Abstract
Astaxanthin (Ax), a carotenoid ubiquitously distributed in microorganisms, fish, and crustaceans, has been known to be a potent antioxidant and hence exhibit various physiological effects. We attempted in these studies to evaluate clinical toxicity and efficacy of long-term administration of a new Ax product, by measuring biochemical and hematological blood parameters and by analyzing brain function (using CogHealth and P300 measures). Ax-rich Haematococcus pluvialis extracts equivalent to 4, 8, 20 mg of Ax dialcohol were administered to 73, 38, and 16 healthy adult volunteers, respectively, once daily for 4 weeks to evaluate safety. Ten subjects with age-related forgetfulness received an extract equivalent to 12 mg in a daily dosing regimen for 12 weeks to evaluate efficacy. As a result, no abnormality was observed and efficacy for age-related decline in cognitive and psychomotor functions was suggested.
Keywords: astaxanthin, bioreactor, brain function, clinical efficacy, clinical safety
Introduction
Astaxanthin (Ax) is a fat-soluble compound classified into xanthophylls that are oxygenated derivatives of carotenoids. In nature, Ax is a naturally-occurring red pigment and is widely found in microorganisms (e.g., bacteria, microalgae, yeast), crustaceans (e.g., lobster, krill, shrimp), fish (e.g., salmon, trout) and some birds (e.g., flamingo, quail) [1–4]. Despite the ubiquitous distribution of Ax, animals cannot synthesize the carotenoid de novo, while microorganisms and plants are able to produce it. Ax, particularly the chemically-synthesized version, has therefore been used in feed additives as a color enhancer in aquaculture and chicken egg farms. Recently, attention has been focused on the possible use of Ax in human health management, because of its unique structural and chemical properties, including strong antioxidant activity [1–8]. For this market, a green microalga Haematococcus pluvialis, a red yeast Phaffia rhodozyma and crustacean byproducts are commercially available as natural sources of the pigment. Of these three sources, H. pluvialis is known to be the richest source of natural Ax (1.5–3.0% of the dry biomass) and various algal cultivation systems (mainly outdoor systems) have been developed at an industrial scale [2, 4, 5, 9–11]. As pointed out by Margalith [12], light intensity and temperature are the most critical factors affecting both algal growth and Ax accumulation. He also noted that a selective culture environment to prevent bacterial and/or protozoal overgrowth is a key factor to successful algal cultivation, but no such environment is currently available for this alga. A culturing technology which enables both higher Ax production and greater sanitary control is therefore desired.
We have recently developed an indoor cultivation system, namely the “YAMAHA High-efficiency Photobioreactor” to manufacture Ax-containing H. pluvialis algal biomass. The YAMAHA bioreactor is a vertical flat-plate type reactor known to have excellent light illumination efficiency, leading to superior algal growth [13]. The bioreactors are illuminated continuously with strong synthetic light and maintained at a desired temperature to maximize Ax accumulation in algal cells. In order to minimize the risk of contamination, the reactors are isolated in a Class 10,000 cleanroom; cultivation and all operations are performed in this environment. High-quality and high-quantity Ax-containing algal oil has thus been produced using these bioreactors since October 2006, and the manufacturing process was given GMP certification by the Japanese Institute for Health Food Standards (JIHFS) in July 2007.
In the present study, we investigated the clinical safety of this new Ax-rich oil, since it is qualitatively and quantitatively different from other existing Ax-containing H. pluvialis oil products. Possible efficacy in the treatment of age-related forgetfulness was also studied.
Methods
Clinical study design
All study protocols and informed consent documents were reviewed and approved by the ethics committee of the Yamaha Motor Co., Ltd. and Anti-Aging Science. All study subjects provided written informed consent prior to the start of study participation. The protocols were carried out under the provisions of the Declaration of Helsinki.
