Abstract
The transcription factors Emx2 and Pax6 are expressed in the proliferating zones of the developing rodent neocortex, and gradients of expression interact in specifying caudal and rostral identities. Pax6 is also involved in corticoneurogenesis, being expressed by radial glial progenitors that give rise to cells that also sequentially express Tbr2, NeuroD and Tbr1, genes temporally downstream of Pax6. In this study, using in situ hybridization, we analysed the expression of EMX2, PAX6, TBR2, NEUROD and TBR1 mRNA in the developing human cortex between 8 and 12 postconceptional weeks (PCW). EMX2 mRNA was expressed in the ventricular (VZ) and subventricular zones (SVZ), but also in the cortical plate, unlike in the rodent. However, gradients of expression were similar to that of the rodent at all ages studied. PAX6 mRNA expression was limited to the VZ and SVZ. At 8 PCW, PAX6 was highly expressed rostrally but less so caudally, as has been seen in the rodent, however this gradient disappeared early in corticogenesis, by 9 PCW. There was less restricted compartment-specific expression of TBR2, NEUROD and TBR1 mRNA than in the rodent, where the gradients of expression were similar to that of PAX6 prior to 9 PCW. The gradient disappeared for TBR2 by 10 PCW, and for NEUROD and TBR1 by 12 PCW. These data support recent reports that EMX2 but not PAX6 is more directly involved in arealization, highlighting that analysis of human development allows better spatio-temporal resolution than studies in rodents.
Keywords: arealization, development, neurogenesis, subventricular zone
Introduction
Cortical arealization controls the differentiation of the early embryonic cortical primordium, a neuroepithelial sheet that lacks any apparent regional-specific morphology or function, into the complex, regionally diverse mature cerebral cortex. Although stimuli arising from subcortical structures affect cortical differentiation, cortical-intrinsic influences drive the early phases of arealization. The genes controlling neocortical arealization are assumed to be expressed in graded or restricted patterns in order to be able to specify regional identities (Job & Tan, 2003; Mallamaci & Stoykova, 2006).
Two genes meeting these criteria in the rodent are Emx2 and Pax6 (O’Leary & Nakagawa, 2002). Pax6 encodes a protein containing a paired domain and a homeodomain, and is mutated in patients with aniridia and in the mouse mutant small eye, sey (Ton et al., 1991 1992), while Emx2 is a homeobox-containing gene encoding a transcription factor that is one homologue of the anterior-head-specific Drosophila empty spiracles head gene (Simeone et al., 1992). In mice, both Pax6 and Emx2 expression are found in the telencephalon during neurogenesis (E10.5–18.5; Walther & Gruss, 1991; Simeone et al., 1992). In Pax6 sey/sey mutant mice, studies of the expression of areal markers such as Cad6, Cad8 and Id2, have indicated a reduction in size of rostro-lateral areas and expansion of caudo-medial regions (Bishop et al., 2000 2002). In Emx2 null mice, a reduction in size of caudal/medial cortical regions, together with an enlargement of those with rostral/lateral identity (Bishop et al., 2000) is seen. Emx2 shows a complementary expression pattern to Pax6, and they downregulate each other in the cortex (Muzio et al., 2002). Pax6 also controls glutamatergic neuronal cell fate in rodents (Kroll & O’Leary, 2005), and that the expression pattern of the T-box domain containing transcription factor Tbr2, a gene expressed by basal progenitors in the subventricular zone (SVZ), exhibits a similar high rostro-lateral, low caudo-medial expression pattern as that of Pax6 (Bulfone et al., 1999). Glutamatergic projection neurons and their progenitors sequentially express Pax6 followed by Tbr2 during development. This sequence of expression is followed by the basic helix-loop-helix, pro-neural gene NeuroD and the Tbr2-related layer VI marker, Tbr1 (Hevner et al., 2006).
In the rodent, Emx2 and Pax6 are expressed in the ventricular zone (VZ) of the dorsal telencephalon that gives rise to cortical neurons, along two complementary tangential gradients. Although many mechanisms involved in rodent cortical development are shared in common with humans (Monuki & Walsh, 2001), the human cortex is composed of different and more complex local area identities that reflect differences in structure and function (Hill & Walsh, 2005). These differences need to be considered when extending findings from rodents to humans, and an important first step is to analyse expression patterns directly in humans. Therefore we analysed the temporal and spatial mRNA expression patterns of EMX2 and PAX6, as well TBR2, NEUROD and TBR1 from 8–12 postconceptional weeks (PCW). This relates to an early period of cortical development before innervation of thalamocortical fibres (Kostovic & Rakic, 1990; Meyer et al., 2000).
Materials and methods
All reagents were purchased from VWR International (Lutterworth, UK) unless otherwise stated.
Human tissue
Brains were dissected from human foetal and embryonic terminations of pregnancies obtained from the MRC-Wellcome Trust Human Developmental Biology Resource at Newcastle University (HDBR, http://www.hdbr.org). Tissue from ages between 8 and 12 PCW (8 PCW, n= 5; 9 PCW, n= 3; 10–10.5 PCW, n= 3; 12–12.5 PCW, n= 3) were used with maternal consent and approval of the local University Hospital Ethical Review Committees. Age was estimated from measurements of foot length and heel to knee length. These were compared with a standard growth chart (Hern, 1984). Prior to sectioning, brains were fixed for 24 h at 4°C in phosphate-buffered saline (PBS) containing 4% paraformaldehyde (PFA), and then transferred to 70% ethanol for storage at 4°C. Samples were processed and then embedded in paraffin. Eight-micron-thick sections were cut, mounted on slides and used for in situ hybridization (ISH).
