Abstract
We estimated the intake of individual flavonoids in a cross sectional study and clarified the major sources contributing to the flavonoid levels in the middle-aged Japanese women by a 24-h weighed dietary record study. The subjects included in the study were 516 free-living women. Each subject completed a 24-h weighed dietary record and received a health check-up. We used the Functional Food Factor database for estimating the intake of 5 major flavonoid intakes, i.e. flavan-3-ols, isoflavones, flavonols, flavanones and flavones. The mean intake of flavan-3-ols, isoflavones, flavonols, flavanones and flavones was 1277, 216, 58, 31 and 15 µmol/d, respectively. The richest source of flavan-3-ols was green tea. The 3 major food sources of isoflavone were the processed soy foods and those of flavonol were the onion, moroheiya (nalta jute) and Japanese radish leaves. Grapefruit and citrus fruit juices were the major sources of flavanones, and tsurumurasaki (malabar spinach), green pepper and grapefruit were the main sources of flavone. Furthermore, analysis of sub-samples from middle-aged Japanese women indicated that there may be an association between flavonoid intake and the levels of oxidized LDL, which might be related to the incidence of cardiovascular diseases.
Keywords: 24-hour weighed dietary record, functional food factor database, flavonoids, oxidized LDL, cardiovascular diseases
Introduction
Several epidemiological studies show that flavonoid intake might decrease the risk of coronary heart disease (CHD) in elderly people [1–4]. It has been suggested that flavonoids scavenge free radicals, chelate metal ions and prevent the oxidation of low density lipoprotein (LDL) in vitro. Oxidized LDL plays an important role in the formation of atherosclerotic plaques. Animal studies [5, 6] and some human intervention studies [7, 8] have reported that flavonoids delay the lag time of LDL to oxidation.
Fruits and vegetables are the main sources of flavonoids. Flavonoids have 2 aromatic rings that are bound by an oxygenated heterocyclic ring. On the basis of their chemical structure, they are divided into several subclasses: flavones, flavonols, flavanones, flavan-3-ols, anthocyanins and isoflavones. In our diet, flavones and flavonols are found in leaf vegetables. Flavanones are mainly found in citrus fruits. Tea and cacao are the richest sources of flavan-3-ols. Soy and soy products are the main sources of isoflavones [9].
In the 1970’s, Kuhnau estimated the flavonoid intake to be up to 1 g/d [10]. More recently, some studies in Western coutries have assessed the subclasses of flavonoid intakes with the food frequency questionnaire (FFQ). These studies estimated the total flavonoid intake to be 1~200 mg/d in Western countries [11, 12]. However, there are few reports that assessed the intake of other flavonoid subclasses, besides isoflavones, in Japan [13, 14]. Therefore, we estimated the intake of individual flavonoids, namely, flavones, flavonols, flavanones, flavan-3-ols and isoflavones, in a cross sectional study. Furthermore, we clarified major food sources contributing to the flavonoid levels in middle-aged Japanese women by a 24-h weighed dietary record (DR) study in order to understand of weighed DR-FFQ relationship.
Methods
Survey sample and brief methodology
This cross-sectional study was carried out from 1996 to 2005 in the northern part of Japan. This study included 569 female volunteers, who agreed to participate in the study and provided their written informed consent. The study design was approved by the ethical committee of the Tokyo University of Agriculture. The subjects were asked to record the weight of all the food they ingested for 1 d as well as cooking methods adopted on that day. The DRs were checked the next day through interviews with trained dieticians.
The anthropometric data and blood samples for biochemical analysis were obtained on the same day as dietary recording. Anthropometric data, including the height, weight, and blood pressure were measured. Casual blood pressure was measured in the sitting position after a 5-min rest by the auscultatory method. The body mass index (BMI, kg/m2) was calculated from the height and weight.
After an overnight fast, blood samples were collected and used for biochemical analysis of total cholesterol (TC), triacylglycerol (TG) and high density lipoprotein cholesterol (HDL-C). Biochemical analysis was performed with a clinical analyzer (Hitachi 7250, Hitachi, Tokyo, Japan). The low density lipoprotein cholesterol (LDL-C) concentration in the plasma was calculated using the Friedewald formula. Other measurements obtained by a health questionnaire included lifestyle variables such as the smoking status, alcohol intake and use of medication.
We examined the association between the flavonoid intake and oxidized LDL levels in the sub-samples. The oxidized LDL in 39 postmenopausal subjects was measured in 2006. The monoclonal antibody DLH3 was used to quantify the concentration of oxidized LDL in the plasma. This assay was carried out using a commercially available enzyme-linked immunosorbent assay (ELISA) kit according to manufacturer’s instructions (MX, Kyowa Medex, Tokyo, Japan). Other biochemical analysis and DRs were collected in a similar manner as mentioned above. Normal LDL-C levels were noted in the case of 25 subjects, and 14 subjects had hyper LDL cholesterolemia. Subjects with an LDL-C concentration of >140 mg/dl, were divided into 2 groups on the basis of the oxidized LDL levels in the plasma: a low plasma oxidized LDL group and high plasma oxidized LDL group.
Each recorded food item was coded, and the consumption dose and frequency were determined. Food nutrients were calculated with the Standard Tables of Food Composition in Japan (5th revised edition).
The intake of flavonoids was estimated with the Functional Food Factor (FFF) database [15–17]. This database calculated 44 polyphenols, 6 carotenoids, and 7 sulfur compounds. However, we estimated the intake of major flavonoids (flavan-3-ols, epicatechin, epigallocatechin and catechin: isoflavones, daidzein and genistein: flavonols, quercetin, kaempferol, myricetin and fisetin: flavones, luteolin and apigenin, and flavanones, hesperetin and narigenin). In the FFF database, the values of epicatchin gallate and epigallocatechin gallate are converted into non-gallate type values.
