Abstract
Oxidative stress is frequently considered as a central mechanism of hepatocellular injury in non-alcoholic steatohepatitis (NASH). The aim of this study was to investigate the effects of fermented green tea extracts (FGTE) on NASH. Rats were fed a choline-deficient high-fat diet for 4 weeks to nutritionally generate fatty livers. NASH was induced chemically by oxidative stress using repeated intraperitoneal injections of nitrite. Rats with NASH developed steatohepatitis and liver fibrosis after 6-week of such treatment. At 10 weeks, blood and liver samples were collected from anesthetized animals and assessed for extent of OS injury and effects of FGTE, by biochemical, histological and histochemical analyses. FGTE reduced serum levels of liver enzymes, lipid peroxidation and production of mitochondrial reactive oxygen species. In addition, FGTE showed inhibition of progressions of cirrhosis. Our findings suggest that our FGTE have strong radical scavenging activity and may be beneficial in the prevention of NASH progression.
Keywords: non-alcoholic steatohepatitis, antioxidant, oxidative stress, hepatoprotection
Introduction
Non-alcoholic steatohepatitis (NASH) is a chronic liver disease characterized by necroinflamatory activity with nonspecific inflammatory infiltrates, hepatocyte ballooning with Mallory’s hyaline and sometimes fibrosis [1]. The “two hit theory” provides the most widely accepted explanation describing the progression of NASH. This hypothesis states that fatty liver, or an excessive fat accumulation in liver hepatocytes, marks the first stage of NASH development. The “second hit” is attributed to a factor such as cytokine or oxidative stress (OS), leading to liver inflammation and fibrosis [2].
We described previously the development of an animal model of NASH (Patent application No. PCT/JP2007/52477), and demonstrated that OS cause extensive hepatic fibrosis and cirrhosis in this model rats [3]. Therefore, we suggest that induction of OS as a “second hit” in rats with fatty liver leads to the development of a NASH-like condition.
There is no effective drug therapy for prevention or treatment of fatty liver disease, although exercise and diet improvement to reduce obesity have been suggested. Therefore, the development of novel supplements or drugs that prevent non-alcoholic fatty liver disease (NAFLD) or NASH is urgently required. Only recently has OS been postulated to contribute to NASH development. Depletion of antioxidants such as reduced glutathione (GSH), vitamins C and E was suggested to occur in NASH patients [4, 5]. To our knowledge, however, there are few published studies that have investigated the potential efficacy of antioxidant supplements against NASH progression. Information described in reports published by other groups, together with the data presented in our previous papers, prompt us to select an excellent antioxidant that could potentially halt the progression of NASH.
Green tea (Camellia sinensis) is a widely consumed beverage whose antioxidant activity and other beneficial health effects have gained considerable notoriety in the recent years [6]. In the liver diseases, green tea has been shown to affect hepatic ischemia-reperfusion injury [7], liver fibrosis [8, 9] and alcohol-induced liver injury [10]. Our green tea water extracts were microbially fermented and developed to improve flavor, taste, and medicinal function. The main polyphenols in our fermented green tea extract (FGTE) preparation were (−)-epigallocatechin (EGC), gallic acid (GA) and (−)-gallocatechin (GC) as shown in Table 1. The aim of this study was to determine whether FGTE prevents NASH progression and to test whether OS provides “a second hit” and contribute to the development of the condition.
Table 1.
Composition of polyphenols in green tea and FGTE
| Components | % | |
|---|---|---|
| Green tea | FGTE | |
| (−)-Catechin (C) | — | — |
| (−)-Epicatechin-3-gallate (ECG) | 1.3 | 1.1 |
| (−)-Epigallocatechin (EGC) | 13.7 | 10.2 |
| (−)-Epigallocatechin-3-gallate (EGCG) | 9.1 | 0.6 |
| (−)-Gallocatechin (GC) | 1.0 | 6.9 |
| Total catechin | 25.1 | 18.9 |
| Caffein | 6.1 | 5.1 |
| Gallic acid | 0.6 | 7.2 |
Data are percentages of solid contents in water extracts.
