Abstract
In this study, we investigated the mechanistic role of the caspase cascade in extrinsic and intrinsic apoptosis induced by apigenin, which has been targeted as a candidate in the development of noncytotoxic anticancer medicines. Treatment with apigenin (1–100 µM) significantly inhibited the proliferation of MDA-MB-453 human breast cancer cells in a dose- and time-dependent manner with IC50 values of 59.44 and 35.15 µM at 24 and 72 h, respectively. This inhibition resulted in the induction of apoptosis and the release of cytochrome c in cells exposed to apigenin at its 72 h IC50. Subsequently, caspase-9, which acts in mitochondria-mediated apoptosis, was cleaved by apigenin. In addition, apigenin activated caspase-3, which functions downstream of caspase-9. The apigenin-induced activation of caspase-3 was accompanied by the cleavage of capases-6, -7, and -8. These results are supported by evidence showing that the activity patterns of caspases-3, -8, and -9 were similar. The present study supports the hypothesis that apigenin-induced apoptosis involves the activation of both the intrinsic and extrinsic apoptotic pathways.
Keywords: apigenin, apoptosis, caspases cascade, human breast cancer MDA-MB-453 cells
Introduction
Recently, flavonoids have been suggested as being potential chemotherapeutic agents owing to their biological activities, which include effects on cell cycle arrest and the induction of apoptosis [1–4]. Apigenin (4',5,7-trihydroxyflavone), a member of the flavone subclass of flavonoids, is present in fruits and vegetables [5] and is believed to have a number of biological functions including anti-inflammatory, anticancer, and free-radical scavenging properties [6–9]. Studies of human malignant cancer cell lines have shown that apigenin inhibits cancer cell growth via the promotion of cell cycle arrest and apoptosis [10, 11]. Moreover, apigenin is reportedly nonmutagenic and of low toxicity compared to related flavonoids [12]. Thus, apigenin may be a good candidate compound for anticancer drugs.
Apoptosis, or programmed cell death, is a multi-step process that is important in controlling cell number and proliferation as part of normal development; however, cancer cells tend to inactivate the cell cycle checkpoints and the subsequent progression of apoptosis. Consequently, the induction of apoptosis has been emphasized in anticancer strategies [13, 14]. Regarding the initiation and execution of cell death, two apoptosis pathways have been identified: the extrinsic (Fas death receptor-mediated) and intrinsic (mitochondrial) pathways. Both pathways involve the activation of caspases, a family of cysteine proteases that are activated in a sequential cascade of cleavage by other caspase family members [15, 16].
Evidence that several flavonoids activate the proteolytic activities of caspases supports their value as potential anticancer agents for controlling tumor growth [17–21]. One major pathway of caspase-activated apoptosis is triggered by the release of cytochrome c from the mitochondria to the cytoplasm. Previously our study, apigenin treatment results in apoptotic cell death via up-regulation of cytochrome c expression in human breast SK-BR-3 cells [22]. It has been reported that apigenin is a candidate therapeutic for neuroblastoma that likely acts by regulating a p53-Bax-caspase-3 apoptotic pathway [23].
However, the mechanisms underlying the effects of apigenin in the induction of apoptosis in human breast cancer cells are still unknown. Therefore, the present study was conducted to investigate whether the initiator caspase in the extrinsic or intrinsic pathway is activated during the induction of apoptosis by apigenin in MDA-MB-453 human breast cancer cells.
Materials and Methods
The cells culture and apigenin treatment
Human breast cancer MDA-MB-453 cells were purchased from the KCLB (Korean Cell Line Bank, Seoul, Korea). Cells was routinely maintained in RPMI 1640 (Invitrogen [Molecular Probes], Gibco, Carlsbad, CA), supplemented with 10% FBS and antibiotics (50 U/ml of penicillin and 50 µg/ml streptomycin, Gibco) at 37°C in a humidified atmosphere containing 5% CO2. For cell proliferation assay, cells were treated with apigenin ranging from 1 to 100 µM and incubated for 24 and 72 h. Then after, to investigate the apoptosis induction by apigenin, cells were exposed to either vehicle or apigenin (at the IC50 concentration) and incubated for 72 h. Apigenin was purchased from Sigma and dissolved in DMSO (final concentration 0.1% in medium, Sigma-Aldrich Corp., St. Louis, MO).