For this analysis, we excluded women who did not fill in the health questionnaire and those with an implausibly high (>4000 kcal) or low (<1000 kcal) total energy intake. After these exclusions, 514 (91%) women were included in the analysis.
Statistics
The mean intake with standard devitation (SD) and median intake with the interquartile range are presented for each flavonoid. The percentage intake of individual flavonoids from different food sources was also presented. Data manipulation and statistical analysis of the data were conducted using MS Excel and the statistical package for social sciences (SPSS) for Windows version 12.0. Differences in flavonoid intake among the groups were compared using Wilcoxon’s rank sum tests.
Results
Anthropometric data and biochemical analysis data are shown in Table 1, and the mean energy and nutrient intake are shown in Table 2. The percentage of subjects who did not consume flavan-3-ol was 22.5%; flavonol, 8.3%; flavanone, 56.2% and flavone, 89.1%. All study participants consumed isoflavone from soy products (data not shown). The average intake of flavonoids is shown in Table 3.
Table 1.
Anthoropometric and plasma biochemical characteristics of this study population
| AVE | SD | Percentile |
||||
|---|---|---|---|---|---|---|
| 10 | 50 | 90 | ||||
| Age | (years) | 58 | 10 | 45 | 59 | 71 |
| HT | (cm) | 152 | 6 | 145 | 152 | 159 |
| BW | (kg) | 54 | 8 | 45 | 53 | 65 |
| BMI | (kg/m2) | 23 | 3 | 20 | 23 | 28 |
| Body fat | (%) | 31 | 6 | 23 | 30 | 38 |
| Systolic BP | (mmHg) | 125 | 17 | 104 | 126 | 146 |
| Diastolic BP | (mmHg) | 74 | 10 | 62 | 74 | 86 |
| TG | (mg/dL) | 97 | 58 | 45 | 79 | 167 |
| TC | (mg/dL) | 222 | 39 | 173 | 219 | 271 |
| HDL-C | (mg/dL) | 62 | 15 | 45 | 60 | 83 |
| LDL-C | (mg/dL) | 141 | 35 | 98 | 137 | 185 |
HT, hegiht; BW, body weight; BMI, body mass index; BP, blood pressure; TG, triacylglycerol; TC, total cholesterol; HDL-C, HDL-cholesterol; LDL-C, LDL-cholesterol.
Table 2.
Energy, nutrients and foods intake in middle aged Japanese women
| AVE | SD | Percentile |
||||
|---|---|---|---|---|---|---|
| 10 | 50 | 90 | ||||
| Energy | (kcal) | 1898 | 412 | 1382 | 1880 | 2416 |
| Protein | (%E) | 16 | 3 | 12 | 16 | 20 |
| Fat | (%E) | 23 | 6 | 16 | 23 | 32 |
| Carbohydrate | (%E) | 60 | 7 | 51 | 61 | 70 |
| Fiber | (g) | 19 | 7 | 12 | 18 | 27 |
| β-carotene | (µg) | 4919 | 3529 | 1355 | 4177 | 9365 |
| α-tocopherol | (mg) | 8 | 4 | 4 | 8 | 13 |
| Vitamin C | (mg) | 126 | 60 | 59 | 116 | 207 |
| Beans | (g) | 91 | 80 | 4 | 73 | 200 |
| Fruits | (g) | 169 | 162 | 0 | 135 | 359 |
| Colored vegetables | (g) | 178 | 130 | 38 | 156 | 348 |
| Other vegetables | (g) | 222 | 116 | 85 | 204 | 384 |
Table 3.
Distribution of flavonoid intake (µmol/day) among middle aged Japanese women
| n = 516 µmol | AVE | SD |
Percentile |
||
|---|---|---|---|---|---|
| 10 | 50 | 90 | |||
| Flavonols | 58.4 | 62.7 | 0.5 | 39.3 | 141.5 |
| quercetin | 52.4 | 58.6 | 0.0 | 35.3 | 131.7 |
| kaempferol | 5.8 | 14.0 | 0.0 | 0.0 | 19.4 |
| myricetin | 0.1 | 0.3 | 0.0 | 0.0 | 0.6 |
| rutin | 0.0 | 0.3 | 0.0 | 0.0 | 0.0 |
| fisetin | 0.0 | 0.1 | 0.0 | 0.0 | 0.0 |
| Isoflavones | 215.7 | 147.3 | 50.4 | 191.6 | 407.1 |
| genistein | 123.4 | 84.7 | 28.5 | 109.9 | 237.0 |
| daidzein | 92.3 | 63.9 | 20.0 | 81.1 | 176.5 |
| Flavanonols | 1277 | 1403 | 0 | 1009 | 2884 |
| (−)-epicatechin | 287 | 315 | 0 | 229 | 658 |
| (−)-epigallocatechin | 984 | 1095 | 0 | 795 | 2224 |
| catechin | 5.6 | 12.9 | 0.0 | 0.0 | 22.0 |
| Flavanones | 30.5 | 145.8 | 0.0 | 0.0 | 7.9 |
| hesperidin | 15.5 | 107.9 | 0.0 | 0.0 | 0.0 |
| narigenin | 15.0 | 90.0 | 0.0 | 0.0 | 0.0 |
| Flavones | 15.0 | 51.6 | 0.0 | 0.0 | 20.0 |
| luteolin | 2.0 | 4.2 | 0.0 | 0.0 | 7.0 |
| apigenin | 13.0 | 51.6 | 0.0 | 0.0 | 14.5 |
Table 4 shows the important sources of flavonoid. The main food sources of flavonol were onion, moroheiya (nalta jute) and Japanese radish leaves; those of isoflavone were soy processed food, such as tofu, natto and miso; that of flavan-3-ol was tea; those of flavanones were grapefruits and citrus fruits juices and those of flavones were tsurumurasaki (malabar spinach), green pepper and grapefruits.
