Abstract
AIMS
To assess the efficacy, pharmacodynamics, safety and tolerability of a range of doses of cetilistat, a novel inhibitor of gastrointestinal lipases, in healthy volunteers.
METHODS
Three Phase I, randomized, placebo-controlled, parallel-group studies were conducted. Enrolled subjects in the three studies (n = 99) received a controlled calorie diet (total intake 2160 calories daily, 30% from fat). Twenty-four subjects were randomized to placebo and 66 were randomized to the following cetilistat doses: 50 mg three times daily [t.i.d. (n = 7)], 60 mg t.i.d. (n = 9), 100 mg t.i.d. (n = 7), 120 mg t.i.d. (n = 9), 150 mg t.i.d. (n = 16), 240 mg t.i.d. (n = 9) and 300 mg t.i.d. (n = 9). Nine subjects received the approved orlistat dose (120 mg t.i.d.). Treatment was for 5 days, with a 2-day run-in period and 1-day post-treatment follow-up. The primary outcome measure was daily faecal fat excretion. Secondary outcomes included plasma lipid levels, tolerability [gastrointestinal adverse events (AEs)] and safety.
RESULTS
Cetilistat increased faecal fat excretion relative to baseline at all doses. Cetilistat was well tolerated, with gastrointestinal AEs the most common (51%). Steatorrhoea (oily stool) was more frequent in the orlistat group (4.11 events per subject) than in any cetilistat dose group (0.14–1.81 events per subject). Most AEs (98%) were mild or moderate in intensity.
CONCLUSIONS
Cetilistat increased dietary fat excretion in healthy volunteers receiving a controlled calorie diet. Cetilistat was well tolerated at all doses examined and tolerability appeared to be improved relative to orlistat. Faecal fat excretion in the cetilistat groups was at least comparable to the orlistat 120 mg t.i.d. group.
Keywords: cetilistat, lipase inhibitor, obesity, orlistat
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Obesity is a significant and growing problem for which many patients seek pharmacological intervention.
Orlistat, a lipase inhibitor, is licensed for the treatment of obesity.
In combination with diet, orlistat is an effective product, but is associated with a number of adverse gastrointestinal adverse events.
WHAT THIS STUDY ADDS
This study demonstrates that cetilistat is an effective inhibitor of gastrointestinal lipases, substantially increasing the amount of faecal fat excreted at all doses studied.
In addition, cetilistat is well tolerated, and a comparison with orlistat suggests an improved tolerability profile.
Introduction
Obesity is a chronic, complex, multifactorial disorder that results in accelerated morbidity and mortality. Obese individuals are at increased risk for developing many medical problems, including insulin resistance and Type 2 diabetes mellitus, hypertension, dyslipidaemia, cardiovascular disease, stroke and osteoarthritis, and certain cancers are also associated with obesity [1].
In many industrialized nations, the incidence of obesity is increasing, and in many has reached epidemic proportions [2]. It has been shown that even a modest loss of 5% of initial body weight can reduce, eliminate or prevent the incidence of comorbidity in a large proportion of overweight patients [3]. However, the success of lifestyle modification, the cornerstone of management strategies for this disorder, is limited. Whereas drug treatment is often indicated, there are currently few well-tolerated drugs available that have proven long-term efficacy in maintaining bodyweight loss. Pharmacological approaches currently under investigation include gut hormones, such as peptide PYY(3–36) and cholecystokinin that normally signal satiety, centrally-acting agents such as serotonin agonists, cannabinoid receptor antagonists and drugs that act on other peptide neurotransmitter systems such as NPY and melanocortins [4, 5].
