Abstract
The cardiac transient outward current Ito is regulated by thyroid hormone (T3). However, it remains unclear whether T3 directly modulates underlying gene transcription and which thyroid receptor (TR) isoform might be responsible for gene transactivation. To clarify this situation, we analysed the role of T3 and its receptors α1 (TRα1) and β1 (TRβ1) in regulation of KCNA4, KCND2, KCND3 and KCNIP2 transcription in rat cardiomyocytes. Initial results demonstrated a T3-mediated increase of Ito current density. T3 stimulation enhanced KCND2 and KCND3 expression and decreased KCNA4 transcription, while KCNIP2 remained unaffected. To dissect the role of TRα1 and TRβ1 in T3-dependent Ito modulation, TRα1 and TRβ1 were overexpressed in cardiomyocytes by adenovirus-mediated gene transfer. TRα1 increased Ito, while TRβ1 significantly reduced Ito in size, which was associated with TRα1-mediated increase and TRβ1-mediated reduction of KCND2/3 transcription. To further evaluate a possible direct interaction of TRα1 and TRβ1 with the KCND3 promoter, TR expression vectors were cotransfected with a construct containing 2335 bp of the KCND3 5′-flanking sequence linked to a luciferase reporter into ventricular myocytes. While the TRα1 aporeceptor enhanced KCND3 transcription, the TRβ1 aporeceptor suppressed KCND3 expression, with both effects exhibiting ligand-dependent amplification upon T3 stimulation. Deletion of the KCND3 5′-flanking region localized the suppressible promoter sequence for TRβ1 to within −293 bp and the activating promoter sequence for TRα1 to within −2335 to −1654 bp of the transcription start site. Disruption of putative TR binding sites by mutagenesis abolished the TRα1- (G-1651T) and TRβ1- (G-73T) mediated effects, indicating that TRα1 and TRβ1 response elements map to different regions of the KCND3 promoter. Thus, Ito is modulated by diverse T3-dependent regulation of underlying gene transcription. TRα1 and TRβ1 exhibit distinct effects on KCND3 transactivation with TRα1 enhancing and TRβ1 suppressing KCND3 transcription.
The heart is one of the major target organs of thyroid hormone (TH) regulation with increasing amounts of TH enhancing cardiac contractile function, accelerating heart rate and abbreviating action potential duration (APD) (Klein & Ojamaa, 2001). These effects are mediated through the activation of TH receptors (TRs), members of the nuclear receptor family. Although both tetraiodothyronine (T4) and triiodothyronine (T3) bind to TRs, T3 is thought to be the biologically relevant TH molecule in cardiac myocytes, as in other cells, due to its higher receptor affinity. The relative expression of TR isoforms varies among tissues (Strait et al. 1990). TR α1 (TRα1) is the major isoform expressed in the heart, although β1 receptor (TRβ1) is expressed also at lower levels (Kinugawa et al. 2001b). TRs form homodimers or heterodimers with other nuclear receptors, which bind to specific DNA recognition sequences, termed thyroid response elements (TRE), in the promoter of T3 target genes (Cheng, 2000; Harvey & Williams, 2002). The ligand T3 binds to the C-terminal domain of TRs, to either activate or suppress transcription of T3-regulated genes. The fact that DNA binding of TRs is not hormone dependent also raises the possibility of a biological activity of the unliganded receptors, or aporeceptors (Hu & Lazar, 2000).
Thyroid hormone signalling has been reported to be abnormal in patients with congestive heart failure and after acute myocardial infarction (Franklyn et al. 1984; Stevenson et al. 1990). Both acute and chronic cardiac dysfunctions are associated with low T3 serum levels and T3-induced alterations in cardiac contractile proteins and cellular electrophysiology. Hyperthyroid conditions shorten the APD, whereas hypothyroid ventricular myocytes exhibited a significantly prolonged APD (Yonemochi et al. 2000; Watanabe et al. 2003). In most mammalian species and in humans the Ca2+-independent transient outward current Ito is most important for early cardiac repolarization. Either directly or indirectly by setting the plateau voltage, Ito plays a prominent role in AP shaping (Hoppe et al. 2000). In most species studied to date, Ito comprises a fast and slow component encoded by KCND2/3 (Kv4.2/3), KCNIP2 (KChIP2) and KCNA4 (Kv1.4), respectively (Dixon et al. 1996; Wickenden et al. 1997; Kaab et al. 1998; Kassiri et al. 2002).
Although it is well recognized that T3 is involved in regulation of voltage-activated K+ channels, it remains unclear whether T3 directly affects transcription and which TR isoform might be responsible for transactivation. Alternatively, indirect effects of T3 on cardiac function and gene expression by changing the autonomic tone and activating the local renin–angiotensin system in the heart have been suggested (Klein & Hong, 1986; Kobori et al. 1999; Basset et al. 2001). To clarify this situation, we aimed to determine whether T3-dependent changes of native Ito size resulted from modification of underlying gene transcription and to analyse the role of TRα1 and TRβ1 in transactivation. Secondly, using chimeric constructs containing 2335 bp of the KCND3 gene 5′-flanking sequence linked to a luciferase reporter we evaluated TR target regions within the promoter. Our results demonstrate divergent regulation of gene transcription in cardiomyocytes by the two TRs probed.
Methods
The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) and animals were killed by decapitation using a protocol approved by the institution.
Plasmid construction and adenovirus preparation
The pGL3-luciferase basic vector (Promega, Madison, WI, USA) inserted with the 2335 bp DNA fragment upstream of the rat KCND3 coding region (pGL3-2335KCND3) was kindly provided by Drs K. Takimoto and E. S. Levitan (Zhang et al. 2001). Plasmids encoding various lengths of KCND3 fragments upstream of the coding region were generated by restriction digestion and religation (AvrII, PvuII, and XhoI corresponding to pGL3-1654KCND3, pGL3-661KCND3 and pGL3-293KCND3, respectively). The point mutations G-1651T, G-707T, and G-73T were introduced into pGL3-2335KCND3, pGL3-1654KCND3 and pGL3-293KCND3, respectively, by site-directed mutagenesis to disrupt putative TREs, creating the vectors pGL3-2335KCND3mut, pGL3-1654KCND3mut and pGL3-293KCND3mut (Fig. 1). The adenovirus shuttle vector pAdCGI has been described (Hoppe et al. 2000). The full-length coding sequences of the murine TRα1 and rat TRβ1, kindly provided by Dr F. Flamant, were cloned into the multiple cloning site of pAdCGI, to generate pAdCGI-TRα1 and pAdCGI-TRβ1, respectively. Adenovirus vectors were generated as previously described (Hoppe et al. 2000, 2001; Er et al. 2003). RL-TK reporter vector encoding the Renilla luciferase gene used as internal control was obtained from Promega. Control cells were infected with adenoviral vectors expressing EGFP alone.
