Abstract
AIMS
To evaluate the incidence and severity of injection pain caused by microemulsion propofol and lipid emulsion propofol in relation to plasma bradykinin generation and aqueous free propofol concentrations.
METHODS
Injection pain was evaluated in 147 patients. Aqueous free propofol concentrations in each formulation, and in formulation mixtures containing agents that reduce propofol-induced pain, were measured by high-performance liquid chromatography. Plasma bradykinin concentrations in both formulations and in their components mixed with blood sampled from six volunteers were measured by radioimmunoassays. Injection pain caused by 8% polyethylene glycol 660 hydroxystearate (PEG660 HS) was evaluated in another 10 volunteers.
RESULTS
The incidence of injection pain [visual analogue scale (VAS) >30 mm] caused by microemulsion and lipid emulsion propofol was 69.7 and 42.3% (P < 0.001), respectively. The median VAS scores for microemulsion and lipid emulsion propofol were 59 and 24 mm, respectively (95% confidence interval for the difference 12.5, 40.0). The aqueous free propofol concentration of microemulsion propofol was seven times higher than that of lipid emulsion propofol. Agents that reduce injection pain did not affect aqueous free propofol concentrations. Microemulsion propofol and 8% PEG660 HS enhanced plasma bradykinin generation, whereas lipid emulsion propofol and lipid solvent did not. PEG660 HS did not cause injection pain.
CONCLUSIONS
Higher aqueous free propofol concentrations of microemulsion propofol produce more frequent and severe pain. The plasma kallikrein–kinin system may not be involved, and the agents that reduce injection pain may not act by decreasing aqueous free propofol concentrations.
Keywords: injection pain, microemulsion propofol
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT.
Aqueous free propofol in lipid emulsion elicits pain.
No data on the incidence and severity of injection pain for Aquafol™ (Daewon Pharmaceutical Co., Ltd, Seoul, Korea), a lipid-free microemulsion propofol, are available.
Two hypotheses involving plasma bradykinin generation have been proposed to explain propofol-induced pain; one implicates aqueous free propofol, the other implicates the lipid solvent.
WHAT THIS STUDY ADDS.
Microemulsion propofol produces more frequent and severe pain on injection, an effect that may be attributable to the high concentration of aqueous free propofol.
There was no evidence that plasma bradykinin generation caused propofol-induced pain.
In addition, agents known to prevent propofol-induced pain did not decrease aqueous free propofol concentrations.
Introduction
Lipid emulsion propofol (Diprivan®; AstraZeneca, London, UK), an intravenous hypnotic, has been a popular choice for general anaesthesia owing to its rapid onset and short duration of action. However, it has several drawbacks that have prompted the development of altered lipid emulsion or non-emulsion formulations [1, 2]. A particular issue associated with lipid emulsion propofol is the incidence of pain on injection, which is approximately 70% in the absence of other treatment regimes [3]. A lipid-free microemulsion propofol (Aquafol™; Daewon Pharmaceutical Co., Ltd, Seoul, Korea), composed of 1% propofol, 8% polyethylene glycol 660 hydroxystearate (Solutol HS 15; BASF Co. Ltd., Seoul, Korea) and 5% tetrahydrofurfuryl alcohol polyethylene glycol ether (Glycofurol; Roche, Basle, Switzerland), was developed to avoid the risk of lipid solvent-related adverse drug reactions, such as fat embolism, postoperative infection, hypertriglyceridaemia and pancreatitis [4]. However, because lidocaine was used to prevent injection pain in this previous study from our group, the incidence and severity of pain on injection with this microemulsion propofol was not assessed.
The concentration of free propofol in the aqueous phase is known to be associated with the intensity of pain on injection [5, 6]. However, the mechanism by which propofol induces injection-site pain remains unclear. In 1988, Scott et al. speculated that propofol produces injection pain by affecting an enzymatic cascade, possibly the plasma kallikrein–kinin system [7]. There have been two hypotheses that related activation of the plasma kallikrein–kinin system, and hence plasma bradykinin generation, to propofol-induced pain: one proposes that aqueous free propofol [8] is the mediator, the other implicates the lipid solvent [9]. In the former study, which reported more frequent and severe pain on injection with a lipid-free propofol solution of unknown composition, neither the concentration of free propofol in the aqueous phase nor plasma bradykinin generation was measured [8]. A number of studies have shown that lipid emulsion propofol with higher concentrations of lipid solvent causes less pain on injection [5, 10, 11], a result that tends to contradict the latter study [9]. Furthermore, intrinsic properties of a drug (such as structure), type of excipients used, properties of the final formulation (such as pH, temperature, drug concentration, injection volume and osmolality) and the injection procedure itself may contribute to pain on injection [12]. Thus, a clearer understanding of the factors related to injection pain caused by microemulsion propofol requires an evaluation of physicochemical properties (such as pH and osmolarity), free propofol concentration in the aqueous phase and plasma bradykinin generation. Several agents reduce propofol-induced pain upon mixing or pretreatment [13–18]. Many studies have presumed, without experimental evidence, that these compounds act by preventing activation of the plasma kallikrein–kinin system and/or by decreasing the concentration of free propofol in the aqueous phase.
In this study, we assessed the incidence and severity of pain on injection with microemulsion propofol in patients scheduled for elective surgery. We also evaluated microemulsion propofol (in the presence and absence of agents that reduce propofol-induced pain) with respect to plasma bradykinin generation and aqueous free propofol concentrations, both of which have been proposed to be involved in propofol-induced pain.
