Abstract
Endoscopic submucosal dissection (ESD) has the advantage over endoscopic mucosa resection, permitting removal of gastrointestinal neoplasms en bloc, but is associated with relatively high risk of complications. Indications for early gastric cancer (EGC) are expanded: mucosal cancer without ulcer findings irrespective of tumor size; mucosal cancer with ulcer findings ≤3 cm in diameter; and minute submucosal invasive cancer ≤3 cm in size. The indications for early esophageal cancer (EEC) are the tumors confined to the two-third layer of the lamina propria. The EEC lesions spreading more than three-quarter of circumference of the esophagus are at frequent risk of stenosis. The procedures include marking, submucosal injection, circumferential mucosal incision and exforiation of the lesion along the submucosal layer. Complete ESD can achieve a large one-piece resection, allowing precise histological assessment to prevent recurrence. Clinical outcomes of gastric and esophageal ESD have been promising, and the prognosis of EGC patients treated by ESD is likely to be excellent, though further longer follow-up studies are warranted. Notification of perforation risk is essential in particular for esophageal ESD. Bleeding during ESD can be managed with coagulation forceps, and postoperative bleeding may be reduced with routine use of the stronger acid suppressant, proton pump inhibitors.
Keywords: endoscopic submucosal dissection, proton pump inhibitor, early gastric cancer, early esophageal cancer, Barrett’s adenocarcinoma
Introduction
Early gastric cancer (EGC) is defined as gastric cancer that is confined to the mucosa or submucosa (T1 cancer), irrespective of the presence of regional lymph node metastases [1]. Currently, almost 10,000 cases of EGC are being detected every year in Japan, corresponding to 40% to 50% of all gastric cancers [2]. Endoscopic mucosal resection (EMR) is widely accepted as a standard treatment for EGC with nominal risk of lymph node metastasis, as it is minimally invasive, safe, and convenient [3, 4]. The conventional EMR is associated with a high risk of local recurrence in such cases, especially when resections are accomplished in multiple segments or the margins are not clear [5].
Endoscopic submucosal dissection (ESD) has been developed to dissect directly along the submucosal layer using an insulation-tipped diathermy knife (IT knife) [6, 7]. Earlier studies have been documented the advantage of ESD over conventional EMR for removing larger or ulcerated EGC lesions in an en bloc manner [8–10]. Thus, ESD allows precise histological assessment of the resected specimens, and it may prevent residual disease and local recurrence [4, 5, 11]. More recently, ESD has been successfully applied for early esophageal cancer (EEC) including squamous cell carcinoma and Barrett’s adenocarcinoma [12–14].
Although the short-term results of ESD are promising, a higher risk of procedure-related complications remains unresolved in this innovative procedure [5, 9, 10, 12–15]. The complications of endoscopic resection for EGC include abdominal pain, bleeding, and perforation [16]. Bleeding is the most common complication, occurring in up to no less than 7% of patients undergoing ESD [16]. On the other hand, accumulating evidence has documented that bleeding occurs in 1.2–11.6% of EGC patients treated by EMR [16]. Proton pump inhibitors (PPIs) and H2-receptor antagonists (H2RAs) have a significant effect on preventing bleeding from the ulcer and facilitating the ulcer healing [17]. ESD creates larger artificial ulcers with greater risks of bleeding, but whether the stronger acid suppressant, PPIs would reduce incidence of the complication is unknown.
In this review, we sought to outline the endoscopic indications, techniques, clinical outcomes and management of the complications of ESD in the upper gastrointestinal tract, in the era of PPIs, the first choice drug for acid-peptic diseases.
Indications of ESD
EMR is widely accepted as a standard treatment for EGC with nominal risk of lymph node metastasis, as it is minimally invasive, safe, and convenient [3, 4]. However, the snaring procedure is not reliable for lesions larger than 20 mm in diameter or lesions with ulcer findings [5, 8]. The conventional EMR is associated with a high risk of local recurrence in such cases, especially when resections are not accomplished en bloc or the margins are not clear [5]. At present, the guideline criteria for EMR, which were established by the Japanese Gastric Cancer Association, have been generally accepted, and they state that: (1) elevated EGCs less than 2 cm in diameter and (2) small (≤1 cm) depressed EGCs without ulceration are absolutely indicated for EMR [18]. At the same time, these lesions must be differentiated adenocarcinoma confined to the mucosa with no lymphatic or vascular involvement. However, it has been observed clinically that the accepted indications for EMR can be too strict, leading to unnecessary surgery [5, 16].