Figure 1. KCND3 5′-flanking nucleotide sequence with TRE half-site consensus and half-site consensus sequence for retinoid acid receptor (RAR) or vitamin D3 receptor (VDR) binding.
The point mutations G-1651T, G-707T and G-73T were introduced by site-directed mutagenesis to disrupt putative TREs.
Myocyte isolation and adenovirus infection
A standard trypsin dissociation method was used to prepare ventricular myocytes of 1- to 2-day-old neonatal rats. For voltage-clamp experiments, 3- to 5-day-old monolayer cultures were dispersed by trypsin, and re-plated at a low density to study isolated cells within 2–8 h. Infection of neonatal cells was performed 1 to 3 days after plating at a multiplicity of infection (MOI) of 15 to 50 p.f.u. per cell. Cells were incubated for 4 h at 37°C, after which the infection medium was replaced with culture medium. To test the effect of TH culture medium was supplemented with T3 10−7 mol l−1 (Sigma).
Transient transfection and luciferase assay
After cell isolation 106 neonatal myocytes were resuspended in 400 μl of phosphate-buffered saline/0.1% glucose containing 50 μg of one of the luciferase constructs (as indicated), 50 μg of pAdCGI, pAdCGI-TRα1 or pAdCGI-TRβ1 (as indicated), and 5 μg of control pRL-TK DNA. Cells were electroporated with the use of the Equibio EasyjecT (Peqlab, Erlangen, Germany) at 280V/300 μF. After electroporation 105 cells were plated/well in 12-well plates. Twelve hours after plating, media were replaced with growth media supplemented with 10−7 mol l−1 T3 or vehicle. Seventy-two hours after media exchange, luciferase activities were measured using a dual luciferase reporter assay system (Promega).
Reverse transcription-polymerase chain reaction (RT-PCR)
RT-PCR experiments were performed as described previously (Gassanov et al. 2007). Briefly, total cellular RNA was isolated with the Trizol reagent. Primer sequences and PCR conditions are described in Table 1. The PCR products were size fractionated by 1.5% agarose gel electrophoresis and analysed with the BioDocAnalyze Imaging Systems (Biometra). 18S (Applied Biosystems, Germany) was used as internal control, and the mRNA concentrations were normalized with respect to the 18S gene (315 bp).
Table 1.
PCR primers and conditions
| Name | Sequence | Annealing temp. (°C) | Reference |
|---|---|---|---|
| KCNA4 | 5′-CTGGGGGACAAGTCAGAGTATCTA-3′ | 58 | (Song et al. 1998) |
| (434 bp) | 5′-ACTCTCCTCGGGACCACCT-3′ | ||
| KCND2 | 5′-CCGAATCCCAAATGCCAATGTG-3′ | 62 | (Goltz et al. 2007) |
| (252 bp) | 5′-CCTGACGATGTTTCCTCCCGAATA-3 | ||
| KCND3 | 5′-CCGTAAGCTTGCAAGACCACGTCACTCATC-3′ | 57 | (Song et al. 2001) |
| (296 bp) | 5′-ATCCGCGGCC GCAGGTGCGTG GTCTTCTTGCT-3′ | ||
| KCNIP2 | 5′-CAAGTTCACACGCAGAGAGC-3′ | 60 | (Weinberg et al. 1993) |
| (293 bp) | 5′-TTGTGATACAGCCGTCCTTG-3′ |
Electrophysiology
Experiments were carried out using standard microelectrode whole-cell patch clamp techniques with an Axopatch 200B amplifier (Axon instruments, Union City, CA, USA) while sampling at 10 kHz and filtering at 2 kHz. The recording bath solution contained (mmol l−1): NaCl 135, KCl 5, CaCl2 2, glucose 10, MgCl2 1, Hepes 10; pH was adjusted to 7.4 with NaOH. For Ito recordings of cardiomyocytes, BaCl2 2 mmol l−1, and CdCl2 200 μmol l−1 were added to block IK1 and ICaL, respectively. The micropipette electrode solution was composed of (mmol l−1): potassium glutamate 130, KCl 15, NaCl 5, MgCl2 1, Hepes 10, and Mg-ATP 5; pH was adjusted to 7.3 with KOH. Ito was elicited by 500 ms pulses, stepping from a holding potential of −80 mV to test potentials of −40 to +40 mV in 10 mV increments, at a frequency of 1 Hz, as described previously (Hoppe et al. 2000). Ito was measured as the difference between the peak outward current and the steady-state current at the end of 500 ms voltage step. Borosilicate microelectrodes had tip resistances of 2–4 MΩ when filled with the internal recording solution. A xenon arc lamp was used to view EGFP at 488/530 nm (excitation/emission).
Statistical analysis
Pooled data are presented as means ± standard error of the mean (s.e.m.). Comparisons between groups were performed using one-way ANOVA. Significant ANOVAs were followed by post hoc tests applying Bonferroni's correction for multiple comparisons (Michels et al. 2005, 2008). Probability values of P < 0.05 were deemed significant.