Cell proliferation assay
The inhibitory effect of the apigenin on cell proliferation was determined by the MTT assay. The cells were treated with apigenin ranging from 1 to 100 µM and incubated for 24 and 48 h and added to methyl thiazolyl tetrazolium (MTT). Four hours later, DMSO was added to each well to dissolve the resulting formazan crystals and then absorbance was recorded at 490 nm in a microplate reader (SpectraMax Plus; Molecular Devices Corp., Orleans, CA). The value of IC50 (i.e., the concentration of the extract required to inhibit cancer cell proliferation by 50% of the control level, which is each cells treated with only compound solvent) was estimated from the plot.
DAPI staining assay
Apoptotic morphological changes were determined by DAPI (4',6-diamidino-2-phenyl-indole) staining. After harvesting the cells exposed with apigenin for 72 h, the cells were seeded in poly-l-lysine coated slides and fixed with 4% methanol-free formaldehyde solution for 30 min. Then mounting medium with DAPI (Molecular Probes, Eugene, OR) was dispersed over the entire section of slides. Mounted slides were stored at 4°C without light. Each slide was observed under Axio vision 4.0 fluorescence microscopes (Carl Zeiss Inc., Thornwood, NY). Additionally, features of MDA-MB-453 cells exposed to apigenin were also observed using a Nikon inverse phase contrast microscope (Nikon TMS, Nikon, Tokyo, Japan) equipped with an objective (Plan 10/0.30DL/Ph1, Nikon) of ×100 magnification.
Immunoblotting assay
Protein expression was determined by western blotting. Briefly, cells were lysed in RIPA buffer (1% NP-40, 150 mM NaCl, 0.05% DOC, 1% SDS, 50 mM Tris, pH 7.5) containing protease inhibitor for 1 h at 4°C. The supernatant was separated by centrifugation, and protein concentration was determined by Bradford protein assay kit II (Bio-rad Laboratories, Hercules, CA). For detection of cytochrome c release, cytosolic fraction was sedimented from post-nuclear supernatant by centrifugation at 100,000 g for 30 min at 4°C. Proteins (25 µg/well) denatured with sample buffer were separated by 10% SDS-polyacrylamide gel. Proteins were transferred onto nitrocellulose membranes (0.45 µm). The membranes were blocked with a 1% BSA solution for 3 h and washed twice with PBS containing 0.2% Tween-20, and incubated with the respective primary antibodies (Cell Signaling Technology, Inc., Danvers, CA) overnight at 4°C. The next day, the immunoreaction was continued with the secondary goat anti-rabbit horseradish-peroxidase–conjugated antibody after washing for 2 h at room temperature. The specific protein bands were detected by Opti-4CN Substrate kit (Bio-rad).
Colorimetric caspases activity assay
To analyze the role of caspases, caspases activity was measured using colorimetric assay. Briefly, the single-cell suspension was collected by centrifugation at 1,000 rpm and the cells were lysed with lysis buffer (1% Triton X-100, 0.32 M sucrose, 5 mM EDTA, 10 mM Tris-HCl, pH 8.0, 2 mM dithiothreitol, 1 mM PMSF, 1 µg/ml Aprotinin, 1 mg/ml Leupeptin). Thereafter, the lysates were transferred to wells in a 96-well flat-bottom microplate and were incubated with 4 mM substrate (final concentration of 200 uM) specific for caspase-3, -8, and -9 at 37°C for 1 h. Specific substrate was DVED-pNA for caspase-3, IETD-pNA for caspase-8 or LEHD-pNA for caspase-9, respectively. The intensity of the developed color was read at 405 nm in a microplate reader (SpectraMax Plus; Molecular Devices).