Recently, Gotoda et al. analyzed more than 5,000 EGC patients who underwent gastrectomy with meticulous D2 level lymph node dissection; they provided important information on the risks of lymph node metastasis, wherein differentiated gastric cancers (well and moderately differentiated tubular adenocarcinoma and papillary adenocarcinoma) with no lymphatic-vascular involvement, correlating with a nominal risk of lymph node metastasis, were defined [19]. Thus, they proposed the expanded criteria for endoscopic resection: (1) mucosal cancer without ulcer findings irrespective of tumor size; (2) mucosal cancer with ulcer findings ≤3 cm in diameter; and (3) minute (<500 µm from the muscularis mucosae) submucosal invasive cancer ≤3 cm in size [4, 5]. These groups of patients have been shown to have no risk or a lower risk of lymph node metastasis compared with the risk of mortality from surgery. Nowadays, en bloc resection of the tumors that fit the expanded criteria is achievable with ESD. In fact, attempts to expand the indications for ESD to treat EGC are currently underway in many Japanese institutes.
EEC involving the epithelium (m1: carcinoma in situ) or the lamina propria (m2) are candidates for endoscopic therapies including ESD and EMR, because no lymph node metastasis have been reported in EEC confined to these layers [20]. For EEC invading the muscularis mucosa (m3), the lymph node metastasis rate is reported as 9%, and for cancer with minute submucosal invasion the rate is increased with 19% [21]. Therefore, for patients unwilling for esophagectomy or patients with comorbid diseases, endoscopic treatment may be a relative indication for m3 or sm1 cancer. EEC spreading more than three-quarter of circumference of the esophagus are not absolutely indicated even if the invasion depth is limited to m1 or m2 [20, 21]. Although such lesions can be removed en bloc with ESD, they are considered as the relative indication. Nevertheless, intensive balloon dilatations or tentative stent insertion may prevent stricture [12, 20, 22]. Previous studies have suggested a satisfactory prognosis after EMR, and EMR has been used for the treatment of EEC or high-grade dysplasia [23]. Despite its efficacy, this method is sometimes associated with local recurrences, especially when lesions larger than 20 mm are resected in a piecemeal manner. In turn, ESD allows en bloc resection for EEC, irrespective of size. In fact, successful resection of large esophageal cancers by ESD has been reported in relatively small numbers of the case series [12, 23]. When the efficacy of ESD for smaller lesions ≤20 mm was compared with that of EMR, ESD was found to be the best endoscopic resection method even for the smaller EEC [23].
Endoscopic treatment is an alternative to esophagectomy in Barrett’s esophagus patients with superficial adenocarcinoma due to the nominal risk of lymph node involvement or distal metastases [20]. For Barrett’s adenocarcinomas, EMR has limitations with respect to the resectable tumor size; in many cases, piecemeal resection is unavoidable and has been occasionally linked to local recurrence [20]. Recently, ESD has been used to remove the esophagogastric junction tumors including Barrett’s neoplasms with promising results [13, 14]. However, there are no available data about nodal metastases from the large numbers of surgically resected cases of Barrett’s adenocarcinoma at an early stage. Indeed, there is no or nominal risk of nodal metastasis for the intramucosal Barrett’s adenocarcinoma, but the tumors with massive sm involvement are associated with considerable risk for metastatic disease [20]. There is no consensus whether one should apply to Barrett’s adenocarcinomas confined within the upper third of the submucosa the same criteria for gastric epithelial neoplasms or esophageal squamous cell neoplasms [20].