One potential therapeutic approach is the induction of a negative energy balance through the inhibition of nutrient, particularly fat, absorption. Orlistat, a synthetic derivative of a product made by Streptomyces toxytricini[6], is a selective inhibitor of gastrointestinal lipases involved in triglyceride hydrolysis that has been approved by the US (Food and Drug Administration) and the European (European Medicines Agency) drug registration authorities for weight reduction. A number of studies have shown that treatment with orlistat is associated with reductions in bodyweight beyond those achieved with diet alone [7–12]. However, treatment with orlistat is also associated with a number of gastrointestinal adverse events (AEs) that are exacerbated among patients who do not adhere to the recommended low-fat diet.
Cetilistat is an inhibitor of pancreatic and gastrointestinal lipases, and is chemically distinct from orlistat. Short-term (12-week) therapy with cetilistat produced statistically and clinically significant weight loss in clinically obese patients compared with placebo, as well as improvements in waist circumference, and serum cholesterol and low-density lipoprotein cholesterol levels [13]. Cetilistat was also well tolerated in this study.
We report here the combined results from three Phase I clinical studies designed to investigate the efficacy, pharmacodynamics and tolerability of a range of cetilistat doses compared with placebo or orlistat in healthy volunteers.
Methods
Subjects
Male volunteers, aged 18–45 years, with a body mass index of ≤30 kg m−2, a minimum weight of 60 kg, no clinically relevant abnormalities on a 12-lead electrocardiogram (ECG) and no relevant systemic disease history were considered for inclusion in these studies. Exclusion criteria included: the presence of any clinically relevant symptoms or severe disease within 4 weeks of the start of the study, any history of hepatic dysfunction, any condition that might affect the absorption, distribution, metabolism or excretion of the study drug, diarrhoea (>2 liquid stools per day) or constipation (≥3 days duration) 1 week prior to the start of the study, hypersensitivity to lipase inhibitors, evidence of hepatitis B or C, HIV positivity, smoking (>10 cigarettes/day), history of alcohol or substance abuse, bulimia or laxative abuse.
Study design
The three studies were all double-blind, randomized, placebo-controlled, parallel-group studies in healthy male volunteers resident in a clinical unit. Subjects were randomized to receive placebo, one of a range of cetilistat doses, or orlistat for 5 days, depending on the study (Table 1), and were maintained on a strictly controlled diet for the duration of the study. Each subject received three standardized meals daily with 30% of calories derived from fat. Each study comprised an initial screening visit within 4 weeks prior to the start of the study, with a 2-day run-in period, 5-day treatment period and a 1-day post-treatment follow-up visit.
Table 1.
Study details and subject characteristics
| Study | Study medication (mg t.i.d.) | Subjects (n = 99) | Age (years) | Height (cm) | Weight (kg) |
|---|---|---|---|---|---|
| 1* | Placebo | 6 | 34.5 | 179.8 | 76.0 |
| 50 | 7 | 29.0 | 184.1 | 80.1 | |
| 100 | 7 | 31.4 | 187.0 | 82.1 | |
| 150 | 7 | 30.1 | 177.7 | 72.1 | |
| 2* | Placebo | 9 | 32.6 | 177.7 | 76.2 |
| 150 | 9 | 34.4 | 181.9 | 82.0 | |
| 300 | 9 | 32.9 | 180.3 | 78.2 | |
| 120 mg t.i.d. Orlistat | 9 | 35.3 | 180.1 | 81.6 | |
| 3* | Placebo | 9 | 34.1 | 180.0 | 79.3 |
| 60 | 9 | 31.2 | 180.6 | 78.1 | |
| 120 | 9 | 33.9 | 181.2 | 82.0 | |
| 240 | 9 | 36.7 | 178.0 | 77.5 | |
| Mean | – | 33.1 | 180.6 | 78.9 |
Study 1: ATL962/012/CL (564/ALZ); Study 2: ATL962/080/CL (605/ALZ); Study 3: ATL962/081/CL (657/ALZ).