Study 1
An open-label clinical study was conducted in 127 subjects between 20 to 60 years of age. The subjects were divided into three groups, low-dose (Group A: 51 men, 22 women), middle-dose (Group B: 21 men, 17 women), and high-dose (Group C: 12 men, 4 women). The subjects ingested Ax-rich H. pluvialis oil (Puresta®, Yamaha Motor Co., Ltd.; Iwata, Shizuoka, Japan) equivalent to 4 mg (Group A), 8 mg (Group B), or 20 mg (Group C) of Ax dialcohol contained in soft capsules, once daily for 4 weeks. Blood biochemical and hematological examinations were completed before (baseline) and after 4 weeks of dosing. Differences between baseline and post-dosing values were tested using Wilcoxon’s Signed Rank test (using SPSS for Windows; SAS Institute, Cary, NC), where p<0.1 was considered statistically significant.
Study 2
An open-label clinical study was conducted using 10 otherwise healthy male subjects (50–69 years of age) who complained of age-related forgetfulness. The subjects ingested Ax-rich H. pluvialis oil (Puresta®, Yamaha Motor Co., Ltd.) equivalent to 12 mg of Ax dialcohol contained in soft capsules once daily for 12 weeks. Cognitive function was evaluated before administration (at baseline) and again every 6 weeks during the study, using either the CogHealth tool (CogState; Melbourne, Australia) or the event-related P300 recognition response elicited by an auditory task [14]. Differences between baseline and post-baseline values were analyzed using Dunnett’s multiple comparison test, where p<0.05 was considered statistically significant and p<0.1 was considered as tendency toward significance.
Results
Study 1
The results of the blood pressure and hematological examinations are shown in Table 1. Findings from the blood biochemical examinations are shown in Table 2. Among vital signs and laboratory analysis (hematology, hepatic, and renal function tests) changes from baseline, no statistically significant changes were noted for any of the dose groups. There were no adverse effects or laboratory abnormalities observed for any dose group. In addition, no adverse event attributed to the administration of the test material was reported in the subject interviews expect for ‘red stool’, which was associated with the color of the test material.
Table 1.
Changes in blood pressure and hematological parameters (mean ± SD) before and after administration of Puresta®
| Parameter | Group A | Group B | Group C |
|---|---|---|---|
| HBP (mm Hg) | |||
| before | 121 ± 14 | 118 ± 14 | 119 ± 17 |
| after | 125 ± 12 | 122 ± 15 | 116 ± 13 |
| LBP (mm Hg) | |||
| before | 76 ± 12 | 74 ± 12 | 72 ± 14 |
| after | 78 ± 12 | 74 ± 13 | 68 ± 11 |
| WBC (×109/liter) | |||
| before | 5.7 ± 1.7 | 6.2 ± 1.6 | 5.5 ± 1.6 |
| after | 6.2 ± 2.1 | 6.0 ± 1.7 | 5.3 ± 0.8 |
| RBC (×1012/liter) | |||
| before | 4.7 ± 0.4 | 4.8 ± 0.6 | 4.8 ± 0.4 |
| after | 4.8 ± 0.6 | 4.9 ± 0.5 | 4.9 ± 0.5 |
| Hb (g/liter) | |||
| before | 144 ± 15 | 137 ± 21 | 148 ± 12 |
| after | 146 ± 15 | 139 ± 21 | 150 ± 13 |
| Hct (%) | |||
| before | 45 ± 4 | 43 ± 5 | 45 ± 3 |
| after | 46 ± 4 | 44 ± 5 | 46 ± 3 |
| MCV (fL) | |||
| before | 94 ± 6 | 90 ± 7 | 95 ± 4 |
| after | 95 ± 6 | 91 ± 7 | 95 ± 4 |
| MCH (pg) | |||
| before | 30.5 ± 2.4 | 28.9 ± 3.2 | 30.7 ± 1.6 |
| after | 30.2 ± 2.4 | 28.7 ± 3.3 | 30.6 ± 1.7 |
| MCHC (%) | |||
| before | 32 ± 1 | 32 ± 2 | 33 ± 1 |
| after | 32 ± 1 | 32 ± 2 | 32 ± 1 |
| Platelets (×109/liter) | |||
| before | 258 ± 50 | 279 ± 59 | 237 ± 52 |
| after | 256 ± 51 | 280 ± 58 | 239 ± 56 |
Group A = Puresta dose equivalent to 4 mg Ax/day, Group B = Puresta dose equivalent to 8 mg Ax/day, Group C = Puresta dose equivalent to 20 mg Ax/day.