Statistical analyses
All data were expressed as percent compared with vehicle-treated control cells, which were arbitrarily assigned 100%. Data were analyzed by one-way analysis of variance followed by Dunnett’s multiple comparison test (Sigma Stat, Jandel, San Rafael, CA). For all comparisons, differences were considered statistically significant at p<0.05.
Results
Apigenin inhibited the cell proliferation
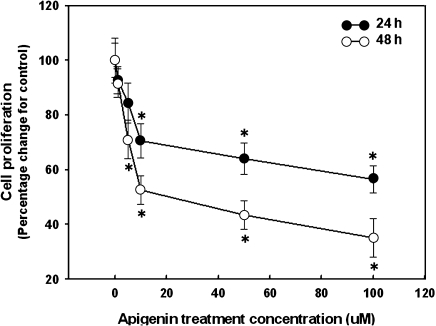
The effects of apigenin on cell proliferation were measured with the MTT assay, using human breast cancer MDA-MB-453 cells exposed to between 1 and 100 µM apigenin for 24 and 72 h (Fig. 1). Statistical differences in cell proliferation were first exhibited as inhibited cell proliferation at 10 and 5 µM after 24 and 48 h of apigenin treatment, respectively (p<0.05). Apigenin exhibited antiproliferative effects against human breast cancer MDA-MB-453 cells with the IC50 values of 59.44 and 35.15 µM at 24 and 72 h, respectively. Cell proliferation dramatically decreased up to 35.10% when treated with 100 µM apigenin for 72 h compared to the control.
Fig. 1.
Effect of apigenin on cell proliferation of human breast cancer MDA-MB-453 cells. Cells were exposed to either vehicle (0.1% DMSO in medium) or apigenin (1–100 µM) and incubated for 24 and 72 h. All data are reported as the percentage change in comparison with the vehicle-only group, which were arbitrarily assigned 100% viability. Values are Mean ± SD (n = 6). *p<0.05, significantly different from the vehicle-only group (0.1% DMSO in medium, that is, apigenin concentration = “0”).
Apigenin induced the apoptosis
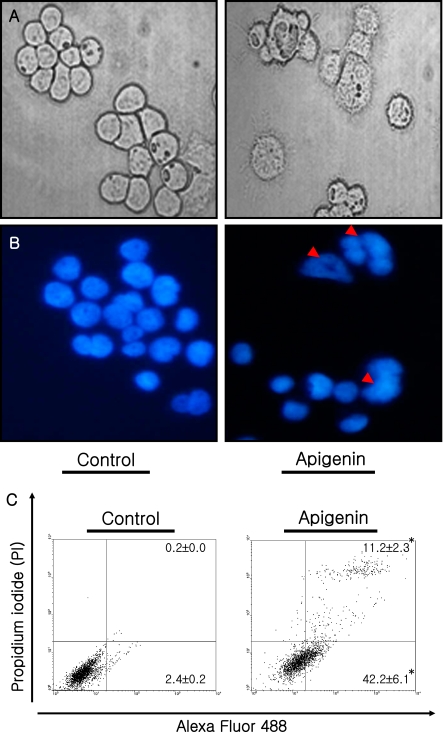
To verify that anticancer activity of apigenin in human breast cancer MDA-MB-453 cells, we observed the apoptotic feature and performed the DAPI staining as apoptosis index after exposing cells to apigenin at IC50 concentration (Fig. 2A, B). DAPI stains DNA specifically by permeating the cell membrane to bind with DNA. DAPI staining assays revealed that apoptotic morphological features such as cell shrinkage and dot-shaped nuclear fragments were observed in exposing cells to apigenin. Furthermore, these results are supported by Annexin-based flow cytometry (Fig. 2C). Apigenin increased significantly the total number of apoptotic cells in the MDA-MB-453 cells treated at its IC50 concentration for 72 h (11.2 and 42.2% in early and late apoptotic cell population, respectively, p<0.05).
Fig. 2.