Results
T3-mediated effect on native Ito
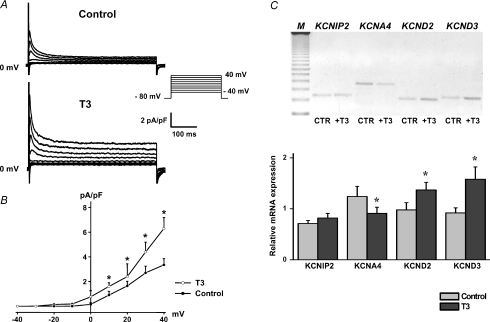
In an initial set of experiments we determined the effect of T3 on native Ito current size in neonatal rat cardiomyocytes. Treatment with T3 significantly increased Ito peak current density (6.3 ± 0.9 pA pF−1 at +40 mV, n= 13) compared to control cells (3.4 ± 0.5 pA pF−1, n= 12; P < 0.05) (Fig. 2A and B). To correlate these patch-clamp findings with the underlying gene expression, we analysed KCNA4, KCND2, KCND3 and KCNIP2 transcription levels by RT-PCR. As demonstrated in Fig. 2C, T3 stimulation enhanced KCND3 and KCND2 expression and diminished KCNA4 mRNA in neonatal myocytes with the most pronounced effect observed for KCND3 transcription. In contrast, KCNIP2 expression was largely unaffected by exogenous T3.
Figure 2. Effect of T3 on native Ito and underlying KCNA4, KCND2, KCND3 and KCNIP2 expression.
Families of Ito currents (A) and current density–voltage relations (B) demonstrate that T3 treatment significantly increased Ito current density. Values are means ±s.e.m.C, increased KCND2, KCND3 and diminished KCNA4 mRNA levels were found in T3-stimulated cardiomyocytes. In contrast, KCNIP2 transcript was not affected by T3. Gene expression was normalized to the ribosomal 18S signal. M, molecular weight (123 bp DNA ladder). *P < 0.05 versus control.
Divergent effects of TRα1 and TRβ1 on native Ito
To dissect a possible role of TRα1 and TRβ1 on native cardiac Ito, we overexpressed TRα1 and TRβ1 in neonatal rat cardiomyocytes by adenovirus-mediated gene transfer. All vectors were bicistronic, also expressing EGFP under control of a single CMV promoter for easy identification of infected cells. Control cells were infected with adenoviral vectors expressing EGFP alone.
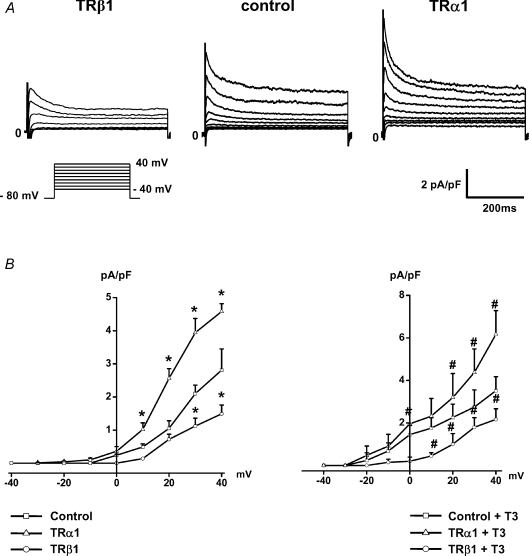
Expression of exogenous TRα1 in rat neonatal cardiomyocytes resulted in a marked increase of native Ito size. Representative current records (Fig. 3A) and current density–voltage relations of each group (Fig. 3B) showed that Ito peak current density was significantly increased in TRα1-infected myocytes (4.6 ± 0.2 pA pF−1 at +40 mV, n= 15) compared to control (2.8 ± 0.6 pA pF−1, n= 17; P= 0.001). Treatment with T3 further increased Ito current amplitude in TRα1-infected myocytes (6.2 ± 1.1 pA pF−1, n= 10; P < 0.001 versus control) (Fig. 3B).
Figure 3. TRα1 increased native Ito in cardiomyocytes, while overexpression of TRβ1 resulted in a significant reduction of Ito.
Representative original current traces recorded in infected myocytes (A) and current density–voltage relations of each group (B) demonstrated that expression of exogenous TRα1 increased Ito current density which was even more pronounced upon T3 treatment. Conversely, overexpression of TRβ1 significantly suppressed Ito1 current size. Control cells were infected with adenoviral vectors expressing EGFP alone. *P < 0.05 versus control, #P < 0.05 compared with control+T3.
Conversely, expression of exogenous TRβ1 significantly decreased mean Ito current density both in untreated (1.5 ± 0.3 ms at +40 mV, n= 12; P < 0.05 versus corresponding control) and in T3-stimulated myocytes (2.2 ± 0.5 ms at +40 mV, n= 10; P < 0.05 versus 3.5 ± 0.7 pA pF−1, n= 12, in T3-treated control cells), confirming the inhibitory properties of TRβ1 on Ito current size (Fig. 3B).
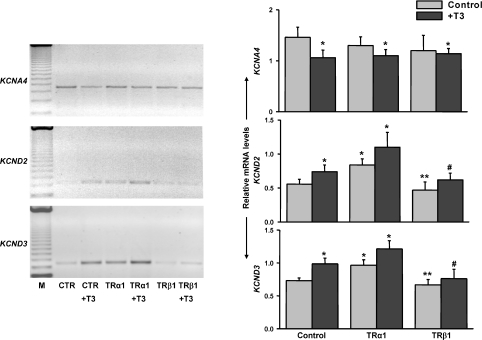
Consistent with Ito current recordings, TRα1-overexpression enhanced both KCND2 and KCND3 mRNA levels, while TRβ1-expressing cardiomyocytes exhibited decreased KCND2/3 expression as illustrated in Fig. 4. T3-mediated reduction of KCNA4 transcription was not further altered by exogenous TRα1 or TRß1 overexpression.
Figure 4. RT-PCR revealed abundant KCND2 and KCND3 expression in TRα1-infected cardiac cells, whereas diminished KCND2 and KCND3 levels were detected in TRβ1-expressing myocytes.
KCNA4 expression was reduced in T3-treated control cells. However, KCNA4 mRNA was largely unaffected by exogenous TRα1 or TRß1 overexpression. Representative ethidium bromide-stained agarose gels of RT-PCR products (left) and densitometric analysis of band intensities (right) from at least three independent experiments. *P < 0.05 versus control, **P < 0.05 compared with TRα1, #P < 0.05 compared with TRα1+T3.
