Abstract
Lipid droplets (LD) consist of accumulations of triacylglycerols and have been proposed to be markers of ischaemic but viable tissue. Previous studies have described the presence of LD in myocardium surviving an acute coronary occlusion. We investigated whether LD may be protective against cell death secondary to ischaemia–reperfusion injury. The addition of oleate–bovine serum albumin complex to freshly isolated adult rat cardiomyocytes or to HL-1 cells resulted in the accumulation of intracellular LD detectable by fluorescence microscopy, flow cytometry and 1H-nuclear magnetic resonance spectroscopy. Simulated ischaemia–reperfusion of HL-1 cells (respiratory inhibition at pH 6.4 followed by 30 min of reperfusion) resulted in significant cell death (29.7 ± 2.6% of total lactate dehydrogenase release). However, cell death was significantly attenuated in cells containing LD (40% reduction in LDH release compared with control cells, P= 0.02). The magnitude of LD accumulation was inversely correlated (r2= 0.68, P= 0.0003) with cell death. The protection associated with intracellular LD was not a direct effect of the fatty acids used to induce their formation, because oleate added 30 min before ischaemia, during ischaemia or during reperfusion did not form LD and did not protect against cell death. Increasing the concentration of free oleate during reperfusion progressively decreased the protection afforded by LD. HL-1 cells labelled with fluo-4, a Ca2+-sensitive fluorochrome, fluorescence within LD areas increased more throughout simulated ischaemia and reperfusion than in the cytosolic LD-free areas of the same cells. As a consequence, cells with LD showed less cytosolic Ca2+ overload than control cells. These results suggest that LD exert a protective effect during ischaemia–reperfusion by sequestering free fatty acids and Ca2+.
Intracellular cytosolic lipid droplets (LD), also known as lipid particles or lipid bodies, consist of a highly hydrophobic core of neutral lipids, mainly triacylglycerols and/or steryl esters, surrounded by a phospholipid monolayer. At their surface, proteins can also be found, mainly adipose differentiation-related protein and perilipin (Murphy & Vance, 1999). The well-known function of these lipid particles is the storage of neutral lipids as an energy source or as building blocks for membrane biogenesis. Typically, LD are found in adipocytes, acting as fat storage globules (Murphy & Vance, 1999). Although cardiomyocytes do not normally accumulate LD, they can do it during fasting (Suzuki et al. 2001) or in pathological conditions, such as ischaemia (Jodalen et al. 1985; Greve et al. 1990) or obesity (McGavock et al. 2006). In the regionally ischaemic heart, LD accumulate mainly in the periphery of the area at risk (Jodalen et al. 1985; Straeter-Knowlen et al. 1996), and it has been suggested that they tend to concentrate in areas where the ischaemic insult is milder.
Intracellular LD can be non-invasively detected in vivo by the use of 1H-nuclear magnetic resonance (NMR) spectroscopy (Barba et al. 1999; Pérez et al. 2002). Techniques requiring the destruction of the sample do not allow the detection of LD because they result in the mixing of lipids from intracellular droplets and membranes. Straeter-Knowlen et al. (1996) found that after 24 h of coronary occlusion in a dog model, the 1H-NMR lipid signals from the myocardium at risk were higher than those from the non-ischaemic control region, while the intensity of the lipid signals in the infarct region was intermediate. The amount of triacylglycerol-containing LD correlated with the intensity of 1H-NMR lipid signals, and tissue chemical analysis showed an increase in the content of total triacylglycerol (Straeter-Knowlen et al. 1996). Other studies (Evanochko et al. 1987) have described a similar 1H-NMR pattern in the lipid signal without differences in the tissue total lipid content and suggested that 1H-NMR lipid signals could be used as markers of reversibly injured myocardium.
Studies of pathology have shown that postreperfusion infarcts secondary to transient myocardial ischaemia are composed of hypercontracted myocytes, forming a pattern of contraction band necrosis (Miyazaki et al. 1987; Barrabes et al. 1996). This type of cell death occurs during the first minutes of reperfusion and is characterized by an extreme distortion of cell geometry and sarcolemmal rupture (Ruiz-Meana et al. 1995). The mechanisms involved in its genesis are not completely established, but the presence of high cytosolic Ca2+ when ATP synthesis is restored is one of the main determinants of reperfusion-induced hypercontracture (Ladilov et al. 1999; Siegmund et al. 1990). Also, extreme elevation of cytosolic Ca2+ activates calpain-mediated degradation of the membrane cytoskeleton (Inserte et al. 2005), favouring cytoskeletal fragility that eventually leads to cell death. Other factors have been described to play a role in modulating cell survival after an ischaemic insult. It has been known for a long time that free fatty acids are detrimental during ischaemia–reperfusion injury (for review see Hendrickson et al. 1997). Most reports in the literature link the accumulation of free fatty acids with adverse effects, which is known as lipotoxicity (Schaffer 2003). In contrast, more recent data show that the accumulation of LD, initially formed by the addition of free fatty acids, can protect against apoptotic cell death by channelling free fatty acids into triglyceride pools (Listenberger et al. 2003).
In the present study, we hypothesized that LD offer protection against cell death secondary to simulated ischaemia–reperfusion by sequestering excess free fatty acids and free Ca2+.
Methods
Cell culture
Experiments were performed in freshly isolated adult rat cardiomyocytes and in HL-1 cardiomyocyte cultures (claycomb medium (Gibco, Carlsbad, USA) Grown in 5% CO2, 95% air), a cell line of mouse atrium-derived cardiac myocytes cultured as previously described (Claycomb et al. 1998; Ruiz-Meana et al. 2004) and plated at 70–80% confluency.