Apigenin induced apoptosis of human breast cancer MDA-MB-453 cells. Cells were exposed to either vehicle or apigenin (at its IC50 concentration) and incubated for 72 h. (A) MDA-MB-453 cells exposed to apigenin were observed using a Nikon inverse phase contrast microscope. (B) To observe the apoptotic morphological changes, cells exposed to apigenin were fixed and stained by DAPI. (C) Apoptotic population was determined by Annexin-V assay. Early apoptotic cells; right bottom, Late apoptotic cells; right top, Live cells; left bottom. Values are Mean ± SD (n = 6). *p<0.05, significantly different from the vehicle-only group (0.1% DMSO in medium, that is, apigenin concentration = “0”).
Apigenin mediated the caspases cascade
Under the same conditions described above, apigenin-induced caspase-dependent pathway of apoptotic signal transduction was evaluated in human breast MDA-MB-453 cells. Caspases are synthesized as relatively inactive zymogens, which become activated upon cleavage, a common mechanism for most protease zymogens. Thus, we observed both the zymogen and the cleaved forms of caspases.
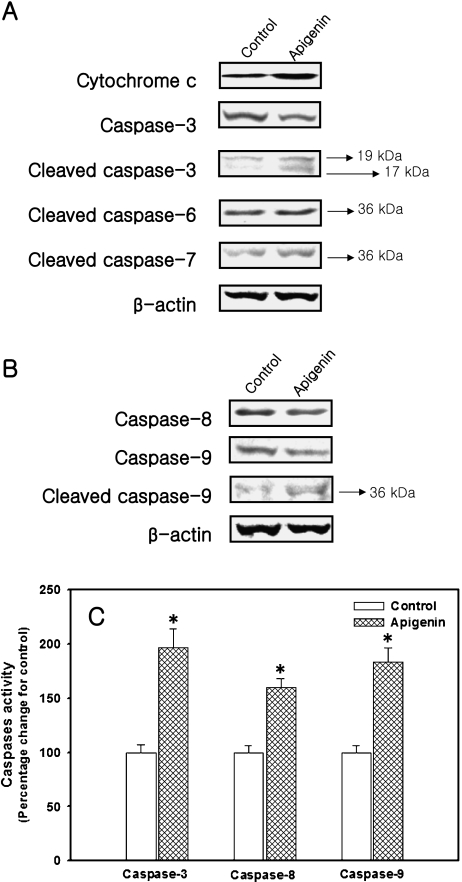
As shown in Fig. 3A, the release of cytochrome c in the cytosolic fraction increased remarkably by apigenin treatment. Moreover, Apigenin activated the caspase-3 significantly, as evidenced by a decrease in capase-3 accompanied by an increase of cleaved caspase-3. In parallel with this result, the activation of caspase-3 in cells exposed to apigenin induced increased cleavage of caspase-6 and -7. Beside, the caspase-8 and -9 were decreased significantly together with an increase of cleaved caspase-9 (Fig. 3B).
Fig. 3.
Effect of apigenin on caspase cascade of human breast cancer MDA-MB-453 cells. Cells were exposed to either vehicle or apigenin at its IC50 concentration and incubated for 72 h. (A, B) Cytochrome c and various caspases protein expressions were determined by Immunoblotting assay. (C) Caspase-3, -8, and -9 activity was measured using colorimetric assay. Data obtained from caspases activity are reported as the percentage change in comparison with the vehicle-only group, which were arbitrarily assigned 100% viability. Values are Mean ± S.D. (n = 6). *p<0.05, significantly different from the vehicle-only group (0.1% DMSO in medium, that is, apigenin concentration = “0”).
To confirm the caspase activations, caspase-3, -8, and -9 were determined using a colorimetric assay. Although three caspase activities increased significantly by apigenin treatment, compared to the control, the caspase-3 activity displayed a greater increase than did capase-8 and -9. The caspase-3 activity increased by more than 2-fold compared to the control, while caspase-8 and -9 increased by 1.6- and 1.8-fold, respectively (p<0.05, Fig. 3C).