Materials and Methods
Chemicals
5,5-Dimethyl-l-pyrroline N-oxide (DMPO) was purchased from Labotech Co. (Tokyo, Japan). 2-(5,5-Dimethyl-2-oxo-2λ5-[1,3,2]dioxaphosphinan-2-yl (CYPMPO)-2-methyl-3,4-dihydro-2H-pyrrole 1-oxide was purchased from Radical Research Inc. (Tokyo, Japan). Dodecyl maltoside (DM) was from Dojin Laboratories (Kumamoto, Japan). Benzamidine hydrochloride and sodium nitrate were purchased from Nacalai Tesque Inc. (Kyoto, Japan). β-NADH was purchased from Oriental Yeast Co. (Osaka, Japan). Other reagents were obtained from Wako Pure Chemical Industries (Osaka, Japan). All chemicals used in our study were of the highest grade available.
Fermented green tea extract (FGTE) preparation
Ikeda Tohka Industries Co. (Fukuyama, Japan) provided the green tea leaves used in the present study. After pasteurizing fresh green tea leaves at 80°C for one hour, the mixture was fermented using filamentous fungi (Aspergillus oryzae IFO5238) at 30°C for seven days. The resulting aqueous green tea extract was dried using a spray drying process. The resulting powder was stored at −20°C until analysis.
Animals and experimental design
Male Wistar rats (Shimizu Experimental Animals, Shizuoka, Japan), weighing 160–170 g and six weeks of age (purchased from Shimizu Experimental Animals, Shizuoka, Japan) were used in this study. They were housed in the Animal Research Center of Okayama University in a temperature-controlled room (22 ± 1°C) with a relative humidity of 50 ± 10% and a 12 h light/dark cycle (lights on at 8:00 a.m.). This study was performed in accordance with the Guide for Animal Experimentation of the Faculty of Pharmaceutical Science, Okayama University.
We induced fatty liver in rats by feeding them a choline-deficient high fat (CDHF) diet (Oriental Yeast) for 4 weeks. The rats were divided at random into five groups: the Normal group (n = 6) was fed standard chow for four weeks), the CDHF group (n = 6) was fed CDHF diet only for 4 weeks; the NASH group (n = 6) was fed CDHF diet for 4 weeks followed by intraperitoneal (i.p.) injections of nitrite (30 mg/kg/day) for 6 weeks; the FGTE 100 group (n = 6) was fed CDHF diet for 4 weeks followed by i.p. injections of nitrite at 30 mg/kg/day for 6 weeks, and also orally administered FGTE at 100 mg/kg/day (low dose) for 6 weeks; the FGTE 300 group (n = 6) was fed CDHF diet for 4 weeks followed by i.p. injections of nitrite at 30 mg/kg/day for 6 weeks, as well as orally administered FGTE at 300 mg/kg/day (high dose). After six weeks, the rats were fasted overnight and sacrificed under deep anesthesia. The samples were prepared to determine OS injury and efficacy of FGTE administration, by using biochemical and OS markers and assessment of histological changes. The liver samples were fixed in 20% formalin or snap-frozen in liquid nitrogen for histological examination and for our analyses of lipid peroxidation and antioxidant activity.
Biochemical analyses
The activities of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in plasma were measured using commercial enzyme assay kits (Wako Pure Chemical Industries, Osaka, Japan). The level of plasma alkaline phosphatase (ALP) was determined using standard assays.
Liver triglycerides
Total lipids were extracted with chloroform-methanol (2:1) from 100 mg of liver tissue according to the method of Folch [11]. The extracted lipids were resuspended in t-butyl-alcohol-methanol-TritonX-100 (2:1:1) and submitted for measurement using a commercial enzyme assay kit (Wako Pure Chemical Industries, Osaka, Japan).
Lipid peroxidation in liver homogenates
Liver tissue (100 mg) was homogenized in 10 volumes of ice-cold Tris-HCl buffer containing 0.25 M sucrose. The resulting homogenate was centrifuged at 3000 × g for 10 min at 4°C. The supernatant was used for assays of malondialdehyde (MDA) + 4-hexanonenal (4-HNE). MDA + 4-HNE was measured using a commercial enzyme assay kit (LPO-586) purchased from OXIS Health Products, Inc. (Portland, OR).
Plasma SOD-like activity
Plasma superoxide dismutase (SOD)-like activity was measured using electron paramagnetic resonance (ESR) spectroscopy. Superoxide radicals were generated using a hypoxanthine-xanthine oxidase system. CYPMPO was used as an ESR spectroscopic spin-trapping reagent. The signal intensity was confirmed by the ratio of the height of the internal manganese standard signal and the fourth of eight peaks from the CYPMPO-OOH spin adducts. The conditions used were as follows: magnetic field, 331.5 mT; power, 8 mW; modulation frequency, 9.41 GHz; modulation amplitude, 1 × 0.1 mT; response time, 0.1 s; amplitude, 790; sweep width, 10 mT; sweep time, 4 min; room temperature [12–14].