Methods
Investigational drugs
The lipid emulsion propofol formulation used was 1% Diprivan®, while the microemulsion propofol formulation was Aquafol™, composed of 1% propofol, 8% polyethylene glycol 660 hydroxystearate and 5% tetrahydrofurfuryl alcohol polyethylene glycol ether [4].
Study design and objectives
This study consisted of three in vitro experiments and two clinical trials. First, a multicentre, single-blinded, randomized, active-controlled, parallel, clinical trial was undertaken to compare the incidence and severity of microemulsion propofol-induced pain with that of lipid emulsion propofol-induced pain in patients scheduled for elective surgery under general anaesthesia. Second, pH and osmolarity of microemulsion propofol were measured to evaluate intrinsic properties that might be associated with injection pain. Third, aqueous free propofol concentrations in microemulsion or lipid emulsion propofol, and in formulation mixtures containing agents that reduce propofol-induced pain were measured to evaluate the relationship between aqueous free propofol concentrations and the incidence and severity of propofol-induced pain, and to determine if agents that reduce propofol-induced pain change the aqueous free propofol concentrations of lipid emulsion and microemulsion propofol. Fourth, plasma bradykinin generation was assessed by radioimmunoassay (RIA) to determine if propofol-induced pain is associated with activation of the plasma kallikrein–kinin system. Fifth, a randomized, single-blinded, two-period crossover clinical trial was performed to assess pain on injection with any vehicle of microemulsion propofol that enhanced plasma bradykinin generation when mixed with human blood.
Assessment of pain on injection with microemulsion and lipid emulsion propofol
After obtaining Institutional Review Board approval (Asan Medical Centre and Inje University Hospital, Seoul, Chungnam National University Hospital, Daejeon, Korea) and written informed consent, we recruited 150 American Society of Anesthesiologists 1 or 2 patients aged 20–65 years scheduled for elective surgery under general anaesthesia. Patients were randomly allocated to receive a 30-mg test dose of propofol in a microemulsion (n = 76) or lipid emulsion (n = 74) formulation to assess pain on injection. Subjects had no medical problems or abnormal laboratory test results. The sample size calculation was based on previous studies, which showed that the incidence difference of injection pain for different formulations of propofol ranged from 27% [8] to 49% [19]. With 72 patients per group, there is a 90% chance of detecting a 27% difference in the incidence of pain on injection with microemulsion and lipid emulsion propofol formulations, with a type I error of 0.05 (χ2 test).
An investigator explained a visual analogue scale for injection pain (VAS; 0 mm, no pain, 100 mm, worst pain imaginable) to patients on the first preoperative day. Patients fasted from midnight onwards, and were not premedicated. On arrival at the operating room, patients were monitored using electrocardiography and pulse oximetry, and tested for end-tidal carbon dioxide concentration. After an intravenous bolus administration of a 30-mg dose of microemulsion or lipid emulsion propofol over 5–10 s through a 20-G catheter placed in the cephalic vein at the wrist, pain of moderate to severe intensity was assessed using a VAS (>30 mm) [20]. Topical anaesthetic was not applied to the skin before intravenous cannulation.
pH and osmolarity of microemulsion propofol
The pH and osmolarity of microemulsion propofol (nine samples) were measured using a pH meter (720A; Thermo Orion, Washington, DC, USA) and cryoscopic osmometer (Osmomat 030; Gonotec GmbH, Berlin, Germany), respectively.
Measurement of free propofol concentrations
As described in an earlier study [21], propofol preparations were dialysed using a dialysis membrane (Dialysis tubing benzoylated®; Sigma-Aldrich Co., St Louis, MO, USA) with a molecular weight cut-off of approximately 3500–4000 Da. A solution of 2.25% (w/v) glycerin (LG household & Health Care, Seoul, Korea) in water was utilized as the release medium. Microemulsion and lipid emulsion propofol (5 ml) were prepared to measure the free propofol concentration in the aqueous phase of each formulation. The agents that reduce propofol-induced pain were as follows: lidocaine (Lidocaine®; Jeil Pharmaceutical Co., Ltd, Seoul, Korea), ketamine (Ketalar®; Yuhan Pharmaceutical Co., Ltd, Seoul, Korea), metoclopramide (Macperan®; Donghwa Pharmaceutical Co., Ltd, Seoul, Korea), ondansetron (Zofran®; GlaxoSmithKline, Seoul, Korea), thiopental (Pentotal®; Choongwae Pharmaceutical Co., Ltd, Seoul, Korea) and ephedrine (Ephedrine®; Jeil Pharmaceutical Co.). Six agent mixtures with corresponding control mixtures were prepared, as shown in Table 1. The volume of each agent mixed with propofol formulations was determined based on the amounts that had been shown to be effective in reducing propofol-induced pain in previous studies [13, 14, 16–18, 22].
Table 1.
Mixtures of lipid emulsion and microemulsion propofol containing agents that reduce propofol-induced pain
| Agents | Volumes of agents mixed with propofol (ml) | Total volumes of mixtures (ml) | Concentrations of agents in mixtures (mg ml−1) |
|---|---|---|---|
| Lidocaine (20 mg ml−1) | 0.5 | 5.5 | 1.81 |
| Ketamine (50 mg ml−1) | 0.2 | 5.2 | 1.92 |
| Metoclopramide (5 mg ml−1) | 1.0 | 6 | 0.83 |
| Ondansetron (2 mg ml−1) | 2.0 | 7 | 0.57 |
| Thiopental (25 mg ml−1) | 2.0 | 7 | 7.14 |
| Ephedrine (5 mg ml−1) | 0.4 | 5.4 | 0.37 |
All agents that reduce propofol-induced pain were mixed with 5 ml of each propofol formulation (agent mixture). For controls, pain-reducing agents were replaced with an equal volume of saline. The numbers of agent and control mixtures for each propofol formulation were all 10 per agent.