Probe manufacture
For PAX6 and EMX2, two sets of RNA probes were used yielding similar results. One set of RNA probes was used for each of TBR1, NEUROD and TBR2. In the case of PAX6 and EMX2, sense and antisense probes were synthesized by transcribing linearized plasmid (pGEM3Z) containing 1200-bp (Emx2) and 720-bp (Pax6) fragments [nucleotides 730–1930 of GenBank accession no. NM_004098NM_004098 (Emx2) and nucleotides 416–1136 of GenBank accession no. BC011953 (Pax6)] with T7 or SP6 RNA polymerases. Additionally, a DNA template for polymerase chain reaction (PCR) was prepared for the production of the second set of probes from a reverse transcription first-strand synthesis reaction from RNA that had already been extracted from a 5-mm slice dissected from the caudal part of a human foetal cortex (9 PCW). PCR was carried out using primers (MWG, Ebersberg, Germany) specific for either EMX2 or PAX6 flanked by consensus sequences for T7 (anti-sense primer) and SP6 (sense primer) RNA polymerases. Probes for TBR2, NEUROD and TBR1 were also manufactured in a similar manner by PCR from a cDNA template. The following primers were used (gene-specific sequence underlined): EMX1 T7 AS: 5′-TAA GTT AAT ACG ACT CAC TAT AGG GCG AGT CAT TGG AGG TGA CAT CGA TGT CC;EMX1 SP6 S: 5′-AAT ACG ATT TAG GTG ACA CTA TAG AAT ACC GCT GAC CGT GCA TCC GGC GCA C;EMX2 T7AS: 5′-TAA GTT AAT ACG ACT CAC TAT AGG GCG AGG CTG AGG CTG TGT GCC AGC TGC; EMX2 SP6S: AAT ACG ATT TAG GTG ACA CTA TAG AAT ACC AAG CGC TGC TTC ACC ATC GAG TC; PAX6 T7AS: TAA GTT AAT ACG ACT CAC TAT AGG GCG ATA GTG CAT GTT GTT CCA GGTT; PAX6 SP6S: AAT ACG ATT TAG GTG ACA CTA TAG AAT ACC TTC ACA TCT GGC TCC ATG TT;TBR1 T7AS: 5′-TAAGT TAA TAC GAC TCA CTA TAG GGC GA CAC CAT CTG CCC ATT GTT ATT TGA;TBR1 SP6S: 5′-AATACG ATT TAG GTG ACA CTA TAG AA TAC TAC CAA GGA GCT CCG TTC TAC CAG;TBR2 T7AS: 5′-TAAGT TAA TAC GAC TCA CTA TAG GGC GA CTA GTT TGT TGG TCC CAG GTT GCT;TBR2 SP6S: 5′-AATACG ATT TAG GTG ACA CTA TAG AA TAC AAT ACC AAC CCC GAC TGC ATA TTG;NEUROD T7AS: 5′-TAAGT TAA TAC GAC TCA CTA TAG GGC GA ATC TCC GAC AGA GCC CAG ATG TAG;NEUROD SP6S: 5′-AATACG ATT TAG GTG ACA CTA TAG AA TAC TGA CCA AAT CGT ACA GCG AGA GTG. The particular gene-specific regions were selected to ensure that standard PCR conditions could be used as follows: 40 cycles of 95°C 15 s, 65°C for 30 s and 72°C for 45 s. The PCR product was electrophoresed on a 1.5% agarose gel, and bands were cut out and purified with a gel extraction kit following manufacturers’ instructions (Qiagen, Hilden, Germany). Purified product was diluted in water (1: 100) and used as a template in a second round of PCR (with similar conditions). The PCR product was subjected to electrophoresis through a 1.5% agarose gel, bands were cut out and purified as before. Identity of the product was confirmed by sequencing. Seventy-five nanograms of purified PCR product or 1 μg of linearized plasmid served as a template for the labelling reaction. Digoxigenin (DIG)-labelled RNA probes were manufactured using a DIG RNA labelling kit according to manufacturers’ instructions (Roche, Lewes, UK). The labelled RNA was purified by centrifuging through ProbeQuant G-50 micro columns (Amersham Biosciences, Chalfont St Giles, UK). Labelling efficiency was determined with a dot blot with control labelled RNA (DIG labelling kit; Roche).
ISH
ISH was carried out as previously described (Moorman et al., 2001), with some modifications. Briefly, slides were de-waxed in xylene, gradually hydrated in decreasing ethanol concentrations before incubation for 8 min in proteinase K (20 μg/mL) at room temperature. Sections were fixed for 20 min in 4% PFA/PBS, washed in PBS, treated for 10 min in 0.1 m triethanolamine (Sigma-Aldrich, pH 8.0)/0.25% acetic anhydride/0.2% HCl, dehydrated in increasing concentrations of ethanol and air-dried by filtered air stream. Labelled probe (300 ng) was used per 100 μL Dig Easy Hyb Mix (Roche). Probe/Hyb Mix (200 μL) was used to cover each slide. Slides were incubated in a hybridization chamber overnight at 68°C, washed in 50% formamide/2× standard sodium citrate (SSC) for 20 min at 65°C, followed by four washes with decreasing SSC concentrations at 50°C (2, 2× SSC washes and 2, 0.2× SSC washes, the final at room temperature). After briefly washing in 0.1 m Tris (pH 7.6)/0.15 m NaCl (Buffer 1), and blocking 10% foetal calf serum (FCS; Invitrogen)/Buffer1 for 1 h, sections were incubated with anti-DIG antibody (Roche; diluted 1: 1000 in 2% FCS/Buffer 1) at 4°C overnight. The slides were washed in Buffer 1 for 6 × 30 min, and DIG antibody was visualized with NBT/BCIP solution (Roche; 20 μL/mL) in 0.1 m Tris (pH 9.5)/0.1 m NaCl (Buffer 2). Developing was stopped by rinsing slides in Buffer 2 then distilled H2O followed by 1%HCl/methanol and dH2O. Sections were mounted using Aquamount. Comparison of staining between sense and anti-sense probes was carried out to ensure specificity (see Supporting information, Fig. S1).
Immunohistochemistry
Paraffin sections were de-waxed in two changes of xylene and re-hydrated in decreasing concentrations of ethanol in water. Sections were then treated with 3% hydrogen peroxidase (Sigma-Aldrich) for 10 min, and boiled in 10 mm citrate buffer before incubation with primary antibody [Pax6: diluted 1: 300; Covance, Princeton NJ, USA; Emx2: 1: 200; Sigma-Aldrich; in 0.3% PBS with 0.3% Triton X-100 (PBS-T) and 3% horse serum; Vector Laboratories, Peterborough, England]. Sections were incubated in a moist chamber at 4°C overnight, washed in PBS-T and incubated with a corresponding biotinylated secondary antibody (Vector Laboratories; 1: 300 in PBS-T) at 4°C for 2 h. After a further PBS-T wash, slides were incubated for 1 h with streptavidin-horseradish peroxidase (Vector Laboratories; 1: 200 in PBS-T). Antibody–epitope interactions were visualized with 0.05% diaminobenzidine/0.003% hydrogen peroxidase in PBS (Sigma-Aldrich) for 10–20 min. Sections were then dehydrated in increasing ethanol concentrations, cleared in xylene and mounted using histamount (Vector Laboratories).
Results
The localization and gradient expression patterns of EMX2 and PAX6 mRNA were analysed by nonradioactive ISH (sense controls are shown in supporting Fig. S1). Furthermore, the localization and gradients of TBR2, NEUROD and TBR1, genes downstream of PAX6, which are thought to be important in the neurogenesis of cortical projection neurons, were also examined.