Preparation of mitochondria
The liver mitochondria were prepared by differential centrifugation of the tissue homogenates [15]. In brief, rats were transcardially perfused with ice-cooled 1.15% KCl buffer containing 5 mM benzamidine (pH 7.4) via the inferior vena cava. The liver tissue was then removed (6 g) and homogenized in 18 volumes ice-cooled 5 mM Tris-HCl buffer (pH 7.4) containing 0.25 mM sucrose and 100 mM KCl. The mixture was centrifuged at 2,500 rpm for 10 min at 4°C. The supernatant was collected and centrifuged at 9,000 × g for 20 min at 4°C. The resulting pellet containing the mitochondrial fraction was resuspended in the buffer and stored at −80°C until analysis. The protein concentration was determined using a BCA kit (Pierce, Rockford, IL) with bovine serum albumin as a standard.
Reactive oxygen species (ROS) from rat liver mitochondria
DMPO was used for the determination of ROS produced by rat liver mitochondria. Rat liver mitochondria isolates (500 µg) were mixed in 0.1 ml potassium phosphate buffer (pH 7.4) containing 0.1% DM, 5 mM glutamate, 5 mM malate, 0.1 mM succinate, 0.92 mM DMPO, and 0.1 mM NADH, incubated for 5 min at 37°C and then measured. Analyses were performed at room temperature under ambient atmosphere. Using a manganese signal as an internal standard, the relative peak height of the second signal obtained from the DMPO-OH adducts was evaluated. The conditions were as follows: microwave power, 8 mW; modulation amplitude width, 0.1 mT; response time, 0.1 s; scanning time, 4 min; magnetic field, 336.0 ± 5 mT [16].
Histological examination
Paraffin-embedded tissues were sectioned at 4 µm thickness and stained with hematoxylin and eosin (H&E), Masson trichrome for fibrosis. Histological assessment of tissue morphology was performed using an Olympus light microscope (Olympus, Tokyo, Japan).
Statistical analyses
Statistical analysis was performed using GraphPad Prism (Version 4.03; Graph Pad Software, La Jolla, CA) on untransformed data. Data were expressed as mean ± standard error of the mean (SEM). Differences among the groups were evaluated using Dunnett’s test as a post hoc test. A p value<0.05 denoted the presence of statistically significant difference.
Results
Histological evaluation of experimental liver samples
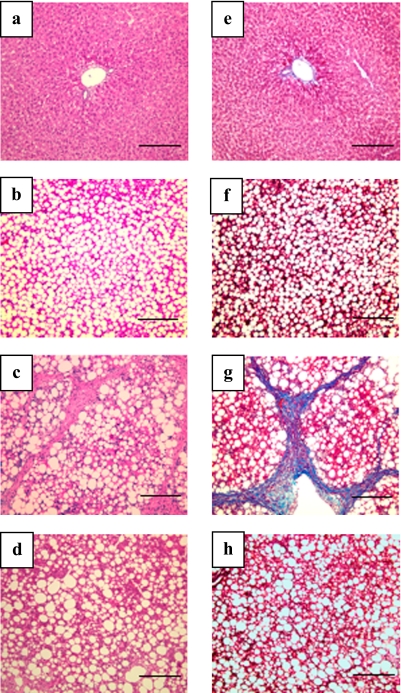
Representative histological sections of livers from each group are shown in Fig. 1. Severe liver atrophy was observed in NASH livers. The livers of CDHF-fed rats were significantly greater in size compared to MF-chow fed rats. Conversely, adding FGTE prevented this effect, but had no effect on the features of fatty liver (data not shown). Histopathological examination of liver tissues stained with H&E showed hepatic macrovesicular steatosis in all CDHF-fed rats (Fig. 1a–d). We evaluated the effects of FGTE on liver fibrogenesis in the different treatment groups. No fibrosis was noted in the livers of rats fed standard chow (Normal) compared with those fed CDHF alone. In the NASH rats, steatosis, liver fibrosis, and necrosis were quite advanced. Liver fibrosis was clearly attenuated in all groups treated with FGTE (Fig. 1e–h).