The primary efficacy variable in all three studies was daily faecal fat excretion. Secondary end-points were the excretion of triglycerides, long-chain fatty acids (LCFA), cholesterol, total faecal fats, and short-chain fatty acids (SCFA), plasma lipid levels, specifically high-density lipoprotein-cholesterol (HDL-C), low-density lipoprotein-cholesterol (LDL-C), cholesterol, free fatty acids and triglycerides, safety (AEs, ECG, vital signs and clinical laboratory parameters) and tolerability (gastrointestinal AEs).
The three studies were conducted in accordance with the ethical principles laid out in the Declaration of Helsinki, and each was approved by the appropriate ethics committees, according to local regulations. All subjects gave written informed consent prior to enrolment.
Assessments
Faecal samples were collected for faecal fat analysis as follows: in Study 1 at day −2 or day −1 to obtain a baseline value, and then at day 3, 4 or 5; in Studies 2 and 3 daily from the start of the run-in period (day −2) until day 5. Blood samples for plasma lipid analysis (one prior to breakfast, one 2 h post breakfast and one 4 h post breakfast) were taken on day −1, 2 and 5 in all three studies.
Blood samples for safety analysis were taken at the screening visit, on day −1 and day 5. AEs were assessed daily from day −2 to day 7 of the study. Subjects were informed of the potential side-effects of lipase inhibitors. Routine clinical laboratory tests (clinical chemistry and haematology) were performed at screening, on day 1 (before the first dose of drug) and day 6 at the final examination. For one study this included assessment of fat soluble vitamin levels (A, D, E, K and β-carotene). Urinalysis was performed at screening and at the final examination on day 6. ECGs were performed at screening and on day −1 and day 6, whereas vital signs were assessed at screening and on each day of the study.
Statistical analysis
Statistical tests were performed using a 5% significance level. All statistical tests were regarded as descriptive, and no adjustment for multiplicity of testing was performed. Differences between treatment groups in the primary and secondary efficacy variables were assessed using analysis of variance (anova), whereas differences between active treatment groups relative to placebo were analysed using the t-test. Dunnett's procedure was applied to adjust for multiple testing for the primary pharmacodynamic variable. No statistical comparisons of cetilistat vs. orlistat were performed.
Results
Subjects
A total of 99 subjects were randomized. Sixty-six received doses of cetilistat, nine received orlistat 120 mg three times per day (t.i.d.) and 24 received placebo (Table 1). Baseline characteristics were similar between the placebo and treatment groups in each of the three studies, and were also similar between the three studies (Table 1). Three subjects withdrew from the studies prematurely (see Adverse events).
Faecal fat excretion
Each of the doses of cetilistat assessed in the three studies increased mean faecal fat excretion relative to both baseline and placebo (Figure 1). In Study 1, faecal fat excretion as a proportion of total faecal mass increased relative to placebo by day 3, 4 or 5 in all three cetilistat dose groups. However, this increase was significant, compared with placebo, only in the 150 mg t.i.d. dose group (1.8 vs. 9.2%; P = 0.0186) (data not shown). In Studies 2 and 3, faecal fat excretion was assessed daily. The results of Study 2, comparing cetilistat 150 and 300 mg t.i.d. with orlistat 120 mg t.i.d. are shown in Figure 2. Faecal fat excretion was higher in the two cetilistat dose groups, and appeared to increase in a dose-dependent manner. In Study 3, the response to treatment was more variable, with no clear dose-dependent response (data not shown). However, faecal fat excretion was significantly higher than in the placebo group by day 5 in both the 120 (0.98 vs. 8.38 g 24 h−1; P = 0.0123) and 240 mg t.i.d. (0.98 vs. 8.29 g 24 h−1; P = 0.0221) groups.
Figure 1.
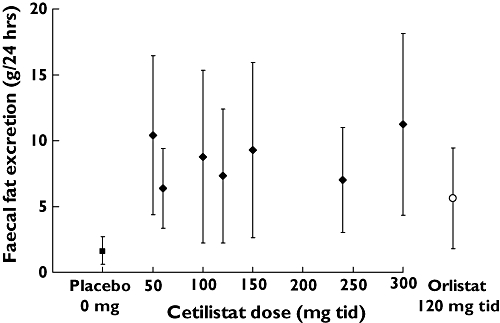
Faecal fat excretion on days 3–5 for each treatment group in the three studies
Figure 2.