Laminar expression of EMX2 and PAX6 during early human cortical development
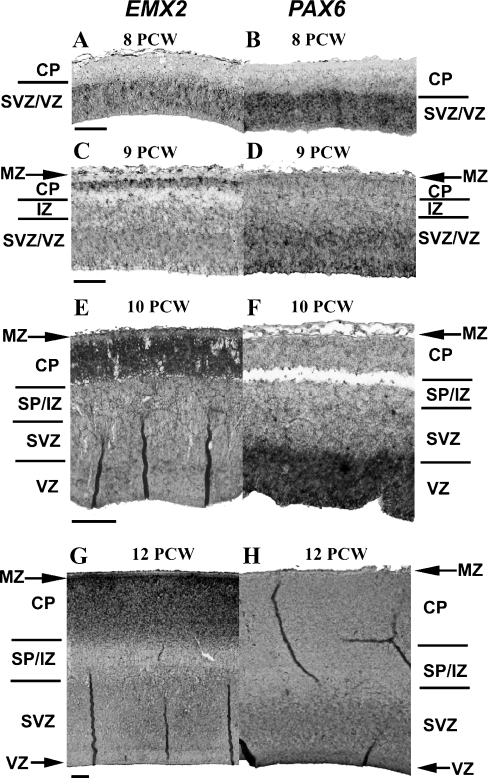
At 8 PCW, tissue ISH revealed expression of EMX2 and PAX6 in the VZ and SVZ of the dorsal forebrain (Fig. 1A and B); however, by 9 PCW, expression of EMX2 was also observed in the newly forming cortical plate (CP; Fig. 1C), where EMX1 was also found to be expressed (in supporting Fig. S2), while PAX6 remained in the proliferative layers (Fig. 1D). From 10 PCW, EMX2 expression had switched to be predominantly within the CP, although lower levels were detected within the VZ/SVZ (Fig. 1E). By 12 PCW intense staining for EMX2 mRNA was evident in the CP, particularly in the newly-forming outer layers, while showing a restricted pattern of distribution in the VZ (Fig. 1G). It is worth noting that EMX2 protein was generally found to localize in the same locations as the EMX2 RNA (in supporting Fig. S3). PAX6 RNA and protein expression was limited to the proliferative zones throughout all stages analysed (Fig. 1D,F and H; in supporting Fig. S3). Thus, EMX2 showed clear differences in its expression between human (where it is detected in both the proliferating zones and in the CP) and mouse (where it is detected primarily in proliferating zones). In contrast, PAX6 expression follows a more similar pattern to that seen in rodents throughout development, being mainly localized to the proliferative layers.
Fig. 1.
Laminar localization of EMX2 and PAX6 during early foetal development of the human neocortex. ISH revealed a changing laminar distribution of EMX2 mRNA between 8 and 12 postconceptional weeks (PCW). Expression was observed only in the subventricular and ventricular zones (SVZ/VZ) at 8 PCW (A). However, by 9 PCW expression was also noted in the cortical plate (CP) (C). Subsequently, expression of EMX2 intensified in the CP at 10 PCW (E) and 12 PCW (G), while still present in the SVZ and VZ at lower intensities. At 12 PCW, the highest level of EMX2 mRNA expression was found in the CP most proximal to the marginal zone (MZ). PAX6 was observed predominantly in the proliferative zones (SVZ/VZ) of the developing cortex (B, D, F, H). PAX6 mRNA was observed most intensely at 8 PCW in the VZ (A), after which a decrease was observed at 9 PCW (C). Staining intensity of PAX6 decreased at 10 PCW and 12 PCW (F and H, respectively) due to the decrease in the relative thickness of the VZ. Sections for EMX2 and PAX6 are taken from the caudal and rostral poles respectively. Scale bars: 100 μm (A, C); 200 μm (E, G). IZ, intermediate zone; SP, subplate.
EMX2 and PAX6 gradients in the developing human cortex
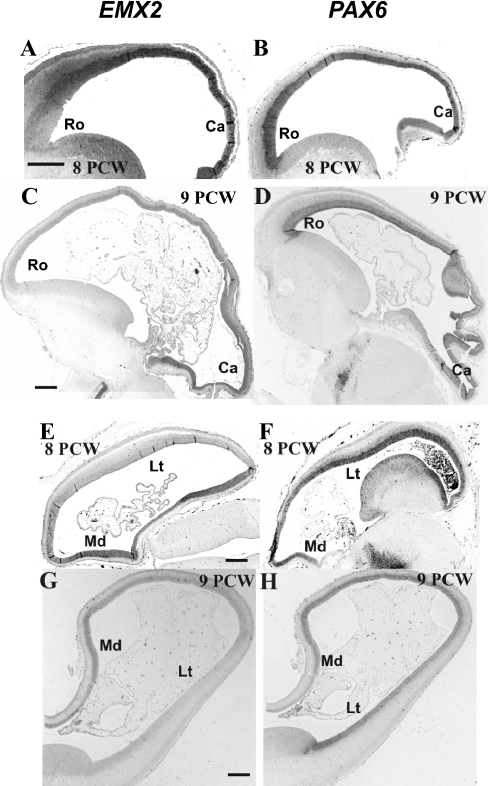
In rodents, Pax6 and Emx2 exhibit reciprocal and opposing caudal-rostral medial-lateral gradients that are instrumental in setting up cortical maps that control the expression of areal markers (Bishop et al., 2000). Here we show that at 8 PCW both genes display similar gradients of expression in human (Fig. 2A,B,E and F).
Fig. 2.
EMX2 and PAX6gradients in the developing human cortex. ISH analysis of sagittal sections at 8 postconceptional weeks (PCW; A, B) revealed that EMX2 and PAX6 mRNA were expressed in reciprocal rostro-caudal gradients. While EMX2 was expressed high caudally and low rostrally, PAX6 showed an opposite gradient of expression. In sagittal sections at 9 PCW (C, D), EMX2 mRNA maintained a similar caudal-rostral gradient to that seen at 8 PCW. The previously observed PAX6 expression gradient, however, disappeared. Similarly, in horizontal sections at 8 PCW, EMX2 and PAX6 exhibited reciprocal opposing medial-lateral gradients (E, F). EMX2 expression was observed high medially and low laterally with the opposite for PAX6. While the EMX2 gradient still persisted at 9 PCW (G), the PAX6 medial-gradient disappeared (H). Scale bars: 500 μm. Ca, caudal; Lt, lateral; Md, medial; Ro, rostral.
At 8 PCW ISH for PAX6 mRNA revealed a high rostral and lateral expression of the gene, with low caudal and medial expression (Fig. 2B and F). Conversely, EMX2 showed a high caudo-medial to low rostro-lateral gradient in expression (Fig. 2A and E). The observed gradient of PAX6 had disappeared by 9 PCW (Fig. 2D and H), although the EMX2 gradient was maintained at 9 weeks (Fig. 2C and G) and at all other stages analysed (data not shown), even though EMX2-expressing cells had predominately translocated to the CP.