No significant differences noted from baseline to end of treatment for any of the treatment groups.
Table 2.
Changes in blood biochemical parameters (mean ± SD) before and after administration of Puresta®
| Parameter | Group A | Group B | Group C |
|---|---|---|---|
| BUN (mg/dl) | |||
| before | 12.7 ± 3.1 | 12.2 ± 2.5 | 12.1 ± 1.3 |
| after | 13.3 ± 3.1 | 12.6 ± 2.7 | 12.6 ± 1.7 |
| Creatinine (mg/dl) | |||
| before | 0.8 ± 0.1 | 0.8 ± 0.2 | 0.8 ± 0.1 |
| after | 0.9 ± 0.1 | 0.9 ± 0.2 | 0.9 ± 0.1 |
| Fasting glucose (mg/dl) | |||
| before | 92 ± 13 | 95 ± 19 | 85 ± 12 |
| after | 93 ± 9 | 95 ± 15 | 91 ± 9 |
| AST (U/liter) | |||
| before | 23 ± 8 | 22 ± 5 | 24 ± 8 |
| after | 22 ± 8 | 22 ± 7 | 22 ± 8 |
| ALT (U/liter) | |||
| before | 25 ± 14 | 24 ± 13 | 32 ± 17 |
| after | 25 ± 13 | 23 ± 12 | 31 ± 21 |
| γ-GTP (U/liter) | |||
| before | 43 ± 33 | 34 ± 26 | 37 ± 23 |
| after | 46 ± 38 | 33 ± 22 | 33 ± 20 |
Group A = Puresta dose equivalent to 4 mg Ax/day.
Group B = Puresta dose equivalent to 8 mg Ax/day.
Group C = Puresta dose equivalent to 20 mg Ax/day.
No significant differences noted from baseline to end of treatment for any of the treatment groups.
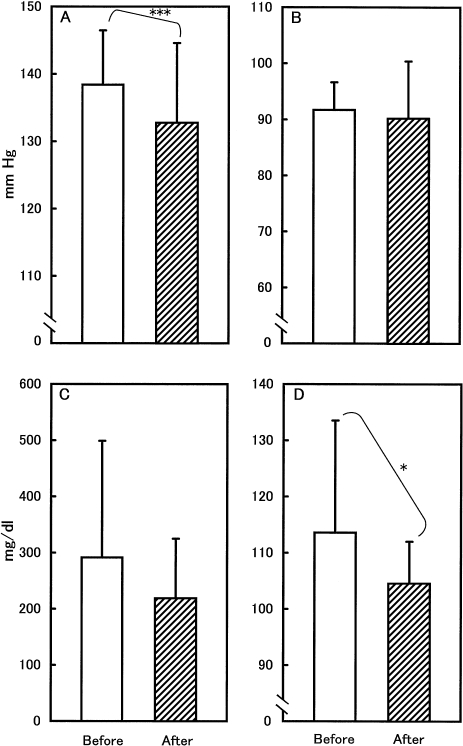
We then attempted to predict clinical efficacy of Ax in the treatment of metabolic syndrome. Subjects whose starting systolic blood pressure (SBP), diastolic blood pressure (DBP), triglyceride (TG) and fasting glucose (FG) values meet the diagnosis of metabolic syndrome (SBP≥130 mmHg, DBP≥85 mmHg, TG≥150 mg/dl, and FG≥100 mg/dl; [15]) were selected from Group A. Mean baseline values were calculated and compared to those after treatment (Fig. 1). As shown in Fig. 1A, a significant (p<0.01) decrease in SBP was observed when subjects were administered 4 mg Ax orally once daily for 4 weeks. No such decrease was apparent in DBP (Fig. 1B). The mean TG value after treatment (218 mg/dl) was much lower than the baseline value (292 mg/dl), although there was no statistical significance between them (Fig. 1C). Fasting glucose decreased with a tendency toward significance (p<0.1) after treatment (Fig. 1D).