TR-dependent effect on KCND3 transcription
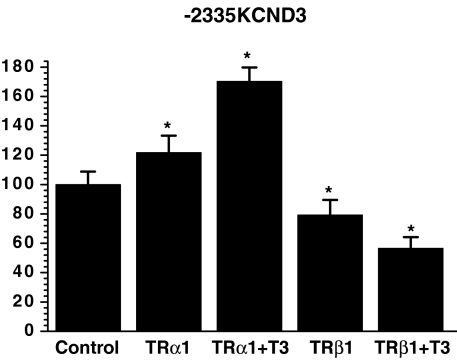
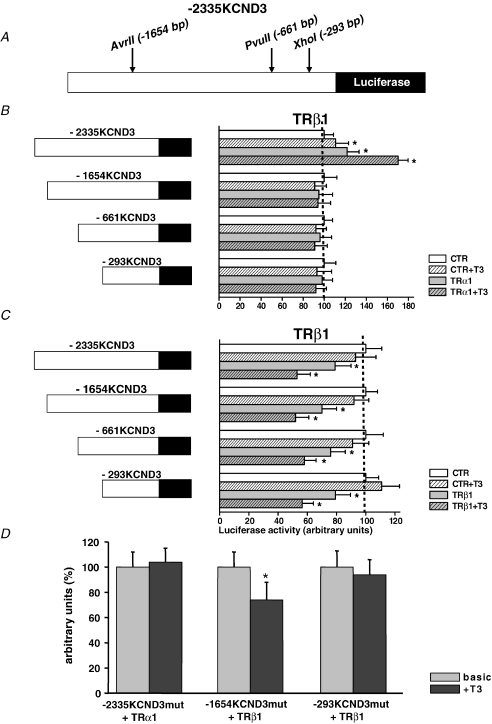
Given (i) distinct modulation of KCND2/3 transcription by the TR isoforms, (ii) potential TREs in the 5′-non-coding sequence of KCND3 (Fig. 1) but not of KCND2/KCNA4 (Negulescu et al. 1998; Jia & Takimoto, 2003; Jang et al. 2004), and (iii) that KCND3 is the predominant molecular component of human cardiac Ito (Shimoni et al. 1997; Oudit et al. 2001), we further analysed potential direct TR-mediated effects on the KCND3 promoter. For this purpose, chimeric constructs containing 2335 bp of the KCND3 5′-flanking sequence linked to a luciferase reporter were transfected into rat neonatal ventricular myocytes. Possible variations in transfection efficacy were corrected by the use of a dual-reporter assay system. To dissect the role of TRα1 and TRβ1 in T3-mediated KCND3 transactivation, TR expression vectors were cotransfected with pGL3-2335KCND3. As shown in Fig. 5, coexpression of the −2335KCND3 reporter construct with exogenous TRα1 led to a significant increase in luciferase activity (121.8 ± 11.4%; P= 0.002 versus control), which was further enhanced upon T3 treatment (170.3 ± 9.4%; P < 0.001). Conversely, cotransfection with a TRβ1 expression vector significantly repressed reporter activity (79.2 ± 10.3%; P < 0.05). This effect was also more pronounced following stimulation with T3 (56.5 ± 7.7%; P < 0.0001). Neither TRα1 nor TRβ1 had an effect on the promoterless reporter construct. These results indicated repression of KCND3 transcription by the TRβ1 aporeceptor, while TRα1 enhanced KCND3 transcription, with both effects exhibiting ligand-dependent amplification.
Figure 5. Enhancement of KCND3 transcription by the TRα1 aporeceptor, while the TRβ1 aporeceptor suppressed KCND3 expression, with both effects exhibiting ligand-dependent amplification.
TR expression vectors were cotransfected with pGL3-2335KCND3 into neonate cardiocytes. Results are expressed as fold activation compared to basal luciferase activity in pGL3-2335KCND3-transfected cells and represent means ±s.e.m. of three to nine separate experiments done in triplicate; *P < 0.05 versus control.
TRα1 and TRβ1 response elements map to different KCND3 promoter regions
In an attempt to define the structural requirements for TRα1 activation and TRβ1 inhibition of the KCND3 promoter, respectively, we compared the ability of a number of 5′-fragments of the KCND3 promoter to signal TR-dependent transcriptional regulation. As shown in Fig. 6A and B truncation of the KCND3 promoter from −2335 bp to −1654 bp of the 5′-flanking sequence eliminated the TRα1-dependent increase in luciferase activity. Cotransfection with TRβ1, on the other hand, resulted in an inhibition of luciferase reporter activity with all KCND3 promoter fragments tested (Fig. 6C).
Figure 6. TRα1 and TRβ1 response elements map (A–C) and bind (D) to different regions of the KCND3 promoter.
A, TR expression vectors were cotransfected with various 5′-fragments of the KCND3 promoter linked to the luciferase reporter into neonate cardiocytes. B, truncation of the KCND3 promoter to −1654 bp, −661 bp and −293 bp of the 5′-flanking sequence eliminated the TRα1-dependent increase in luciferase activity. C, conversely, cotransfection with TRβ1 resulted in an inhibition of luciferase reporter activity with each of the KCND3 promoter fragments tested. Results are expressed as fold activation compared to basal luciferase activity in pGL3-1654KCND3, pGL3-661KCND3, and pGL3-293KCND3 transfected cells, respectively. D, TR expression vectors were cotransfected with constructs carrying mutations of putative TREs of the KCND3 promoter linked to the luciferase reporter into cardiocytes. Mutation of nucleotide G-1651T (pGL3-2335KCND3mut) of the KCND3 promoter eliminated the TRα1-dependent increase in luciferase activity upon T3 stimulation. Mutation of nucleotide G-707T (pGL3-1654KCND3mut) of the 5′-fragment of the KCND3 promoter did not alter the suppressible effect of T3-liganded TRβ1. Conversely, mutation of nucleotide G-72T (pGL3-293KCND3mut) abolished the reduction of luciferase activity by TRβ1. Results are expressed as fold activation following T3 exposure compared to basal luciferase activity, and represent means ±s.e.m. of three to nine separate experiments done in triplicate; *P < 0.05 versus control.
To identify potential TREs we disrupted putative binding sites for TRα1 in pGL3-2335KCND3 and for TRβ1 in pGL3-1654KCND3 and pGL3-293KCND3 by site-directed mutagenesis (Fig. 1). Indeed, the mutation G-1651T (pGL3-2335KCND3mut) undermined the TRα1-mediated increase of luciferase activity upon T3 stimulation, as virtually no change in the luciferase activity was observed following T3 administration (Fig. 6D). While the mutation G-707T (pGL3-1654KCND3mut) did not affect reduction of reporter activity by T3-liganded TRβ1, mutation G-73T (pGL3-293KCND3mut) abolished the suppressible effect of T3-liganded TRβ1. These findings imply that the TRα1 and TRβ1 response elements map to different regions of the KCND3 promoter.