ESD Technique
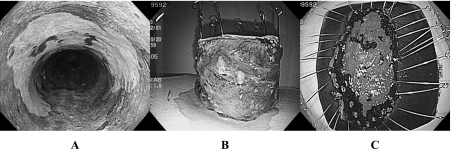
ESD, which is performed with special endoscopic knives such as IT knife, has been developed for en bloc resection with a standard single-channel gastroscope [6]. This innovative technique has the great advantage for resections of larger and/or ulcerated lesions in one-piece over EMR [5, 6]. Under written informed consent, the ESD procedures, which are described previously in detail [24], are done with the following steps. EGC is first identified and demarcated using white-light endoscopy and chromoendoscopy with indigo-carmine solution, and then marking around the lesions is carried out with spotty cautery. A 10% glycerin plus 5% fructose in 0.9% saline solution (Glyceol; Chugai Pharmaceutical Co., Tokyo, Japan) was injected into the submucosal layer to lift the mucosa. A circumferential mucosal incision is made around the lesion using the IT knife (Olympus Optical Co., Tokyo, Japan). A high-frequency generator (ICC200; ERBE Elektromedizin GmbH, Tübingen, Germany) is used during incision of the mucosa set the Endo cut mode at Effect 3, output 80W. The ceramic ball at the tip of IT knife prevents perforation of the muscle layer. Then, submucosal dissection is performed for exforiation of the lesion using the IT-knife and/or the Hook-knife (Olympus Optical Co.) in technically difficult situations. The solution can be injected into the submucosa at any time to raise and confirm the submucosal layer. A cap attachment (Olympus Optical Co.) is frequently useful for creating countertraction, making it easier to exfoliate the submucosal tissue directly. Complete endoscopic submucosal dissection can achieve a large one-piece resection. The ESD procedures for EEC are performed in the similar way to those for EGC with several modifications for the esophagus. A mucosal incision is made circumferentially around the lesion using the FlushKnife (Fujinon-Toshiba ES System Co., Omiya, Japan). A submucosal injection solution is prepared by mixing 1 volume of Suvenyl (a 10 mg/mL solution of 1900 kD hyaluronic acid in physiologic saline, Chugai Pharmaceutical Co.) with 3 volumes of the Glyceol. The mucosal incision employs the ENDOCUT mode, effect 2 (output 60W). The distal half of the mucosal incision is completed first. The proximal half is incised after the submucosal layer is dissected slightly, as described in the following steps. Before incising all the way around the markings, dissection of the submucosa is started beneath the area where the mucosal incision is made. This is done to avoid flattening the remaining area lifted by the cushioning solution as time passes. The submucosal dissection is done using the Flush knife and/or the Hook knife, under the forced coagulation mode (output 40W), until the lesion is detached. With this highly skilled technique, curative resection (en bloc resection with the tumor-free lateral/vertical margins) was achieved in circumference for a case of semicircular esophageal squamous cell carcinoma, approximately 7 cm wide (Fig. 1). Even if the invasion depth of EECs spreading more than three-quarter of circumference of the esophagus is limited to m1 or m2, they are considered as the relative indication due to the unavoidable stricture.
Fig. 1.
Endoscopic submucosal dissection in a case of semicircular esophageal squamous cell carcinoma, approximately 7 cm wide. Choromoendoscopy with an iodine solution revealed the iodine unstained area spreading three-quarter of circumference of the esophagus (A). En bloc removal of the lesion was achieved in circumference (B). Resected specimen removed by ESD showed complete resection with the tumor-free margin (C).
To control bleeding during ESD or to prevent possible bleeding from visible vessels in the artificial ulcer immediately after the resection, a hemostatic forceps (Coagrasper, Olympus Optical Co.) is employed in the soft coagulation mode (60W for EGC and 50W for EEC).
All patients are sedated by intravenous injection of 5–7.5 mg diazepam (Cercine; Takeda Pharmaceutical Co., Osaka, Japan) and 15 mg pentazocine (Pentazin, Daiichi-Sankyo Pharmaceutical Co., Tokyo, Japan), and 2.5 mg diazepam was additionally given for sedation as needed throughout the procedure.
The excised specimens were fixed in 10% buffered formalin, paraffin-embedded, sectioned perpendicularly at 2-mm intervals and stained with hematoxylin and eosin. Macroscopic appearance, histological type, the tumor size, the depth of invasion, the presence of ulcerative changes, lymphatic and vascular involvement, and tumor involvement to the lateral and vertical margins are assessed. En bloc resection refers to a resection in one-piece. When the lesion had to be removed in multiple segments, the piecemeal-resected specimens were reconstructed as completely as possible. Resections were deemed curative when removal is achieved with tumor-free lateral and vertical margins. By definition, there should be no lymphatic and vascular involvement. In addition, there should be no submucosal invasion deeper than 500 µm from the muscularis mucosae for EGC or be limited to the m1 or m2 in cases of EEC.