Discussion
It was previously demonstrated that apigenin causes cell cycle arrest via the regulation of CDK1 and p21Cip1 and the induction of apoptosis [22]. These findings led to the hypothesis that apigenin has potential as an anticancer compound. In addition, several studies have shown that apigenin-induced apoptosis involves both the intrinsic and extrinsic apoptotic pathways [24, 25]. Thus, in the present study, we investigated the modulation of the initiator caspase in the extrinsic or intrinsic pathway during apigenin-induced apoptosis.
We first examined the antiproliferative effect of apigenin at various concentrations (1–100 µM) on MDA-MB-453 human breast cancer cells following 24 or 72 h of incubation. Apigenin significantly inhibited cellular proliferation in a dose- and time-dependent manner. This finding is consistent with previous data showing that apigenin may be an effective agent against cell growth in some cancer cell types [26, 27].
To investigate whether this inhibition induces apoptosis, cells exposed to apigenin at its IC50 for 72 h were stained with DAPI. Typical morphological features of apoptosis such as membrane blebbing were observed. Therefore, we next investigated the apigenin-induced caspase-dependent apoptotic signaling pathway. The expression of several apoptosis-related genes was evaluated in MDA-MB-453 cells treated as described above. Apigenin treatment caused the dramatic release of cytochrome c into the cytosol, which is consistent with previous data showing that the exposure of SK-BR-3 cells to apigenin significantly increased the release of cytochrome c [22]. Thus, it appears that apigenin induces apoptosis in MDA-MB-453 cells via the mitochondrial apoptotic pathway.
We next analyzed the caspase pathway involved in the interaction between apigenin and mitochondria. In mammalian cells, apoptosis is mediated by cysteine proteases, which are divided into initiators (e.g., caspase-8, -9, -10 and -12) and executors (e.g., caspase-2, -3, -6 and -7). The initiators cleave and activate the executors. The extrinsic apoptotic pathway is initiated by the ligation of a transmembrane death receptor with its ligand, which activates membrane-proximal caspases (e.g., caspases-8 and -10), whereas the intrinsic apoptotic pathway is initiated by the release of cytochrome c. Caspase-8 mediates signal transduction downstream of death receptors located on the plasma membrane [28–31]. In this study, apigenin-induced apoptosis was associated with the extrinsic and intrinsic apoptotic pathways as evidenced by the activation of caspases-9 and -3, as well as caspase-8. These results are supported by the observed activity patterns of caspases-3, -8, and -9, which were similar to their expression patterns.
Recently, apoptosis has been identified as a useful target in the development of anticancer therapies. Caspase-mediated apoptosis is a major focus in the field of cancer growth inhibition, because activation of the proteolytic caspase cascade is a critical component in the execution of apoptotic cell death. Our current results support the hypothesis that apigenin-induced apoptosis involves the activation of both the intrinsic and extrinsic apoptotic pathways. It is clear that apigenin may be a very useful anticancer drug candidate for chemotherapy and, possibly, cancer prevention. Because natural phytochemicals, such as apigenin, have been reported to act on multiple molecular and cellular targets, a better understanding of the mechanism of apigenin-induced apoptosis is crucial.
Acknowledgement
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD, KRF-2006-311-F00127 & KRF-2005-005-J13001).