Table 4.
Contribution of food stuff to flavonoid intakes by 24 h weighed dietary record in middle aged Japanese women
| Percentages contributed | Cumulative percentages contributed | |
|---|---|---|
| Flavones | (%) | (%) |
| Malabar spinach | 79.5 | 79.5 |
| Green pepper | 12.3 | 91.8 |
| Grapefruits | 3.6 | 95.4 |
| Parsley | 2.2 | 97.6 |
| Celery | 1.1 | 98.7 |
| Flavonols | ||
| Oninon | 58.8 | 58.8 |
| Mroheya | 15.9 | 74.7 |
| Radish leaves | 5.6 | 80.3 |
| Komatsuna | 4.8 | 85.1 |
| Okra | 4.3 | 89.4 |
| Flavanols | ||
| Grapefruits | 38.6 | 38.6 |
| Orange juice | 27.9 | 66.5 |
| Mandarin orange juice | 11.8 | 78.3 |
| Mandarin orange | 8.9 | 87.2 |
| Orange | 6.3 | 93.5 |
| Flavan-3-ols | ||
| Tea | 98 | 98 |
| Apples | 1.9 | 99.5 |
| Peach | 0.2 | 100 |
| Plum | 0.1 | 100 |
| Strawberry | 0.1 | 100 |
| Isoflavones | ||
| Tofu | 42.2 | 42.2 |
| Natto | 28.7 | 70.9 |
| Miso | 16.3 | 87.2 |
| Fried tofu | 2.6 | 89.8 |
| Kinako | 2.1 | 91.9 |
Table 5 shows the specific food intakes according to the quintile of individual total flavonoid consumption. Onion intake was 0 g/d in the lowest quintile of quercetin intake, but in the highest quintile, it was approximately 70 g/d. Similarly, tofu and natto intakes in the lowest quintile of daidzein intake were lower than that in the highest quintile of isoflavone intake. Tofu intake in the lowest quintile of isoflavone was 8 g/d, but in the highest quintile, it was about 132 g/d. Natto intake in the lowest quintile of isoflavone was almost 0 g/d, but it was 35 g/d in the highest quintile. However, the miso intake did not differ in each category.
Table 5.
Specific food intakes according to quintile of individual total flavonoid consumption
| (g/day/person) | Lowest | 2nd | 3rd | 4th | Highest |
|---|---|---|---|---|---|
| Contributors of flavonol intake | |||||
| Onion | 0 | 3.6 | 21.1 | 35.5 | 71.3 |
| Moroheya | 0 | 0 | 0.9 | 6.9 | 21.2 |
| Radish leaves | 0 | 0.1 | 0.7 | 0.5 | 5 |
| Contributors of isoflavone intake | |||||
| Tofu | 7.8 | 33.7 | 49 | 71.1 | 132.4 |
| Natto | 0.4 | 6.3 | 17.1 | 25.6 | 34.8 |
| Miso paste | 13.7 | 17.6 | 17.4 | 19.4 | 19.9 |
| Contributors of flavan-3-ols intake | |||||
| Green tea | 0 | 83.6 | 220.9 | 360 | 763.4 |
We conducted a stratified analysis of subjects with LDL cholesterol concentrations >140 mg/dL. Twenty five subjects showed normal LDL-C level (normal group) and 14 subjects exhibited hyper LDL-cholesterolemia. The subjects with hyper LDL-cholesterolemia were divided into 2 groups based on the oxidized LDL levels in the plasma, namely, the low oxidized LDL group and high oxidized LDL group. The level of oxidized LDL in the case of the low oxidized LDL group was similar to that in the case of the normal group. Table 6 shows the anthropometric measurements, serum lipids levels, energy and energy-adjusted nutrient intakes. In physical characteristics, the height was significantly lower in the high oxidized LDL group than in the normal and low oxidized LDL groups. The waist circumference was higher in the high oxidized LDL group than in the normal group. TC was significantly higher in the high oxidized LDL group than in the normal group. HDL-C was lower in the low oxidized LDL group than in the normal group. LDL-C was significantly higher in the low and high oxidized LDL groups than in the normal group.
Table 6.