Management of Complications during ESD and Clinical Course after ESD
The complications of endoscopic resection for EGC include pain, bleeding, and perforation [16]. Pain after resection is typically mild. Bleeding is the most common complication, occurring in up to 1.2–11.6% of patients undergoing standard EMR and in up to 7% of patients undergoing ESD [16]. During ESD, minor bleeding is commonly seen but can be successfully treated by grasping the bleeding vessels with Coagrasper as described above. Endoclips are sometimes needed for aggressive bleeding. Thereafter, a sodium alginate powder (Alto, Kaigen Co., Osaka, Japan) or sucrose-aluminum hydroxide gel (Chugai Pharmaceutical Co.) is sprayed onto the artificial ulcer base [22]. Delayed bleeding, manifested by hematemesis or melena at 0–30 days after the procedure, may require emergent endoscopy. In the era of the stronger acid suppressant, PPIs, in order to prevent postoperative bleeding and promote ulcer healing, the standard dose of PPI is administered for patients with EGC or EEC treated by ESD. Even large ulcers after ESD have recently been reported to heal within 8 weeks after resection under the antacid treatment [16]. Nevertheless, most delayed bleeding (75%) occurs within 12 h after the procedure [16], and patients are typically placed on fasting for 1 day, followed by liquid diet on the second day, and a soft diet for another 3 days. The patients remain hospitalized for at least 8 days. In cases of semicircular or circumferential EECs, intraluminal stenosis of the esophagus often occurs postoperatively [22], and such patients should undergo repeated (i.e., twice per week in our hospital) mechanical dilation with the specialized balloon catheter (Boston Scientific Japan Co., Tokyo, Japan), and hence, would be discharged several weeks after ESD.
PPIs and H2RAs have a significant effect on preventing bleeding from the ulcer and facilitating the ulcer healing after gastric EMR [17, 25]. ESD creates larger artificial ulcers with greater risks of bleeding, but there is little information on whether PPIs would reduce incidence of the complication [25]. In order to investigate whether a PPI, rabeprazole (Eisai Pharmaceutical Co., Tokyo, Japan) more effectively prevents bleeding after ESD for EGC, Uedo et al. conducted a prospective randomized controlled trial, where a total of 143 patients with EGC who underwent ESD were randomly assigned to either rabeprazole 20 mg/day (PPI group) or cimetidine 800 mg/day (H2RA group) on the day before ESD and continued for 8 weeks [25]. Bleeding occurred in 4 patients in the PPI group and 11 in the H2RA group; multivariate analysis revealed that treatment with the PPI significantly reduced the risk of bleeding (adjusted hazard ratio 0.47, 95% confidence interval, 0.22–0.92). One delayed perforation was experienced in the H2RA group. Thus, PPI may prevent bleeding from the ulcer created after ESD more effectively than H2RA. In our hospital, patients receive oral lansoprazole (Takeda Pharmaceutical Co.) 30 mg/day for 2 weeks prior to ESD in cases of EGC with ulcer finding. On the day of ESD, the PPI is given intravenously at the same dose every 12 h for 2 days, and then oral lansoprazole are continued with 30 mg/day for 8 weeks.
Perforation is rare with EMR but is seen not so uncommonly with ESD. The risk of perforation during ESD for EGC is about 4% in a large series from the Japanese Cancer institute [16]. Gastric perforation during endoscopic resection can be conservatively treated by complete endoscopic closure with endoclips (HX-600-090, Olympus Optical Co.) without peritoneal dissemination of the cancer cells. In addition, nasogastric suction is applied, and a broad spectrum antibiotic is efficiently given for 2–3 days. If abdominal fullness due to air leakage from the perforated lesion is severe, decompression of the pneumoperitoneum by puncture needle must be performed. On the other hand, a delayed perforation due to the artificial ulcers following ESD is rare but may require surgical intervention.
More recently, we conducted statistical analysis for the clinicopathological factors related to ESD-related bleeding and perforation in the largest consecutive series with more than 700 EGCs [24]. The procedure-related bleeding after ESD was defined as bleeding that required transfusion or surgical intervention, or bleeding that caused the hemoglobin level to fall by 2 g/dL [15]. Perforation was diagnosed endoscopically or by the presence of free air on an abdominal plain radiograph or computed tomogram (CT). The procedure-related bleeding was rarely seen in 1.8% of the cases. All hemorrhagic episodes were successfully treated by endoscopic clipping or coagulation. Perforations related to ESD occurred in 4.5% and could also be managed by conservative medical treatment after endoscopic closure with clipping. The bleeding was not associated with any clinicopathological characteristics including age, gender, tumor size, tumor location, and macroscopic appearance of EGC while the location and tumor size had significant impact on the ESD-related perforation. Gotoda et al. reported that tumors located in the upper third of the stomach and those with ulcer findings were at significantly higher risk of perforation [16].