To obtain isolated rat cardiomyocytes, the principles of laboratory animal care (Guide for the Care and Use of Laboratory Animals published by the United States National Institute of Health, NIH Publication no. 85-23, revised 1996) were followed, and the procedure was approved by the Research Commission on Ethics of the Hospital Vall d'Hebron. Rat cardiomyocytes were isolated as previously described (Ruiz-Meana et al. 1995). A total of four adult male Sprague–Dawley rats weighting 300 g were deeply anaesthetized by an intraperitoneal injection of sodium pentobarbitone (Laboratory Dr Carreras, Barcelona, Spain) (150 mg kg−1) until total loss of pedal reflex. After thoracotomy, the heart was rapidly excised, cannulated by the aorta in a Langendorff system and retrogradely perfused for 20 min with a modified Krebs buffer (pH 7.4 bubbled with 5% CO2–95% air, in mmol l−1: 110 NaCl, 2.6 KCl, 1.2 KH2PO4, 1.2 MgSO4, 25 NaHCO3 and 11 glucose) containing 25 μmol l−1 CaCl2 and 0.03% collagenase (type II, Heidelberg, Serva, Germany). Cells from dissociated tissue were subjected to a progressive normalization of Ca2+ levels to a final concentration of 1 mmol l−1. Rod-shaped cells were selected by gravity sedimentation in a 4% bovine serum albumin (BSA) gradient (fraction V, Boehringer Mannheim, Germany) and plated on laminin-coated glass-bottomed culture dishes with medium 199/Hepes (Sigma, St Louis, MO, USA) and 4% fetal calf serum (Carlsbad, Gibco, USA).
For the HL-1 cardiomyocyte cultures, oleate was loaded overnight to reach final concentrations of 0.05–2 mmol l−1, using a 35 mmol l−1 stock solution of oleate prepared with 0.2 mg ml−1 BSA (Callies et al. 1993). When using adult rat cardiomyocytes, the loading period with oleate was reduced to 4 h to prevent cell culture de-differentiation. Ischaemia was simulated for 30 min by replacing the growth medium with a buffer containing (in mmol l−1): 140 NaCl, 20 Hepes, 3.6 KCl, 1.2 MgSO4, 1.3 CaCl2, 2 KCN and 20 2-deoxy-glucose, at pH 6.4 adjusted with HCl. Simulateous with the symulated ischaemia, hypoxia (1% O2, 5% CO2 and 94% N2) was induced by placing the culture plates in a Ruskin in vivo 2 chamber (Ruskin Technologies, Guiseley, UK). Reperfusion was induced by changing the buffer to one containing glucose 5 mmol l−1 and pH to 7.4 for 15 min. In some experiments, the reperfusion buffer was supplemented with 5 mmol l−1 glycine to inhibit the mitochondrial permeability transition pore (Ruiz-Meana et al. 2004); in others, 0.1 μmol l−1 perhexiline was added during the oleate–BSA loading protocol to inhibit mitochondrial carnitine palmitoyltransferase-1, therefore preventing free fatty acids from entering mitochondria to undergo β-oxidation.
Free oleate was prepared by sonication of a mixture of oleic acid and distilled water until a milky suspension was obtained. It was added to the culture wells at the specified final concentrations.
Fluorescence microscopy of lipid droplets
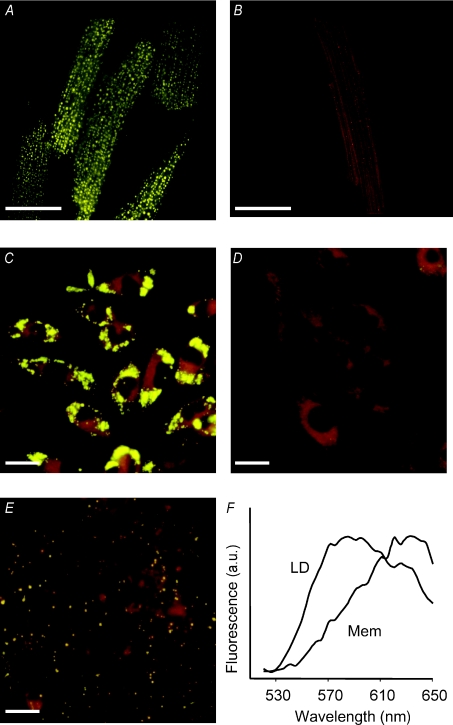
Growth medium was removed after each treatment, and cells were stained with Nile red at a final concentration of 1 μg ml−1 as described previously (Barba et al. 1999). Culture plates were placed on the stage of an inverted microscope (Nikon TE2000-S) equipped with a digital camera (Nikon, Barcelona, Spain). Excitation wavelength was set at 450–490 nm, and emitted light was collected above 520 nm using a 505 nm cut-off filter. Emission spectral data were obtained with a Nikon C1-Si microscope by exciting the sample with a 488 nm laser and acquiring the spectra in 32 bins of 5 nm each. The emission spectra of Nile red corresponding to LD areas (Fig. 1F) showed a clear shift to yellow fluorescence compared with the fluorescence arising from membranes. This was used to distinguish between LD and membranes by fluorescence microscopy and flow cytometry of Nile red stained cells.
Figure 1. Fluorescent imaging of LD loaded cells.
Fluorescence micrographs of adult rat cardiomyocytes supplemented with oleate–BSA (2 mmol l−1; A) or BSA alone (B). Micrographs C, D and E correspond to HL-1 cardiomyocytes supplemented with oleate–BSA, BSA alone and after an overnight hypoxic incubation in 1% O2 without oleate–BSA, respectively. Cells were stained with Nile red at a final concentration of 1 μmol l−1. Lipid droplets appear as bright yellow dots. Scale bars represent 25 μm in A–C and 20 μm in D and E. F shows the emission spectra recorded from areas corresponding to lipid droplets (LD) or membranes (Mem) in HL-1 cells loaded with oleate–BSA.
Flow cytometry
The flow cytometry protocol was based on a previously published method (Awonusonu et al. 1999). Briefly, after each experiment cells were removed from the culture plates by trypsinization and stained with Nile red at a final concentration of 0.1 μg ml−1 in phosphate-buffered saline. Stained cells were analysed on a FACScalibur flow cytometer (Becton Dickinson Inc., San Jose, CA, USA), calibrated weekly for fluorescence and light scatter using fluorescent beads. The HL-1 cells (10 000 per sample) were identified on the basis of forward and sideways scatter parameters in the logarithmic mode. Nile red excitation was obtained by a 488 nm argon laser lamp, and its fluorescence was collected on the FL1 and FL3 detectors on a linear scale. Data were analysed with CELLQuest software (Becton Dickinson). Positive cells showed a shift to higher green fluorescence (FL1) while maintaining a constant red (FL3) fluorescence intensity.