All samples were transferred to a dialysis membrane bag, and release media was added to produce a total volume of 10 ml. After sealing with a closer, the bag was immersed in 40 ml of release medium and shaken for 24 h at 100 cycles min−1 in a 20°C water bath (Figure 1).
Figure 1.
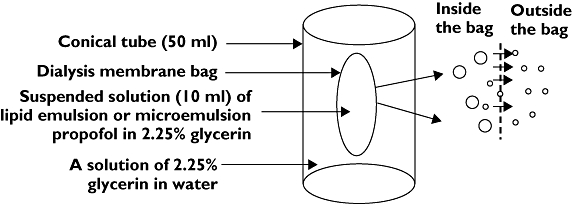
An illustration of the dialysis approach used to measure the concentration of free propofol in the aqueous phase of microemulsion and lipid emulsion propofol. Because their molecular sizes are greater than the molecular weight cut-off (MWCO) of the dialysis tubing, microemulsion and lipid emulsion propofol remain inside the membrane bag, whereas free propofol, which is below the MWCO, escapes into the release medium. After reaching saturation, the concentration of free propofol in a solution of 2.25% glycerine was measured by high-performance liquid chromatography. Lipid emulsion or microemulsion propofol (○); Free propofol (∘)
All laboratory personnel were blinded with respect to sample identity. Free propofol concentrations in the aqueous phase were measured by high-performance liquid chromatography (Agilent 1100 series; Agilent Technologies, Inc., Santa Clara, CA, USA) using a C18 column (Xterra RP18, 5 µm, 4.6 × 150 mm; Waters Corp., Milford, MA, USA) and a tetrahydrofuran–water mixture as the mobile phase [23]. The flow rate was 0.7 ml min−1, and components of the column effluent were monitored using an ultraviolet detector at a wavelength of 275 nm. The lower limit of quantification was 5 µg ml−1. The coefficients of variation for intra-assay were <2.4%. The coefficients of variation for within-day and between-day interassay were <7.3% and 4.6%, respectively. Intra-assay accuracy values were 89.9–101.6%, whereas interassay accuracy values were 90.3–101.5% of the nominal value.
Ex vivo and in vitro detection of plasma bradykinin generation by RIA
After obtaining approval from the Institutional Review Board (Asan Medical Centre) and written informed consent, six adult healthy volunteers (M/F = 5/1) aged 29–39 years with no medical history or medication were enrolled.
Seven plastic syringes were prepared to contain the following samples at room temperature: (i) 1.5 ml saline (control), (ii) lipid emulsion propofol, (iii) 10% lipid solvent (Intralipid; Kabi Pharmacia AB, Stockholm, Sweden), (iv) microemulsion propofol, (v) 8% polyethylene glycol 660 hydroxystearate, (vi) 5% tetrahydrofurfuryl alcohol polyethylene glycol ether, and (vii) a mixture of other ingredients in microemulsion propofol minus propofol, polyethylene glycol 660 hydroxystearate and tetrahydrofurfuryl alcohol polyethylene glycol ether. In cases where the concentration of bradykinin was increased and there was evidence that the increase in bradykinin was the main cause of pain on injection with microemulsion or lipid emulsion propofol, the effects of agents that reduce propofol-induced pain on the generation of bradykinin were also considered.
A 20-G angio-catheter was placed in a vein of the antecubital area, and 3.5 ml venous blood was aspirated over 10 s from the catheter using the above-prepared syringes. All samples were transferred to plain tubes containing 0.5 ml of an inhibition solution composed of aprotinin (10 000 kIU ml−1), soybean trypsin inhibitor (800 µg ml−1) and polybrene (4 mg ml−1) to inactivate plasma and glandular kallikrein and other kinin-producing enzymes, and 1,10-phenanthroline (10 mg ml−1) and ethylenediamine tetraaceticacid (EDTA; 20 mg ml−1), to inactivate kinin-destroying enzymes [24]. This inhibition solution allows bradykinin generation by propofol formulations and their components to be measured.
After shaking gently for 20 s, samples were centrifuged at 1600 g for 15 min at 4°C. The plasma was collected and stored at −70°C until assay [25]. All laboratory personnel were blinded with respect to sample identity. Peptides were extracted from plasma according to the following procedure: plasma (2 ml) was acidified with an equal volume of buffer A (1% trifluoroacetic acid; Sigma-Aldrich, Inc.) and centrifuged at 15 000 g for 20 min at 4°C. The supernatant was loaded onto a pretreated separation column (Strata C18-E; Phenomenex, Inc., Torrance, CA, USA), which was slowly washed with buffer A (3 ml, twice). Peptides were slowly eluted with 3 ml Buffer B (Buffer A containing 60% acetonitrile; Fisher Scientific, Pittsburgh, PA, USA), and the eluent was collected in a polypropylene tube. The organic layer in the eluent was removed using a centrifugal concentrator (Savant Speedvac SPD2010; Thermo Fisher Scientific, Inc., Waltham, MA, USA) for 15 min, and the remaining sample was freeze-dried overnight using a lyophilizer (Freeze Dry System; Labconco Corp., Kansas City, MO, USA).