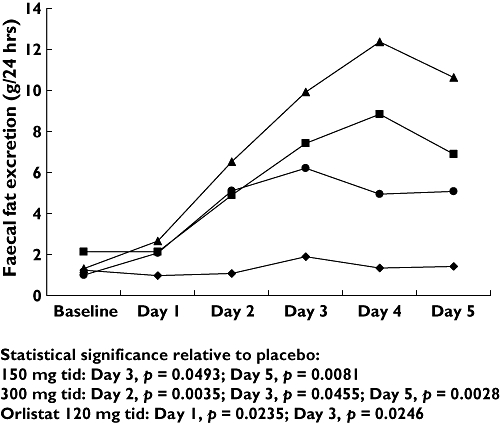
Changes in faecal fat excretion over 5 days following administration of placebo, orlistat 120 mg t.i.d., cetilistat 150 mg t.i.d. and cetilistat 300 mg t.i.d. (Study 2). Orlistat 120 mg tid (•); Cetilistat 150 mg tid ( ); Cetilistat 300 mg tid (
); Cetilistat 300 mg tid ( ); Placebo (♦)
); Placebo (♦)
A comparison of faecal fat excretion on days 3–5 across all three studies is shown in Figure 1. Cetilistat and orlistat dose groups show increased faecal fat excretion compared with placebo. The days 3–5 faecal fat excretion for the orlistat 120 mg t.i.d. was similar to that in the cetilistat dose groups.
The excretion of triglycerides, as a proportion of total faecal mass, was increased in all cetilistat groups relative to placebo (Table 2). These increases were significant in the cetilistat 120, 150, 240, and 300 mg t.i.d. dose groups. A significant increase was also seen in the orlistat 120 mg t.i.d. dose group.
Table 2.
Percentage triglyceride excretion as a proportion of total faecal mass at baseline and day 5
| Cetilistat dose (mg t.i.d.) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study 1* | Study 2* | Study 3* | Orlistat† | |||||||||
| Placebo (n= 6) | 50 (n = 7) | 100 (n = 7) | 150* (n = 7) | Placebo (n = 9) | 150 (n = 9) | 300 (n = 9) | Placebo (n = 9) | 60 (n = 9) | 120 (n = 9) | 240 (n = 9) | 120 (n = 9) | |
| Baseline Mean(%)(±s) | 0.3 (±0.2) | 0.3 (±0.1) | 0.4 (±0.2) | 0.2 (±0.1) | 0.4 (±0.3) | 0.5 (±0.5) | 0.5 (±0.5) | 0.1 (±0.1) | 0.1 (±0.0) | 0.1 (±0.1) | 0.2 (±0.2) | 0.3 (±0.1) |
| Day 5 Mean (%) (±SD) | 0.4 (±0.2) | 3.3 (±2.5) | 2.5 (±0.9) | 5.9 (±6.7) | 0.3 (±0.2) | 2.3 (±1.2) | 3.9 (±3.1) | 0.1 (±0.1) | 0.9 (±1.1) | 2.1 (±1.6) | 2.1 (±1.4) | 2.7 (±1.3) |
| P-value‡ | 0.0223 | 0.0003 | 0.0143 | – | 0.0053 | – | 0.0117 | 0.0274 | 0.0063 | |||
Study 1: ATL962/012/CL (564/ALZ); Study 2: ATL962/080/CL (605/ALZ); Study 3: ATL962/081/CL (657/ALZ).
The orlistat group was in Study 2.
P-value for between-group comparison vs. placebo.