Nuclear magnetic resonance spectroscopy
The HL-1 cells from one T-75 flask were harvested by trypsinization and washed once in deuterated phosphate-buffered saline at 4°C. Nuclear magnetic resonance spectroscopy of the cell pellet was started immediately after harvesting. The NMR spectra were acquired at 400 MHz on a Bruker Avance spectrometer (Bruker, Madrid, Spain) equipped with a High Resolution-Magic Angle Spinning (HR-MAS) probe. The sample was kept at 4°C during the acquisition and spun at 3200 Hz at the magic angle. Each spectrum was the result of the accumulation of 64 scans with a one-dimensional NOESY sequence with presaturation of the water signal which lasted for 6 min.
Measurement of Ca2+ by confocal microscopy
Changes in intracellular Ca2+ concentration were continuously monitored in HL-1 cardiomyocytes during simulated ischaemia–reperfusion both in the cytosolic area and within the area previously defined by light microscopy as LD. Briefly, cells were loaded with 5 μmol l−1 fluo-4 (Molecular Probes, Carlsbad, USA), and imaged using a confocal Ar–Kr laser system (CSU10, Yokogawa, Tokyo, Japan, Nipkow spinning disk) set on an Olympus IX70 (×60, NA 1.4) as previously described (Ruiz-Meana et al. 2007). Fluo-4-loaded cells were excited at 488 nm, and changes in 520 nm light emission from defined regions within the cells were monitored over time using commercially available software (VoxCell Scan, VisiTech, Sunderland, UK). Changes in fluorescence, reflecting increases in intracellular Ca2+ concentration, were expressed with respect to the initial arbitrary units obtained immediately before simulated ischaemia–reperfusion.
In a subset of experiments, cells were loaded with TNS (2-p-toluidinyl-naphatalene-6-sulphonate), a non-polar fluorescent probe that shows an increase in emission fluorescence upon Ca2+ binding (Hernando et al. 2003). The HL-1 cardiomyocytes were harvested and washed in phosphate-buffered saline, stained with 1 μmol l−1 TNS (prepared from a stock solution of 1 mmol l−1 in methanol) and resuspended in simulated ischaemia buffer. Excitation was performed at 320 nm using a fast-speed monochromator, and emission light was collected at 440 nm by means of a CCCD camera (VisiTech). Changes in fluorescence were monitored over time using commercially available software (QuantiCell 2000, Visitech).
Cell death
Release of lactate dehydrogenase (LDH) into the extracellular medium was measured after simulated ischaemia and reperfusion periods as an index of sarcolemmal disruption (Sarri et al. 2006). Previous studies have documented a correlation between LDH release as evaluated by propidium iodide or Trypan blue uptake (Mirabet et al. 2005). At the end of the experiment, extracellular buffer was replaced by 1 ml of 0.1% Triton X-100 in distilled water, and left for at least 30 min to induce sarcolemmal disruption and promote the release of the remaining LDH. Cell death was determined as the LDH activity of the buffer at each stage of the experiment relative to total LDH (sum of LDH activities of buffer and Triton X-100 solutions).
Statistics
Experiments were performed between three and six times, with triplicates for every experiment. All data are presented as means ±s.e.m. Comparisons were done using Student's paired t test and were considered significant when P < 0.05.
Results
Induction of lipid droplet accumulation
Both in freshly isolated rat cardiomyocytes and in HL-1 cells, incubation with oleate–BSA complex resulted in the accumulation of intracellular LD that were seen as bright yellow dots in fluorescence micrographs of Nile red stained freshly isolated cardiomyocytes and HL-1 cardiomyocytes (Fig. 1A and C, respectively). There were no detectable LD in cells incubated with the equivalent amount of BSA alone (Fig. 1B and D). Other than the presence of LD, there were no apparent changes in the cell or nuclear morphology. Hypoxia per se also induced intracellular LD accumulation in HL-1 cardiomyocytes (Fig. 1E). In hypoxic conditions, LD started to appear after 3 h of exposure. The number of cells positive for LD measured by flow cytometry increased from 3% in basal conditions to 12% at 3 h and 20% at 5 h hypoxia. After an overnight hypoxic treatment, all the cells contained LD; at this time point, there were no morphological changes in the cells, although LD were smaller than those observed after induction with oleate–BSA.
Flow cytometry of Nile red stained cells could quantitatively differentiate between control and oleate–BSA-loaded HL-1 cells. There was no difference in red (FL3) fluorescence arising from membranes but there was a clear shift to higher green (FL1) fluorescence when LD were present (Fig. 2A and B). By fitting the dose–fluorescence response to an exponential curve (Fig. 2E), it was possible to show that it would reach saturation at concentrations of oleate close to 2 mmol l−1; this concentration was not tested because it caused cell damage during loading. Half of the maximal fluorescence intensity was obtained at 0.2 mmol l−1 oleate. Figure 2E shows that flow cytometry- and NMR-based curves of dose dependency after overnight oleate–BSA loading are in close agreement.
Figure 2. Flow citometry and H-NMR of LD loaded cells.
Flow cytometry dot plots of control HL-1 cardiomyocytes (A) and HL-1 cardiomyocytes treated overnight with 0.2 mmol l−1 oleate–BSA (B). Spectra in C and D correspond to control HL-1 cells and HL-1 cells loaded with 0.2 mmol l−1 oleate–BSA, respectively, and were acquired with a NOESY presat one-dimenstional sequence. Vertical scale in C is increased twofold. E shows the increase in intracellular lipids in response to varying overnight loading concentrations of oleate–BSA measured by flow cytometry (▪) and lipid-to-creatine ratio (◊) in the NMR spectra. F shows an NMR spectrum of an oleate standard acquired with the same sequence as C and D, in which ‘a’ indicates protons from methyl groups of fatty acyl chains –CH3, ‘b’ methylene protons –CH2–, ‘c’ methylene protons close to the ester bond O=C–CH2–CH2–, ‘d’ methylene groups close to a double bond –CH2–CH= and ‘e’ methylene next to the ester bond O=C–CH2–.
One-dimensional 1H HR-MAS NMR spectra of HL-1 cardiomyocytes loaded overnight with 0.2 mmol l−1 oleate–BSA showed a severalfold increase in the lipid peaks compared with control cells (Fig. 2C and D). This lipid pattern was similar to what was found in liquid state NMR spectra of a myeloma-derived cell line loaded with oleate–BSA (Callies et al. 1993).