The bradykinin concentration in samples was measured using an RIA kit (Phoenix Pharmaceuticals, Inc., Burlingame, CA, USA). Peptide standards were reconstituted with RIA buffer provided by the manufacturer. Concentrated (lyophilized) bradykinin powder was dissolved in 250 µl RIA buffer and divided into duplicate 100-µl aliquots. Serial 1:2 dilutions of standard peptides (1280, 640, 320, 160, 80, 40, 20 and 10 pg ml−1), assayed in quadruplicate, were used to generate a standard curve. Aliquoted samples were assayed in duplicate. Assays were performed in 12 × 75 mm polystyrene tubes. Following the addition of primary antibody, each tube was vortexed and incubated for 16–24 h at 4°C. 125I-peptide was then added to each tube, and the procedure was repeated. After adding goat antirabbit IgG and normal rabbit serum, each tube was then vortexed and incubated at room temperature for 90 min. RIA buffer was added and centrifuged at 1700 g for 20 min after gentle vortexing. The supernatant was aspirated, and peptide content in assay tubes, in terms of counts per minute, was evaluated using a gamma counter (Packard Cobra Gamma Counters, Downers Grove, IL, USA). A standard curve (r2 ≥ 0.993), obtained by plotting standard peptide concentrations, was used to determine bradykinin concentrations. The measured bradykinin concentration was converted into plasma bradykinin (pg ml−1) by applying a factor of eight. Specifically, lyophilized powder was made from 2 ml plasma and dissolved into 250 µl RIA buffer. Samples were diluted or concentrated as necessary in cases where the measured bradykinin concentration was above or below the range of the standard curve. The lower limit of quantification was 0.81 pg ml−1. The intra- and interassay coefficients of variation were 2.7–6.8 and 1.4–7.1%, respectively. Intra-assay accuracy values were 88.2–97.5%, and interassay accuracy values were 91.8–96.6%.
Assessment of pain on injection with 8% polyethylene glycol 660 hydroxystearate
After obtaining Institutional Review Board approval (Asan Medical Centre) and written informed consent, 10 adult healthy volunteers with no history of medical problems and who were not receiving medication were enrolled to assess pain on injection with 8% polyethylene glycol 660 hydroxystearate. The pH and osmolarity of 8% polyethylene glycol 660 hydroxystearate were adjusted to values similar to those of microemulsion propofol using an NaOH solution (Yakuri Pure Chemicals Co., Ltd. Kyoto, Japan) and glycerine (LG household & Health Care, Seoul, Korea), respectively. After explaining the VAS score for pain on injection, an investigator administered injections of 3 ml normal saline and 8% polyethylene glycol 660 hydroxystearate to each patient. Solutions were administered in a crossover fashion separated by a 15-min wash-out period; the order of administration was randomized. The injections were administered to the veins on the dorsum of both hands at the rate of 1 ml s−1. Topical anaesthetic was not applied to the skin before intravenous cannulation.
Statistical analysis
Statistical analyses were conducted using SigmaStat for Windows version 3.11 (Systat Software Inc., San Jose, CA, USA). Patient characteristics were compared using a two-sample t-test, Mann–Whitney rank sum test or χ2 test, as appropriate. The incidence and severity of pain on injection with lipid emulsion and microemulsion propofol were compared using χ2 and Mann–Whitney rank sum tests, respectively. Free propofol concentrations in the aqueous phase of lipid emulsion and microemulsion propofol were compared using a two-sample t-test or Mann–Whitney rank sum test. Free propofol concentrations in the aqueous phase in the absence and presence of agents that reduce propofol-induced pain were compared using a two-sample t-test or a Mann–Whitney rank sum test, as appropriate. The bradykinin concentration in plasma was evaluated using a one-way analysis of variance (anova), and multiple comparisons were made using the Holm–Sidak method. VAS for pain on injection with normal saline and 8% polyethylene glycol 660 hydroxystearate were evaluated using paired t-tests. Values are expressed as mean ± SD or median (25%, 75%). A P-value <0.05 was considered to be statistically significant.
Results
Assessment of pain on injection with microemulsion and lipid emulsion propofol
The results of pain on injection are based on data from 147 patients. Three patients receiving lipid emulsion propofol were excluded from the analysis owing to deep sedation, which precluded the possibility of measuring VAS. Patient characteristics are shown in Table 2.
Table 2.
Patient characteristics in lipid emulsion and microemulsion propofol groups
| Lipid emulsion (n = 71) | Microemulsion (n = 76) | |
|---|---|---|
| Age, years | 46.4 ± 10.4 | 48.3 ± 11.1 |
| Weight, kg | 64.4 ± 11.4 | 62.9 ± 11.8 |
| Height, cm | 164.3 ± 8.0 | 163.4 ± 8.6 |
| Sex (M/F) | 43/28 | 45/31 |
| ASA PS (I/II) | 52/19 | 54/22 |
Values are presented as mean ± SD or count, as appropriate. There were no significant differences between the two groups. ASA PS, American Society of Anesthesiologists Physical Status.
The incidence of pain on injection with microemulsion and lipid emulsion propofol was 69.7 and 42.3%, respectively (P < 0.001). These incidence values were estimated from the ratio of patients experiencing pain on injection (VAS >30 mm) to all patients in each group. The median (25%, 75%) VAS scores for pain on injection with microemulsion and lipid emulsion propofol were 59 (25, 85) and 24 (0, 50) mm, respectively (P < 0.001). The 95% confidence interval for the difference in VAS scores for pain was 12.5, 40.0. The distribution of the VAS scores is shown in Figure 2.