Fig. 1.
Histological evaluation of liver samples of rats of various groups. Effects of FGTE on liver structure were examined by hematoxylin and eosin staining in Normal (a), CDHF (b), NASH (c), and NASH + FGTE 300 mg/kg (d) rats. Effects of FGTE on liver fibrosis were examined by Masson Trichrome staining in Normal (e), CDHF (f), NASH (g), and NASH + FGTE 300 mg/kg (h). Data show typical results. Scale bar = 200 µm.
Effects of FGTE on body weight, liver index, and plasma biochemical parameters
There were no differences in body weights between NASH and FGTE-treated rats. In contrast, the body weights of rats fed normal or CDHF alone were greater during the entire experimental period. Wet liver weights and liver indexes of NASH rats were lower than those of the FGTE group and CDHF alone group (p<0.05 vs Normal rats, p<0.01 vs CDHF-fed rats). Food consumption was about 20 g/day while water intake was about 20 ml/day.
With regard to liver function test, AST, ALT, and ALP levels were significantly higher in NASH rats compared with rats of the CDHF group. In the FGTE 100 group, the liver enzymes improved compared with NASH, but the changes were not significant. FGTE supplementation at 300 mg/kg significantly improved AST and ALP compared with the NASH group, although these parameters were still higher than the normal and CDHF groups (Table 2).
Table 2.
Effects of FGTE on body weight, wet liver weight and biochemical markers
| Normal | CDHF | NASH | |||
|---|---|---|---|---|---|
| 0 | 100 | 300 | |||
| FGTE (mg/kg/day, p.o.) | |||||
| Body weight (g) | 356.0 ± 28.3 | 249.0 ± 25.9 | 241.5 ± 29.0 | 225.1 ± 4.9 | 250.3 ± 8.8 |
| Wet liver weiht (g) | 9.4 ± 4.6 | 14.4 ± 3.0 | 11.2 ± 2.6# | 10.4 ± 0.5 | 14.3 ± 2.0* |
| Liver index (%) (liver wet weight/body × 100) | 2.6 ± 0.8 | 5.6 ± 0.6 | 4.9 ± 0.9# | 4.6 ± 0.2 | 5.7 ± 0.4* |
| Biochemical marker (plasma) | |||||
| AST (IU/L) | 54.2 ± 2.5 | 178.8 ± 23.3 | 290.7 ± 7.9# | 243.8 ± 18.2 | 235.2 ± 21.7* |
| ALT (IU/L) | 15.0 ± 1.1 | 21.9 ± 4.6 | 33.91 ± 2.4# | 24.8 ± 1.3 | 14.1 ± 1.3 |
| ALP (Released p-nitrophenol, mM) | 0.1 ± 0.1 | 0.3 ± 0.1 | 0.5 ± 0.1# | 0.5 ± 0.1 | 0.3 ± 0.1** |
Each value is the mean ± SEM of 6 rats.
*p<0.05, **p<0.01, compared with NASH. #p<0.01, compared with Normal.
FGTE: fermented green tea extract, CDHF: choline-deficient high-fat. NASH: CDHF + OS, FGTE 100: NASH + FGTE 100 mg/kg, FGTE 300: NASH + FGTE 300 mg/kg.
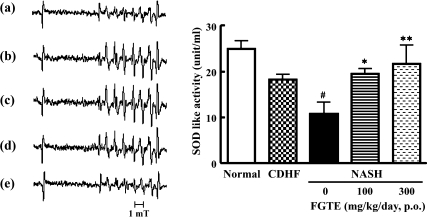
Effects of FGTE on plasma antioxidant activity
The levels of plasma SOD-like activity were significantly lower in NASH rats (p<0.01, vs Normal). NASH rats supplemented with FGTE at 300 mg/kg showed a significant improvement and had high plasma SOD-like activity that was comparable to that found in NASH rats. This was not the case, however, for rats supplemented with 100 mg/kg FGTE (p<0.01 vs NASH rats, Fig. 2).
Fig. 2.
Effects of FGTE on plasma SOD-like activity in Normal, CDHF, NASH, FGTE 100 and FGTE 300 groups using an ESR. ESR spectra of the CYPMPO-OOH spin adducts obtained from hypoxanthine-xanthine oxidase systems in Normal (a), CDHF (b), NASH (c), NASH + FGTE 100 mg/kg (d) and NASH + FGTE 300 mg/kg (e) rats. Each value is the mean ± SEM of 6 rats. *p<0.05, **p<0.01, compared with NASH. #p<0.01, compared with Normal.
