interrupted by raising the sensitivity of the RyR to Ca2+. We also demonstrate that alternans brought about by inhibition of L-type Ca2+ current conforms to the same pattern we have previously found, i.e. fragmentation of the Ca2+ release profile and propagation of mini-waves.
The relationship between pulse amplitude and alternans
The results show that the larger alternans ratio produced by smaller depolarizing pulses is associated with a reduced number of Ca2+ release initiation sites. An obvious explanation for this result is that the smaller the depolarizing pulses, the fewer L-type Ca2+ channels open. The smaller responses are smaller because fewer SR release sites are activated, and therefore Ca2+ efflux is less. This means that the SR Ca2+ content rises more before the larger release takes place, and this results in a high alternans ratio. Under normal conditions Ca2+ release is roughly proportional to the third power of SR Ca2+ content (Trafford et al. 2000). When the size of the depolarizing pulse is made very small then the relationship becomes much steeper and is essentially ‘all’ (i.e. the whole cell releases Ca2+ in a wave) or ‘none’ (where no propagation results). As the pulse size is increased the L-type Ca2+ current will increase and, at a given SR content, more Ca2+ release will be triggered directly. This will decrease the slope of the relationship and, in particular, will increase the amount of Ca2+ released on the small responses in the alternating pairs. As a consequence the SR Ca2+ content before the larger response will be decreased and the alternans ratio will decrease with increasing pulse size.
Of particular interest is the observation that the effects of changing the amplitude of the depolarizing pulse take several pulses to develop. This shows that a factor other than the number of L-type channels that open must also be involved in determining whether alternans develops. At first sight the effects of changing depolarisation amplitude on the development of alternans appear rather complicated. They can, however, be understood if the L-type Ca2+ current has two effects on SR function. (1) It triggers Ca2+ release from the SR and (2) it loads the SR with Ca2+ (Fabiato, 1985; Trafford et al. 2001). Depending on the relative importance of these two effects, a given change of L-type Ca2+ current can lead to an increase or decrease of SR Ca2+ content (Trafford et al. 2001). As shown in Fig. 4A, changing from a large pulse (with no alternans, e.g. 40 mV) to the very small pulses (e.g. 20 mV) required to produce alternans leads to a gradual decrease of the amplitude of the Ca2+ transient before alternans develops. However, changing from a medium pulse (with no alternans e.g. 30 mV) to a very small pulse (e.g. 20 mV) to produce alternans results in a gradual increase of the amplitude of the Ca2+ transient before alternans develops. These apparently diverse results can be reconciled by the calculated changes of SR Ca2+ content. When the depolarization is initially 40 mV there is a large L-type Ca2+ current and large SR Ca2+ content. When the size of the depolarizing pulse is then reduced to 20 mV the SR Ca2+ content is still large enough that Ca2+ waves can propagate through the cell and large Ca2+ transients are seen. These large Ca2+ transients will produce a Ca2+ efflux from the cell which will now be much larger than the greatly decreased entry of Ca2+ into the cell on the L-type Ca2+ current. Consequently the SR Ca2+ content will decrease. This will continue until the SR content falls to a sufficiently low level that Ca2+ waves no longer propagate. On the first pulse that this occurs, Ca2+ entry will now exceed efflux and the SR Ca2+ content will increase and on the next pulse Ca2+ waves will propagate and a large response will be seen. Alternans will then continue by the mechanism described in previous work (Díaz et al. 2004). In contrast when the cell is initially stimulated with medium size pulses (30 mV) the SR Ca2+ content is less (Fig. 4). The main effect of decreasing the pulse size is to decrease the trigger for Ca2+ release from the SR. With a 30 mV pulse the Ca2+ influx through the L-type current is small in comparison with the background Ca2+ entry and therefore the loading component of the L-type current is less important. The SR Ca2+ content is initially too small for Ca2+ waves to propagate. The decrease of trigger will produce an immediate decrease of the Ca2+ transient and thereby of Ca2+ efflux from the cell. If this decrease of efflux is less than the decrease of influx the SR Ca2+ content will increase until a level at which it is high enough to support Ca2+ wave propagation. This behaviour is shown in Fig. 4A.