Figure 2.
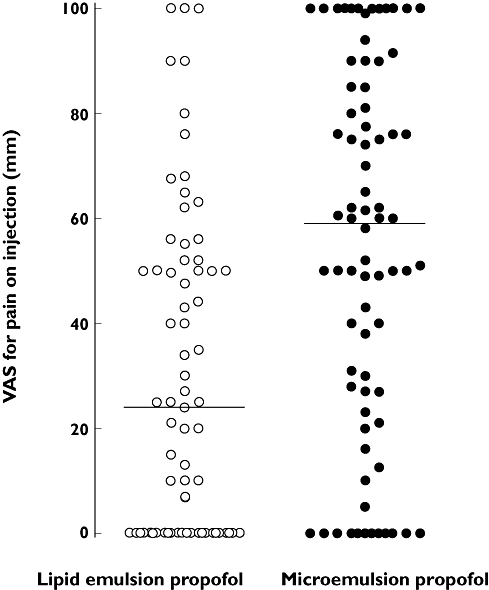
The distribution of visual analogue scale (VAS) scores for pain on injection with an intravenous bolus (30 mg) of microemulsion (•) or lipid emulsion (○) propofol in 147 patients (VAS, 0 mm = no pain, 100 mm = worst pain imaginable). The solid horizontal lines represent median VAS scores for pain on injection with microemulsion and lipid emulsion propofol (59 and 24 mm, respectively, P < 0.001 by Mann–Whitney rank sum test)
pH and osmolarity of microemulsion propofol
The pH and osmolarity of microemulsion propofol were 7.51 ± 0.01 and 280.0 ± 0.7 mOsm l−1, respectively, which are very similar to physiological values.
Measurement of free propofol concentrations
Free propofol concentrations in the aqueous phases of various samples are shown in Table 3. The data on free propofol concentrations in the lipid emulsion formulation (both control and test samples) correspond to the values in our recent study [26], which represent the results of assays on a reformulated microemulsion that were performed contemporaneously with assays of lipid emulsion formulation (1% Diprivan®) and microemulsion formulation tested in the current study. The concentration of free propofol in the aqueous phase was seven times greater for microemulsion propofol than for lipid emulsion propofol. Regardless of the propofol formulation, none of the various agents that reduce propofol-induced pain affected the concentration of free propofol in the aqueous phase.
Table 3.
Free propofol concentrations in the aqueous phase of lipid emulsion and microemulsion propofol and the effects of agents known to reduce propofol-induced pain on aqueous free propofol concentrations
| Agents mixed with 5 ml of propofol formulations (concentration, volume) | Lipid emulsion (µg ml−1) | Microemulsion (µg ml−1) | ||
|---|---|---|---|---|
| Control* | Test† | Control* | Test† | |
| None‡ | – | 12.4 ± 0.7 | – | 83.9 ± 0.6 |
| Lidocaine (20 mg ml−1, 0.5 ml) | 12.3 ± 0.5 | 12.3 ± 0.6 | 84.2 ± 0.6 | 84.0 ± 0.7 |
| Ketamine (50 mg ml−1, 0.2 ml) | 12.4 (12.1, 12.5) | 12.3 (11.6, 13.2) | 83.9 ± 0.5 | 83.8 ± 0.7 |
| Metoclopramide (5 mg ml−1, 1.0 ml) | 12.2 ± 0.6 | 11.9 ± 0.6 | 84.0 ± 0.8 | 84.1 ± 0.8 |
| Ondansetron (2 mg ml−1, 2.0 ml) | 12.4 ± 0.7 | 12.5 ± 0.7 | 83.8 ± 0.9 | 83.9 ± 0.8 |
| Thiopental (25 mg ml−1, 2.0 ml) | 12.4 ± 0.7 | 12.2 ± 0.6 | 83.8 ± 0.9 | 83.8 ± 0.7 |
| Ephedrine (5 mg ml−1, 0.4 ml) | 12.3 ± 0.6 | 12.4 ± 0.7 | 83.9 ± 0.7 | 83.9 ± 0.8 |
Mixture of each propofol formulation (5 ml) and saline (0.2, 0.4, 0.5, 1.0 and 2.0 ml). For controls, pain-reducing agents were replaced with an equal volume of saline.
Control and test samples in each propofol formulation were compared to test the effects of agents known to reduce propofol-induced pain on the free propofol concentrations in the aqueous phase (P > 0.05 for all pairwise comparisons).
The free propofol concentrations in the aqueous phase of lipid emulsion and microemulsion propofol were compared (P < 0.001). n = 10 for all control and test samples. Data are presented as mean ± SD or median (25%, 75%).
Ex vivo and in vitro detection of plasma bradykinin generation by RIA
The concentrations of bradykinin in plasma upon mixing with 1.5 ml saline, lipid emulsion propofol, 10% lipid solvent, microemulsion propofol, 8% polyethylene glycol 660 hydroxystearate, 5% tetrahydrofurfuryl alcohol polyethylene glycol ether, or a mixture of other ingredients in microemulsion propofol minus propofol, polyethylene glycol 660 hydroxystearate and tetrahydrofurfuryl alcohol polyethylene glycol ether are shown in Figure 3. The data on the concentration of bradykinin in plasma for normal saline (control), lipid emulsion propofol and 10% lipid solvent (Intralipid) in Figure 3 refer to the values published in our recent study [26], which represent the results of assays on a reformulated microemulsion that were performed contemporaneously with assays of lipid emulsion formulation (1% Diprivan®) and microemulsion formulation in this study.