There have been few reports on the complications of ESD in the esophagus. Fujishiro et al. reported that perforation occurred in 6.9% (4/58) patients with esophageal squamous cell neoplasms during the ESD, whereas there was no evidence of significant bleeding [12]. The patients with the complication were managed by conservative medical treatments after endoscopic closure of the perforation. Here, notification of risk of perforation in esophageal ESD is essential, since it may cause severe or even life-threatening conditions including mediastinal emphysema and mediastinitis [12].
The incidence of local recurrence of EGC after EMR varies between 2% and 35% [5]. On the other hand, local recurrence of gastric cancer after ESD would be nominal when curative resection is achieved, whereas about 10% of patients with non-curative resection had local recurrence [24]. This implies that EGC patients with non-curative ESD require close follow-up surveillance for cancer recurrence for at least 2 years, as the recurrent tumors developed 13 to 24 months after ESD in the follow-up study [24]. Preliminary outcomes of ESD for EGC documented 14 (6.2%) metachronous lesions among 225 EGCs at unknown stages following ESD [11]. The incidence of metachronous gastric cancer varies from 1.8% to 8.1% after EMR [26], and, thus, the necessity for continued surveillance for recurrent and/or new lesions as well as metastatic disease is an intrinsic drawback of endoscopic therapies, irrespective of curability. As for the follow-up in our hospital, endoscopic examinations were scheduled at 1, 3, 6, and 12 months after ESD and then annually thereafter. Biopsy specimens during each follow-up endoscopy were taken from the treatment-related scar or any other suspicious abnormalities to assess the presence of local recurrent tumor or metachronous cancer of the stomach. To detect lymph node and distant metastases, contrast-enhanced CT and ultrasound sonography of the abdomen and chest X-rays were performed annually.
Outcomes of ESD
In the recent largest case series with EGC, en bloc resection was achieved in 94.9% (559/589) with ESD. 550 of 581 lesions (94.7%) were deemed to have undergone curative resection [24]. En bloc resection of ESD provides much higher curative resection rates than piecemeal resection. Using logistic regression analysis, we have assessed the impact of various factors on the curability of ESD. On univariate basis, piecemeal resection and ulcer findings interfered with curative resection. Oka et al. also showed that ulceration prevented complete removal of EGCs, notably in lesions larger than 21 mm [8]. A similar tendency was seen in EGC patients treated by ESD for recurrent EGC after previous EMR [27]. Nevertheless, multivariate analysis revealed that en bloc resection was the sole significant contributor to curative ESD [24]. Oda et al. reported that upper and middle location, tumor size more than 21 mm, and positive ulcer findings were associated with piecemeal resection [15]. Oka et al. reported a marked decrease in en bloc resection rates from 92.9% for EGC without ulcer findings to 19.2% for tumors with ulceration [9]. Only 9.1% of the ulcerative lesions greater than 21 mm in size were resected en bloc [9]. On the multivariate analysis, there was a significant association of tumor size with lesion resectability. Collectively, larger and/or ulcerative EGCs could be at higher risk of piecemeal resection; therefore, their treatment requires a high level of expertise and experience.
Despite the increasing use of ESD for EGC, the long-term clinical outcomes have not been fully evaluated. The 3-year overall survival after ESD seems excellent, with the rate being nearly 99% [24]. In a multicenter study of endoscopic resection for EGC, Oda et al. reported a comparable 3-year overall survival between the EMR and ESD groups (99.7% and 98.5%, respectively) [28]. The 5-year survival rate after ESD reached 97.1%, which was equivalent to those after EMR documented in previous reports [26, 29]. Of note, both the 3-year and 5-year disease-specific survival rates after ESD were 100% [24], similar to those after EMR in 12 major Japanese institutions [26]. In general, EGC has excellent clinical outcomes, with 10- and 20-year survival rates after gastrectomy with removal of lymph nodes as high as 95% [26]. Confirmation of whether ESD can equal surgery will require further long-term prospective studies.