Discussion
The present study demonstrates diverse T3-mediated effects on transcription of ion channel genes encoding the channels conducting Ito in cardiomyocytes. Notably, TRα1 and TRβ1 exhibit distinct modulation of KCND2/3 transactivation, with TRα1 enhancing and TRβ1 suppressing expression. Moreover, response elements of TR isoforms map to different regions of the KCND3 promoter.
TH has profound effects on the heart and vascular system by interacting with intracellular TRs. Previously, effects of T3 on Ito and non-Ito Kv currents, like increased levels of KCNA5 and KCNB1 and decreased KCNA2 expression were demonstrated (Shimoni et al. 1997; Nishiyama et al. 1998; Ma et al. 2003; Watanabe et al. 2003). Data obtained from mice with disruption of either TRα or TRβ genes have led to the conclusion that the effect of T3 on the heart is mediated predominantly by TRα1 (Wikstrom et al. 1998; Gloss et al. 2001; O'Shea & Williams, 2002). The physiological relevance of TR knockout models, however, is unclear since modification of TR functions that is not under control of a regulated expression system is may lead to adaptive changes of other genes in transgenic animals during development, and thus may have unpredictable effects on target gene expression. To circumvent any possible adaptive interference, in the present study we made the first systematic comparison of TR function on gene expression encoding Ito in isolated myocytes and found by two complimentary approaches distinct TR isoform effects.
Ito is composed of a rapid and slow component with varying expression levels in different cardiac regions, during development and in disease states (Näbauer et al. 1996; Wickenden et al. 1997; Guo et al. 1998; Kääb et al. 1998; Kassiri et al. 2002). Given our electrophysiological protocol with a stimulation frequency of 1 Hz we recorded and measured Ito,f, which was increased upon T3 treatment consistent with observations by others (Wickenden et al. 1997; Kassiri et al. 2002). While Wickenden and coworkers showed a corresponding significant higher expression of KCND3 and a trend to higher transcript levels of KCND2 (Wickenden et al. 1997), in the present experiments and data of Shimoni and Guo T3-mediated transcriptional increase of both genes reached statistical significance (Shimoni et al. 1997; Guo et al. 1998). We now extend this previous work by excluding any additional effect of T3 on KCNIP2 transactivation. Moreover, a significant reduction of KCNA4 by T3 was obtained in line with T3-induced reduction of Ito,s (Shimoni et al. 1997; Wickenden et al. 1997; Guo et al. 1998). While KCNA4 expression was not further altered by exogenous overexpression of TR isoforms, we detected opposite effects of TRα1 and TRβ1 on Ito,f current size and corresponding KCND2/3 transcription.
Amplification of native Ito current size in TRα1-infected myocytes compared to control cells, which was further enhanced upon T3 exposure, implicates that the level of TRα1 expression is normally limiting for TH-dependent Ito increase. Notably, we obtained the same pattern of TRα1 aporeceptor function on luciferase activity mediated by the KCND3 promoter. TRα1-induced enhancement of reporter activity was further increased upon T3 stimulation. It has been postulated that the TRα1 aporeceptor might play a role during cardiac fetal and postnatal development (Mai et al. 2004). While in that study the TRα1 aporeceptor was shown to repress expression of other cardiac genes, in our experiments the aporeceptor, similar to the holoreceptor, activated KCND3 promoter transactivation.
Conversely, the TRβ1 aporeceptor downregulated KCND3 expression. T3-liganded TRβ1 further inhibited cotransfected −2335KCND3 reporter activity. T3 also significantly reduced native Ito current density in TRβ1-infected cells compared to T3-treated controls, though the difference was slightly less pronounced than following overexpression of the KCND3 promoter. Apparently, endogenous TRα1 to some extent blunted the suppressive effect of exogenous TRβ1, possibly due to a higher endogenous abundance of TRα1 in cardiac tissue or a higher affinity for TREs. These observations support the notion, that physiologically, as for other cardiac genes, T3 effects on Ito are predominantly transferred by TRα1, thus resulting in an increase of the fast recovering component of Ito (Wikstrom et al. 1998; Gloss et al. 2001; O'Shea & Williams, 2002).
Most of genes regulated by TH possess TREs in their promoter region, and are regulated by both unliganded and liganded TRs (Desvergne, 1994; Munoz & Bernal, 1997; Harvey & Williams, 2002). Beside different TR affinities for TREs, distinct TR isoform effects on various promoters might be explained by isoform-specific interactions with cofactors or diverse TRE structures. TRs can bind to TREs as a monomer, homodimer, or heterodimer (Desvergne, 1994; Munoz & Bernal, 1997; Harvey & Williams, 2002). In the present study, deletion analysis of the 5′-flanking sequence localized the suppressible promoter sequence for TRβ1 to within −293 bp and the activating promoter sequence for TRα1 to within −2335 to −1654 bp of the transcription start site of KCND3. For TR to bind as a monomer, only one nuclear hormone receptor half-site is necessary. The optimized consensus for the half-site binding motif, KRRGGT(C/R)A is an octamer that includes a 5′-stabilizing extension (White et al. 2001). Analysis of the genomic sequence of the KCND3 promoter revealed that nucleotides −1655 to −1648 (TAGGGTGA) and nucleotides −711 to −704 (TAGGGTAA) both form perfect consensus sites (Fig. 1). Mutation of the nucleotides G-1651T prevented a TRα1-mediated increase of KCND3 promoter transactivation indicating that this region provides a binding site for TRα1, while disruption of the second consensus site did not alter TRβ1 binding. Moreover, we noted the presence of a TRE half-site consensus from nucleotides −74 to −69 (GGGTCA) followed by a half-site consensus sequence for retinoid acid receptor or vitamin D3 receptor binding, with a 1 bp spacer. Mutation of this TRE half-site (G-73T) abolished the suppressible effect of TRβ1, suggesting that this region functions as a TRβ1 binding sequence. While it might be intuitive that the 5′-non-coding sequence of KCND2 and possibly KCNA4 exhibit the same/similar TREs compared to the KCND3 promoter, no optimized TRE consensus half-sites nor TRE half-site consensus associated with known binding sequences for other nuclear receptors could be identified in the 5′-flanking regions of KCND2/KCNA4 (Negulescu et al. 1998; Jia & Takimoto, 2003; Jang et al. 2004), indicating that KCND2 and KCNA4 may not be regulated by TR via TREs or might be regulated via TRE sequences which have not yet been identified.