Although we have shown (Díaz et al. 2004) that our alternans is not associated with delayed effects on L-type Ca2+ current (such as facilitation/defacilitation) it should be pointed out that if such a thing were to occur it might lead to stable alternans of systolic release. This does not appear to be the mechanism responsible for alternans in our hands.
These experiments suggest that when the depolarizing pulse is increased from very small levels, SR Ca2+ content passes through a minimum (at about 20–25 mV) before increasing into a range where alternans is possible (Fig. 4B). The ‘U’ shape of this relationship means that a given SR Ca2+ content can be reached at two pulse sizes: at one of these alternans of systolic Ca2+ may be seen (e.g. point ii of Fig. 4B) and at the other no alternans will be seen (point iv). If the pulse size is switched between these two levels, alternans will develop immediately as there is no need for changes of SR Ca2+ content. This explains the immediate onset of alternans in Fig. 4C.
As we have seen, intermediate pulses are associated with a minimum level of SR Ca2+; however, as pulse size becomes increasingly large, the influx of Ca2+ increases, loading the SR with Ca2+. At some point SR Ca2+ will be high enough to allow alternans (we know this because with large pulses SR Ca2+ has to fall before alternans appear). What prevents the appearance of alternans at this larger pulse? Alternans produced by the mechanism we propose requires a fragmented Ca2+ release profile to allow propagation. The higher number of sites at which Ca2+ release is directly stimulated by L-type channels prevents this because release occurs throughout the cell thereby removing the possibility of propagated release required to produce alternans. A clear example of this is shown in Fig. 4C where presumably only the fragmentation of the release profile is required for alternans to proceed.
It appears that the increased number of wave initiation sites as depolarisation amplitude increases takes place against a background of falling SR Ca2+ content that ought to militate against propagation. One thing that may help explain this is the number of L-type Ca2+ channels (and/or sparks) activated as depolarisation increases. What effect might this have? Influx of Ca2+ (and/or sparks, which themselves do not initiate propagation) might raise cytosolic [Ca2+] sufficiently to facilitate initiation of wave propagation. That this might be happening can be seen in Fig. 2A. There is a clear, generalised increase of [Ca2+]i following depolarisation by 16 mV that is much less obvious in the corresponding 14 mV depolarisation image. Therefore, the lower SR Ca2+ content, which would not favour propagation of release, may be compensated for by this increase of [Ca2+]i as depolarisation amplitude increases. Thus the interaction between the number of L-type channels activated, the prevailing [Ca2+]i and SR Ca2+ content seems to determine whether alternans occurs by determining whether CICR can propagate. So, as the number of L-type channels active increases, the possibility of propagation is influenced in a number of ways: it is reduced as there is less space into which release can propagate; reduced as SR Ca2+ falls (as greater mean release depletes the SR i.e. from ii to iii in Fig. 4B); and increased as the increased number of sparks facilitate propagation.
Interestingly, the data shown in Fig. 1B also indicate a substantial influx of Ca2+ independent of L-type current i.e. the intercept with the y-axis. As the voltage step is reduced the mean efflux of Ca2+ generated on NCX tail current appears to reach a stable value of around 3.3 μmol l−1 cell volume. Thus as the L-type current disappears this remaining efflux must be required to balance some other source of influx that under these conditions constitutes the total Ca2+ influx. The identity of this background influx is unknown (see Kupittayanant et al. 2006 for further discussion).
Inhibition of L-type Ca2+ current and systolic alternans
The appearance of mini-waves of CICR is, as we have seen, of primary importance to the presence of alternans in our experimental model. However, if this is also to be the mechanism for the clinical phenomenon of pulsus alternans, it is essential that we demonstrate a similar mechanism at work whenever L-type Ca2+ current is inhibited. The alternans of Ca2+ release in Fig. 5 has been brought about by simply inhibiting L-type Ca2+ current with 200 μm Cd2+ but there is obvious fragmentation of the release profile. Similar subcellular heterogeneity of Ca2+ release during alternans has been shown in cells in intact rat hearts (Aistrup et al. 2006) and, recently, in canine ventricular myocytes (Cordeiro et al. 2007). All of the subsequent mechanisms we have previously proposed will then follow i.e. depletion of SR Ca2+ by a large, propagating release that prevents propagation on the next stimulus, leading to alternans of systolic release. It should also be noted that it has been shown that systolic Ca2+ alternans can occur without changes of SR Ca2+ content adding further complexity (Picht et al. 2006) and that this may be due to changes in the refractory period of RyR (Restrepo et al. 2008).