As for esophageal ESD, 41 cases of esophageal neoplasms consisting of 26 superficial squamous cell carcinomas, 13 severe dysplasia and 2 intramucosal Barrett’s adenocaricnomas underwent ESD in our hospital between April 2006 and October 2008. On the whole, en bloc resection was achieved in all patients, and the rate of en bloc with the tumor-free lateral/vertical margins was 88% (36/41). There was no perforation and bleeding, but intramural stricture occurred in 4 of the 41 cases. All the 4 lesions extended more than three-quarter of circumference of the esophagus. The 4 patients with non-curative resection underwent additional treatment (3 radical surgery and 1 chemoradiation), the one with severe dysplasia positive for the lateral margin has been under follow-up without local recurrence. Fujishiro et al. reported that the rate of en bloc resection was 100% (58/58) for esophageal squamous cell neoplasms, and en bloc resection with tumor-free lateral/basal margins was achieved in 78% (45/58) [12]. Of 40 lesions occurring in 31 patients fulfilling the criteria of node-negative tumors (mean follow-up, 17 months), one lesion resected by en bloc resection with nonevaluable tumor-free lateral margins recurred locally 6 months after ESD, which was treated successfully by a second ESD procedure.
For 30 lesions of esophagogastric tumors including Barrett’s adenocarcinoma treated by ESD, the en bloc resection with the tumor-free lateral/vertical margins was achieved in 97% (29/30) [13]. Histological evaluation of the resected specimens revealed five cases of angiolymphatic invasion and five cases of submucosal invasion deeper than 500 µm. Local recurrence was not observed in any patient during follow-up (mean 14.6 months, range 6–31 months) in their study. In another study, the en bloc resection rate was 100% in 25 superficial adenocarcinoma located at the esophagogastric junction. Seventeen lesions (72%) were judged as curative resection with the tumor-free lateral/vertical margins and showed no local or distant recurrence during a median follow-up period of 30.1 months [14]. Thus, ESD can be safely and effectively performed for the esophagogastric junction tumors, albeit the study numbers of these preliminary studies were limited.
Conclusions
Endoscopic resection of EGC and EEC is well established as a standard therapy in Japan and is increasingly becoming accepted and regularly used in the other countries. ESD, an innovative application modality of EMR, has been developed to allow the resection of larger lesions in an en bloc manner; the earlier results so far have been promising in EGC, EEC and esophagogastric junction tumors including Barrett’s adenocarcinoma. It is feasible to assess the histopathological curability of the resected specimens precisely, reducing recurrence. ESD still has relatively high complication rates; notification of perforation risk is essential in particular when performing esophageal ESD. Bleeding during ESD can be managed by endoscopic closure with endoclips, and delayed bleeding is rare with the use of oral and/or intravenous PPIs. The relatively long-term outcomes may be excellent in EGC after ESD. Nevertheless, continued surveillance is necessary for recurrent tumors in cases of non-curative resection and for metachronous cancers even after curative ESD.
Abbreviations
- ESD
endoscopic submucosal dissection
- EMR
endoscopic mucosal resection
- EGC
early gastric cancer
- EEC
early esophageal cancer
- PPI
proton pump inhibitor
- H2RA
H2-receptor antagonist
- CT
computed tomography
- IT knife
insulation-tipped diathermy knife
References
- 1.Sano T., Kobori O., Muto T. Lymph node metastasis from early gastric cancer: endoscopic resection of tumour. Br. J. Surg. 1992;79:241–244. doi: 10.1002/bjs.1800790319. [DOI] [PubMed] [Google Scholar]