Anthropometric, serum lipids and energy and energy adjusted nutrient intakes
| Hyper-LDL-cholesterolemia | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal (n = 25) |
ox-LDL LOW (n = 7) |
ox-LDL HIGH (n = 7) |
||||||||||||||
| AVE | SD | Percentile |
AVE | SD | Percentile |
AVE | SD | Percentile |
||||||||
| 10 | 50 | 90 | 10 | 50 | 90 | 10 | 50 | 90 | ||||||||
| Physical Characteristics | ||||||||||||||||
| Age | (yr) | 67 | 8 | 55 | 68 | 79 | 64 | 6 | 57 | 61 | 74 | 70 | 9 | 57 | 69 | 84 |
| HT | (cm) | 152 | 6a | 143 | 151 | 160 | 151 | 5a | 145 | 152 | 160 | 145 | 6b | 137 | 144 | 156 |
| BT | (kg) | 51 | 7 | 42 | 51 | 60 | 55 | 8 | 46 | 56 | 69 | 53 | 9 | 43 | 51 | 65 |
| BMI | (kg/m2) | 22.3 | 2.7 | 17.6 | 22.9 | 26.0 | 23.9 | 2.8 | 20.8 | 22.8 | 28.9 | 25.3 | 4.0 | 19.5 | 26.2 | 31.2 |
| Waist | (cm) | 82 | 8a | 72 | 81 | 91 | 86 | 11a,b | 73 | 81 | 105 | 91 | 10b | 77 | 91 | 101 |
| Systolic BP | (mmHg) | 130 | 15 | 112 | 126 | 151 | 126 | 23 | 100 | 126 | 166 | 139 | 11 | 124 | 142 | 150 |
| Diastolic BP | (mmHg) | 79 | 10 | 62 | 80 | 91 | 79 | 11 | 70 | 76 | 100 | 81 | 7 | 68 | 84 | 90 |
| Serum Lipids | ||||||||||||||||
| TG | (mg/dL) | 75 | 31 | 41 | 70 | 123 | 116 | 59 | 46 | 119 | 222 | 75 | 19 | 51 | 80 | 94 |
| TC | (mg/dL) | 209 | 26 | 169 | 217 | 238 | 232 | 10 | 223 | 225 | 248 | 241 | 24 | 219 | 232 | 291 |
| HDL-C | (mg/dL) | 73 | 20 | 42 | 75 | 103 | 54 | 9 | 40 | 57 | 67 | 64 | 8 | 54 | 65 | 79 |
| LDL-C | (mg/dL) | 121 | 14a | 101 | 122 | 137 | 155 | 11b | 141 | 150 | 168 | 162 | 17b | 144 | 156 | 196 |
| ox-LDL | (U/mL) | 9.1 | 5.8a | 4.9 | 6.9 | 13.6 | 7.7 | 1.7a | 5.3 | 7.4 | 10.7 | 17.7 | 8.7b | 10.7 | 16.2 | 35.7 |
| Energy & Energy Adjusted Nutrient Intakes | ||||||||||||||||
| Energy | (kcal) | 1814 | 324 | 1391 | 1730 | 2331 | 1644 | 135 | 1466 | 1652 | 1824 | 1999 | 401 | 1190 | 2070 | 2507 |
| Protein | (%E) | 17.6 | 4.0 | 13.2 | 17.3 | 24.6 | 16.7 | 3.1 | 13.6 | 15.9 | 23.3 | 16.6 | 4.2 | 10.2 | 16.4 | 22.5 |
| Fat | (%E) | 23.6 | 6.5 | 15.4 | 23.6 | 34.1 | 23.4 | 3.5 | 17.5 | 22.9 | 27.4 | 26.8 | 10.1 | 13.2 | 29.9 | 39.3 |
| Carbohydrate | (%E) | 58.8 | 7.2 | 48.0 | 59.0 | 69.4 | 59.9 | 6.1 | 49.7 | 60.8 | 68.9 | 56.5 | 11.3 | 44.3 | 54.1 | 76.5 |
| Sodium | (mg) | 2474 | 560 | 1684 | 2383 | 3263 | 2448 | 927 | 1144 | 2721 | 3905 | 2349 | 774 | 1100 | 2575 | 3217 |
| Potassium | (mg) | 1732 | 465 | 1145 | 1624 | 2414 | 1686 | 423 | 1362 | 1592 | 2596 | 1564 | 502 | 829 | 1476 | 2480 |
| Calcium | (mg) | 356 | 103 | 198 | 352 | 513 | 387 | 185 | 178 | 329 | 677 | 330 | 119 | 122 | 363 | 479 |
| Iron | (mg) | 5.2 | 1.2 | 3.8 | 5.0 | 7.0 | 5.4 | 2.2 | 3.8 | 4.7 | 10.2 | 4.9 | 0.6 | 4.1 | 5.0 | 5.8 |
| SFA | (mg) | 6.8 | 2.1 | 4.1 | 7.0 | 9.1 | 6.3 | 2.0 | 4.4 | 6.3 | 9.8 | 7.4 | 3.5 | 2.8 | 7.7 | 13.3 |
| MUFA | (mg) | 8.8 | 3.3 | 5.2 | 8.4 | 13.6 | 8.5 | 1.4 | 6.4 | 8.8 | 10.4 | 10.1 | 4.1 | 4.3 | 11.1 | 14.8 |
| PUFA | (mg) | 6.7 | 2.2 | 4.3 | 6.6 | 10.4 | 6.1 | 1.1 | 4.9 | 6.3 | 7.5 | 7.9 | 3.6 | 2.6 | 8.5 | 12.0 |
| Cholesterol | (mg) | 197 | 122 | 55 | 178 | 341 | 181 | 119 | 60 | 127 | 348 | 208 | 100 | 103 | 199 | 403 |
| Dietary fibre | (g) | 10.1 | 3.2 | 6.9 | 9.2 | 16.3 | 11.4 | 3.8 | 8.0 | 10.2 | 19.3 | 9.4 | 4.1 | 5.0 | 9.5 | 17.6 |
Values with different superscripts are significantly different (p<0.05).
HT, hegiht; BW, body weight; BMI, body mass index; BP, blood pressure; TG, triacylglycerol; TC, total cholesterol; HDL-C, HDL-cholesterol; LDL-C, LDL-cholesterol; ox-LDL, oxidized LDL; SFA, saturated fatty acid; MUFA, mono unsaturated fatty acid; PUFA, poly unsaturated fatty acid
The energy intake was significantly lower in the low oxidized LDL group than in the high oxidized LDL group. The energy-adjusted nutrient intake did not differ among the 3 groups.