Laminar expression of TBR2, NEUROD and TBR1 during early human cortical development
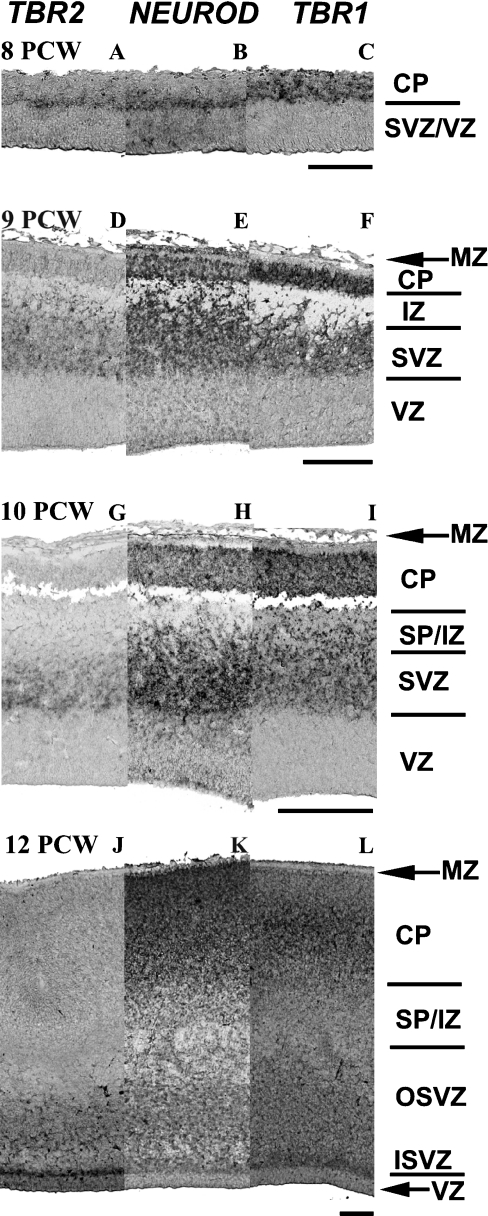
Tbr2, NeuroD and Tbr1 exhibit distinct compartmental-specific localizations in rodents (Hevner et al., 2006). Here we have examined whether similar localizations are observed during the development of the human cortex between 8 and 12 PCW. At 8 PCW, TBR2 RNA was located within a distinct layer between the VZ and the CP, probably corresponding to a newly formed SVZ. In a similar fashion, NEUROD was also found to be present in a discrete layer above the VZ, while TBR1 exhibited a more widespread expression pattern including the SVZ and CP (Fig. 3A–C). It was interesting to note that NEUROD was expressed in the CP at 8 PCW in more lateral locations (see Fig. 4E). By 9 PCW, TBR2 expression was predominantly limited to the SVZ, while both NEUROD and TBR1 exhibited expression additionally in the CP, where most of the expression of TBR1 was observed (Fig. 3D–F). These expression patterns were maintained for all three genes at 10 PCW (Fig. 3G–I). As the SVZ had expanded by 12 PCW, the majority of TBR2 expression remained at the border of the SVZ and VZ, with less intense staining throughout the SVZ but most intense within the inner SVZ (Fig. 3J). NEUROD and TBR1 were both mainly localized to the CP; however, both were expressed at the border of the SVZ and VZ and throughout the SVZ at lower levels (Fig. 3K and L). Taken together with the laminar localization of PAX6, these data show that although TBR2, NEUROD and TBR1 exhibit some degree of compartmental-specific expression, together with PAX6, all four were expressed within the SVZ during early human corticogenesis, unlike in rodents where Tbr1 is absent (Hevner et al., 2006).
Fig. 3.
Laminar localization of TBR2, NEUROD and TBR1 mRNA during early foetal development of the human neocortex. ISH analysis of TBR2, NEUROD and TBR1 mRNA in the developing human cortex between 8 and 12 postconceptional weeks (PCW). At 8 PCW, TBR2 and NEUROD exhibit restricted expression in what is probably the subventricular zone (SVZ) of the developing cortex (A, B), while TBR1 expression additionally includes the cortical plate (CP; C). By 9 PCW, the SVZ is more distinctly identified at the border with the ventricular zone (VZ). All three genes are absent from the majority VZ but possibly present at the SVZ border, TBR2 being restricted to the SVZ (D) while NEUROD and TBR1 are additionally present in the CP (E, F). The expression patterns of these genes are similar at 10 PCW (G–I); however, TBR2 exhibits a more restricted expression close to the SVZ/VZ border. By 12 PCW, all three show high expression in the ISVZ, while NEUROD and TBR1 show more widespread expression in the SVZ and CP. Scale bars: 100 μm (C, F); 200 μm (I, L). ISVZ, inner SVZ; IZ, intermediate zone; MZ, marginal zone; OSVZ, outer SVZ; SP, subplate.
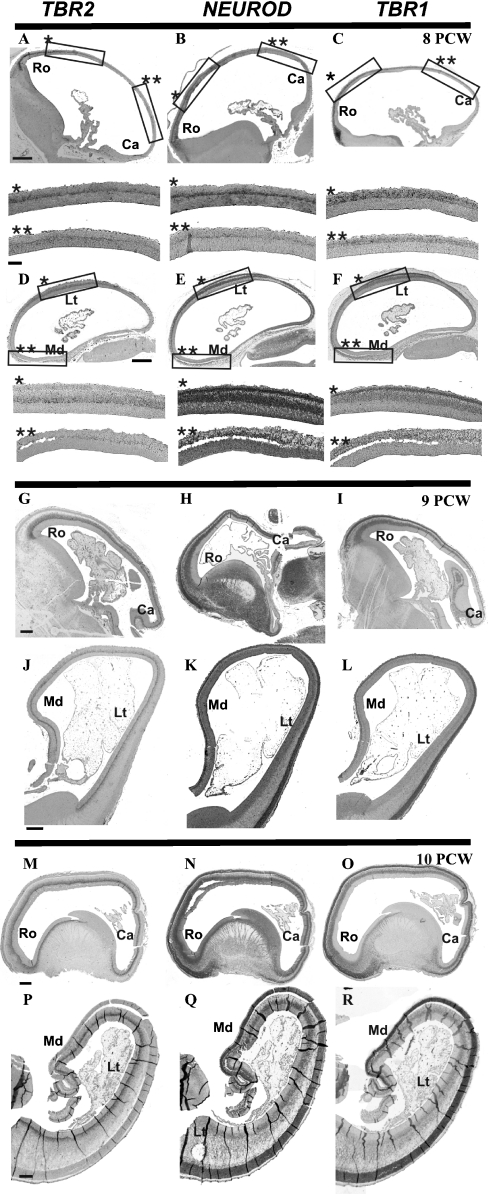
Fig. 4.