- 2.Shimizu S., Tada M., Kawai K. Early gastric cancer: its surveillance and natural course. Endoscopy. 1995;27:27–31. doi: 10.1055/s-2007-1005628. [DOI] [PubMed] [Google Scholar]
- 3.Rembacken B.J., Gotoda T., Fujii T., Axon A.T. Endoscopic mucosal resection. Endoscopy. 2001;33:709–718. doi: 10.1055/s-2001-16224. [DOI] [PubMed] [Google Scholar]
- 4.Soetikno R., Kaltenbach T., Yeh R., Gotoda T. Endoscopic mucosal resection for early cancers of the upper gastrointestinal tract. J. Clin. Oncol. 2005;23:4490–4498. doi: 10.1200/JCO.2005.19.935. [DOI] [PubMed] [Google Scholar]
- 5.Gotoda T. Endoscopic resection of early gastric cancer. Gastric Cancer. 2007;10:1–11. doi: 10.1007/s10120-006-0408-1. [DOI] [PubMed] [Google Scholar]
- 6.Gotoda T., Kondo H., Ono H., Saito Y., Yamaguchi H., Saito D., Yokota T. A new endoscopic mucosal resection procedure using an insulation-tipped electrosurgical knife for rectal flat lesions: report of two cases. Gastrointest. Endosc. 1999;50:560–563. doi: 10.1016/s0016-5107(99)70084-2. [DOI] [PubMed] [Google Scholar]
- 7.Ono H., Kondo H., Gotoda T., Shirao K., Yamaguchi H., Saito D., Hosokawa K., Shimoda T., Yoshida S. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001;48:225–229. doi: 10.1136/gut.48.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oka S., Tanaka S., Kaneko I., Mouri R., Hirata M., Kanao H., Kawamura T., Yoshida S., Yoshihara M., Chayama K. Endoscopic submucosal dissection for residual/local recurrence of early gastric cancer after endoscopic mucosal resection. Endoscopy. 2006;38:996–1000. doi: 10.1055/s-2006-944780. [DOI] [PubMed] [Google Scholar]
- 9.Oka S., Tanaka S., Kaneko I., Mouri R., Hirata M., Kawamura T., Yoshihara M., Chayama K. Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest. Endosc. 2006;64:877–883. doi: 10.1016/j.gie.2006.03.932. [DOI] [PubMed] [Google Scholar]
- 10.Takeuchi Y., Uedo N., Iishi H., Yamamoto S., Yamamoto S., Yamada T., Higashino K., Ishihara R., Tatsuta M., Ishiguro S. Endoscopic submucosal dissection with insulated-tip knife for large mucosal early gastric cancer: a feasibility study (with videos) Gastrointest. Endosc. 2007;66:186–193. doi: 10.1016/j.gie.2007.03.1059. [DOI] [PubMed] [Google Scholar]
- 11.Takenaka R., Kawahara Y., Okada H., Hori K., Inoue M., Kawano S., Tanioka D., Tsuzuki T., Yagi S., Kato J., Uemura M., Ohara N., Yoshino T., Imagawa A., Fujiki S., Takata R., Yamamoto K. Risk factors associated with local recurrence of early gastric cancers after endoscopic submucosal dissection. Gastrointest. Endosc. 2008;68:887–894. doi: 10.1016/j.gie.2008.03.1089. [DOI] [PubMed] [Google Scholar]
- 12.Fujishiro M., Yahagi N., Kakushima N., Kodashima S., Muraki Y., Ono S., Yamamichi N., Tateishi A., Shimizu Y., Oka M., Ogura K., Kawabe T., Ichinose M., Omata M. Endoscopic submucosal dissection of esophageal squamous cell neoplasms. Clin. Gastroenterol. Hepatol. 2006;4:688–694. doi: 10.1016/j.cgh.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 13.Kakushima N., Yahagi N., Fujishiro M., Kodashima S., Nakamura M., Omata M. Efficacy and safety of endoscopic submucosal dissection for tumors of the esophagogastric junction. Endoscopy. 2006;38:170–174. doi: 10.1055/s-2005-921039. [DOI] [PubMed] [Google Scholar]
- 14.Yoshinaga S., Gotoda T., Kusano C., Oda I., Nakamura K., Takayanagi R. Clinical impact of endoscopic submucosal dissection for superficial adenocarcinoma located at the esophagogastric junction. Gastrointest. Endosc. 2008;67:202–209. doi: 10.1016/j.gie.2007.09.054. [DOI] [PubMed] [Google Scholar]
- 15.Oda I., Gotoda T., Hamanaka H., Eguchi T., Saito Y., Matsuda T., Bhandari P., Emura F., Saito D., Ono H. Endoscopic submucosal dissection for early gastric cancer: Technical feasibility, operation time and complications from a large consecutive series. Dig. Endosc. 2005;17:54–58. [Google Scholar]