Figure 3.
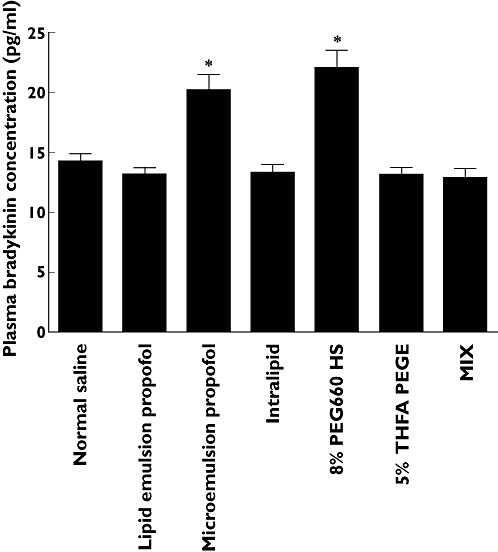
The concentration of bradykinin in plasma obtained after mixing 3.5 ml venous blood, sampled from six healthy volunteers, with 1.5 ml normal saline [control, 14.2 ± 1.6, coefficient of variation (CV) 11.5%, 95% confidence interval (CI) 12.5, 15.9], lipid emulsion propofol (13.2 ± 1.3, CV 10.2%, 95% CI 11.8, 14.6), 10% lipid solvent (Intralipid 13.3 ± 1.6, CV 12.1%, 95% CI 11.6, 15.0), microemulsion propofol (20.2 ± 3.2, CV 16.0%, 95% CI 16.8, 23.6), 8% polyethylene glycol 660 hydroxystearate (PEG660 HS, 22.0 ± 3.6, CV 16.4%, 95% CI 18.3, 25.8), 5% tetrahydrofurfuryl alcohol polyethylene glycol ether (THFA PEGE, 13.2 ± 1.4, CV 10.5%, 95% CI 11.7, 14.6) and a mixture of other ingredients in microemulsion propofol minus propofol, polyethylene glycol 660 hydroxystearate and tetrahydrofurfuryl alcohol polyethylene glycol ether (MIX, 12.9 ± 1.8, CV 14.3%, 95% CI 11.0, 14.9). *P < 0.05 vs. normal saline by the Holm–Sidak method. Data are presented as mean ± SD. CV, SD/mean × 100%; CI, confidence interval of population mean
Lipid emulsion propofol and 10% lipid solvent (Intralipid) had no effect on the concentration of bradykinin in plasma compared with the saline control. In view of these results, it can be concluded that neither propofol nor the lipid solvent activates the plasma kallikrein–kinin system. Accordingly, the effects of agents that reduce propofol-induced pain on the generation of bradykinin in plasma were not evaluated in the context of these formulations. On the other hand, microemulsion propofol and 8% polyethylene glycol 660 hydroxystearate enhanced bradykinin generation in plasma by approximately 1.5-fold relative to the saline control (P < 0.05). This increase in the plasma bradykinin concentration was attributed to 8% polyethylene glycol 660 hydroxystearate. Accordingly, we investigated whether pain was produced on injection with 8% polyethylene glycol 660 hydroxystearate in healthy volunteers.
Assessment of pain on injection with 8% polyethylene glycol 660 hydroxystearate
The mean age and body weight of volunteers (M/F = 7/3) were 28.3 ± 4.9 years and 70.3 ± 14.8 kg, respectively (mean ± SD). The mean VAS scores for pain on injection with 8% polyethylene glycol 660 hydroxystearate and normal saline were 0 and 0.4 mm, respectively. One subject complained of pain on injection with normal saline, which was attributed to the injection technique of a research nurse. These findings indicate that plasma bradykinin generation does not elicit pain.
Discussion
Propofol-induced pain has been ranked by American anaesthesiologists as the seventh most important problem of current clinical anaesthesiology [27]. The nature of pain is extremely aching, burning and crushing. The initial component of propofol-induced pain involves immediate stimulation of nociceptors and free nerve endings and the delayed component of pain, appearing within half a minute, is also believed to result from interaction with nociceptors and free nerve endings [28]. Nakane and Iwama have suggested that lipid solvent aggravates propofol-induced pain owing to the modification of the peripheral vein caused by enhanced plasma bradykinin generation [29]. In our study, lipid emulsion propofol and 10% lipid solvent did not generate bradykinin in plasma when mixed with human blood. This finding suggests that neither propofol itself nor lipid solvent activates the plasma kallikrein–kinin system. Furthermore, no pain was observed on injection with 8% polyethylene glycol 660 hydroxystearate, even though it induced an approximately 1.5-fold increase in plasma bradykinin concentration (22 pg ml−1) relative to saline control (14 pg ml−1). Accordingly, we propose that bradykinin generation via activation of the plasma kallikrein–kinin system is not related to propofol-induced pain. To the best of our knowledge, there are no literature reports to suggest a threshold level of plasma bradykinin that causes injection pain. In the previous study, lipid solvent increased plasma bradykinin up to 17 pg ml−1[29].