References
- 1.Ren W., Qiao Z., Wang H., Zhu L., Zhang L. Flavonoids: promising anticancer agents. Med. Res. Rev. 2003;23:519–534. doi: 10.1002/med.10033. [DOI] [PubMed] [Google Scholar]
- 2.Manthey J.A., Grohmann K., Guthrie N. Biological properties of citrus flavonoids pertaining to cancer and inflammation. Curr. Med. Chem. 2001;8:135–153. doi: 10.2174/0929867013373723. [DOI] [PubMed] [Google Scholar]
- 3.Ramos S. Effects of dietary flavonoids on apoptotic pathways related to cancer chemoprevention. Nutr. Biochem. 2007;18:427–442. doi: 10.1016/j.jnutbio.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Kale A., Gawande S., Kotwal S. Cancer phytotherapeutics: role for flavonoids at the cellular level. Phytother. Res. 2008;22:567–577. doi: 10.1002/ptr.2283. [DOI] [PubMed] [Google Scholar]
- 5.Miean K.H., Mohamed S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J. Agric. Food Chem. 2001;49:3106–3112. doi: 10.1021/jf000892m. [DOI] [PubMed] [Google Scholar]
- 6.Soares R., Azevedo I. Apigenin: is it a pro- or anti-inflammatory agent? Am. J. Pathol. 2006;168:1762–1763. doi: 10.2353/ajpath.2006.060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuchs J., Milbradt R. Skin anti-inflammatory activity of apigenin-7-glucoside in rats. Arzneimittelforschung. 1993;43:370–372. [PubMed] [Google Scholar]
- 8.Singh J.P., Selvendiran K., Banu S.M., Padmavathi R. Protective role of Apigenin on the status of lipid peroxidation and antioxidant defense against hepatocarcinogenesis in Wistar albino rats. Phytomedicine. 2004;11:309–314. doi: 10.1078/0944711041495254. [DOI] [PubMed] [Google Scholar]
- 9.Romanova D., Vachalkova A., Cipak L., Ovesna Z., Rauko P. Study of antioxidant effect of apigenin, luteolin and quercetin by DNA protective method. Neoplasma. 2001;48:104–107. [PubMed] [Google Scholar]
- 10.Bektic J., Guggenberger R., Spengler B., Christiffel V., Pelzer A., Berger A.P., Ramoner R., Bartsch G., Klocker H. The flavonoid apigenin inhibits the proliferation of prostatic stromal cells via the MAPK-pathway and cell-cycle arrest in G1/S. Maturitas. 2006;55s:s37–s46. [Google Scholar]
- 11.Lindenmeyer F., Li H., Menashi S., Soria C., Lu H. Apigenin acts on the tumor cell invasion process and regulates protease production. Nutr. Cancer. 2001;39:139–147. doi: 10.1207/S15327914nc391_19. [DOI] [PubMed] [Google Scholar]
- 12.Czeczot H., Tuek B., Kusztelak J., Szymczyk T., Dobrowolska B., Glinkowska G., Malinowski J., Strzelecka H. Isolation and studies of the mutagenic activity in the Ames test of flavonoids naturally occurring in medical herbs. Mutat. Res. 1990;240:209–216. doi: 10.1016/0165-1218(90)90060-f. [DOI] [PubMed] [Google Scholar]
- 13.Fulda S., Debatin K.M. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006;25:4798–4811. doi: 10.1038/sj.onc.1209608. [DOI] [PubMed] [Google Scholar]
- 14.Prados J., Melguizo C., Boulaiz H., Marchal J.A., Aranega A. Cancer gene therapy: strategies and clinical trials. Cell Mol. Biol. 2005;51:23–36. [PubMed] [Google Scholar]
- 15.Kim R. Recent advances in understanding the cell death pathways activated by anticancer therapy. Cancer. 2005;103:1551–1560. doi: 10.1002/cncr.20947. [DOI] [PubMed] [Google Scholar]
- 16.Philchenkov A., Zavelevich M., Kroczak T.J., Los M. Caspases and cancer: mechanisms of inactivation and new treatment modalities. Exp. Oncol. 2004;26:82–97. [PubMed] [Google Scholar]