- 16.Gotoda T., Yamamoto H., Soetikno R.M. Endoscopic submucosal dissection of early gastric cancer. J. Gastroenterol. 2006;41:929–942. doi: 10.1007/s00535-006-1954-3. [DOI] [PubMed] [Google Scholar]
- 17.Daneshmend T.K., Hawkey C.J., Langman M.J., Logan R.F., Long R.G., Walt R.P. Omeprazole versus placebo for acute upper gastrointestinal bleeding: Randomized double blind controlled trial. B.M.J. 1992;304:143–147. doi: 10.1136/bmj.304.6820.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Japanese Gastric Cancer Association, author. Japanese classification of gastric carcinoma—2nd English Edition—. Gastric Cancer. 1998;1:10–24. doi: 10.1007/s101209800016. [DOI] [PubMed] [Google Scholar]
- 19.Gotoda T., Yanagisawa A., Sasako M., Ono H., Nakanishi Y., Shimoda T., Kato Y. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219–225. doi: 10.1007/pl00011720. [DOI] [PubMed] [Google Scholar]
- 20.Fujishiro M. Perspective on the practical indications of endoscopic submucosal dissection of gastrointestinal neoplasms. World J. Gastroenterol. 2008;14:4289–4295. doi: 10.3748/wjg.14.4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oyama T., Miyata Y., Shimaya S. Lymph nodal metastasis of m3, sm1 esophageal cancer [in Japanese] Stomach and Intestine. 2002;37:71–74. [Google Scholar]
- 22.Fujishiro M., Yahagi N., Kakushima N., Kodashima S., Ichinose M., Omata M. En bloc resection of a large semicircular esophageal cancer by endoscopic submucosal dissection. Surg. Laparosc. Endosc. Percutan. Tech. 2006;16:237–241. doi: 10.1097/00129689-200608000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Kakushima N., Fujishiro M. Endoscopic submucosal dissection for gastrointestinal neoplasms. World J. Gastroenterol. 2008;14:2962–2967. doi: 10.3748/wjg.14.2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Isomoto H., Shikuwa S., Yamaguchi N., Fukuda E., Ikeda K., Nishiyama H., Ohnita K., Mizuta Y., Shiozawa J., Kohno S. Endoscopic submucosal dissection for early gastric cancer: a large-scale feasibility study. Gut. 2009;58(3):331–336. doi: 10.1136/gut.2008.165381. [DOI] [PubMed] [Google Scholar]
- 25.Uedo N., Takeuchi Y., Yamada T., Ishihara R., Ogiyama H., Yamamoto S., Kato M., Tatsumi K., Masuda E., Tamai C., Yamamoto S., Higashino K., Iishi H., Tatsuta M. Effect of a proton pump inhibitor or an H2-receptor antagonist on prevention of bleeding from ulcer after endoscopic submucosal dissection of early gastric cancer: a prospective randomized controlled trial. Am. J. Gastroenterol. 2007;102:1610–1616. doi: 10.1111/j.1572-0241.2007.01197.x. [DOI] [PubMed] [Google Scholar]
- 26.Kojima T., Parra-Blanco A., Takahashi H., Fujita R. Outcome of endoscopic mucosal resection for early gastric cancer: review of the Japanese literature. Gastrointest. Endosc. 1998;48:550–554. doi: 10.1016/s0016-5107(98)70108-7. [DOI] [PubMed] [Google Scholar]
- 27.Yokoi C., Gotoda T., Hamanaka H., Oda I. Endoscopic submucosal dissection allows curative resection of locally recurrent early gastric cancer after prior endoscopic mucosal resection. Gastrointest. Endosc. 2006;64:212–218. doi: 10.1016/j.gie.2005.10.038. [DOI] [PubMed] [Google Scholar]
- 28.Oda I., Saito D., Tada M., Iishi H., Tanabe S., Oyama T., Doi T., Otani Y., Fujisaki J., Ajioka Y., Hamada T., Inoue H., Gotoda T., Yoshida S. A multicenter retrospective study of endoscopic resection for early gastric cancer. Gastric Cancer. 2006;9:262–270. doi: 10.1007/s10120-006-0389-0. [DOI] [PubMed] [Google Scholar]
- 29.Uedo N., Iishi H., Tatsuta M., Ishihara R., Higashino K., Takeuchi Y., Imanaka K., Yamada T., Yamamoto S., Yamamoto S., Tsukuma H., Ishiguro S. Longterm outcomes after endoscopic mucosal resection for early gastric cancer. Gastric Cancer. 2006;9:88–92. doi: 10.1007/s10120-005-0357-0. [DOI] [PubMed] [Google Scholar]