TBR2, NEUROD, and TBR1 gradients in the developing human cortex. Expression analysis of TBR2, NEUROD and TBR1 transcription factors temporally downstream of PAX6 during neurogenesis demonstrates similar gradients of expression. At 8 postconceptional weeks (PCW), all three genes exhibited high rostral-low caudal (A–C), high lateral-low medial (D–F) expression. These gradients persisted at 9 PCW (G–I and J–L, respectively); however, the TBR2 gradient was not as pronounced (G and J). By 10 PCW the TBR2 gradient was absent (M and P), the NEUROD and TBR1 gradients were becoming less pronounced (N, O, Q and R). By 12 PCW, there were no gradients of expression of any of these three transcription factors (data not shown). Higher magnification images of respective boxed areas are indicated by single and double asterisks. Sections shown are in the sagittal plane (A–C, G–I and M–O), horizontal plane (D–F) or coronal plane (J–L and P–R). Scale bars: 200 μm. Ca, caudal; Lt, lateral; Md, medial; Ro, rostral.
TBR2, NEUROD and TBR1 gradients in the developing human cortex
Tbr2, NeuroD and Tbr1 are sequentially expressed temporally downstream of Pax6 by progenitor cells undergoing differentiation and radial migration before finally residing in the CP as cortical glutamatergic projection neurons (Hevner et al., 2006). Previous studies in rodents indicate that Tbr2 is expressed in a high rostral-lateral, low caudal-medial gradient similar to Pax6 (Bulfone et al., 1999). At 8 PCW, TBR2 mRNA, as well as NEUROD, and TBR1 exhibited a gradient similar to PAX6 (Fig. 4A–C, D–F). However, at 9 PCW, when the PAX6 gradient has disappeared, the high rostral-lateral, low caudal-medial gradients of the other three transcription factors were maintained, although the TBR2 gradient was less pronounced (Fig. 4G–I, J–L), and by 10 PCW it had definitely disappeared, and gradients for NEUROD and TBR1 were barely discernible (Fig. 4M–O). By 12 PCW all gradients were absent (data not shown). Therefore the sequential loss of gradients of these transcription factors broadly mirrors the sequence in which these transcription factors are first expressed: PAX6 at 9 PCW; TBR2 at 10 PCW; and NEUROD and TBR1 at 12 PCW.
Discussion
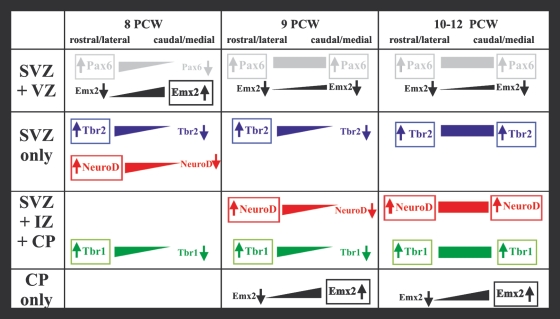
In order to examine the relative roles of PAX6 and EMX2 during arealization, we have examined the expression patterns of these two transcription factors using ISH during the early stages of corticogenesis in human. As we initially observed that the PAX6 gradient disappeared early in corticogenesis, we also analysed the laminar localization and gradients of expression of TBR2, NEUROD and TBR1, genes temporally downstream of PAX6 and expressed by cells during neurogenesis, in order to determine how they compare with PAX6 (all gene expression patterns summarized in Fig. 5).
Fig. 5.
Summary of changes in expression patterns of EMX2, PAX6, TBR2, NEUROD and TBR1 during early development of the human cerebral cortex. At 8 postconceptional weeks (PCW) EMX2 and PAX6 are localized within the proliferative zones of the developing cortex in opposing rostro-lateral/caudo-medial gradients. From 9 to 12 PCW onwards the majority of EMX2 expression is found in the cortical plate (CP), where a similar gradient exists to that observed in the subventricular zone (SVZ)/ventricular zone (VZ). By this time the PAX6 gradient has disappeared and expression is widespread throughout the SVZ/VZ only. However, the mRNA of transcription factors downstream of PAX6 in neurogenesis form PAX6-like gradients in different compartments during this time period. TBR2 exhibits a PAX6-like gradient within the SVZ until 10 PCW. NEUROD, which initially is not expressed in the CP, also forms a gradient within the SVZ at 8 PCW, which extends to the SP/intermediate zone (IZ) and CP at 9 PCW. The TBR1 gradient is also observed from 8 PCW and encompasses all compartments outside of the VZ, most prominently within the CP, but disappears before 12 PCW. Thus, as EMX2-expressing cells migrate from the VZ to the CP, they pass through compartments expressing genes downstream of PAX6 that exhibit PAX6-like gradients.
Laminar localization of Emx2 and Pax6
Previous work in our laboratory has demonstrated the presence of EMX2 mRNA in the proliferative zone of the developing human forebrain, at relatively low levels, from Carnegie stage 18 (CS18; 44 days; 6.5 PCW) until CS21 (52 days; 7.5 PCW; Lindsay et al., 2005; Sarma et al., 2005), when the CP starts to emerge (Meyer et al., 2000). Here we report the expression of EMX2 during the early stages of CP formation, where the functions of this gene are thought to be critical in modulating arealization mechanisms (Cecchi & Boncinelli, 2000). A principal finding of this study is that EMX2 mRNA expression in the developing human cortex differs from that reported in rodents. In the rodent, Emx2 mRNA localized chiefly to the VZ/SVZ (Cecchi, 2002), whereas in the human, expression of EMX2 was present in the VZ/SVZ only during the early stages of CP formation; however, from 10 PCW onwards expression had switched predominantly to the CP. Emx2 has been reported to be expressed at low levels in the rodent CP, but only in the apical dendrites of CP neurons synapsing with the Cajal–Retzius cells of the marginal zone. In rodents, expression of Emx1, a transcription factor that is related to Emx2, is found to be expressed within the CP, but is not thought to be involved in mechanisms regulating cortical arealization (Gulisano et al., 1996). However, double mutants indicate that Emx1 co-operates with Emx2 to regulate cortical size, lamination, neuronal differentiation, development of cortical efferents and thalamocortical path finding (Bishop et al., 2003). Our observations that EMX2 expression in the CP is reminiscent of that of Emx1 in rodents lead to the intriguing possibility that EMX1 in humans may take over EMX2 function in cortical arealization in humans. However, the majority of EMX1 expression is also found in the CP (in supporting Fig. S2) and this is consistent with previous reports in rodents.