Since Ito is important in the early phase of membrane repolarization, changes in its density will alter the time course of the cardiac action potential and susceptibility to arrhythmias (Beuckelmann et al. 1993; Hoppe et al. 1999, 2001). Moreover, a shift of the relative contribution of the fast and slow component of Ito will affect rate-dependent APD shortening (Wickenden et al. 1997). In this respect precise knowledge of the regulation of cardiac Ito by TR isoforms is particularly important as in disease states diverse changes of both TR isoform expression and Ito current size have been demonstrated (Beuckelmann et al. 1993; Näbauer et al. 1996; Kaab et al. 1998; Kinugawa et al. 2001a). In human heart failure TRα1 was shown to be downregulated with no alteration of TRβ1 (Kinugawa et al. 2001a). Moreover, reduction of Ito,f and KCND3 mRNA consistently has been demonstrated in mammalian and human heart failure (Beuckelmann et al. 1993; Näbauer et al. 1996; Kaab et al. 1998). Considering our observations of isoform-specific regulation of cardiac gene expression, i.e. KCND2/3, targeted stimulation of selected receptors might prove useful to restore changes in Ito and treat heart disease (Grover et al. 2003; Ye et al. 2003).
Acknowledgments
This study was supported by a grant from the Deutsche Forschungsgemeinschaft (Ho 2146/3-1), by Köln Fortune, and by the Marga and Walter Boll-Stiftung. We are grateful to N. Henn for technical assistance.
References
- Basset A, Blanc J, Messas E, Hagege A, Elghozi JL. Renin-angiotensin system contribution to cardiac hypertrophy in experimental hyperthyroidism: an echocardiographic study. J Cardiovasc Pharmacol. 2001;37:163–172. doi: 10.1097/00005344-200102000-00004. [DOI] [PubMed] [Google Scholar]
- Beuckelmann DJ, Nabauer M, Erdmann E. Alterations of K+ currents in isolated human ventricular myocytes from patients with terminal heart failure. Circ Res. 1993;73:379–385. doi: 10.1161/01.res.73.2.379. [DOI] [PubMed] [Google Scholar]
- Cheng SY. Multiple mechanisms for regulation of the transcriptional activity of thyroid hormone receptors. Rev Endocr Metab Disord. 2000;1:9–18. doi: 10.1023/a:1010052101214. [DOI] [PubMed] [Google Scholar]
- Desvergne B. How do thyroid hormone receptors bind to structurally diverse response elements? Mol Cell Endocrinol. 1994;100:125–131. doi: 10.1016/0303-7207(94)90291-7. [DOI] [PubMed] [Google Scholar]
- Dixon JE, Shi W, Wang H, McDonald C, Yu H, Wymore RS, Cohen IS, McKinnon D. Role of the Kv4.3 K+ channel in ventricular muscle. A molecular correlate for the transient outward current. Circ Res. 1996;79:659–668. doi: 10.1161/01.res.79.4.659. [DOI] [PubMed] [Google Scholar]
- Er F, Larbig R, Ludwig A, Biel M, Hofmann F, Beuckelmann DJ, Hoppe UC. Dominant-negative suppression of HCN channels markedly reduces the native pacemaker current If and undermines spontaneous beating of neonatal cardiomyocytes. Circulation. 2003;107:485–489. doi: 10.1161/01.cir.0000045672.32920.cb. [DOI] [PubMed] [Google Scholar]
- Franklyn JA, Gammage MD, Ramsden DB, Sheppard MC. Thyroid status in patients after acute myocardial infarction. Clin Sci (Lond) 1984;67:585–590. doi: 10.1042/cs0670585. [DOI] [PubMed] [Google Scholar]
- Gassanov N, Jankowski M, Danalache B, Wang D, Grygorczyk R, Hoppe UC, Gutkowska J. Arginine vasopressin-mediated cardiac differentiation: insight into the role of its receptors and nitric oxide signaling. J Biol Chem. 2007;282:11255–11265. doi: 10.1074/jbc.M610769200. [DOI] [PubMed] [Google Scholar]
- Gloss B, Trost S, Bluhm W, Swanson E, Clark R, Winkfein R, Janzen K, Giles W, Chassande O, Samarut J, Dillmann W. Cardiac ion channel expression and contractile function in mice with deletion of thyroid hormone receptor α or β. Endocrinology. 2001;142:544–550. doi: 10.1210/endo.142.2.7935. [DOI] [PubMed] [Google Scholar]
- Goltz D, Schultz JH, Stucke C, Wagner M, Bassalay P, Schwoerer AP, Ehmke H, Volk T. Diminished Kv4.2/3 but not KChIP2 levels reduce the cardiac transient outward K+ current in spontaneously hypertensive rats. Cardiovasc Res. 2007;74:85–95. doi: 10.1016/j.cardiores.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Grover GJ, Mellstrom K, Ye L, Malm J, Li YL, Bladh LG, Sleph PG, Smith MA, George R, Vennstrom B, Mookhtiar K, Horvath R, Speelman J, Egan D, Baxter JD. Selective thyroid hormone receptor-β activation: a strategy for reduction of weight, cholesterol, and lipoprotein (a) with reduced cardiovascular liability. Proc Natl Acad Sci U S A. 2003;100:10067–10072. doi: 10.1073/pnas.1633737100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Kamiya K, Hojo M, Kodama I, Toyama J. Regulation of Kv4.2 and Kv1.4 K+ channel expression by myocardial hypertrophic factors in cultured newborn rat ventricular cells. J Mol Cell Cardiol. 1998;30:1449–1455. doi: 10.1006/jmcc.1998.0730. [DOI] [PubMed] [Google Scholar]
- Harvey CB, Williams GR. Mechanism of thyroid hormone action. Thyroid. 2002;12:441–446. doi: 10.1089/105072502760143791. [DOI] [PubMed] [Google Scholar]