Fig. 1.
Changes in systolic blood pressure (A), diastolic blood pressure (B), triglyceride (C), and fasting glucose (D) values before and after oral administration of Puresta equivalent to 4 mg of Ax dialcohol once daily for 4 weeks. The number of subjects per group was 20, 16, 12, and 10 in Groups A, B, C, and D, respectively. Error bars represents standard deviations. *** and * represent p<0.01 and p<0.1, respectively.
Study 2
In the present study, we investigated the effect of Ax on brain function in healthy older adults with age-related forgetfulness using the CogHealth test, since CogHealth is a cognitive function test specifically designed to detect changes in healthy or mildly-impaired subjects at an early date [16]. The test evaluates five cognitive domains—psychomotor speed, impulse control, working memory, episodic learning, and attention—by measuring the subject’s response times (RTs) and accuracy (i.e., the hit rate percentage). Table 3 represents mean response time on the following CogHealth tasks—simple reaction, choice reaction, working memory, delayed recall, and divided attention—at baseline, and after 6 and 12 weeks of Ax treatment. The mean accuracy of the ‘working memory’ and ‘delayed recall’ tasks is also shown in the table. Significant reduction in response time was apparent for the ‘divided attention’ task after 6 weeks and on all tasks after 12 weeks. The accuracy on the ‘working memory’ task was improved significantly after 12 weeks of treatment, while no such effect was observed for the ‘delayed recall’ task.
Table 3.
Mean response times and accuracies (±SD) on CogHealth tasks at baseline, and after 6 and 12 weeks of Ax treatment.
| Tasks | Mean ± SD |
||
|---|---|---|---|
| Baseline | 6 weeks | 12 weeks | |
| Response time (ms) | |||
| Simple Reaction | 341.68 ± 94.41 | 303.31 ± 33.80 | 281.76 ± 33.56** |
| Choice Reaction | 504.53 ± 56.84 | 480.63 ± 39.87 | 463.63 ± 26.49** |
| Divided Attention | 494.13 ± 135.57 | 419.52 ± 59.32** | 412.07 ± 51.97** |
| Working Memory | 762.94 ± 141.65 | 732.95 ± 174.83 | 654.83 ± 128.42** |
| Delayed Recall | 1008.19 ± 153.37 | 975.40 ± 190.75 | 916.77 ± 151.04** |
| Accuracy (%) | |||
| Working Memory | 90.46 ± 7.18 | 95.22 ± 5.37 | 96.30 ± 3.94** |
| Delayed Recall | 70.95 ± 6.42 | 71.19 ± 5.98 | 70.71 ± 8.91 |
** p<0.05 vs baseline.
We then evaluated cognitive function using an event-related potential, the P300, which reflects processes involved in memory, response modulation, and contextual updating, and has been used to evaluate mental workload [17, 18]. The mean P300 latency and amplitude values at baseline and after 12 weeks of treatment are shown in Table 4. The P300 peak amplitude tended to increase (p<0.1) after 12 weeks, while no such change was apparent in the latency. It is thought that the amplitude and latency reflect capacity and speed, respectively, of cognitive information processing [19], and that both parameters closely relate to selective attention [20]. The improving effect of Ax on the amplitude of P300 therefore indicates that this carotenoid might increase patient information processing capacity and selective attention.
Table 4.
Mean ( ± SD) P300 latency and amplitude values at baseline and after 12 weeks of Ax treatment.
| Parameter | Mean ± SD |
|
|---|---|---|
| Baseline | 12 weeks | |
| Latency (ms) | 359.40 ± 16.49 | 363.10 ± 29.22 |
| Amplitude (µV) | 7.60 ± 4.05 | 10.54 ± 3.39* |
* p<0.1 vs baseline
Discussion
In the present study, we found that no abnormality was apparent when adult subjects ingested Ax-containing H. pluvialis oil product, Puresta®, up to 20 mg (equivalent to Ax dialcohol) once daily for 4 weeks. The safety of Puresta® has been investigated previously in a clinical study where 17 subjects were administered Puresta® amounts equivalent to 8 mg of Ax dialcohol twice daily for 12 weeks [21]. Our current findings further support the safety of this product at a dose of 20 mg/day, with no negative effects on blood chemistry or hematology results over time, and no subject reports of adverse experiences during treatment. We also found positive effects of this product on metabolic syndrome and cognitive function.