The effects of increasing RyR sensitivity
When caffeine is added to increase the sensitivity of the RyR, the fragmentation of the Ca2+ release profile is prevented. This is accompanied by the abolition of alternans (Fig. 6). This situation is, however, transient as the increased Ca2+ release activates greater Ca2+ efflux (not balanced by extra Ca2+ influx) and so SR Ca2+ content falls. As a result the open probability of the RyR will decrease (Sitsapesan & Williams, 1997; Terentyev et al. 2002) leading to fragmentation of the release profile and allowing alternans to return. This nicely demonstrates the interplay between SR Ca2+ content and responsiveness of the Ca2+ release sites to L-type activity in production of alternans produced by this technique. The redevelopment of alternans during maintained exposure to caffeine is analogous to the situation of Figs 3 and 4 where a loss of SR Ca2+ (Fig. 4C) accompanies the development of alternans when the amplitude of the depolarizing pulse is decreased from 40 to 20 mV (Fig. 4A, left panel).
Limitations of the study and physiological relevance
The study is somewhat limited by the method used for estimation of the SR Ca2+ content. Local measurements of SR Ca2+ are possible and have been published recently (Picht et al. 2006) and such measurements would certainly allow us to be more confident of the relative SR Ca2+ contents that produce systolic alternans.
The results presented here may seem a little artificial and not relevant to the clinical phenomenon of pulsus alternans. However, it is our view that there needs to be a site that initiates alternans and it is the mechanism involved here that we are seeking. In heart failure there is very likely to be regions of myocardium that are Ca2+ overloaded and may have abnormally small Ca2+ currents, e.g. spike and dome action potentials in various species will give a relatively small early Ca2+ current. If we make the assumption that the SR is similar in all ventricular muscle then the mechanism of fragmentation of the release profile and wave propagation is a good candidate for initiation of alternans.
Acknowledgments
The work described in this paper was supported by a grant from the BHF (RG/06/002/21138).
References
- Adler D, Wong AY, Mahler Y. Model of mechanical alternans in the mammalian myocardium. J Theor Biol. 1985;117:563–577. doi: 10.1016/s0022-5193(85)80238-1. [DOI] [PubMed] [Google Scholar]
- Aistrup GL, Kelly JE, Kapur S, Kowalczyk M, Sysman-Wolpin I, Kadish AH, Wasserstrom JA. Pacing-induced heterogeneities in intracellular Ca2+ signaling, cardiac alternans, and ventricular arrhythmias in intact rat heart. Circ Res. 2006;99:E65–E73. doi: 10.1161/01.RES.0000244087.36230.bf. [DOI] [PubMed] [Google Scholar]
- Cordeiro JM, Malone JE, Di Diego JM, Scornik FS, Aistrup GL, Antzelevitch C, Wasserstrom JA. Cellular and subcellular alternans in the canine left ventricle. Am J Physiol Heart Circ Physiol. 2007;293:H3506–H3516. doi: 10.1152/ajpheart.00757.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz ME, Eisner DA, O’Neill SC. Depressed ryanodine receptor activity Increases variability and duration of the systolic Ca2+ transient in rat ventricular myocytes. Circ Res. 2002;91:585–593. doi: 10.1161/01.res.0000035527.53514.c2. [DOI] [PubMed] [Google Scholar]
- Díaz ME, O’Neill SC, Eisner DA. Sarcoplasmic reticulum calcium content fluctuation is the key to cardiac alternans. Circ Res. 2004;94:650–656. doi: 10.1161/01.RES.0000119923.64774.72. [DOI] [PubMed] [Google Scholar]
- Eisner DA, Choi HS, Díaz ME, O’Neill SC, Trafford AW. Integrative analysis of calcium cycling in cardiac muscle. Circ Res. 2000;87:1087–1094. doi: 10.1161/01.res.87.12.1087. [DOI] [PubMed] [Google Scholar]