The significantly higher incidence and severity of pain on injection with microemulsion propofol are associated with a sevenfold increase in the aqueous free propofol concentration. From these findings, we conclude that aqueous free propofol elicits pain in a concentration-dependent manner via a mechanism that is not related to plasma bradykinin generation. The lipid solvent is thought to decrease free propofol concentration in the aqueous phase, which would be expected to reduce rather than aggravate propofol-induced pain. This interpretation is consistent with the observation that injection pain was higher with the Cremophor EL preparation of propofol than with a lipid emulsion formulation [30]. Wang et al. postulated that the more stable microemulsion propofol might generate less free propofol outside the nanoparticles due to a reduction in leakage or cracking, and thereby reduce pain at the injection site [31]. The results of our study do not support this hypothesis, suggesting instead that small particle size and improved stability of propofol formulation are not associated with a reduction in propofol-induced pain.
The two classic pathways responsible for the generation of kinins are the plasma system, also known as the contact system, and the tissue kallikrein–kinin system [32]. Contact of plasma with a negatively charged surface leads to the release of bradykinin [33]. In vitro, nonphysiological substances activate the contact system of plasma [34], but the physiological surface responsible for this phenomenon in vivo remains unknown. Activation of this intrinsic coagulation/kinin-forming cascade occurs continuously at a finite rate, but is controlled by plasma inhibitors [33]. Another mechanism for activating the plasma kallikrein–kinin system depends on the binding of components of the contact activation cascade to the surface of cells such as leucocytes, platelets, endothelial cells and myocytes [35]. One limitation of the ex vivo and in vitro plasma bradykinin generation experiments in this study, as also in a previous study [29], is that neither was capable of evaluating the contribution of vascular endothelial cells to the activation of the plasma kallikrein–kinin system.
The reason for the apparent discrepancy of plasma bradykinin generation by lipid emulsion propofol and 10% lipid solvent between this and the previous study [29] is currently not clear. The volumes of saline, lipid emulsion propofol, 10% lipid solvent, microemulsion propofol and its components, and the volumes of human blood mixed with these agents were the same in both studies. One potential difference is that we added the inhibition solution to the samples (human blood mixed with saline, lipid emulsion propofol, 10% lipid solvent, microemulsion propofol and its components) immediately after blood sampling, whereas Nakane and Iwama gently agitated the samples for 20 s before adding the inhibition solution [29]. Propofol-induced pain is usually observed within 10 s of injection in clinical settings, so it follows that bradykinin must be generated within a similar time frame experimentally using syringes containing various agents, provided that bradykinin is related to propofol-induced pain. Thus ideally, plasma kallikrein and other kinin-producing enzymes, as well as kinin-destroying enzymes, should be inhibited immediately after completion of blood sampling. Shimamoto and Iimura have suggested that blood sampling should be performed using a syringe prefilled with inhibition solution because the inhibition of kinin-destroying enzymes precedes that of kinin-producing enzymes [24]. Using this approach, they found that plasma kinin levels were approximately 6 pg ml−1; however, when blood samples were mixed with inhibitor solution after sampling rather than during collection, plasma kinin levels increased markedly (approximately 25 pg ml−1) [24]. In our study, we used the latter method to evaluate drug-induced plasma bradykinin generation, which might account for the increased plasma concentrations of bradykinin (14.2 ± 1.6 pg ml−1) in the saline controls. However, it is not clear why plasma concentrations of bradykinin in the saline control (approximately 10 pg ml−1) in the study of Nakane and Iwama [29] were lower than those reported here, considering that the inhibition solution was added to the blood samples 20 s later in the previous study.
Additional methodological differences, such as the composition of the inhibition solution and the amount of each agent included, may also be crucial to plasma bradykinin concentration measurements, but these were not described in detail by Nakane and Iwama [29]. As reported by Shimamoto and Iimura [24], we used aprotinin, soybean trypsin inhibitor and polybrene to inactivate plasma and glandular kallikreins and other kinin-producing enzymes, and 1,10-phenanthroline and EDTA to inhibit kinin-destroying enzymes. Although Nakane and Iwama used aprotinin and trypsin inhibitor to inactivate kinin-producing enzymes, they did not include polybrene, and they used edetic acid to inhibit kinin-destroying enzymes instead of 1,10-phenanthroline and EDTA. The extent to which these differences in the composition of inhibition solutions influence the measurements of plasma bradykinin concentration is uncertain, however, because there are no literature reports that directly address this question.
To maximize accuracy and reproducibility, we used bradykinin RIA kits that had relatively high levels of isotope binding (42–51%) in the absence of bradykinin, and only included data from concentration determinations in which the positive controls were within the ranges indicated by the manufacturer. The coefficients of variation of plasma bradykinin concentrations in this study ranged from 10.2 to 16.4%, which suggests that the level of measurement precision was acceptable.
Of the several agents that reduce propofol-induced pain, lidocaine is that most commonly used in clinical practice. In an earlier study, lidocaine decreased the pH of lipid emulsion propofol, which in turn decreased free propofol concentration in the aqueous phase [36]. However, the free propofol concentrations in microemulsion and lipid emulsion propofol mixed with lidocaine were not different from the saline controls in our study. Similarly, none of the other agents tested, including ketamine, metoclopramide, ondansetron and ephedrine, influenced free propofol concentration in microemulsion or lipid emulsion propofol. Thus, earlier literature reports that attributed the underlying pain-eliciting mechanism of propofol to a decrease in free propofol concentration or prevention of bradykinin release [13, 18] may have been incorrect.