We have previously demonstrated the early expression of the PAX6 gene during human development, specifically within the dorsal forebrain at 6.5–7 PCW (Kerwin et al., 2004; Lindsay et al., 2005) by ISH, and from 8 to 12 PCW during early cortical development by immunohistochemistry (Bayatti et al., 2008). We have extended these observations by analysing the extent and localization of PAX6 mRNA expression during early human neocortical development. Unlike EMX2, the general laminar expression of PAX6 in humans resembled that described in the rodent, being confined to the proliferative zones at all ages studied. The observation that PAX6 and EMX2 laminar-specific expression diverged as early as 9 PCW implies differing roles for these transcription factors during cortical development, whilst EMX2 and PAX6 may be important in regulating processes leading to the initial formation of the CP (at/or before 8 PCW; Gotz & Huttner, 2005). Additionally, PAX6 expressed in the human SVZ has been implicated in controlling the neurogenesis of γ-aminobutyric acid (GABA)ergic and glutamatergic neurons (Letinic et al., 2002; Mo & Zecevic, 2008). Recently, analysis of a transgenic mouse model that carries several copies of the human PAX6 locus, expressing up to three times more protein than the wild-type, provided evidence that PAX6 may not be directly involved in arealization. These mice exhibited no changes in Emx2 protein localization, and expression of the areal markers Id2 and Ephrin-B2 were also unaffected. However, reductions were seen in thickness of cortical layers, probably due to a reduced rate of proliferation in overexpressing cells (Manuel et al., 2007).
A study identifying genes regulated by Emx2 in neuronal precursors in vitro has identified a gene profile that would be consistent with conferring maturation, i.e. differentiation of early precursors and/or induction of a migratory specification to these normally resident cells (Gangemi et al., 2006). Given its expression gradient and location within the CP in humans, EMX2 may also be involved in the early stages of arealization and may control expression of areal markers directly. Recently, a study using nestin-Emx2 transgenic mice observed rostro-lateral shifts in the primary sensory and motor areas (Hamasaki et al., 2004). As these mice do not show defects in thalamocortical pathfinding, regional-specific loss in cortical tissue or differences in Fgf8 expression, which previously obscured interpretation of the function of Emx2 in Emx2 null mice, the authors concluded that Emx2 is directly involved in the mapping of primary cortical areas. Whilst our findings support these conclusions, we propose that in humans, due to its extended presence, EMX2 acts predominantly in the CP, in contrast to rodents where Emx2 in progenitors in the VZ is thought to impart positional identity to neuronal progeny.
EMX2 and PAX6 gradients during early human cortical development
It has been proposed that Emx2 and Pax6 are two key regulatory genes that control neocortical arealization through expression in graded, restricted patterns in the embryonic cortex (O’Leary & Nakagawa, 2002; Job & Tan, 2003). This study demonstrates that in the developing human neocortex, EMX2 and PAX6 are expressed in counter caudomedial/rostrolateral gradients, in a similar manner to that observed in rodents. However, this pattern of expression is restricted to the period of development up to 9 PCW. Following this, EMX2 maintains a gradient whereas PAX6 becomes uniformly expressed throughout the proliferating zones of the cortex (Fig. 3D and H, respectively). If EMX2 and PAX6 play a role in cortical arealization in human development they are only able to interact up to the time the CP is initially formed and very soon thereafter.
PAX6 may therefore affect arealization indirectly. The first cells to populate the CP form layer VI and contribute cells to the subplate (Kostovic & Rakic, 1990). These cells are crucial in guiding thalamic afferents to different regions of the cortex, as they send out axons to the thalamus that meet incoming thalamic axons, providing guidance cues (Molnar & Blakemore, 1995; Hevner et al., 2002). The regional identities of primary areas of the cortex are influenced by the thalamic innervation they eventually receive. Pax6 mutant mice exhibit defects in this thalamic innervation as well as in reciprocal cortical pathfinding (Hevner et al., 2002), as axons do not reach their targets suggesting that Pax6 is important in the cortex for such events. As the first CP cells do not express Pax6, Pax6-dependent processes such as pathfinding may be mediated by other transcription factors such as Tbr1 and NeuroD that are expressed in differing compartments downstream of Pax6 during the process of progenitor migration and differentiation (Hevner et al., 2006).
Localization and gradients of PAX6, TBR2, NEUROD and TBR1
Although PAX6 mRNA does not exhibit a gradient of expression from 9 PCW onwards in humans, it is possible that downstream transcription factors may be responsible for further arealization mechanisms in differentiating neurons migrating towards the pial surface. The compartmental localization of TBR2 and TBR1 mRNA is consistent with previous studies of the protein expression (Bayatti et al., 2007). Our results further indicate that in addition to TBR2, NEUROD and TBR1 form gradients similar to that of PAX6 in compartments through which progenitors and subsequent immature neurons migrate during cortical development (summarized in Fig. 5). TBR2 forms a gradient in the SVZ from 8 to 10 PCW, whilst NEUROD and TBR1 also exhibit gradients in this compartment. Interestingly these gradients are also present in the CP for TBR1 and NEUROD from 8 and 9 PCW, respectively, until at least 10 PCW. During this period TBR1, which is expressed by ‘early-born’ neurons including SP neurons and those in layer VI (Bulfone et al., 1995; Hevner et al., 2003), is expressed throughout the CP. Our previous observations show that the layer V marker Er81 appears later than 12 PCW in the human cortex (Bayatti et al., 2007). All four of these transcription factors are localized within the SVZ, suggesting that PAX6–TBR2–NEUROD–TBR1 transitions all occur within this compartment. Considering the relative size and degree of differentiation of the human SVZ during development compared with that of the rodent, this observation supports a fundamental role for this compartment during corticogenesis. This study has highlighted that the location of TBR2 is a particularly good marker for the SVZ at early stages. In particular it predominantly localizes to the inner subventricular zone (ISVZ) characterized in the macaque at E46, before the differentiation of the SVZ and appearance of an outer subventricular zone (OSVZ) (Smart et al., 2002). The majority of TBR2 expression is maintained in the ISVZ at later stages and corresponds to the junction between the ISVZ and VZ. Future experiments should characterize whether the SVZ contributes to arealization mechanisms, and if EMX2 and these transcription factors are expressed within the same cells during their migration and development. In addition, putative interactions between these transcription factors and EMX2 should be analysed, in a similar manner to that which PAX6 and EMX2 regulate each other in the VZ of rodents (Muzio et al., 2002).