The excretion of SCFA was found to be highly variable in active treatment groups (data not shown), with only the cetilistat 300 mg t.i.d. group exhibiting a significant increase relative to placebo (368.0 µmol g−1vs. 173.1 µmol g−1, P = 0.0031). LCFA excretion as a proportion of faecal mass had increased in all active treatment groups in all three studies by day 5. In the cetilistat 300 mg t.i.d. dose group, there was a sevenfold increase in LCFA excretion relative to baseline, a significant increase relative to placebo (3.9% vs. 0.6%, P < 0.0001). There were also significant increases in LCFA excretion at day 5 in the cetilistat 60 mg t.i.d. (1.7% vs. 0.5%, P = 0.0421), 120 mg t.i.d. (2.3% vs. 0.5%, P = 0.0113) and 240 mg t.i.d. (2.1% vs. 0.5%, P = 0.0120) dose groups. Excretion of cholesterol at day 5 increased in all active dose groups, although the effects were small.
Plasma lipids
Reductions from baseline to day 5 were seen in total cholesterol levels, which were statistically significant compared with placebo in the majority of dose groups, but there was no evidence of a dose relationship and the levels generally remained within the normal ranges. There were no consistent or clinically significant changes in mean triglyceride levels at any dose.
Adverse events
A total of 319 AEs were observed in 89/99 subjects enrolled in the three studies. Sixty-six AEs were associated with orlistat (n = 9 subjects), 69 were associated with placebo (n = 24) and 184 AEs were associated with cetilistat (n = 66). Almost all (98%) of the AEs reported were mild or moderate in intensity, and 65% were considered possibly or probably related to the study medication. Three subjects developed a maculopapular rash (two severe; one moderate) and were withdrawn prematurely from Study 2 (one in the cetilistat 150 mg t.i.d. group; two in the cetilistat 300 mg t.i.d. group). Two of the three subjects were tested for hypersensitivity for cetilistat and a metabolite by prick testing and cutaneous occlusion testing. No reactions were observed.
Most AEs related to the gastrointestinal system, with 160 gastrointestinal AEs (50.2%) reported across the three studies (Table 3). The most common gastrointestinal AE was steatorrhoea (oily stool), with 98 reported events (60.5% of all gastrointestinal AEs). In general, the incidence of steatorrhoea in the cetilistat dose groups was less than one episode per subject, in comparison with 4.11 episodes per subject in the orlistat group, where eight out of nine subjects were affected (Figure 3 and Table 3). The only exceptions were the 50 mg t.i.d. (1.14 episodes per subject) and 150 mg t.i.d. (1.81 episodes per subject) dose groups. Furthermore, there was no correlation between daily faecal fat excretion and the incidence of steatorrhoea.
Table 3.
No. of subjects reporting gastrointestinal AEs
| Cetilistat dose (mg t.i.d.) | Orlistatmg tid | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| No. subjects | Placebo(n = 24) | 50(n = 7) | 60(n = 9) | 100(n = 7) | 120(n = 9) | 150(n = 16) | 240(n = 9) | 300(n = 9) | 120(n = 9) |
| Number of subjects (% Subjects) Number of adverse events | |||||||||
| Abdominal pain | 5 (20.8) 7 | 0 | 0 | 1 (14.3) 1 | 1 (11.1) 1 | 3 (18.8) 3 | 2 (22.2) 2 | 0 | 0 |
| Change in bowel habit | 0 | 0 | 1 (11.1) 1 | 0 | 1 (11.1) 2 | 0 | 1 (11.1) 1 | 0 | 0 |
| Constipation | 3 (12.5) 3 | 0 | 2 (22.2) 2 | 0 | 0 | 0 | 1 (11.1) 1 | 0 | 0 |
| Defaecation urgency | 1 (4.2) 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Diarrhoea | 0 | 0 | 0 | 1 (14.3) 1 | 0 | 4 (25.0) 4 | 1 (11.1) 1 | 0 | 1 (11.1) 1 |
| Dysphagia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (11.1) 1 |
| Dry mouth | 1 (4.2) 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Faeces discoloured | 0 | 0 | 0 | 0 | 0 | 1 (6.2) 1 | 1 (11.1) 1 | 0 | 0 |
| Flatulence | 8 (33.4) 8 | 0 | 3 (33.3) 3 | 0 | 3 (33.3) 3 | 3 (33.3) 3 | 3 (33.3) 3 | 1 (11.1) 1 | 2 (22.2) 2 |
| Melaena | 1 (4.2) 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Steatorrhoea | 4 (16.7) 8 | 1 (14.3) 8 | 2 (22.2) 2 | 1 (14.3) 1 | 3 (33.3) 3 | 10 (62.5) 29 | 2 (22.2) 3 | 3 (33.3) 7 | 8 (88.9) 37 |
| Vomiting/nausea | 1 (4.2) 1 | 0 | 0 | 0 | 0 | 1 (6.2) 1 | 0 | 0 | 0 |
Figure 3.