Intracellular lipid droplets and tolerance to energy deprivation
The presence of LD induced by incubation of the cells with oleate–BSA complex had a significant protective effect against cell death both in adult rat cardiomyocytes and in HL-1 cardiomyocytes exposed to simulated ischaemia–reperfusion (Fig. 3). This protective effect was concentration dependent, increasing between 0.05 and 0.5 mmol l−1 oleate (Fig. 3B). The reduction in cell death reached 34 and 40% of that observed in control cells supplemented with 0.2 and 0.5 mmol l−1 oleate, respectively. In the case of freshly isolated cardiomyocytes, protection reached 25% when supplemented with 0.2 mmol l−1 oleate; there was no effect of oleate on cell survival during the ischaemic period, which reached 13% for freshly isolated cardiomyocytes and was below the detection threshold in HL-1 cells. There was a strong correlation between intracellular LD, as detected by flow cytometry, and cellular protection (r2= 0.68, P < 0.01). When HL-1 cardiomyocytes were pre-incubated with palmitate–BSA, a trend towards a protective effect against simulated ischaemia–reperfusion was observed, similar to that obtained with oleate–BSA, but it did not reach a statistically significant level.
Figure 3. Effect of LD on ischemia–reperfusion induced cell death.
A, bar graph showing LDH release during normoxia (Nx), simulated ischaemia (SI) and reperfusion (R) in adult rat cardiomyocytes loaded with BSA (open bars) or oleate–BSA (grey bars). B, cellular death during reperfusion in HL-1 cardiomyocytes after different overnight oleate loading concentrations. Results are expressed as the percentage of cell death compared with control cultures. *p < 0.05 compared to BSA loaded cells.
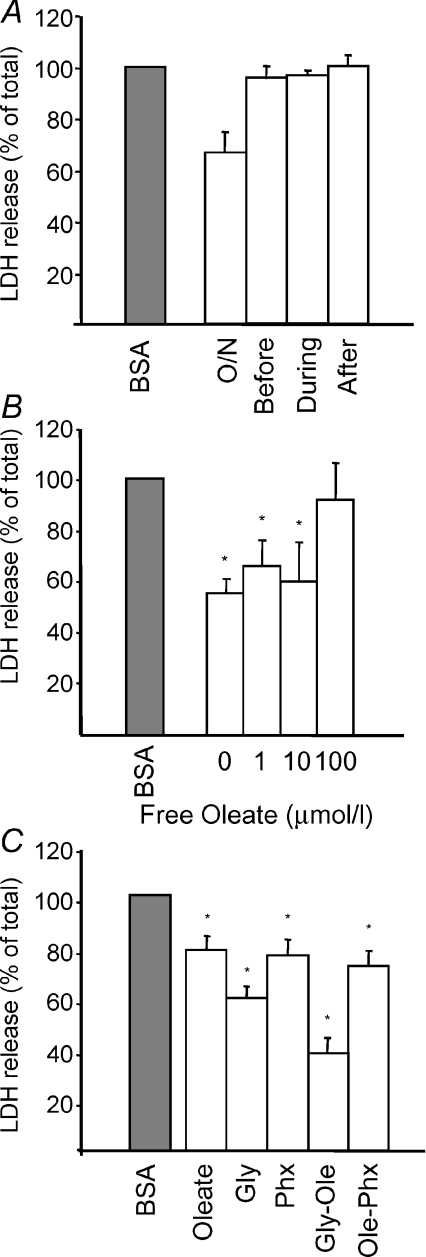
In order to establish whether the protective effect of LD was due to the presence of residual oleate or to the intracellular LD, we performed experiments in which 0.2 mmol l−1 oleate–BSA complex was added either during the simulated ischaemia and reperfusion periods or for 30 min prior to the ischaemia–reperfusion period. When using such short periods of incubation, there were no LD visible within the cells. Cell death rates were 95, 96 and 100% of control values, respectively, compared with 66% observed in cells treated overnight (Fig. 4A). These results clearly suggest that the protection obtained in HL-1 cardiomyocytes loaded with oleate–BSA is the result of the intracellular accumulation of LD and not of the presence of free fatty acids.
Figure 4. Effect of different interventions on reperfusion induced cell death.
A, LDH release in HL-1 cells after simulated ischaemia–reperfusion when 0.2 mmol l−1 oleate–BSA was added 30 min before ischaemia (before), during ischaemia (during) or during reperfusion (after) compared with control BSA-loaded cells (grey bar) and cells loaded overnight with oleate–BSA (O/N). B, cell death at increasing concentrations of free oleate added during reperfusion in cells when lipid droplets had been induced by overnight incubation with oleate–BSA. C, cell death during reperfusion, comparing the effects of LD (overnight oleate–BSA loading), 5 mmol l−1 glycine to inhibit mitochondria transition pore opening, LD and glycine and cells with LD and perhexiline to inhibit the use of fatty acids as energy source. *P < 0.05 compared with control BSA-loaded cells (grey bar).
Mechanism of the cardioprotective effect of intracellular lipid droplets
To investigate whether sequestration of free fatty acids into LD is one of the mechanisms by which LD could exert its protection against cell death, we performed additional simulated ischaemia–reperfusion experiments in the presence of free oleate (without BSA) during the reperfusion period in cells treated overnight with oleate–BSA. The protection offered by LD decreased with increasing concentrations of free oleate added during reperfusion (Fig. 4B).
Glycine, a drug that has been described to exert a strong protective effect against immediate reperfusion-induced cell death through the inhibition of the mitochondrial permeability transition pore (Ruiz-Meana et al. 2004), was used to investigate the potential mechanism of protection of LD. The results show that the protection afforded by glycine was more potent than that of LD, and both interventions appeared to have an additive effect. In the case of cells treated with perhexiline as well as BSA–oleate during the overnight incubation, protection was similar to that of cells treated with BSA–oleate alone (Fig. 4C).