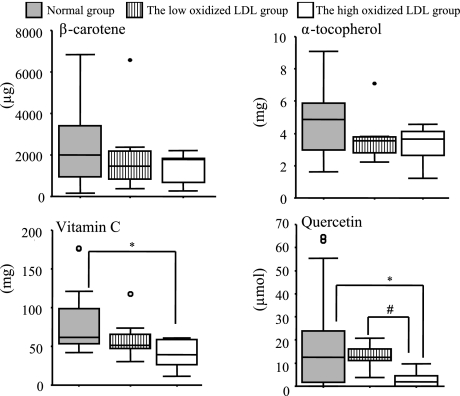
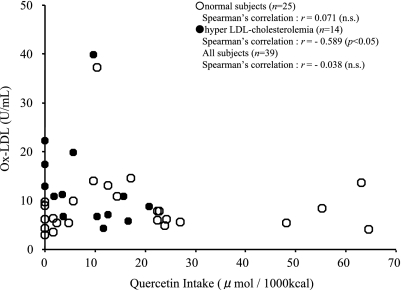
Fig. 1 shows the energy-adjusted antioxidant vitamins and quercetin intake. There were no significant differences in the antioxidant vitamins between the low and high oxidized LDL groups. The quercetin intake was higher in the low oxidized LDL group than in the high oxidized LDL group. Fig. 2 shows correlation between oxidized LDL and quercetin intake. In subjects with hyper LDL cholesterolemia, there was a significant inverse correlation between the intake of quercetin and the oxidized LDL levels, but this was not the case in normal LDL-C subjects.
Fig. 1.
Energy adjusted antioxidant vitamins and quercetin intakes in the three groups.
*Normal group vs The high oxidized group (p<0.05).
#Normal group vs The low oxidized LDL group (p<0.05).
Fig. 2.
Correlation between oxidized LDL and energy-adjusted quercetin intake
Discussion
In the present study, we assessed the mean intake of total flavonoids in middle-aged Japanese women with the Functional Food Factor database. Of the total flavnoid, the mean intake of flavan-3-ols was the highest followed by isoflavones, flavonols, flavanones and flavones. The intake of flavonols in this study was similar to that reported in an Australian study (50 µmol/d, 15.3 mg/d, in the case of women with ages ranging from 45 to 64 years) [18]. Arts et al. reported that the average flavan-3-ol intake was 160 µmol/d (50 mg/d) in 6200 Dutch men and women aged 1 to 97 years old [19]. In our study, flavan-3-ol levels were 10 times higher level than those in previous study. The flavan-3ol levels were higher in green tea than in black tea [20]. The flavanone intake in our study populations was 31 µmol/d; this was lower than that in the case of a previous report from U.S.A., where the values ranged from 30 to 100 µmol/d (10 to 30 mg/d) [21]. The consumption of flavones was closed to 0 µmol, and other reports showed the same values. Flavones are much less common as compared to the flavonoids present in vegetables and fruits. Soy products are rarely consumed in Western countries. The isoflavone intake in these countries was ranged 0 to 10 µmol/d (< 1 mg/d) [12]. In this study, all subjects consumed soy products and the isolfavone intake was 20 times higher in this Japanese population than in populations in Western countries.
Additionally, we demonstrated the sources of flavonoid in the diet of middle-aged Japanese women. The major dietary sources of total flavonoids were tea, onions and soy products in our study population. The results of this study differed from that of previous studies because the Japanese traditional diet includes many soy products such as tofu, natto and miso paste. In the Long Island Breast Cancer Study Project (LIBCSP), the major dietary sources of total flavonoids intake were tea, citrus fruit juices and citrus fruits [12]. Similarly, Ock Kyoung Chun et al. reported that the main sources of flavonoid intake were tea, citrus fruit juices, wine and citrus fruits in the National Health and Nutrition Examination Surveys (NHANES) study [21].
The main sources of flavan-3ols and flavanones, namely, tea or citrus fruits and citrus fruits juices, were similar to those reported in the previous study by Ock Kyoung Chun et al. [21]. The main sources of isoflavones are soy products. Several soy products, such as natto, tofu and miso, were sources of isoflavone and contributed to up to 80% of the isoflavone intake in our study population. Quercetin, which is the main flavonol, commonly occurs in various food sources. It is abundantly present in onion, which is the main source of flavonol in Dutch diets [19]. Our results were similar to those of the previous study. The major sources of flavonol intake were not only onion but also leafy vegetables, such as moroheiya (nalta jute), Japanese radish leaves, komatsuna and okra. Flavones composed less than 1% of the total flavonoids ingested by participants in this study. Intake of flavones is much less compared to other flavonoids present in fruits and vegetables [22]. Parsley and celery were reported to be important sources of flavones in a previous study [11]. In this study, these foodstuffs were not considered to be major sources of flavones because they are consumed in lesser quantities compared to tsurumurasaki (malabar spinach) and green peppers.
The 24-h weighed DR method was used as a gold standard to estimate the total flavonoid intake in this study population. This is the most reliable method for estimation of the absolute intake of nutrients compared to any other methods of dietary assessment. By the 24-h weighed DR method, 5 major contributors of each flavonoids were about up to 90% of subclasses of flavonoid intakes. Estimating the total flavonoid intake and the major food sources contributing to the intake of each flavonoid sub-class was the first step in the understanding the DR-FFQ relationship. FFQ is used for estimating dietary nutrient intakes in epidemiologic studies. This method is cost effective and can assess diet over long periods of time. Yamamoto et al. adopted the FFQ to assess the isoflavone intake in the Japanese population [23]. The FFQ provides results that are both valid and reproducible, and hence, it can be used in epidemiological studies. They estimated isoflavone intakes from FFQ included in 8 major contributors of isoflavone intake and DR and measured the blood and urine isoflavone in from 215 subjects. The mean intake estimated from the FFQ, DR, serum concentrations and urine excretion in the case of daidzein were 71 µmol/d (18 mg/d), 59 µmol/d (15 mg/d), 120 nmol/L, 170 µmol/L, respectively, and in the case of genistein were 115 µmol/d (31 mg/d), 85 µmol/d (23 mg/d), 475 nmol/L, 14 µmol/L, respectively. Correlations between the daidzein intake values obtained from the FFQ and those from DR, serum and urine excretion were 0.64 (p<0.05), 0.31 (p<0.05) and 0.43 (p<0.05), respectively. They also demonstrated that a shorter version of the FFQ with 3 items showed a similar correlation as that with 8 items. In Western countries, a modified FFQ was used to estimate the daily intake of quercetin and naringenin [24]. The original questionnaire specified 38 food stuffs that were quercetin sources and 16 food stuffs as naringenin sources. The estimated mean intakes of quercetin and naringenin were 97 µmol/d (29 mg/d) and 214 µmol/d (58 mg/d), respectively. The mean urinary excretion of quercetin was 0.2 µmol/d (60 µg/d) and that of naringenin was 2 µmol/d (560 µg/d). The correlation between FFQ and urinary excretion of quercetin and naringenin was 0.82 (p<0.05) and 0.25 (p<0.05), respectively. Two original FFQs for assessing some flavonoid intakes have sufficient levels for epidemiologic studies.