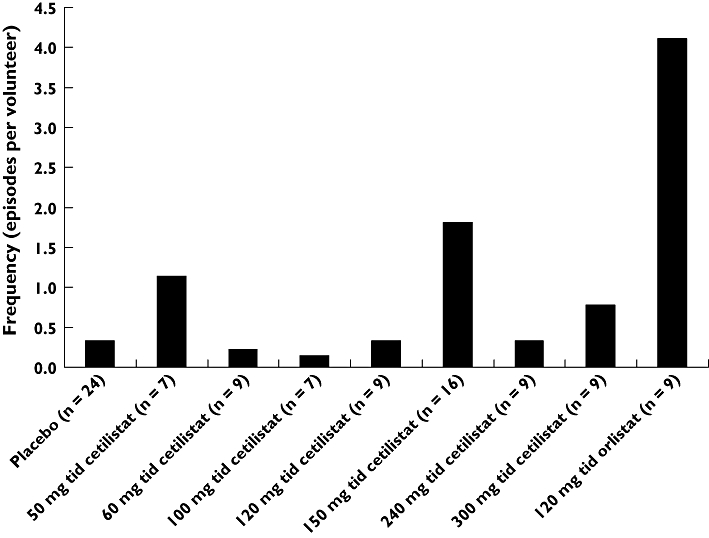
The frequency of steatorrhoea across the placebo and active treatment groups over the 5-day study period
Treatment with cetilistat had no clinically relevant effects on routine clinical chemistry and haematology parameters or on levels of fat-soluble vitamins. No clinically relevant abnormalities were detected in the medical examinations, and no clinically significant changes from baseline in vital signs were observed during any of the studies.
Discussion
The three studies demonstrate, in healthy subjects receiving a controlled calorie diet, that treatment with cetilistat is effective in reducing the absorption of dietary fat, as measured by increased faecal fat excretion. In this population, treatment with cetilistat was also well tolerated, with most AEs being mild or moderate in intensity.
Administration of cetilistat clearly inhibits the absorption of dietary fats, as indicated by the substantial and, in many cases, significant increases in faecal fat excretion relative to placebo observed at all doses of cetilistat. However, the standard deviations for each mean are large. There are several factors complicating the determination of a definite dose–response effect in this instance. First, the studies were of relatively short duration, and second, each study recruited only small sample numbers. This was particularly true in Study 3, where the response to treatment was variable to the extent that it precluded any firm conclusions being drawn.
In order to assess the relative effects of cetilistat and orlistat on faecal fat excretion, one study included a treatment arm with subjects receiving the approved dose of orlistat (120 mg t.i.d.). When faecal fat excretion with orlistat was compared with all the cetilistat doses investigated in the three studies, orlistat was found to produce a level of faecal fat excretion similar to that achieved with a cetilistat dose between 60 mg t.i.d. and 120 mg t.i.d. Given that the efficacy of orlistat 120 mg t.i.d. relative to placebo with regard to weight loss has been demonstrated in a number of controlled, randomized studies [7–12], it is reasonable to speculate that, based on these results, cetilistat would be equally effective at similar doses. Indeed, this has recently been demonstrated in a 12-week Phase 2, placebo-controlled study with cetilistat, in which significant weight loss relative to placebo was achieved in obese patients treated with 60, 120 or 240 mg t.i.d. doses of cetilistat [13].