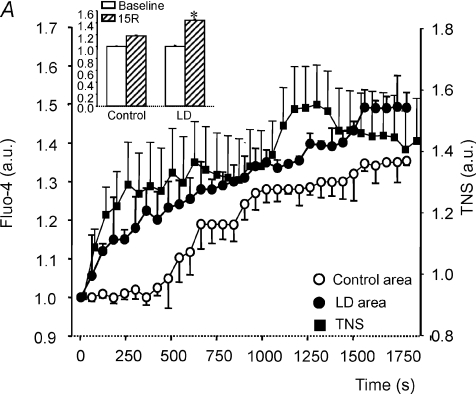
Since LD are able to sequester free fatty acids, we investigated whether free cytosolic Ca2+, one of the main determinants of reperfusion injury, could also get trapped by lipid vesicles. In HL-1 cardiomyocytes submitted to simulated ischaemia–reperfusion, the areas corresponding to LD within the cells experienced a progressive and sustained increase in the fluo-4 fluorescence, reflecting an increase in Ca2+ concentration, whereas in the cytosolic, LD-free areas, the increase in fluo-4 was substantially attenuated (Fig. 5A). In TNS-labelled HL-1 cardiomyocytes, there was a steady increase in fluorescence during the first 20 min of simulated ischaemia, levelling thereafter (Fig. 5A), probably as a result of saturation of Ca2+-binding capacity. This result further supports the idea that LD can bind Ca2+, most probaby to the phospholipids that form the outer layer of LD or to the proteins found there. Interestingly, the onset of Ca2+ increase occurred earlier in the LD areas compared with the cytosolic areas (Fig. 5A). Upon reperfusion, the Ca2+ concentration within LD areas remained higher than that measured in the cytosolic areas of the same cells (Fig. 5A).
Figure 5. Effect of LD on Ca2+ overload during ischemia–reperfusion.
A, increase in Ca2+ concentration in HL-1 cardiomyocytes submitted to 30 min of simulated ischaemia and 15 min of reperfusion, measured by changes in fluo-4 fluorescence in areas of LD (•), previously defined by light microscopy, versus cytosolic areas devoid of LD (○) within the same cells; the inset represents the relative Ca2+ increase at the end of reperfusion (15R) in those areas. Filled squares indicate whole-cell TNS fluorescence. B, cytosolic area of control cells versus cytosolic area of cells containing LD throughout simulated ischaemia (SI) and reperfusion (R) periods. Results are expressed as means ±s.e.m. of n= 10–14 cells in each group.
This effect of LD on Ca2+ accumulation was associated with a reduction in the global Ca2+ increase in the cytosolic compartment throughout simulated ischaemia in cells containing LD compared with control cells without LD (Fig. 5B), and with a subsequent reduced cytosolic Ca2+ overload upon reperfusion.
Discussion
The present study shows that the induction of intracellular LD in cardiomyocytes (freshly isolated and HL-1 cardiomyocytes) is associated with a significant reduction in cell death after transient simulated ischaemia–reperfusion. This protective effect decreased upon the addition of increasing concentrations of free fatty acids. Free Ca2+ concentration in the cell areas corresponding to LD experienced a sustained and significant increase throughout the simulated ischaemia period and remained high upon reperfusion, whereas in the lipid-free cytosolic areas, Ca2+ rise was attenuated. As a consequence, cells presenting LD had an attenuated cytosolic Ca2+ overload during simulated ischaemia–reperfusion. These results are consistent with the hypothesis that LD may have a protective effect against cell death by a mechanism involving sequestration of both toxic free fatty acids and free Ca2+.
Accumulation of lipid droplets in cultured cells
Accumulation of LD in adult cardiomyocytes was successfully achieved by either short (4 h) or overnight incubation with oleate–BSA complex. de Vries et al. (1997) showed a similar LD accumulation pattern in neonatal rat ventricular myocytes after 48 h incubation with an oleate–BSA. These authors also found an increase in triacyglycerol when cardiomyocytes were supplemented with mono-unsaturated fatty acids.
Oleate–BSA loading up to a concentration of 0.5 mmol l−1 oleate does not seem to have any detrimental effect on HL-1 cardiomyocytes because there were no morphological differences and no reduction in the total LDH release (reflecting sarcolemmal integrity) with respect to control cells. Nevertheless, clear morphological differences and total LDH reduction was observed at concentrations of 2 mmol l−1 and higher. The large variation observed in cell protection when the oleate–BSA loading was 1 mmol l−1 (Fig. 3B) could be due to the fact that some detrimental effects start to be apparent at this concentration. Hence, we consider a safe loading concentration of oleate to be 0.5 mmol l−1 or lower, in close agreement with previously published studies (Callies et al. 1993; de Vries et al. 1997; Listenberger et al. 2003).
The 1H-NMR pattern of HL-1 cardiomyocytes loaded with oleate–BSA was very similar to what has been reported for the area of mild ischaemia in dog hearts (Miyazaki et al. 1987; Straeter-Knowlen et al. 1996). Although hypoxia per se can induce the accumulation of LD within the cells, the quantity of LD and intensity 1H-NMR lipid signals in the case of overnight hypoxia was lower than in the case of oleate–BSA-loaded cells. Accumulation of LD due to a hypoxic environment could be clinically relevant during episodes of transient myocardial ischaemia secondary to coronary occlusion; unfortunately, it is not possible to obtain LD-free hypoxic cells, which precludes investigation of the effect of BSA–oleate loading in those cells however interesting it may be.
Lipid droplets and cell survival during ischaemia–reperfusion
In the present study, the presence of NMR-visible LD induced by the overnight incubation with BSA–oleate had a protective effect against cell death following simulated ischaemia–reperfusion, and the degree of protection was dependent on the oleate loading dose. The protective effect seen after the overnight addition of oleate to the cells was the result of the intracellular LD formation and not of the fatty acids used during the incubation because when oleate, but not LD, was present during reperfusion no protective effect could be seen.
It has been shown that in non-overloading conditions, LD tend to accumulate during growth arrest without inducing an increase in total lipid content (Quintero et al. 2007). Nuclear magnetic resonance-visible LD have been suggested to be markers of growth-arrested neural progenitor cells (Manganas et al. 2007). Recent reports showed that, during growth, LD are not located within but external to the endoplasmic reticulum membranes (Robenek et al. 2006) and also associated to plasma membranes, which facilitates lipid influx (Robenek et al. 2005). Also, LD can grow in size by the action of diacylglycerol acyltransferase 2 that is present inside the LD (Kuerschner et al. 2008).