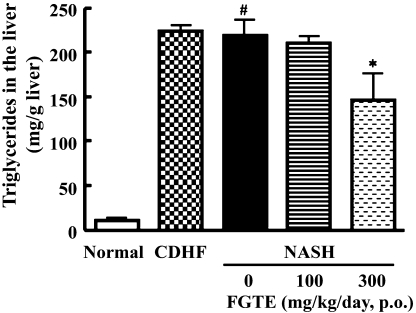
Effects of FGTE on liver triglyceride level
Triglyceride levels were higher in the livers of NASH rats and rats fed CDHF alone, compared with the control (Fig. 3). However, supplementation of 300 mg/kg FGTE, but not 100 mg/kg FGTE, decreased these levels in such rats (p<0.01 vs NASH rats).
Fig. 3.
Effects of FGTE on triglycerides level in the liver of Normal, CDHF, NASH, NASH + FGTE 100 mg/kg and NASH + FGTE 300 mg/kg groups. Each value is the mean ± SEM of 6 rats. *p<0.05, compared with NASH. ##p<0.01, compared with Normal.
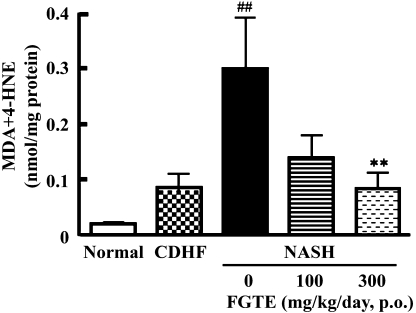
Effects of FGTE on lipid peroxidation in liver homogenates
To investigate whether FGTE can prevent lipid peroxidation, we measured the formation of MDA and 4-HNE in liver tissue. The levels of MDA and 4-HNE were significantly higher in NASH rats (p<0.01, vs the CDHF group, p<0.01 versus the CDHF group). However, they were not different between CDHF and Normal rats. Supplementation of 300 mg/kg FGTE resulted in reduction of MDA and 4-HNE levels (p<0.01 vs NASH rats, Fig. 4).
Fig. 4.
Effects of FGTE on lipid peroxide levels in the liver of Normal, CDHF, NASH, NASH + FGTE 100 mg/kg and NASH + FGTE 300 mg/kg groups. Each value is the mean ± SEM of 6 rats. **p<0.01, compared with NASH. ##p<0.01, compared with Normal.
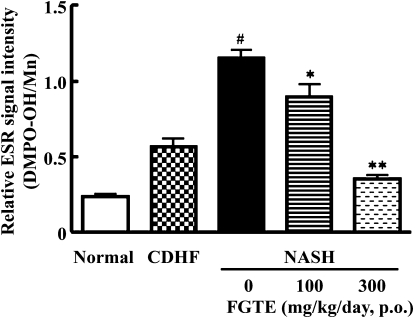
Effects of FGTE on mitochondrial ROS production
Finally, we evaluated the effects of FGTE on mitochondrial ROS production. Signals derived from DMPO-OH adducts in our ESR analysis were detected in the liver mitochondria of all groups. The highest signal intensities, however, were found in rats of the NASH group. In contrast, no significant difference was found between CDHF-fed rats and Normal group. FGTE (300 mg/kg) administration, however, caused a significant decrease in detectable levels of liver mitochondrial ROS (p<0.01 vs NASH rats, Fig. 5).
Fig. 5.
Effects of FGTE on liver mitochondrial ROS production of the Normal, CDHF, NASH, NASH + FGTE 100 mg/kg and NASH + FGTE 300 mg/kg group. Each value is the mean ± SEM of 6 rats. *p<0.05, **p<0.01, compared with NASH. #p<0.01, compared with Normal.