Increases in faecal fat excretion were mirrored by increases in triglyceride excretion with all cetilistat doses >100 mg t.i.d., suggesting that cetilistat is successfully inhibiting triglyceride hydrolysis. Excretion of LCFA was also markedly increased with cetilistat administration. The effects of treatment on plasma lipid levels were variable. Although there were some significant reductions in cholesterol levels, these generally remained within the normal range.
Cetilistat was generally well tolerated, and virtually all the AEs experienced were classed as being mild or moderate in intensity. More than half of the AEs observed involved the gastrointestinal system; however, considering the mechanism of action of lipase inhibitors, this observation is not unexpected. Data from clinical studies demonstrate that treatment with orlistat is associated with a range of gastrointestinal AEs, including oily spotting, flatus with discharge, and steatorrhoea (oily stool) [7–12]. A comparison of AEs between cetilistat and orlistat was conducted using the number of reported events of steatorrhoea, one of the more commonly occurring gastrointestinal events during orlistat treatment, occurring with a frequency of around 20% [14]. In the three studies detailed here, 66 subjects were treated with cetilistat, and nine with orlistat. Approximately 40% of the episodes of steatorrhoea occurred in the nine subjects receiving orlistat. The number of episodes per subject in the orlistat group (4.11) was 2.5-fold greater than that of the cetilistat dose with the highest number of events per subject (1.81 in the 150 mg t.i.d. group). Many of the common gastrointestinal AEs observed with orlistat are thought to result from the decreased absorption of fat – a consequence of the mechanism of action of orlistat [15]. However, when the incidence and frequency of steatorrhoea was compared with faecal fat excretion in these studies, no evidence of a relationship was observed, suggesting that other factors in addition to faecal fat content may determine the incidence of some of these gastrointestinal events.
It is possible that it is the physical form of the fat in the intestine, rather than the amount of fat, that is important in terms of tolerability. Cetilistat and orlistat have quite different chemical structures, in terms of hydrophilic and lipidophilic components, which may influence the way in which the molecules interact with fat micelles in the intestine. It may be that cetilistat acts more like a detergent, whereas orlistat may promote the coalescence of micelles, leading to oils and increased gastrointestinal adverse events.
In conclusion, cetilistat is an effective inhibitor of gastrointestinal lipases, substantially increasing the level of faecal fat excreted in healthy volunteers at all doses studied. In addition, cetilistat is well tolerated across a wide range of doses, and comparison with orlistat suggests improved tolerability.
Competing interests
AB and CD are employees and shareholders of Alizyme Therapeutics Ltd. SdlM is an employee of Harrison Clinical Research (HCR). HCR was contracted by Alizyme to perform the studies that are published here.