It is likely that LD formation constitutes an endogenous mechanism of protection because LD are metabolically active and follow a reversible accumulation pattern (Madden et al. 1993). Diacylglycerol acyltransferase 2 within LD could incorporate acylated free fatty acids into diacylglycerol to form triacylglycerol without the need of endoplasmic reticulum or cytoplasmic membrane involvement. This mechanism would require energy, explaining why LD are found mainly at the periphery of the areas at risk after myocardial infarction. Disappearance of ischaemia should then be followed by reversion to the normal condition; in contrast, lipotoxicity associated with certain conditions (e.g. obesity) is characterized by a marked chronic increase in the cellular lipid content (McGavock et al. 2006).
Mechanism of protection
Previous studies have shown that free fatty acids are toxic to the cell because of their protonophoric action (Wojtczak & Schonfeld, 1993) and proapoptotic effect (Listenberger et al. 2003). When free palmitate was added to HL-1 cardiomyocytes during reperfusion there was an increase in cell death, confirming that in our model, the presence of saturated free fatty acids is also toxic.
The observation that the protection afforded by intracellular LD decreased with increasing concentration of free oleate added during reperfusion (Fig. 4B) could suggest that the main mechanism of action of LD is the sequestration of free fatty acids into their hydrophobic core, although with a limited capacity, which would result in an exhaustion of their protective effect at high free fatty acid concentrations. This mechanism of action would reduce the toxic load of free fatty acids in a similar way to what has been described for Chinese Hamster Ovary (CHO) cells, in which LD protected against apoptotic death by channelling palmitate into triglyceride pools and removing it from the pathways involved in the apoptotic cascade (Listenberger et al. 2003). Thus, the mechanisms of sequestration of free fatty acids proposed for the protective effect of LD against apoptotic cell death would also apply for necrosis induced by an ischaemic insult.
The toxicity of free fatty acids is well established, although the mechanisms involved are not completely understood. It has been described that they can promote the opening of the mitochondrial permeability transition pore (Wieckowiski & Wojtczak, 1998; Wieckowiski et al. 2000). If this was the case in our model, the protection afforded by LD against reperfusion-induced cell death could be seen downstream of free fatty acid sequestration. However, the experiments carried out with the permeability transition pore inhibitor glycine during reperfusion were not conclusive, since both interventions, addition of glycine and induction of LD accumulation, had additive protective effects against cell death, suggesting a rather independent mechanism of action.
Perhexiline shifts metabolism from free fatty acids to glucose utilization and has been used for the treatment of heart failure (Lee et al. 2005). In our hands, when it was used in combination with oleate–BSA there were no changes in lipid droplet loading or cell death compared with cells treated with oleate–BSA alone. This suggests that the protection against cell death offered by the presence of LD is not due to higher energy availability in lipid-loaded cells because it was not modified by inhibition of β-oxidation with perhexiline.
The deleterious effect of cytosolic Ca2+ rise during energy deprivation on the rate of cardiomyocyte death upon subsequent re-energization has been extensively documented and related to contractile activation (Garcia-Dorado et al. 1992; Piper et al. 1998) and calpain-mediated degradation of membrane cytoskeleton (Inserte et al. 2005). Previous studies have suggested the importance of the Ca2+-buffering role of intracellular organelles in shaping the spatio-temporal pattern of cytosolic Ca2+ rise and reducing cell death in cardiomyocytes (Bianchi et al. 2004; Ruiz-Meana et al. 2006). This buffering capacity is expected to play a relevant role under pathophysiological conditions, such as energy deprivation, in which cytosolic Ca2+ homeostasis is disrupted owing to an increase in intracellular Na+ and the reverse activity of the sarcolemmal Na+–Ca2+ exchanger (Ladilov et al. 1999). In the present study, accumulation of Ca2+ by LD, probably in the polar heads of the phopholipid layer surrounding the LD or in lipid droplet-associated proteins, may have indirect beneficial effects by reducing the detrimental consequences of cytosolic Ca2+ overload during the first minutes of reperfusion. Previous studies on neuroepithelial cells showed that LD also serve as Ca2+-storage and -releasing sites (Bush et al. 1992), further supporting the idea of LD being able to buffer cytosolic Ca2+ during simulated ischaemia–reperfusion.
The protective effect offered by LD may be endo-genously stimulated in certain conditions, such as during hypoxia, in which spontaneous formation of LD is observed, and it could be speculated that this mechanism may contribute to the survival of the cells from the area at risk in the ischaemic myocardium.
In conclusion, we have shown that LD are protective against ischaemia–reperfusion injury in freshly isolated adult rat cardiomyocytes and reproduced the findings in a cell culture model. The primary mechanism for the protection afforded by LD is likely to be the reduction of cytosolic free fatty acids and Ca2+. Hence, the presence of intracellular LD could be described as an endogenous mechanism of protection for at risk but still viable cells.
Acknowledgments
Financial support for this work was obtained from the Comisión Interministerial de Ciencia y Tecnología (CICYT-SAF/2002-0759) and the Spanish Ministry of Health (Red de Enfermedades Cardiovasculares RECAVA) and FIS-P1060996.