Metabolic syndrome is a common growing problem in human health in industrialized countries. Hussen reported that administration of astaxanthin decreased the blood pressure of the SHR rat (a hypertensive rat model) [22]. However our study is the first instance to show efficacy in humans. We observed previously that HbA1c and TNF-α levels decreased significantly, while adiponectin levels increased significantly when volunteers with borderline metabolic syndrome were administered Puresta® equivalent to 8 mg of Ax dialcohol twice daily for 12 weeks [21]. The decreases in systolic blood pressure, triglyceride, and fasting glucose values observed in the present study may support that the product has a beneficial effect in patients with borderline diabetes mellitus or persons at risk for metabolic syndrome.
The findings from our second study using CogHealth and P300 suggest that administration of Ax might improve higher brain function including cognition, attention, memory, information processing, and resultant behaviors in older persons. Little information is available in animal models in regards to improvements in brain function with this food supplement. Hussein et al. [22] reported that Ax showed some memory-improving effects in ischemic mice. Administration of H. pluvialis powder also significantly improved memory function in wild-type mice [23]. However, there had been no study investigating effect on Ax on higher cognitive function of human brain. To our knowledge, the present study is the first report suggesting the beneficial effects of Ax on cognitive function in humans. All tasks investigated by CogHealth and P300 are indispensable for skillful performance in the daily life activities. For example, driving is a complex form of activity involving especially cognitive and psychomotor functions. Age-related decreases in these tasks are therefore a serious problem causing increased number of death of old persons by traffic accident. Ax would be a one of the promising food factors that contribute to ameliorate those social problems. Further clinical studies remain to be carried out to confirm these possibilities.
References
- 1.Miki M. Biological functions and activities of animal carotenoids. Pure Appl. Chem. 1991;63:141–146. [Google Scholar]
- 2.Higuera-Ciapara I., Félix-Valenzuela L., Goycoolea F.M. Astaxanthin: A review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006;46:185–196. doi: 10.1080/10408690590957188. [DOI] [PubMed] [Google Scholar]
- 3.Hussein G., Sankawa U., Goto H., Matsumoto K., Watanabe H. Astaxanthin, a carnotenoid with potential in human health and nutrition. J. Nat. Prod. 2006;69:443–449. doi: 10.1021/np050354+. [DOI] [PubMed] [Google Scholar]
- 4.Johnson E.A., An G.H. Astaxanthin from microbial sources. Crit. Rev. Biotechnol. 1991;11:297–326. [Google Scholar]
- 5.Guerin M., Huntley M.E., Olaizola M. Haematococcus astaxanthin: applications for human health and nutrition. Trends Biotechnol. 2003;21:210–216. doi: 10.1016/S0167-7799(03)00078-7. [DOI] [PubMed] [Google Scholar]
- 6.Palozza P., Krinsky N.I. Astaxanthin and canthaxanthin are potent antioxidants in a membrane model. Arch. Biochem. Biophys. 1992;297:291–295. doi: 10.1016/0003-9861(92)90675-m. [DOI] [PubMed] [Google Scholar]
- 7.Di Mascio P., Murphy M.E., Sies H. Antioxidant defense systems: the role of carotenoids, tocopherols, and thiols. Am. J. Clin. Nutr. 1991;53:194S–200S. [PubMed] [Google Scholar]
- 8.Naguib Y.M.A. Antioxidant activities of astaxanthin and related carotenoids. J. Agric. Food Chem. 2000;48:1150–1154. doi: 10.1021/jf991106k. [DOI] [PubMed] [Google Scholar]