REFERENCES
- 1.National Task. Force on the Prevention and Treatment of Obesity. Overweight, obesity, and health risk. Arch Intern Med. 2000;160:898–904. doi: 10.1001/archinte.160.7.898. [DOI] [PubMed] [Google Scholar]
- 2.Silventoinen K, Sans S, Tolonen H, Monterde D, Kuulasmaa K, Kesteloot H, Tuomilehto J. Trends in obesity and energy supply in the WHO MONICA Project. Int J Obes Relat Metab Disord. 2004;28:710–18. doi: 10.1038/sj.ijo.0802614. [DOI] [PubMed] [Google Scholar]
- 3.Pasanisi F, Contaldo F, de Simone G, Mancini M. Benefits of sustained moderate weight loss in obesity. Nutr Metab Cardiovasc Dis. 2001;11:401–6. [PubMed] [Google Scholar]
- 4.Wilding J. Clinical evaluation of anti-obesity drugs. Curr Drug Targets. 2004;5:325–32. doi: 10.2174/1389450043490479. [DOI] [PubMed] [Google Scholar]
- 5.Bays H, Dujovne C. Anti-obesity drug development. Expert Opin Investig Drugs. 2002;11:1189–204. doi: 10.1517/13543784.11.9.1189. [DOI] [PubMed] [Google Scholar]
- 6.Hadvary P, Lengsfeld H, Wolfer H. Inhibition of pancreatic lipase in vitro by the covalent inhibitor tetrahydrolipstatin. Biochem J. 1988;256:357–61. doi: 10.1042/bj2560357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finer N, James WP, Kopelman PG, Lean ME, Williams G. One-year treatment of obesity: a randomized, double-blind, placebo-controlled, multicentre study of orlistat, a gastrointestinal lipase inhibitor. Int J Obes Relat Metab Disord. 2000;24:306–13. doi: 10.1038/sj.ijo.0801128. [DOI] [PubMed] [Google Scholar]
- 8.Sjöström L, Rissanen A, Andersen T, Boldrin M, Golay A, Koppeschaar HP, Krempf M. Randomised placebo-controlled trial of orlistat for weight loss and prevention of weight regain in obese patients. Lancet. 1998;352:167–72. doi: 10.1016/s0140-6736(97)11509-4. [DOI] [PubMed] [Google Scholar]
- 9.Drent ML, Larsson I, William-Olsson T, Quaade F, Czubayko F, von Bergmann K, Strobel W, Sjostrom L, van der Veen EA. Orlistat (Ro 18-0647), a lipase inhibitor, in the treatment of human obesity: a multiple dose study. Int J Obes Relat Metab Disord. 1995;19:221–6. [PubMed] [Google Scholar]
- 10.Davidson MH, Hauptman J, DiGirolamo M, Foreyt JP, Halsted CH, Heber D, Heimburger DC, Lucas CP, Robbins DC, Chung J, Heymsfield SB. Weight control and risk factor reduction in obese subjects treated for 2 years with orlistat: a randomized controlled trial. JAMA. 1999;281:235–42. doi: 10.1001/jama.281.3.235. [DOI] [PubMed] [Google Scholar]
- 11.Muls E, Kolanowski J, Scheen A, Van Gaal L. The effects of orlistat on weight and on serum lipids in obese patients with hypercholesterolemia: a randomized, double-blind, placebo-controlled, multicentre study. Int J Obes Relat Metab Disord. 2001;25:1713–21. doi: 10.1038/sj.ijo.0801814. [DOI] [PubMed] [Google Scholar]
- 12.Van Gaal LF, Broom JI, Enzi G, Toplak H. Efficacy and tolerability of orlistat in the treatment of obesity: a 6-month dose-ranging study. Orlistat Dose-Ranging Study Group. Eur J Clin Pharmacol. 1998;54:125–32. doi: 10.1007/s002280050433. [DOI] [PubMed] [Google Scholar]
- 13.Kopelman P, Bryson A, Hickling R, Rissanen A, Rossner S, Toubro S, Valensi P. Cetilistat (ATL-962), a novel lipase inhibitor: a 12-week randomized, placebo-controlled study of weight reduction in obese patients. Int J Obes. 2007;31:494–9. doi: 10.1038/sj.ijo.0803446. [DOI] [PubMed] [Google Scholar]
- 14.Roche Laboratories Inc. Xenical® Product Information Sheet. Nutley, NJ: Roche Laboratories Inc; 2003. [Google Scholar]
- 15.Filippatos TD, Derdemezis CS, Gazi IF, Nakou ES, Mikhailidis DP, Elisaf MS. Orlistat associated adverse events and drug interactions – a critical review. Drug Saf. 2008;31:53–65. doi: 10.2165/00002018-200831010-00005. [DOI] [PubMed] [Google Scholar]