- Hoppe UC, Johns DC, Marban E, O’Rourke B. Manipulation of cellular excitability by cell fusion: effects of rapid introduction of transient outward K+ current on the guinea pig action potential. Circ Res. 1999;84:964–972. doi: 10.1161/01.res.84.8.964. [DOI] [PubMed] [Google Scholar]
- Hoppe UC, Marban E, Johns DC. Molecular dissection of cardiac repolarization by in vivo Kv4.3 gene transfer. J Clin Invest. 2000;105:1077–1084. doi: 10.1172/JCI8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe UC, Marban E, Johns DC. Distinct gene-specific mechanisms of arrhythmia revealed by cardiac gene transfer of two long QT disease genes, HERG and KCNE1. Proc Natl Acad Sci U S A. 2001;98:5335–5340. doi: 10.1073/pnas.091239098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Lazar MA. Transcriptional repression by nuclear hormone receptors. Trends Endocrinol Metab. 2000;11:6–10. doi: 10.1016/s1043-2760(99)00215-5. [DOI] [PubMed] [Google Scholar]
- Jang GM, Leong LE, Hoang LT, Wang PH, Gutman GA, Semler BL. Structurally distinct elements mediate internal ribosome entry within the 5'-noncoding region of a voltage-gated potassium channel mRNA. J Biol Chem. 2004;279:47419–47430. doi: 10.1074/jbc.M405885200. [DOI] [PubMed] [Google Scholar]
- Jia Y, Takimoto K. GATA and FOG2 transcription factors differentially regulate the promoter for Kv4.2 K+ channel gene in cardiac myocytes and PC12 cells. Cardiovasc Res. 2003;60:278–287. doi: 10.1016/s0008-6363(03)00528-5. [DOI] [PubMed] [Google Scholar]
- Kaab S, Dixon J, Duc J, Ashen D, Nabauer M, Beuckelmann DJ, Steinbeck G, McKinnon D, Tomaselli GF. Molecular basis of transient outward potassium current downregulation in human heart failure: a decrease in Kv4.3 mRNA correlates with a reduction in current density. Circulation. 1998;98:1383–1393. doi: 10.1161/01.cir.98.14.1383. [DOI] [PubMed] [Google Scholar]
- Kääb S, Dixon J, Duc J, Ashen D, Näbauer M, Beuckelmann DJ, Steinbeck G, McKinnon D, Tomaselli GF. Molecular basis of transient outward potassium current downregulation in human heart failure. A decrease in Kv4.3 mRNA correlates with a reduction in current density. Circulation. 1998;98:1383–1393. doi: 10.1161/01.cir.98.14.1383. [DOI] [PubMed] [Google Scholar]
- Kassiri Z, Hajjar R, Backx PH. Molecular components of transient outward potassium current in cultured neonatal rat ventricular myocytes. J Mol Med. 2002;80:351–358. doi: 10.1007/s00109-002-0325-7. [DOI] [PubMed] [Google Scholar]
- Kinugawa K, Minobe WA, Wood WM, Ridgway EC, Baxter JD, Ribeiro RC, Tawadrous MF, Lowes BA, Long CS, Bristow MR. Signaling pathways responsible for fetal gene induction in the failing human heart: evidence for altered thyroid hormone receptor gene expression. Circulation. 2001a;103:1089–1094. doi: 10.1161/01.cir.103.8.1089. [DOI] [PubMed] [Google Scholar]
- Kinugawa K, Yonekura K, Ribeiro RC, Eto Y, Aoyagi T, Baxter JD, Camacho SA, Bristow MR, Long CS, Simpson PC. Regulation of thyroid hormone receptor isoforms in physiological and pathological cardiac hypertrophy. Circ Res. 2001b;89:591–598. doi: 10.1161/hh1901.096706. [DOI] [PubMed] [Google Scholar]
- Klein I, Hong C. Effects of thyroid hormone on cardiac size and myosin content of the heterotopically transplanted rat heart. J Clin Invest. 1986;77:1694–1698. doi: 10.1172/JCI112488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med. 2001;344:501–509. doi: 10.1056/NEJM200102153440707. [DOI] [PubMed] [Google Scholar]
- Kobori H, Ichihara A, Miyashita Y, Hayashi M, Saruta T. Local renin-angiotensin system contributes to hyperthyroidism-induced cardiac hypertrophy. J Endocrinol. 1999;160:43–47. doi: 10.1677/joe.0.1600043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma ML, Watanabe K, Watanabe H, Hosaka Y, Komura S, Aizawa Y, Yamamoto T. Different gene expression of potassium channels by thyroid hormone and an antithyroid drug between the atrium and ventricle of rats. Jpn Heart J. 2003;44:101–110. doi: 10.1536/jhj.44.101. [DOI] [PubMed] [Google Scholar]
- Mai W, Janier MF, Allioli N, Quignodon L, Chuzel T, Flamant F, Samarut J. Thyroid hormone receptor alpha is a molecular switch of cardiac function between fetal and postnatal life. Proc Natl Acad Sci U S A. 2004;101:10332–10337. doi: 10.1073/pnas.0401843101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels G, Brandt MC, Zagidullin N, Khan IF, Larbig R, van Aaken S, Wippermann J, Hoppe UC. Direct evidence for calcium-conductance of HCN channels and human native If at physiological calcium concentrations. Cardiovasc Res. 2008;78:466–475. doi: 10.1093/cvr/cvn032. [DOI] [PubMed] [Google Scholar]
- Michels G, Er F, Khan IF, Südkamp M, Herzig S, Hoppe UC. Single-channel properties support a potential contribution of HCN channels and If to cardiac arrhythmias. Circulation. 2005;111:399–404. doi: 10.1161/01.CIR.0000153799.65783.3A. [DOI] [PubMed] [Google Scholar]
- Munoz A, Bernal J. Biological activities of thyroid hormone receptors. Eur J Endocrinol. 1997;137:433–445. doi: 10.1530/eje.0.1370433. [DOI] [PubMed] [Google Scholar]
- Näbauer M, Beuckelmann DJ, Uberfuhr P, Steinbeck G. Regional differences in current density and rate-dependent properties of the transient outward current in subepicardial and subendocardial myocytes of human left ventricle. Circulation. 1996;93:168–177. doi: 10.1161/01.cir.93.1.168. [DOI] [PubMed] [Google Scholar]