Epidemiologic studies have indicated that the flavonoid intake is inversely associated with CHD. The Zutphen Elderly study demonstrated that catechin intake was significantly associated with reduced risks of ischaemic heart disease and CHD mortality, and there were 68% reductions in the mortality risk [25]. On the other hands, recently published data in a large prospective cohort of women from the Nurses’ Health Study does not support an inverse association between flavonol intake and CHD risk [26]. Epidemiological studies have not yet provided a consensus of the association between flavonoid intake and CHD diseases.
Our results showed that high intake of quercetin was associated with lower levels of circulating oxidized LDL in postmenopausal women who had an LDL-C concentration >140 mg/dL. Oxidized LDL is thought to play an important role in atherosclerosis. In fact, oxidized LDL has been recognized as a risk factor for CHD [27]. Pistavas et al. showed that the traditional Mediterranean diet, which includes antioxidant vitamins and phenolic compounds, is associated with lower levels of circulating oxidized LDL and higher total antioxidant capacity [28]. The traditional Mediterranean diet might help in decreasing the levels of circulating oxidized LDL. Recently published data indicated that an association exists between the antioxidant capacity of flavan-3-ols and suppression of oxidized LDL via intervention study. Baba et al. conducted a study in humans and reported that the cocoa polyphenol, which contains flavan-3-ol, might increase the resistance against LDL oxidation [29]. It has been suggested that the antioxidant capacity of flavonoids might contribute to their potentially protective role in the supression of LDL oxidation.
Quercetin has antioxidant capacities in vitro according to the 2,3-double bond in the C ring and the 4-oxo function and belongs to hydroxyl groups in positions 3, 5, 7, 3', 4'. [30]. Furthermore, quercetin conjugates also have antioxidative efficacies. Quercetin is converted to the conjugated metabolites by phase II enzyme. The major quercetin metabolites are the quercetin-3-glucronide and quercetin 3'-glucronoide in human and rat plasma [31, 32]. It is likely that quercetin-3-glucronide has an effect of antioxidant capacity in blood plasma LDL due to retaining its cathecol structure in positions 3' and 4' [31]. In addition, quercetin-3'-O-mehtylquercetin delayed the copper induced oxidation of human plasma LDL [7]. Quercetin conjugates are about half to two-thirds antioxidant activity of the aglycone [7, 31]. Moreover, quercetin conjugates retain some biological effects in vivo in human intervention studies [33]. Our results suggested that querectin could have a potentially protective role in suppression of LDL oxidation regardless of the effect of antioxidant vitamins. Therefore, a high consumption of quercetin may have a protective effect for chronic diseases such as CHD and CVD due to LDL oxidation.
There are some limitations to this study. First, although we estimated the flavonoid intake by the weighed DR method, which is a gold standard method, our sample study population was small. Second, because our study population included only middle-aged women, we have not considered age or sex-related variations in flavonoid intake. Third, we have not taken into account the seasonal variations in flavonoid intake. Seasonal variations play an important part in the Japanese diet. Since this factor influence the vegetable and fruit intake in Japan, the food sources of flavonoids would differ with the seasons. Fourth, flavonoid intake is provisional. Our data might have overestimated the flavonoid intake. Contents of flavonoids in foods are influenced by storage conditions, processing and cooking methods. Frying and microwaving cause 30% to 70% flavonoid degradation [34, 35]. By boiling, about 30% loss quercetin glycosides transfer to the boiling water [36]. Additionally, the flavonoid contents of food differ between the inner parts and outer parts of foods. Our Functional Food Factor database has taken these factors into consideration. Therefore, we estimated the flavonoids in the case of fresh-food intake by using the DR method. Finally, the values of epicatechin and epigallocatechin are included in non-gallate type in the FFF database. Tea contains epicatechin gallate and epigallocatechin gallate [20]. Therefore, the FFF database should distinguish catechins from garic acid esters of catechins.
In conclusion, we assessed the flavonoid intake and demonstrated the major food sources contributing to the levels of flavonoid in middle-aged Japanese women. The total flavonoid intake was higher in our study population compared to that in previous reports, and the sources of total flavonoid differed from those in Western countries. This is the first step towards understanding the DR-FFQ relationship in case of Japanese middle-aged women. Furthermore, analysis of the sub-samples from middle-aged Japanese women indicated that there may be an association between flavonoid intake and oxidized LDL, which might to be related to the incidence of CHD and CVD.