References
- Awonusonu F, Srinivasan S, Strange J, Al-Jumaily W, Bruce MC. Developmental shift in the relative percentages of fibroblast subsets: role of apoptosis postseptation. Am J Physiol Lung Cell Mol Physiol. 1999;277:L848–L859. doi: 10.1152/ajplung.1999.277.4.L848. [DOI] [PubMed] [Google Scholar]
- Barba I, Cabanas ME, Arus C. The relationship between nuclear magnetic resonance-visible lipids, lipid droplets, and cell proliferation in cultured C6 cells. Cancer Res. 1999;59:1861–1868. [PubMed] [Google Scholar]
- Barrabes JA, Garcia-Dorado D, Ruiz-Meana M, Piper HM, Solares J, Gonzalez MA, Oliveras J, Herrejon MP, Soler J. Myocardial segment shrinkage during coronary reperfusion in situ. Relation to hypercontracture and myocardial necrosis. Pflugers Arch. 1996;431:519–526. doi: 10.1007/BF02191898. [DOI] [PubMed] [Google Scholar]
- Bianchi K, Rimessi A, Prandini A, Szabadkai G, Rizzuto R. Calcium and mitochondria: mechanisms and functions of a troubled relationship. Biochim Biophys Acta. 2004;1742:119–131. doi: 10.1016/j.bbamcr.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Bush KT, Lee H, Nagele RG. Lipid droplets of neuroepithelial cells are a major calcium storage site during neural tube formation in chick and mouse embryos. Experientia. 1992;48:516–519. doi: 10.1007/BF01928178. [DOI] [PubMed] [Google Scholar]
- Callies R, Sri-Pathmanathan RM, Ferguson DYP, Brindle KM. The appearance of neutral lipid signals in the 1H NMR spectra of a myeloma cell line correlates with the induced formation of cytoplasmic lipid droplets. Mag Res Med. 1993;29:546–550. doi: 10.1002/mrm.1910290418. [DOI] [PubMed] [Google Scholar]
- Claycomb WC, Lanson NA, Jr, Stallworth BS, Egeland DB, Delcarpio JB, Bahinksy A, Izzo NJ., Jr HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci USA. 1998;95:2979–2984. doi: 10.1073/pnas.95.6.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries JE, Vork MM, Roemen THM, de Jong YF, Clutjens JPM, van de Vusse GJ, Bilsen M. Saturated but not mono-unsaturated fatty acids induce apoptotic cell death in neonatal rat ventricular myocytes. J Lipid Res. 1997;38:1384–1394. [PubMed] [Google Scholar]
- Evanochko WT, Reeves RC, Sakai TT, Canby RC, Pohost GM. Proton NMR spectroscopy in myocardial ischemic insult. Mag Res Med. 1987;5:23–31. doi: 10.1002/mrm.1910050104. [DOI] [PubMed] [Google Scholar]
- Garcia-Dorado D, Théroux P, Duran JM, Solares J, Alonso J, Sanz E, Munoz R, Elizaga J, Botas J, Fernández-Avilés F, Soriano J, Esteban E. Selective inhibition of the contractile apparatus. A new approach to modification of infarct size, infarct composition, and infarct geometry during coronary artery occlusion and reperfusion. Circulation. 1992;85:1160–1174. doi: 10.1161/01.cir.85.3.1160. [DOI] [PubMed] [Google Scholar]
- Greve G, Rotevatn S, Svendby K, Grong K. Early morphologic changes in cat heart muscle cells after acute coronary artery occlusion. Am J Pathol. 1990;136:273–283. [PMC free article] [PubMed] [Google Scholar]
- Hendrickson SC, St Louis JD, Lowe JE, Abdel-aleem S. Free fatty acid metabolism during myocardial ischemia and reperfusion. Mol Cell Biochem. 1997;166:85–94. doi: 10.1023/a:1006886601825. [DOI] [PubMed] [Google Scholar]
- Hernando V, Rieutord A, Brion F, Prognon P. Evidence for lipids-calcium ions interactions using fluorescent probing in paediatric nutrition admixtures. Talanta. 2003;60:543–554. doi: 10.1016/S0039-9140(03)00104-8. [DOI] [PubMed] [Google Scholar]
- Inserte J, Garcia-Dorado D, Hernando V, Soler-Soler J. Calpain-mediated impairment of Na+/K+-ATPase activity during early reperfusion contributes to cell death after myocardial ischemia. Circ Res. 2005;97:465–473. doi: 10.1161/01.RES.0000181170.87738.f3. [DOI] [PubMed] [Google Scholar]
- Jodalen H, Stangeland L, Grong K, Vik-Mo H, Lekven J. Lipid accumulation in the myocardium during acute regional ischemia in cats. J Mol Cell Cardiol. 1985;17:973–980. doi: 10.1016/s0022-2828(85)80077-8. [DOI] [PubMed] [Google Scholar]
- Kuerschner L, Moessinger C, Thiele C. Imaging of lipid biosynthesis: how a neutral lipid enters lipid droplets. Traffic. 2008;9:338–352. doi: 10.1111/j.1600-0854.2007.00689.x. [DOI] [PubMed] [Google Scholar]
- Ladilov Y, Haffner S, Balser-Schafer C, Maxeiner H, Piper HM. Cardioprotective effects of KB-R7943: a novel inhibitor of the reverse mode of Na+/Ca2+ exchanger. Am J Physiol Heart Circ Physiol. 1999;276:H1868–H1876. doi: 10.1152/ajpheart.1999.276.6.H1868. [DOI] [PubMed] [Google Scholar]
- Lee L, Campbell R, Scheuermann-Freestone M, Taylor R, Gunaruwan P, Williams L, Ashrafian H, Horowitz J, Frasesr AG, Clarke K, Frenneaux M. Metabolic modulation with perhexiline in chronic heart failure: a randomized, controlled trial of short-term use of a novel treatment. Circulation. 2005;112:3280–3288. doi: 10.1161/CIRCULATIONAHA.105.551457. [DOI] [PubMed] [Google Scholar]
- Listenberger LL, Han X, Lewin SE, Cases S, Farese RV, Ory DS, Schaffer JE. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci USA. 2003;100:3077–3082. doi: 10.1073/pnas.0630588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGavock JM, Victor RG, Unger RH, Szczepaniak LS. Adiposity of the heart, revisited. Ann Intern Med. 2006;144:517–524. doi: 10.7326/0003-4819-144-7-200604040-00011. [DOI] [PubMed] [Google Scholar]
- Madden MC, van Winkle WB, Vaughn JM, Pohost GM, Wolkowicz PE. Morphometric analysis demonstrates that metabolically active cardiac triglycerides are 1H NMR visible. J Mol Cell Cardiol. 1993;25:587–597. doi: 10.1006/jmcc.1993.1068. [DOI] [PubMed] [Google Scholar]