In conclusion, during human neocortical development, EMX2 and PAX6 expression patterns diverge at an early stage, when in rodents they are still presumed to be regulating the initial phases of arealization by reciprocal gradients of expression. However, TBR2, NEUROD and TBR1, temporally downstream genes of PAX6, maintain early PAX6-like gradients until 12 PCW in different compartments of the developing cortex (summarized in Fig. 5). The extended period of gestation in humans, as compared with that in rodents, enables finer spatiotemporal resolution of overlapping gene expression patterns. The majority of neurogenesis in the human forebrain occurs between 8 and 16 PCW (ten Donkelaar, 2000), in comparison to rodents in which similar events occurs within days. All the transcription factors described in this study are likely to possess additional functions during this extended period of human cortical development. Further analysis of the actions of these genes in humans using in vitro models may shed light on their functions during human development.
Acknowledgments
The human embryonic and foetal material was provided by the Joint MRC-Wellcome Trust Human Developmental Biology Resource (http://www.hdbr.org) at the IHG, Newcastle-upon-Tyne, UK. We thank the consenting women who made this study possible and A. Farnworth who gained consent on our behalf. This study was funded by the Wellcome Trust.
Glossary
Abbreviations
- CP
cortical plate
- CS
Carnegie stage
- DIG
digoxigenin
- FCS
foetal calf serum
- ISH
in situ hybridization
- PBS
phosphate-buffered saline
- PBS-T
0.3% PBS with 0.3% Triton X-100
- PCR
polymerase chain reaction
- PCW
postconceptional weeks
- PFA
paraformaldehyde
- SSC
standard sodium citrate
- SVZ
subventricular zone
- VZ
ventricular zone
Supporting information
Additional Supporting Information may be found in the online version of this article:
Fig. S1. Detection of anti sense and sense probes for EMX1, EMX2, PAX6, TBR2, NEUROD and TBR1.
Fig. S2. Laminar EMX1 expression in the early human fetal cortex.
Fig. S3. Laminar localisation of EMX2 and PAX6 protein during early fetal development of the human neocortex.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Bayatti N, Moss JA, Sun L, Ambrose P, Ward JF, Lindsay S, Clowry GJ. A Molecular Neuroanatomical Study of the developing human neocortex from 8 to 17 postconceptional weeks revealing the early differentiation of the subplate and subventricular zone. Cereb. Cortex. 2008;18:1536–1548. doi: 10.1093/cercor/bhm184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop KM, Goudreau G, O’Leary DD. Regulation of area identity in the mammalian neocortex by Emx2 and Pax6. Science. 2000;288:344–349. doi: 10.1126/science.288.5464.344. [DOI] [PubMed] [Google Scholar]
- Bishop KM, Rubenstein JL, O’Leary DD. Distinct actions of Emx1, Emx2, and Pax6 in regulating the specification of areas in the developing neocortex. J. Neurosci. 2002;22:7627–7638. doi: 10.1523/JNEUROSCI.22-17-07627.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop KM, Garel S, Nakagawa Y, Rubenstein JL, O’Leary DD. Emx1 and Emx2 cooperate to regulate cortical size, lamination, neuronal differentiation, development of cortical efferents, and thalamocortical pathfinding. J. Comp. Neurol. 2003;457:345–360. doi: 10.1002/cne.10549. [DOI] [PubMed] [Google Scholar]
- Bulfone A, Smiga SM, Shimamura K, Peterson A, Puelles L, Rubenstein JLR. T-brain-1: a homolog of Brachyury whose expression defines molecularly distinct domains within the cerebral cortex. Neuron. 1995;15:63–78. doi: 10.1016/0896-6273(95)90065-9. [DOI] [PubMed] [Google Scholar]
- Bulfone A, Martinez S, Marigo V, Campanella M, Basile A, Quaderi N, Gattuso C, Rubenstein JL, Ballabio A. Expression pattern of the Tbr2 (Eomesodermin) gene during mouse and chick brain development. Mech. Dev. 1999;84:133–138. doi: 10.1016/s0925-4773(99)00053-2. [DOI] [PubMed] [Google Scholar]
- Cecchi C. Emx2: a gene responsible for cortical development, regionalization and area specification. Gene. 2002;291:1–9. doi: 10.1016/s0378-1119(02)00623-6. [DOI] [PubMed] [Google Scholar]
- Cecchi C, Boncinelli E. Emx homeogenes and mouse brain development. Trends Neurosci. 2000;23:347–352. doi: 10.1016/s0166-2236(00)01608-8. [DOI] [PubMed] [Google Scholar]
- ten Donkelaar HJ. Major events in the development of the forebrain. Eur. J. Morphol. 2000;38:301–308. doi: 10.1076/ejom.38.5.301.7356. [DOI] [PubMed] [Google Scholar]
- Gangemi RM, Daga A, Muzio L, Marubbi D, Cocozza S, Perera M, Verardo S, Bordo D, Griffero F, Capra MC, Mallamaci A, Corte G. Effects of Emx2 inactivation on the gene expression profile of neural precursors. Eur. J. Neurosci. 2006;23:325–334. doi: 10.1111/j.1460-9568.2005.04559.x. [DOI] [PubMed] [Google Scholar]
- Gotz M, Huttner WB. The cell biology of neurogenesis. Nat. Rev. Mol. Cell Biol. 2005;6:777–788. doi: 10.1038/nrm1739. [DOI] [PubMed] [Google Scholar]
- Gulisano M, Broccoli V, Pardini C, Boncinelli E. Emx1 and Emx2 show different patterns of expression during proliferation and differentiation of the developing cerebral cortex in the mouse. Eur. J. Neurosci. 1996;8:1037–1050. doi: 10.1111/j.1460-9568.1996.tb01590.x. [DOI] [PubMed] [Google Scholar]
- Hamasaki T, Leingartner A, Ringstedt T, O’Leary DD. EMX2 regulates sizes and positioning of the primary sensory and motor areas in neocortex by direct specification of cortical progenitors. Neuron. 2004;43:359–372. doi: 10.1016/j.neuron.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Hern WM. Correlation of fetal age and measurements between 10 and 26 weeks of gestation. Obstet. Gynecol. 1984;63:26–32. [PubMed] [Google Scholar]
- Hevner RF, Miyashita-Lin E, Rubenstein JL. Cortical and thalamic axon pathfinding defects in Tbr1, Gbx2, and Pax6 mutant mice: evidence that cortical and thalamic axons interact and guide each other. J. Comp. Neurol. 2002;447:8–17. doi: 10.1002/cne.10219. [DOI] [PubMed] [Google Scholar]
- Hevner RF, Daza RA, Rubenstein JL, Stunnenberg H, Olavarria JF, Englund C. Beyond laminar fate: toward a molecular classification of cortical projection/pyramidal neurons. Dev. Neurosci. 2003;25:139–151. doi: 10.1159/000072263. [DOI] [PubMed] [Google Scholar]