References
- 1.Hertog M.G., Feskens E.J., Hollman P.C., Katan M.B., Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 1993;342:1007–1011. doi: 10.1016/0140-6736(93)92876-u. [DOI] [PubMed] [Google Scholar]
- 2.Hertog M.G., Kromhout D., Aravanis C., Blackburn H., Buzina R., Fidanza F., Giampaoli S., Jansen A., Menotti A., Nedeljkovic S., Pekkarinen M., Simic B.S., Toshima H., Feskens E.J., Hollman P.C., Katan M.B. Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven countries study. Arch. Intern. Med. 1995;155:381–386. [PubMed] [Google Scholar]
- 3.Knekt P., Isotupa S., Rissanen H., Heliövaara M., Järvinen R., Häkkinen S., Aromaa A., Reunanen A. Quercetin intake and the incidence of cerebrovascular disease. Eur. J. Clin. Nutr. 2000;54:415–417. doi: 10.1038/sj.ejcn.1600974. [DOI] [PubMed] [Google Scholar]
- 4.Hirvonen T., Pietinen P., Virtanen M., Ovaskainen M.L., Häkkinen S., Albanes D., Virtamo J. Intake of flavonols and flavones and risk of coronary heart disease in male smokers. Epidemiology. 2001;12:62–67. doi: 10.1097/00001648-200101000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Morand C., Crespy V., Manach C., Besson C., Demigne C., Remesy C. Plasma metabolites of quercetin and their antioxidant properties. Am. J. Physiol. 1998;275:212–219. doi: 10.1152/ajpregu.1998.275.1.R212. [DOI] [PubMed] [Google Scholar]
- 6.Manach C., Morand C., Texier O., Favier M.L., Agullo G., Demigné C., Régérat F., Rémésy C. Quercetin metabolites in plasma of rats fed diets containing rutin or quercetin. J. Nutr. 1995;125:1911–1922. doi: 10.1093/jn/125.7.1911. [DOI] [PubMed] [Google Scholar]
- 7.Manach C., Morand C., Crespy V., Demigne C., Texier O., Regerat F., Remesy C. Quercetin is recovered in human plasma as conjugated derivatives which retain antioxidant properties. FEBS Lett. 1998;426:331–336. doi: 10.1016/s0014-5793(98)00367-6. [DOI] [PubMed] [Google Scholar]
- 8.Chopra M., Fitzsimons P.E., Strain J.J., Thurnham D.I., Howard A.N. Nonalcoholic red wine extract and quercetin inhibit LDL oxidation without affecting plasma antioxidant vitamin and carotenoid concentrations. Clin. Chem. 2000;46:1162–1170. [PubMed] [Google Scholar]
- 9.Manach C., Scalbert A., Morand C., Rémésy C., Jiménez L. Polyphenols: food sources and bioavailability. Am. J. Clin. Nutr. 2004;79:727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 10.Kühnau J. The flavonoids. A class of semi-essential food components: their role in human nutrition. World Rev. Nutr. Diet. 1976;24:117–191. [PubMed] [Google Scholar]
- 11.Sampson L., Rimm E., Hollman P.C., de Vries J.H., Katan M.B. Flavonol and flavone intakes in US health professionals. J. Am. Diet. Assoc. 2002;102:1414–1420. doi: 10.1016/s0002-8223(02)90314-7. [DOI] [PubMed] [Google Scholar]
- 12.Fink B.N., Steck S.E., Wolff M.S., Kabat G.C., Gammon M.D. Construction of a flavonoid database for assessing intake in a population-based sample of women on Long Island, New York. Nutr. Cancer. 2006;56:57–66. doi: 10.1207/s15327914nc5601_8. [DOI] [PubMed] [Google Scholar]
- 13.Adlercreutz H., Honjo H., Higashi A., Fotsis T., Hämäläinen E., Hasegawa T., Okada H. Urinary excretion of lignans and isoflavonoid phytoestrogens in Japanese men and women consuming a traditional Japanese diet. Am. J. Clin. Nutr. 1991;54:1093–1100. doi: 10.1093/ajcn/54.6.1093. [DOI] [PubMed] [Google Scholar]
- 14.Kokubo Y., Iso H., Ishihara J., Okada K., Inoue M., Tsugane S., for JPHC Study Group. Association of dietary intake of soy, beans, and isoflavones with risk of cerebral and myocardial infarctions in Japanese populations: the Japan Public Health Center-based (JPHC) study cohort I. Circulation. 2007;116:2553–2562. doi: 10.1161/CIRCULATIONAHA.106.683755. [DOI] [PubMed] [Google Scholar]
- 15.Melby M.K., Murashima M., Watanabe S. Phytochemical intake and relationship to past health history in Japanese women. Biofactors. 2004;22:265–269. doi: 10.1002/biof.5520220153. [DOI] [PubMed] [Google Scholar]
- 16.Kita J., Tada J., Ito M., Shirakawa M., Murashima M., Zhuo X.G., Watanabe S. Intake of phytochemicals among Japanese, calculated by the new FFF database. Biofactors. 2004;22:259–263. doi: 10.1002/biof.5520220152. [DOI] [PubMed] [Google Scholar]
- 17.Zhuo X.G., Watanabe S. The construction of web database server-client system for functional food factors. Biofactors. 2004;22:329–332. doi: 10.1002/biof.5520220165. [DOI] [PubMed] [Google Scholar]
- 18.Johannot L., Somerset S.M. Age-related variations in flavonoid intake and sources in the Australian population. Public Health Nutr. 2006;9:1045–1054. doi: 10.1017/s1368980006009712. [DOI] [PubMed] [Google Scholar]