- 9.Del Campo J.A., García-González M., Guerrero M.G. Outdoor cultivation of microalgae for carotenoid production: current state and perspectives. Appl. Microbiol. Biotechnol. 2007;74:1163–1174. doi: 10.1007/s00253-007-0844-9. [DOI] [PubMed] [Google Scholar]
- 10.Olaizola M. Commercial production of astaxanthin from Haematococcus pluvialis using 25,000-liter outdoor photobioreactors. J. Appl. Phycol. 2000;12:499–506. [Google Scholar]
- 11.Olaizola M., Huntley M.E. In: Recent advantages in commercial production of astaxanthin from microalgae, in Biomaterials and bioprocessing. Fingerman M., Nagabhushanam R., editors. Science Publishers; Enfield: 2003. pp. 143–164. [Google Scholar]
- 12.Margalith P.Z. Production of ketocarotenoids by microalgae. Appl. Microbiol. Biotechnol. 1999;51:431–438. doi: 10.1007/s002530051413. [DOI] [PubMed] [Google Scholar]
- 13.Zhang K., Miyachi S., Kurano N. Evaluation of a vertical flat-plate photobioreactor for outdoor biomass production and carbon dioxide bio-fixation: effects of reactor dimensions, irradiation and cell concentration on the biomass productivity and irradiation utilization efficiency. Biotechnol. Lett. 2001;23:21–26. doi: 10.1007/s002530000550. [DOI] [PubMed] [Google Scholar]
- 14.A guideline for recording ERPs by the Japanese Society of Clinical Neurophysiology. Nouha to Kindenzu. 1997;25:1–16. (in Japanese) [Google Scholar]
- 15.Committee to evaluate diagnostic standards for metabolic syndrome. Nippon Naika Gakkai Zasshi. 2005;94:794–809. (in Japanese) [PubMed] [Google Scholar]
- 16.Collie A., Maruff P., Darby D.G., McStephen M. The effects of practice on the cognitive test performance of neurologically normal individuals assessed at brief test-retest intervals. J. Int. Neuropsychol. Soc. 2003;9:419–428. doi: 10.1017/S1355617703930074. [DOI] [PubMed] [Google Scholar]
- 17.Donchin E., Kramer A.F., Wickens C.D. In: Applications of brain event-related potentials to problems in engineering psychology, in Psychophysiology: Systems, processes, and applications. Coles M.G.H., Donchin E., Porges S.W., editors. Guilford Press; New York: 1986. pp. 702–718. [Google Scholar]
- 18.Kramer A.F., Weber T. In: Applications of psychophysiology to human factors, in Handbook of psychophysiology (2nd ed.) Cacioppo J.T., Tassinary L.G., Berntson G.G., editors. Cambridge University Press; New York: 2000. pp. 794–814. [Google Scholar]
- 19.Naatanen R. Selective attention and evoked potentials in humans—a critical review. Biol. Psychol. 1975;2:237–307. doi: 10.1016/0301-0511(75)90038-1. [DOI] [PubMed] [Google Scholar]
- 20.Wickens C.D., Kramer A.F., Vanasse L., Donchin E. Performance and concurrent tasks: a psychophysiological analysis of the reciprocity of information-processing resources. Science. 1983;221:1080–1082. doi: 10.1126/science.6879207. [DOI] [PubMed] [Google Scholar]
- 21.Uchiyama A., Okada Y. Clinical efficacy of astaxanthin-containing Haematococcus pluvialis extract for the voluntees at risk of metabolic syndrome. J. Clin. Biochem. Nutr. 2008;43 Suppl. 1:38–43. [Google Scholar]
- 22.Hussein G., Nakamura M., Zhao Q., Iguchi T., Goto H., Sankawa U., Watanabe H. Antihypertensive and neuroprotective effects of astaxanthin in experimental animals. Biol. Pharm. Bull. 2005;28:47–52. doi: 10.1248/bpb.28.47. [DOI] [PubMed] [Google Scholar]
- 23.Zhang X., Pan L., Wei X., Gao H., Liu J. Impact of astaxanthin-enriched algal powder of Haematococcus pluvialis on memory improvement in BALB/c mice. Environ. Geochem. Health. 2007;29:483–489. doi: 10.1007/s10653-007-9117-x. [DOI] [PubMed] [Google Scholar]