Discussion
The goal of this study was to investigate whether FGTE can prevent NASH progression and to propose a novel functional food for the prevention of NASH. Our results clearly showed that supplements of FGTE prevent liver dysfunction, increase plasma antioxidant, decrease liver triglyceride level and suppressing mitochondrial ROS production in NASH model rats. The results suggest that FGTE delays the progression of fatty liver to fibrosis. Consistent with the present study, it has been reported that EGCG attenuates steatohepatitis induced by high fat diet [17]. In addition, green tea extracts protect against obesity-triggered by NAFLD in leptin-deficient obese mice [18]. However, one of the striking differences between the above report and our present study is the contents of EGCG. The EGCG content in their study was high whereas FGTE in our study was not detected by HPLC analysis because of the decrease in the fermentation process. Additionally, the fermentation process caused a 12-fold increase in GA content and a 6.9-fold increase in GC content, but vitamin C levels were lower compared to those in regular green tea extracts. Despite containing less EGCG and vitamin C than green tea extracts, our FGTE had a strong radical scavenging activity, which was at least equal to that of typical tea extracts and vitamin C (data not shown). These results suggest that FGTE seems to prevent NASH progression through mechanism(s) different to those of EGCG.
NAFLD is exacerbated by impaired antioxidant pathways. This effect is more pronounced in NASH than in fatty liver. Furthermore, depletion of antioxidants such as GSH, vitamins C and E, and low plasma antioxidant activity are also reported in NASH [19]. These changes result in increased lipid peroxidation in NASH patients [20]. Therefore, we hypothesized that treatment with an antioxidant may prevent NASH progression. Our results showed that the administration of FGTE increased plasma SOD-like activity and decreased liver concentrations of MDA and 4-HNE (Fig. 2 and 4). These results suggest that FGTE supplementation can reset the balance between pro- and antioxidants. What is the mechanism of FGTE-induced increased plasma antioxidant activity? One potential mechanism is up-regulation of Cu-Zn SOD protein. However, no significant difference was found in Cu-Zn SOD protein expression between NASH and FGTE-treated rats (data not shown). This finding suggests that FGTE could have superior effects over regular green tea extracts. It is possible that the fermentation process of green tea extract changes its components and augments its antioxidative effects. Therefore, so many polyphenols in FGTE can play the role in protect body against NASH.
At present, however, the exact ingredient of FGTE most responsible for the observed effects in our study is unknown. Therefore, further studies are necessary to isolate and identify this ingredient from our FGTE. Nevertheless, the results emphasize the potential beneficial effects of foods with radical scavenging activity in NASH.
To clarify the mechanisms underlying the effects of FGTE on NASH, we examined triglyceride levels in the liver and ROS productions in the liver mitochondria. The high fat accumulations in the liver promote the β-oxidation in mitochondria, and follow to induce excess ROS production and result in mitochondrial dysfunction. Therefore, the mitochondria are the most important intracellular source of ROS in NASH [21]. In this study, FGTE administration reduced liver triglyceride levels (Fig. 3), suggesting that FGTE administration may regulate the excess triglyceride accumulation in the liver of NASH rat. According to the recent report, inhibiting TG synthesis improve hepatic steatosis but exacerbates liver damage and fibrosis in mice model of NASH [22]. Therefore, we consider that it is the most important for the NASH prevention to control both the hepatic steatosis and oxidative stress.
ROS production results in the depletion of mitochondrial DNA, up-regulation of uncoupling protein 2, reduced activity of respiratory chain complexes, impaired mitochondrial β-oxidation, resulting in mitochondrial dysfunction and abnormal mitochondrial morphology [23–25]. We also demonstrated previously increased ROS production by the mitochondria in rats with NASH, indicating that accumulation of excess ROS and fatty acids in the liver could cause damage of the mitochondrial membranes. Accordingly, we expected that administration of FGTE would prevent mitochondrial dysfunction caused by induction of excessive ROS and fatty acids. Our results indicate that FGTE-treated rats with NASH-like disease state showed restoration of normal levels of mitochondrial ROS production (Fig. 5). Moreover, our work indicated that the increased mitochondrial ROS productions might cause progression of fatty liver disease to NASH.
In summary, in NASH rats, oxidative stress damage is induced by pro-excessive ROS production and failure of antioxidant defence mechanisms. It is possible that oxidative stress damage is associated with progression of NASH. At this stage, however, further studies are warranted to assess serial changes in mitochondrial function and liver architecture in NASH liver, determine the mechanisms of NASH improvement by and anti-fibrotic activity of FGTE. Moreover, further studies are required to understand how FGTE prevent mitochondrial dysfunction and fibrosis in advanced NASH.
Conclusion
In conclusion, we demonstrated in the present study that the antioxidant activity of FGTE can prevent the progression of NASH by suppressing the production of mitochondrial ROS.