- Manganas LN, Zhang X, Li Y, Hazel RD, Smith SD, Wagshul ME, Henn F, Benveniste H, Djuric PM, Enikolopov G, Maletic-Savatic M. Magnetic resonance spectroscopy identifies neural progenitor cells in the live human brain. Science. 2007;318:980–985. doi: 10.1126/science.1147851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirabet M, Garcia-Dorado D, Ruiz-Meana M, Barrabés JA, Soler-Soler J. Thrombin increases cardiomyocyte acute cell death after ischemia and reperfusion. J Mol Cell Cardiol. 2005;39:277–283. doi: 10.1016/j.yjmcc.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Miyazaki S, Fujiwara H, Onodera T, Kihara Y, Matsuda M, Wu D, Nakamura Y, Kumada T, Sasayama S, Kawai C, Hamashima Y. Quantitative analysis of contraction band and coagulation necrosis after ischemia and reperfusion in the porcine heart. Circulation. 1987;75:1074–1082. doi: 10.1161/01.cir.75.5.1074. [DOI] [PubMed] [Google Scholar]
- Murphy DJ, Vance J. Mechanisms of lipid-body formation. Trends Biochem Sci. 1999;24:109–115. doi: 10.1016/s0968-0004(98)01349-8. [DOI] [PubMed] [Google Scholar]
- Pérez Y, Laherech H, Cabañas ME, Barnadas R, Sabés M, Rémy C, Arús C. Measurement by nuclear magnetic resonance diffusion of the dimensions of the mobile compartment in C6 cells. Cancer Res. 2002;62:5672–5677. [PubMed] [Google Scholar]
- Piper HM, Garcia-Dorado D, Ovize M. A fresh look at reperfusion injury. Cardiovasc Res. 1998;38:291–300. doi: 10.1016/s0008-6363(98)00033-9. [DOI] [PubMed] [Google Scholar]
- Quintero M, Cabanas ME, Arús C. A possible cellular explanation for the NMR-visible mobile lipid (ML) changes in cultured C6 glioma cells with growth. Biochim Biophys Acta. 2007;1771:31–44. doi: 10.1016/j.bbalip.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Robenek H, Hofnagel O, Buers I, Robenek MJ, Troyer D, Severs NJ. Adipophilin-enriched domains in the ER membrane are sites of lipid droplet biogenesis. J Cell Sci. 2006;119:4215–4224. doi: 10.1242/jcs.03191. [DOI] [PubMed] [Google Scholar]
- Robenek H, Robenek MJ, Buers I, Lorkowski S, Hofnagel O, Troyer D, Severs NJ. Lipid droplets gain PAT family proteins by interaction with specialized plasma membrane domains. J Biol Chem. 2005;280:26330–26338. doi: 10.1074/jbc.M413312200. [DOI] [PubMed] [Google Scholar]
- Ruiz-Meana M, Abellan A, Miro-Casas E, Garcia-Dorado D. Opening of mitochondrial transition pore induces hypercontracture in Ca2+ overloaded cardiac myocytes. Basic Res Cardiol. 2007;102:542–552. doi: 10.1007/s00395-007-0675-y. [DOI] [PubMed] [Google Scholar]
- Ruiz-Meana M, García-Dorado D, González MA, Barrabés JA, Soler-Soler J. Effect of osmotic stress on sarcolemmal integrity of isolated cardiomyocytes following transient metabolic inhibition. Cardiovasc Res. 1995;30:64–69. [PubMed] [Google Scholar]
- Ruiz-Meana M, Garcia-Dorado D, Miró-Casas E, Abellán A, Soler-Soler J. Mitochondrial Ca2+ uptake during simulated ischemia does not affect permeability transition pore opening upon simulated reperfusion. Cardiovasc Res. 2006;71:715–724. doi: 10.1016/j.cardiores.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Ruiz-Meana M, Pina P, Garcia-Dorado D, Rodríguez-Sinovas A, Barba I, Miró-Casas E, Mirabet M, Soler-Soler J. Glycine protects cardiomyocytes against lethal reperfusion injury by inhibiting mitochondrial permeability transition. J Physiol. 2004;558:873–882. doi: 10.1113/jphysiol.2004.068320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarri E, Garcia-Dorado D, Abellan A, Soler-Soler J. Effect of hypoxia, glucose deprivation and acidosis on phosphatidilcholine synthesis in HL-1 cardiomyocytes. CTP:phosphocholine cytidylyltransferase activity correlates with sarcolemal disruption. Biochem J. 2006;394:325–334. doi: 10.1042/BJ20050834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer JE. Lipotoxicity: when tissues overeat. Curr Opin Lipidol. 2003;14:281–287. doi: 10.1097/00041433-200306000-00008. [DOI] [PubMed] [Google Scholar]
- Siegmund B, Koop A, Klietz T, Schwartz P, Piper HM. Sarcolemmal integrity and metabolic competence of cardiomyocytes under anoxia-reoxygenation. Am J Physiol Heart Circ Physiol. 1990;258:H285–H291. doi: 10.1152/ajpheart.1990.258.2.H285. [DOI] [PubMed] [Google Scholar]
- Straeter-Knowlen IM, Evanochko WT, den Hollander JA, Wolkowicz PE, Balschi JA, Caulfield JB, Ku DD, Pohost GM. 1H NMR spectroscopic imaging of myocardial triglycerides in excised dog hearts subjected to 24 hours of coronary occlusion. Circulation. 1996;93:1464–1470. doi: 10.1161/01.cir.93.7.1464. [DOI] [PubMed] [Google Scholar]
- Suzuki J, Shen W-J, Nelson BD, Patel S, Veerkamp JH, Selwood SP, Murphy GM, Reaven E, Kramer FB. Absence of cardiac lipid accumulation in transgenic mice with heart-specific HSL overexpresion. Am J Physiol Endocrinol Metab. 2001;281:E857–E866. doi: 10.1152/ajpendo.2001.281.4.E857. [DOI] [PubMed] [Google Scholar]
- Wieckowiski MR, Brdiczka D, Wojtczak L. Long-chain fatty acids promote opening of the reconstituted mitochondrial permeability transition pore. FEBS Lett. 2000;484:61–64. doi: 10.1016/s0014-5793(00)02127-x. [DOI] [PubMed] [Google Scholar]
- Wieckowiski MR, Wojtczak L. Fatty acid-induced uncoupling of oxidative phosphorylation is partly due to the opening of the mitochondrial permeability transition pore. FEBS Lett. 1998;423:339–342. doi: 10.1016/s0014-5793(98)00118-5. [DOI] [PubMed] [Google Scholar]
- Wojtczak L, Schonfeld P. Effect of fatty acids on energy coupling processes in mitochondria. Biochim Biophys Acta. 1993;1183:41–57. doi: 10.1016/0005-2728(93)90004-y. [DOI] [PubMed] [Google Scholar]