- 17.Ramos S. Effects of dietary flavonoids on apoptotic pathways related to cancer chemoprevention. J. Nutr. Biochem. 2007;18:427–442. doi: 10.1016/j.jnutbio.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Gercel-Taylor C., Feitelson A.K., Taylor D.D. Inhibitory effect of genistein and daidzein on ovarian cancer cell growth. Anticancer Res. 2004;24:795–800. [PubMed] [Google Scholar]
- 19.Hedlund T.E., van Bokhoven A., Johannes W.U., Nordeen S.K., Ogden L.G. Prostatic fluid concentrations of isoflavonoids in soy consumers are sufficient to inhibit growth of benign and malignant prostatic epithelial cells in vitro. Prostate. 2006;66:557–566. doi: 10.1002/pros.20380. [DOI] [PubMed] [Google Scholar]
- 20.Rassi C.M., Lieberherr M., Chaumaz G., Pointillart A., Cournot G. Down-regulation of osteoclast differentiation by daidzein via caspase 3. J. Bone Miner. Res. 2002;17:630–638. doi: 10.1359/jbmr.2002.17.4.630. [DOI] [PubMed] [Google Scholar]
- 21.Totta P., Acconcia F., Virgili F., Cassidy A., Weinberg P.D., Rimbach G., Marino M. Daidzein-sulfate metabolites affect transcriptional and antiproliferative activities of estrogen receptor-beta in cultured human cancer cells. J. Nutr. 2005;135:2687–2693. doi: 10.1093/jn/135.11.2687. [DOI] [PubMed] [Google Scholar]
- 22.Choi E.J., Kim G.H. Apigenin causes G(2)/M arrest associated with the modulation of p21(Cip1) and Cdc2 and activates p53-dependent apoptosis pathway in human breast cancer SK-BR-3 cells. J. Nutr. Biochem. 2008 doi: 10.1016/j.jnutbio.2008.03.005. in press, [DOI] [PubMed] [Google Scholar]
- 23.Torkin R., Lavoie J.F., Kaplan D.R., Yeger H. Induction of caspase-dependent, p53-mediated apoptosis by apigenin in human neuroblastoma. Mol. Cancer Ther. 2005;4:1–11. [PubMed] [Google Scholar]
- 24.Abu-Yousif A.O., Smith K.A., Getsios S., Green K.J., Van Dross R.T., Pelling J.C. Enhancement of UVB-induced apoptosis by apigenin in human keratinocytes and organotypic keratinocyte cultures. Cancer Res. 2008;68:3057–3065. doi: 10.1158/0008-5472.CAN-07-2763. [DOI] [PubMed] [Google Scholar]
- 25.Xu L., Zhang L., Bertucci A.M., Pope R.M., Datta S.K. Apigenin, a dietary flavonoid, sensitizes human T cells for activation-induced cell death by inhibiting PKB/Akt and NF-kappaB activation pathway. Immunol Lett. 2008 doi: 10.1016/j.imlet.2008.08.004. Epub ahead of print, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Q., Zhao X.H., Wang Z.J. Flavones and flavonols exert cytotoxic effects on a human oesophageal adenocarcinoma cell line (OE33) by causing G2/M arrest and inducing apoptosis. Food Chem. Toxicol. 2008;46:2042–2053. doi: 10.1016/j.fct.2008.01.049. [DOI] [PubMed] [Google Scholar]
- 27.Wang W., Heideman L., Chung C.S., Pelling J.C., Koehler K.J., Birt D.F. Cell-cycle arrest at G2/M and growth inhibition by apigenin in human colon carcinoma cell lines. Mol. Carcinog. 2002;28:102–110. [PubMed] [Google Scholar]
- 28.Kruidering M., Evan G.I. Caspase-8 in apoptosis: the beginning of “the end”? IUBMB Life. 2000;50:85–90. doi: 10.1080/713803693. [DOI] [PubMed] [Google Scholar]
- 29.Kim P.K., Mahidhara R., Seol D.W. The role of caspase-8 in resistance to cancer chemotherapy. Drug resis. Updates. 2001;4:293–296. doi: 10.1054/drup.2001.0223. [DOI] [PubMed] [Google Scholar]
- 30.Philchenkov A., Zavelevich M., Kroczak T.J., Los M. Caspases and cancer: mechanisms of inactivation and new treatment modalities. Exp. Oncol. 2004;26:82–97. [PubMed] [Google Scholar]
- 31.Kim R. Recent advances in understanding the cell death pathways activated by anticancer therapy. Cancer. 2005;103:1551–1560. doi: 10.1002/cncr.20947. [DOI] [PubMed] [Google Scholar]