Acknowledgments
This work was supported by a Grant-in-Aid for young Scientists B from the Ministry of Education, Culture, Sports, Science and Technology, Japan (No. 20700550). We thank Ms. Kei Morita for the excellent technical assistance.
Abbreviations
- AST
aspartate aminotransferase
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- CDHF
choline-deficient high-fat
- CYPMPO
2-(5,5-Dimethyl-2-oxo-2λ5-[1,3,2]dioxaphosphinan-2-yl)-2-methyl-3,4-dihydro-2H-pyrrole 1-oxide
- DMPO
5,5-dimethyl-l-pyrroline N-oxide
- EGC
(−)-epigallocatechin
- EGCG
(−)-Epigallocatechin gallate
- ESR
electron spin resonance
- FGTE
fermented green tea extracts
- GA
gallic acid
- GC
(−)-gallocatechin
- GSH
reduced glutathione
- H&E
hematoxylin and eosin
- 4-HNE
4-hexanonenal
- MDA
malondialdehyde
- NAFLD
non-alcoholic fatty liver disease
- NASH
non-alcoholic steatohepatitis
- OS
oxidative stress
- ROS
reactive oxygen spieces
- SOD
superoxide dismutase
References
- 1.Ludwig J., Viggiano T.R., McGill D.B., Oh B.J. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin. Proc. 1980;55:434–438. [PubMed] [Google Scholar]
- 2.Day C.P., James O. Steatohepatitis: a tale of “two hits’’? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 3.Takayama F., Hobara N., Nakamoto K., Ohro M., Yoshida N., Takeda A., Kawasaki H., Egashira T. The 79th Annual Meeting of The Japanese Pharmacological Society, Yokohama, Japan, March 8–10. Vol. 100. 2006. Construction of a non-alcoholic steatohepatitis model induced by fatty liver and nitrite administration. Proceedings; p. 164. [Google Scholar]
- 4.Leclercq I.A. Antioxidant defence mechanisms: new players in the pathogenesis of non-alcoholic steatohepatitis? Clin. Sci. 2004;106:235–237. doi: 10.1042/CS20030368. [DOI] [PubMed] [Google Scholar]
- 5.Videla L.A., Rodrigo R., Orellana M., Fernandez V., Tapia G., Quiñones L., Varela N., Contreras J., Lazarte R., Csendes A., Rojas J., Maluenda F., Burdiles P., Diaz J.C., Smok G., Thielemann L., Poniachik J. Oxidative stress-related parameters in the liver of non-alcoholic fatty liver disease patients. Clin. Sci. 2004;106:261–268. doi: 10.1042/CS20030285. [DOI] [PubMed] [Google Scholar]
- 6.Higdon J.V., Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit. Rev. Food Sci. Nutr. 2003;43:89–143. doi: 10.1080/10408690390826464. [DOI] [PubMed] [Google Scholar]
- 7.Zhi Z., Froh M., Connor H.D., Li X., Conzelmann L.O., Mason R.P., Lemasters J.J., Thurman R.G. Prevention of hepatic ischemia-reperfusion injury by green tea extract. Am. J. Physiol. Gastrointest. Liver Physiol. 2002;283:957–964. doi: 10.1152/ajpgi.00216.2001. [DOI] [PubMed] [Google Scholar]
- 8.Zhi Z., Froh M., Lehnert M., Schoonhoven R., Yang L., Lind H., Lemasters J.J., Thurman R.G. Polyphenols from Camellia sinenesis attenuate experimental cholestasis-induced liver fibrosis in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2003;285:1004–1013. doi: 10.1152/ajpgi.00008.2003. [DOI] [PubMed] [Google Scholar]
- 9.Li Y.M., Zhang X.G., Zhou H.L., Chen S.H., Zhang Y., Yu C.H. Effects of tea polyphenols on hepatic fibrosis in rats with alcoholic liver disease Hepatobiliary Pancreat. Dis. Int. 2004;3:577–579. [PubMed] [Google Scholar]
- 10.Arteel G.E., Uesugi T., Bevan L.N., Gäbele E., Wheeler M.D., McKim S.E., Thurman R.G. Green tea extract protects against early alcohol-induced liver injury in rats. Biol. Chem. 2002;383:663–670. doi: 10.1515/BC.2002.068. [DOI] [PubMed] [Google Scholar]