- Eisner DA, Li Y, O’Neill SC. Alternans of intracellular calcium: Mechanism and significance. Heart Rhythm. 2006;3:743–745. doi: 10.1016/j.hrthm.2005.12.020. [DOI] [PubMed] [Google Scholar]
- Eisner DA, Nichols CG, O’Neill SC, Smith GL, Valdeolmillos M. The effects of metabolic inhibition on intracellular calcium and pH in isolated rat ventricular cells. J Physiol. 1989;411:393–418. doi: 10.1113/jphysiol.1989.sp017580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Simulated calcium current can both cause calcium loading in and trigger calcium release from the sarcoplasmic reticulum of a skinned canine cardiac purkinje cell. J Gen Physiol. 1985;85:291–320. doi: 10.1085/jgp.85.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupittayanant P, Trafford AW, Diaz ME, Eisner DA. A mechanism distinct from the L-type Ca current or Na-Ca exchange contributes to Ca entry in rat ventricular myocytes. Cell Calcium. 2006;39:417–423. doi: 10.1016/j.ceca.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Myles RC, Burton FL, Cobbe SM, Smith GL. The link between repolarisation alternans and ventricular arrhythmia: Does the cellular phenomenon extend to the clinical problem? J Mol Cell Cardiol. 2008;45:1–10. doi: 10.1016/j.yjmcc.2008.03.024. [DOI] [PubMed] [Google Scholar]
- O’Neill SC, Eisner DA. A mechanism for the effects of caffeine on Ca2+ release during diastole and systole in isolated rat ventricular myocytes. J Physiol. 1990;430:519–536. doi: 10.1113/jphysiol.1990.sp018305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picht E, Desantiago J, Blatter LA, Bers DM. Cardiac alternans do not rely on diastolic sarcoplasmic reticulum calcium content fluctuations. Circ Res. 2006;99:740–748. doi: 10.1161/01.RES.0000244002.88813.91. [DOI] [PubMed] [Google Scholar]
- Restrepo JG, Weiss JN, Karma A. Calsequestrin-mediated mechanism for cellular calcium transient alternans. Biophys J. 2008;95:3767–3789. doi: 10.1529/biophysj.108.130419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitsapesan R, Williams AJ. Regulation of current flow through ryanodine receptors by luminal Ca2+ J Membr Biol. 1997;159:179–185. doi: 10.1007/s002329900281. [DOI] [PubMed] [Google Scholar]
- Tao T, O’Neill SC, Diaz ME, Li YT, Eisner DA, Zhang H. Alternans of cardiac calcium cycling in a cluster of ryanodine receptors: a simulation study. Am J Physiol Heart Circ Physiol. 2008;295:H598–H609. doi: 10.1152/ajpheart.01086.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terentyev D, Viatchenko-Karpinski S, Valdivia HH, Escobar AL, Gyorke S. Luminal Ca2+ controls termination and refractory behavior of Ca2+-induced Ca2+ release in cardiac myocytes. Circ Res. 2002;91:414–420. doi: 10.1161/01.res.0000032490.04207.bd. [DOI] [PubMed] [Google Scholar]
- Trafford AW, Díaz ME, Eisner DA. A novel, rapid and reversible method to measure Ca buffering and timecourse of total sarcoplasmic reticulum Ca content in cardiac ventricular myocytes. Pflügers Archiv. 1999;437:501–503. doi: 10.1007/s004240050808. [DOI] [PubMed] [Google Scholar]
- Trafford AW, Díaz ME, Eisner DA. Coordinated control of cell Ca2+ loading and triggered release from the sarcoplasmic reticulum underlies the rapid inotropic response to increased L-type Ca2+ current. Circ Res. 2001;88:195–201. doi: 10.1161/01.res.88.2.195. [DOI] [PubMed] [Google Scholar]
- Trafford AW, Díaz ME, Sibbring GC, Eisner DA. Modulation of CICR has no maintained effect on systolic Ca2+: simultaneous measurements of sarcoplasmic reticulum and sarcolemmal Ca2+ fluxes in rat ventricular myocytes. J Physiol. 2000;522:259–270. doi: 10.1111/j.1469-7793.2000.t01-2-00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss JN, Karma A, Shiferaw Y, Chen PS, Garfinkel A, Qu Z. From pulsus to pulseless: the saga of cardiac alternans. Circ Res. 2006;98:1244–1253. doi: 10.1161/01.RES.0000224540.97431.f0. [DOI] [PubMed] [Google Scholar]