- Negulescu D, Leong LE, Chandy KG, Semler BL, Gutman GA. Translation initiation of a cardiac voltage-gated potassium channel by internal ribosome entry. J Biol Chem. 1998;273:20109–20113. doi: 10.1074/jbc.273.32.20109. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Kambe F, Kamiya K, Seo H, Toyama J. Effects of thyroid status on expression of voltage-gated potassium channels in rat left ventricle. Cardiovasc Res. 1998;40:343–351. doi: 10.1016/s0008-6363(98)00135-7. [DOI] [PubMed] [Google Scholar]
- O'Shea PJ, Williams GR. Insight into the physiological actions of thyroid hormone receptors from genetically modified mice. J Endocrinol. 2002;175:553–570. doi: 10.1677/joe.0.1750553. [DOI] [PubMed] [Google Scholar]
- Oudit GY, Kassiri Z, Sah R, Ramirez RJ, Zobel C, Backx PH. The molecular physiology of the cardiac transient outward potassium current (Ito) in normal and diseased myocardium. J Mol Cell Cardiol. 2001;33:851–872. doi: 10.1006/jmcc.2001.1376. [DOI] [PubMed] [Google Scholar]
- Shimoni Y, Fiset C, Clark RB, Dixon JE, McKinnon D, Giles WR. Thyroid hormone regulates postnatal expression of transient K+ channel isoforms in rat ventricle. J Physiol. 1997;500:65–73. doi: 10.1113/jphysiol.1997.sp021999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M, Helguera G, Eghbali M, Zhu N, Zarei MM, Olcese R, Toro L, Stefani E. Remodeling of Kv4.3 potassium channel gene expression under the control of sex hormones. J Biol Chem. 2001;276:31883–31890. doi: 10.1074/jbc.M101058200. [DOI] [PubMed] [Google Scholar]
- Song WJ, Tkatch T, Baranauskas G, Ichinohe N, Kitai ST, Surmeier DJ. Somatodendritic depolarization-activated potassium currents in rat neostriatal cholinergic interneurons are predominantly of the A type and attributable to coexpression of Kv4.2 and Kv4.1 subunits. J Neurosci. 1998;18:3124–3137. doi: 10.1523/JNEUROSCI.18-09-03124.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson LW, Tillisch JH, Hamilton M, Luu M, Chelimsky-Fallick C, Moriguchi J, Kobashigawa J, Walden J. Importance of hemodynamic response to therapy in predicting survival with ejection fraction less than or equal to 20% secondary to ischemic or nonischemic dilated cardiomyopathy. Am J Cardiol. 1990;66:1348–1354. doi: 10.1016/0002-9149(90)91166-4. [DOI] [PubMed] [Google Scholar]
- Strait KA, Schwartz HL, Perez-Castillo A, Oppenheimer JH. Relationship of c-erbA mRNA content to tissue triiodothyronine nuclear binding capacity and function in developing and adult rats. J Biol Chem. 1990;265:10514–10521. [PubMed] [Google Scholar]
- Watanabe H, Ma M, Washizuka T, Komura S, Yoshida T, Hosaka Y, Hatada K, Chinushi M, Yamamoto T, Watanabe K, Aizawa Y. Thyroid hormone regulates mRNA expression and currents of ion channels in rat atrium. Biochem Biophys Res Commun. 2003;308:439–444. doi: 10.1016/s0006-291x(03)01420-7. [DOI] [PubMed] [Google Scholar]
- Weinberg BA, Miles WM, Klein LS, Bolander JE, Dusman RE, Stanton MS, Heger JJ, Langefeld C, Zipes DP. Five-year follow-up of 589 patients treated with amiodarone. Am Heart J. 1993;125:109–120. doi: 10.1016/0002-8703(93)90063-f. [DOI] [PubMed] [Google Scholar]
- White P, Burton KA, Fowden AL, Dauncey MJ. Developmental expression analysis of thyroid hormone receptor isoforms reveals new insights into their essential functions in cardiac and skeletal muscles. FASEB J. 2001;15:1367–1376. doi: 10.1096/fj.00-0725com. [DOI] [PubMed] [Google Scholar]
- Wickenden AD, Kaprielian R, Parker TG, Jones OT, Backx PH. Effects of development and thyroid hormone on K+ currents and K+ channel gene expression in rat ventricle. J Physiol. 1997;504:271–286. doi: 10.1111/j.1469-7793.1997.271be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikstrom L, Johansson C, Salto C, Barlow C, Campos Barros A, Baas F, Forrest D, Thoren P, Vennstrom B. Abnormal heart rate and body temperature in mice lacking thyroid hormone receptor α1. EMBO J. 1998;17:455–461. doi: 10.1093/emboj/17.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, Li YL, Mellstrom K, Mellin C, Bladh LG, Koehler K, Garg N, Garcia Collazo AM, Litten C, Husman B, Persson K, Ljunggren J, Grover G, Sleph PG, George R, Malm J. Thyroid receptor ligands. 1. Agonist ligands selective for the thyroid receptor β1. J Med Chem. 2003;46:1580–1588. doi: 10.1021/jm021080f. [DOI] [PubMed] [Google Scholar]
- Yonemochi H, Yasunaga S, Teshima Y, Takahashi N, Nakagawa M, Ito M, Saikawa T. Rapid electrical stimulation of contraction reduces the density of β-adrenergic receptors and responsiveness of cultured neonatal rat cardiomyocytes. Possible involvement of microtubule disassembly secondary to mechanical stress. Circulation. 2000;101:2625–2630. doi: 10.1161/01.cir.101.22.2625. [DOI] [PubMed] [Google Scholar]
- Zhang TT, Takimoto K, Stewart AF, Zhu C, Levitan ES. Independent regulation of cardiac Kv4.3 potassium channel expression by angiotensin II and phenylephrine. Circ Res. 2001;88:476–482. doi: 10.1161/01.res.88.5.476. [DOI] [PubMed] [Google Scholar]