- 19.Arts I.C., Hollman P.C., Feskens E.J., Bueno de Mesquita H.B., Kromhout D. Catechin intake and associated dietary and lifestyle factors in a representative sample of Dutch men and women. Eur. J. Clin. Nutr. 2001;55:76–81. doi: 10.1038/sj.ejcn.1601115. [DOI] [PubMed] [Google Scholar]
- 20.Arts I.C., van De Putte B., Hollman P.C. Catechin contents of foods commonly consumed in The Netherlands. 2. Tea, wine, fruit juices, and chocolate milk. J. Agric. Food Chem. 2000;48:1752–1757. doi: 10.1021/jf000026+. [DOI] [PubMed] [Google Scholar]
- 21.Chun O.K., Chung S.J., Song W.O. Estimated dietary flavonoid intake and major food sources of U.S. adults. J. Nutr. 2007;137:1244–1252. doi: 10.1093/jn/137.5.1244. [DOI] [PubMed] [Google Scholar]
- 22.Hertog M.G., Hollman P.C., Katan M.B. Content of potentially anticarcinogenic flavonoids of 28 vegetables and 9 fruits commonly consumed in the Netherlands. J. Agric. Food Chem. 1992;40:2379–2383. [Google Scholar]
- 23.Yamamoto S., Sobue T., Sasaki S., Kobayashi M., Arai Y., Uehara M., Adlercreutz H., Watanabe S., Takahashi T., Iitoi Y., Iwase Y., Akabane M., Tsugane S. Validity and reproducibility of a self-administered food-frequency questionnaire to assess isoflavone intake in a japanese population in comparison with dietary records and blood and urine isoflavones. J. Nutr. 2001;131:2741–2747. doi: 10.1093/jn/131.10.2741. [DOI] [PubMed] [Google Scholar]
- 24.Ranka S., Gee J.M., Biro L., Brett G., Saha S., Kroon P., Skinner J., Hart A.R., Cassidy A., Rhodes M., Johnson I.T. Development of a food frequency questionnaire for the assessment of quercetin and naringenin intake. Eur. J. Clin. Nutr. 2008;62:1131–1138. doi: 10.1038/sj.ejcn.1602827. [DOI] [PubMed] [Google Scholar]
- 25.Arts I.C., Hollman P.C., Feskens E.J., Bueno de Mesquita H.B., Kromhout D. Catechin intake might explain the inverse relation between tea consumption and ischemic heart disease: the Zutphen Elderly Study. Am. J. Clin. Nutr. 2001;74:227–232. doi: 10.1093/ajcn/74.2.227. [DOI] [PubMed] [Google Scholar]
- 26.Lin J., Rexrode K.M., Hu F., Albert C.M., Chae C.U., Rimm E.B., Stampfer M.J., Manson J.E. Dietary intakes of flavonols and flavones and coronary heart disease in US women. Am. J. Epidemiol. 2007;165:1305–1313. doi: 10.1093/aje/kwm016. [DOI] [PubMed] [Google Scholar]
- 27.Steinberg D., Parthasarathy S., Carew T.E., Khoo J.C., Witztum J.L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N. Engl. J. Med. 1989;320:915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- 28.Pitsavos C., Panagiotakos D.B., Tzima N., Chrysohoou C., Economou M., Zampelas A., Stefanadis C. Adherence to the Mediterranean diet is associated with total antioxidant capacity in healthy adults: the ATTICA study. Am. J. Clin. Nutr. 2005;82:694–699. doi: 10.1093/ajcn.82.3.694. [DOI] [PubMed] [Google Scholar]
- 29.Baba S., Natsume M., Yasuda A., Nakamura Y., Tamura T., Osakabe N., Kanegae M., Kondo K. Plasma LDL and HDL cholesterol and oxidized LDL concentrations are altered in normo- and hypercholesterolemic humans after intake of different levels of cocoa powder. J. Nutr. 2007;137:1436–1441. doi: 10.1093/jn/137.6.1436. [DOI] [PubMed] [Google Scholar]
- 30.Rice-Evans C.A., Miller N.J., Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996;20:933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- 31.Moon J.H., Tsushida T., Nakahara K., Terao J. Identification of quercetin 3-O-beta-D-glucuronide as an antioxidative metabolite in rat plasma after oral administration of quercetin. Free Radic. Biol. Med. 2001;30:1274–1285. doi: 10.1016/s0891-5849(01)00522-6. [DOI] [PubMed] [Google Scholar]
- 32.Day A.J., Mellon F., Barron D., Sarrazin G., Morgan M.R., Williamson G. Human metabolism of dietary flavonoids: identification of plasma metabolites of quercetin. Free Radic. Res. 2001;35:941–952. doi: 10.1080/10715760100301441. [DOI] [PubMed] [Google Scholar]
- 33.Williamson G., Barron D., Shimoi K., Terao J. In vitro biological properties of flavonoid conjugates found in vivo. Free Radic. Res. 2005;39:457–469. doi: 10.1080/10715760500053610. [DOI] [PubMed] [Google Scholar]
- 34.van der Sluis A.A., Dekker M., van Boekel M.A. Activity and concentration of polyphenolic antioxidants in apple juice. 3. Stability during storage. J. Agric. Food Chem. 2005;53:1073–1080. doi: 10.1021/jf040270r. [DOI] [PubMed] [Google Scholar]
- 35.Crozier A., Lean M.E.J., McDonald M.S., Black C. Quantitative analysis of the flavonoid content food of commercial tomatoes, onions, lettuce, and celery. J. Agric. Food Chem. 1997;45:590–595. [Google Scholar]
- 36.Ioku K., Aoyama Y., Tokuno A., Terao J., Nakatani N., Takei Y. Various cooking methods and the flavonoid content in onion. J. Nutr. Sci. Vitamiol. 2001;47:78–83. doi: 10.3177/jnsv.47.78. [DOI] [PubMed] [Google Scholar]