- Hevner RF, Hodge RD, Daza RA, Englund C. Transcription factors in glutamatergic neurogenesis: conserved programs in neocortex, cerebellum, and adult hippocampus. Neurosci. Res. 2006;55:223–233. doi: 10.1016/j.neures.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Hill RS, Walsh CA. Molecular insights into human brain evolution. Nature. 2005;437:64–67. doi: 10.1038/nature04103. [DOI] [PubMed] [Google Scholar]
- Job C, Tan SS. Constructing the mammalian neocortex: the role of intrinsic factors. Dev. Biol. 2003;257:221–232. doi: 10.1016/s0012-1606(03)00070-8. [DOI] [PubMed] [Google Scholar]
- Kerwin J, Scott M, Sharpe J, Puelles L, Robson SC, Martinez-de-la-Torre M, Ferran JL, Feng G, Baldock R, Strachan T, Davidson D, Lindsay S. 3 dimensional modelling of early human brain development using optical projection tomography. BMC Neurosci. 2004;5:27. doi: 10.1186/1471-2202-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostovic I, Rakic P. Developmental history of the transient subplate zone in the visual and somatosensory cortex of the macaque monkey and human brain. J. Comp. Neurol. 1990;297:441–470. doi: 10.1002/cne.902970309. [DOI] [PubMed] [Google Scholar]
- Kroll TT, O’Leary DD. Ventralized dorsal telencephalic progenitors in Pax6 mutant mice generate GABA interneurons of a lateral ganglionic eminence fate. Proc. Natl. Acad. Sci. U.S.A. 2005;102:7374–7379. doi: 10.1073/pnas.0500819102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letinic K, Zoncu R, Rakic P. Origin of GABAergic neurons in the human neocortex. Nature. 2002;417:645–649. doi: 10.1038/nature00779. [DOI] [PubMed] [Google Scholar]
- Lindsay S, Sarma S, Martinez-de-la-Torre M, Kerwin J, Scott M, Luis Ferran J, Baldock R, Puelles L. Anatomical and gene expression mapping of the ventral pallium in a three-dimensional model of developing human brain. Neuroscience. 2005;136:625–632. doi: 10.1016/j.neuroscience.2005.06.093. [DOI] [PubMed] [Google Scholar]
- Mallamaci A, Stoykova A. Gene networks controlling early cerebral cortex arealization. Eur. J. Neurosci. 2006;23:847–856. doi: 10.1111/j.1460-9568.2006.04634.x. [DOI] [PubMed] [Google Scholar]
- Manuel M, Georgala PA, Carr CB, Chanas S, Kleinjan DA, Martynoga B, Mason JO, Molinek M, Pinson J, Pratt T, Quinn JC, Simpson TI, Tyas DA, van Heyningen V, West JD, Price DJ. Controlled overexpression of Pax6 in vivo negatively autoregulates the Pax6 locus, causing cell-autonomous defects of late cortical progenitor proliferation with little effect on cortical arealization. Development. 2007;134:545–555. doi: 10.1242/dev.02764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer G, Schaaps JP, Moreau L, Goffinet AM. Embryonic and early fetal development of the human neocortex. J. Neurosci. 2000;20:1858–1868. doi: 10.1523/JNEUROSCI.20-05-01858.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo Z, Zecevic N. Is Pax6 critical for neurogenesis in the human fetal brain? Cereb. Cortex. 2008;18:1455–1465. doi: 10.1093/cercor/bhm181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar Z, Blakemore C. How do thalamic axons find their way to the cortex? Trends Neurosci. 1995;18:389–397. doi: 10.1016/0166-2236(95)93935-q. [DOI] [PubMed] [Google Scholar]
- Monuki ES, Walsh CA. Mechanisms of cerebral cortical patterning in mice and humans. Nat. Neurosci. 2001;4(Suppl):1199–1206. doi: 10.1038/nn752. [DOI] [PubMed] [Google Scholar]
- Moorman AF, Houweling AC, de Boer PA, Christoffels VM. Sensitive nonradioactive detection of mRNA in tissue sections: novel application of the whole-mount in situ hybridization protocol. J. Histochem. Cytochem. 2001;49:1–8. doi: 10.1177/002215540104900101. [DOI] [PubMed] [Google Scholar]
- Muzio L, DiBenedetto B, Stoykova A, Boncinelli E, Gruss P, Mallamaci A. Emx2 and Pax6 control regionalization of the pre-neuronogenic cortical primordium. Cereb. Cortex. 2002;12:129–139. doi: 10.1093/cercor/12.2.129. [DOI] [PubMed] [Google Scholar]
- O’Leary DD, Nakagawa Y. Patterning centers, regulatory genes and extrinsic mechanisms controlling arealization of the neocortex. Curr. Opin. Neurobiol. 2002;12:14–25. doi: 10.1016/s0959-4388(02)00285-4. [DOI] [PubMed] [Google Scholar]
- Sarma S, Kerwin J, Puelles L, Scott M, Strachan T, Feng G, Sharpe J, Davidson D, Baldock R, Lindsay S. 3D modelling, gene expression mapping and post-mapping image analysis in the developing human brain. Brain Res. Bull. 2005;66:449–453. doi: 10.1016/j.brainresbull.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Simeone A, Gulisano M, Acampora D, Stornaiuolo A, Rambaldi M, Boncinelli E. Two vertebrate homeobox genes related to the Drosophila empty spiracles gene are expressed in the embryonic cerebral cortex. EMBO J. 1992;11:2541–2550. doi: 10.1002/j.1460-2075.1992.tb05319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart IH, Dehay C, Giroud P, Berland M, Kennedy H. Unique morphological features of the proliferative zones and postmitotic compartments of the neural epithelium giving rise to striate and extrastriate cortex in the monkey. Cereb. Cortex. 2002;12:37–53. doi: 10.1093/cercor/12.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ton CC, Hirvonen H, Miwa H, Weil MM, Monaghan P, Jordan T, van Heyningen V, Hastie ND, Meijers-Heijboer H, Drechsler M, Royer-Pokora B, Collins F, Swaroop A, Strong LC, Saunders GF. Positional cloning and characterization of a paired box-and homeobox-containing gene from the aniridia region. Cell. 1991;67:1059–1074. doi: 10.1016/0092-8674(91)90284-6. [DOI] [PubMed] [Google Scholar]
- Ton CC, Miwa H, Saunders GF. Small eye (Sey): cloning and characterization of the murine homolog of the human aniridia gene. Genomics. 1992;13:251–256. doi: 10.1016/0888-7543(92)90239-o. [DOI] [PubMed] [Google Scholar]
- Walther C, Gruss P. Pax-6, a murine paired box gene, is expressed in the developing CNS. Development. 1991;113:1435–1449. doi: 10.1242/dev.113.4.1435. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.