- 11.Folch J., Lees M., Sloane Stanly G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 12.Egashira T., Takayama F. Free radicals and oxidative stress: targeted ESR measurement of free radicals. Nippon Yakurigaku Zasshi. 2002;120:229–236. doi: 10.1254/fpj.120.229. [DOI] [PubMed] [Google Scholar]
- 13.Takayama F., Egashira T., Kudo Y., Yamanaka Y. Effects of anti-free radical interventions on phosphatidylcholine hydroperoxide in plasma after ischemia-reperfusion in the liver of rats. Biochem. Pharmacol. 1993;46:1749–1757. doi: 10.1016/0006-2952(93)90579-l. [DOI] [PubMed] [Google Scholar]
- 14.Takayama F., Egashira T., Yamanaka Y. Effect of diclofenac, a non-steroidal anti-inflammatory drug, on lipid peroxidation caused by ischemia-reperfusion in rat liver. Jpn. J. Pharmacol. 1994;64:71–78. doi: 10.1254/jjp.64.71. [DOI] [PubMed] [Google Scholar]
- 15.Hogeboom G.H. Fractionation of cell components of animal tissues. Meth. Enzymol. 1955;1:16–19. [Google Scholar]
- 16.Wang Y., Fang J., Leonard S.S., Rao K.M. Cadmium inhibits the electron transfer chain and induces reactive oxygen species. Free Radic. Biol. Med. 2004;36:1434–1443. doi: 10.1016/j.freeradbiomed.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 17.Kuzu N., Bahcecioglu I.H., Dagli A.F., Ozercan I.H., Ustündag B., Sahin K. Epigallocatechin gallate attenuates experimental non-alcoholic steatohepatitis induced by high fat diet. J. Gastroenterol. Hepatol. 2007;23:465–470. doi: 10.1111/j.1440-1746.2007.05052.x. [DOI] [PubMed] [Google Scholar]
- 18.Bruno R.S., Dugan C.E., Smyth J.A., DiNatale D.A., Koo S.I. Green tea extract protects leptin-deficient, spontaneously obese mice from hepatic steatosis and injury. J. Nutr. 2008;1388:323–331. doi: 10.1093/jn/138.2.323. [DOI] [PubMed] [Google Scholar]
- 19.Sanyal A.J., Campbell-Sargent C., Mirshahi F., Rizzo W.B., Contos M.J., Sterling R.K., Luketic V.A., Shiffman M.L., Clore J.N. Nonalcoholic steatohepatitis: Association of insulin resistance and mitochondorial abnormalities. Gastroenterology. 2001;120:1183–1192. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 20.Robertson G., Leclercq I., Farrell G.C. Nonalcoholic steatosis and steatohepatitis. II. Cytochrome P-450 enzymes and oxidative stress. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;281:1135–1139. doi: 10.1152/ajpgi.2001.281.5.G1135. [DOI] [PubMed] [Google Scholar]
- 21.Pessayre D., Mansouri A., Fromenty B. Nonalcoholic steatosis and steatohepatitis V. Mitochondrial dysfunction in steatohepatitis. Am. J. Physiol. 2002;282:193–199. doi: 10.1152/ajpgi.00426.2001. [DOI] [PubMed] [Google Scholar]
- 22.Yamaguchi K., Yang L., McCall S., Huang J., Yu X.X., Pandey S.K., Bhanot S., Monia B.P., Li Y.X., Diehl A.M. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with no nalcoholic steatohepatitis. Hepatology. 2007;45:1366–1374. doi: 10.1002/hep.21655. [DOI] [PubMed] [Google Scholar]
- 23.Caldwell S.H., Swerdlow R.H., Khan E.M., Iezzoni J.C., Hespenheide E.E., Parks J.K., Parker W.D. Jr. Mitochondrial abnormalities in non-alcoholic steatohepatitis. J. Hepatol. 1999;31:430–434. doi: 10.1016/s0168-8278(99)80033-6. [DOI] [PubMed] [Google Scholar]
- 24.Begriche K., Igoudjil A., Pessayre D., Fromenty B. Mitochondrial dysfunction in NASH: causes, consequences and possible means to prevent it. Mitochondrion. 2006;6:1–28. doi: 10.1016/j.mito.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Wei Y., Rector R.S., Thyfault J.P., Ibdah J.A. Nonalcoholic fatty liver disease and mitochondrial dysfunction. World J. Gastroenterol. 2008;14:193–199. doi: 10.3748/wjg.14.193. [DOI] [PMC free article] [PubMed] [Google Scholar]