Propofol concentration (6% vs. 1%) has no effects on the incidence of injection pain [37]. Intrinsic factors that are associated with pain on injection of lipid emulsion propofol [12] include temperature (either cooling [38] or warming [39]), injection site (e.g. a large vein) [40] and solvent (medium-chain triglyceride/long-chain triglyceride vs. long-chain triglyceride) [41]. Of these factors, warming and medium-chain triglyceride/long-chain triglyceride solvent, as well as lower pH, are known to decrease the free propofol concentration in the aqueous phase, and hence reduce propofol-induced pain. Klement and Arndt have suggested that the injection pain caused by a number of sedative and hypnotic drugs is possibly attributable to formulations with extremely unphysiological osmolality (1.0 Osm kg−1 during infusion and 3.0 Osm kg−1 during rapid injection) or pH (<4 and >11) [42]. The pH and osmolarity/osmolality of microemulsion and lipid emulsion propofol are not extremely unphysiological (pH 6–8.5 and 0.303 Osm kg−1 for lipid emulsion propofol [30]), so these factors are unlikely to contribute to injection pain.
It is possible that the single-blinded experimental design applied to the assessment of pain on injection with microemulsion and lipid emulsion propofol in elective surgical patients, and to the assessment of pain on injection with 8% polyethylene glycol 660 hydroxystearate in healthy volunteers, was a limitation of this study. However, a double-blinded design was not applicable owing to clear differences in colour between microemulsion (colourless) and lipid emulsion propofol (white), and a slight difference in turbidity between 8% polyethylene glycol 660 hydroxystearate and saline. Our finding that microemulsion caused more frequent and severe pain, which coincided with higher concentrations of the aqueous free propofol in microemulsion formulation, suggests that this approach presents the least potential bias for the assessment of VAS scores in clinical trials.
In conclusion, microemulsion propofol produces more frequent and severe pain on injection than lipid emulsion propofol, a difference that may be attributable to the sevenfold higher concentration of aqueous free propofol. There was no evidence that bradykinin generation associated with activation of the plasma kallikrein–kinin system caused propofol-induced pain. In addition, agents known to prevent propofol-induced pain did not decrease aqueous free propofol concentrations.
Acknowledgments
This study was supported by a grant (2006-301) from the Asan Institute for Life Sciences, Seoul, Korea and by a grant (2008-0322) from the Industry Trust Research Service between University of Ulsan College of Medicine and Daewon Pharmaceutical Co., Ltd, Seoul, Korea. The authors are deeply grateful to Se-il Sohn (Manager, Research Laboratory, Daewon Pharmaceutical Co., Ltd, Seoul, Korea) and Tae-won Song (Researcher, Research Laboratory, Daewon Pharmaceutical Co., Ltd, Seoul, Korea) for the measurement of free propofol concentrations in the aqueous phase.
REFERENCES
- 1.Devlin JW, Lau AK, Tanios MA. Propofol-associated hypertriglyceridemia and pancreatitis in the intensive care unit: an analysis of frequency and risk factors. Pharmacotherapy. 2005;25:1348–52. doi: 10.1592/phco.2005.25.10.1348. [DOI] [PubMed] [Google Scholar]
- 2.Baker MT, Naguib M. Propofol: the challenges of formulation. Anesthesiology. 2005;103:860–76. doi: 10.1097/00000542-200510000-00026. [DOI] [PubMed] [Google Scholar]
- 3.Picard P, Tramer MR. Prevention of pain on injection with propofol: a quantitative systematic review. Anesth Analg. 2000;90:963–9. doi: 10.1097/00000539-200004000-00035. [DOI] [PubMed] [Google Scholar]
- 4.Kim KM, Choi BM, Park SW, Lee SH, Christensen LV, Zhou J, Yoo BH, Shin HW, Bae KS, Kern SE, Kang SH, Noh GJ. Pharmacokinetics and pharmacodynamics of propofol microemulsion and lipid emulsion after an intravenous bolus and variable rate infusion. Anesthesiology. 2007;106:924–34. doi: 10.1097/01.anes.0000265151.78943.af. [DOI] [PubMed] [Google Scholar]
- 5.Klement W, Arndt JO. Pain on injection of propofol: effects of concentration and diluent. Br J Anaesth. 1991;67:281–4. doi: 10.1093/bja/67.3.281. [DOI] [PubMed] [Google Scholar]
- 6.Doenicke AW, Roizen MF, Rau J, Kellermann W, Babl J. Reducing pain during propofol injection: the role of the solvent. Anesth Analg. 1996;82:472–4. doi: 10.1097/00000539-199603000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Scott RP, Saunders DA, Norman J. Propofol: clinical strategies for preventing the pain of injection. Anaesthesia. 1988;43:492–4. doi: 10.1111/j.1365-2044.1988.tb06641.x. [DOI] [PubMed] [Google Scholar]
- 8.Dubey PK, Kumar A. Pain on injection of lipid-free propofol and propofol emulsion containing medium-chain triglyceride: a comparative study. Anesth Analg. 2005;101:1060–2. doi: 10.1213/01.ane.0000166951.72702.05. [DOI] [PubMed] [Google Scholar]
- 9.Iwama H, Nakane M, Ohmori S, Kaneko T, Kato M, Watanabe K, Okuaki A. Nafamostat mesilate, a kallikrein inhibitor, prevents pain on injection with propofol. Br J Anaesth. 1998;81:963–4. doi: 10.1093/bja/81.6.963. [DOI] [PubMed] [Google Scholar]
- 10.Song D, Hamza M, White PF, Klein K, Recart A, Khodaparast O. The pharmacodynamic effects of a lower-lipid emulsion of propofol: a comparison with the standard propofol emulsion. Anesth Analg. 2004;98:687–91. doi: 10.1213/01.ane.0000103184.36451.d7. [DOI] [PubMed] [Google Scholar]
- 11.Soltesz S, Silomon M, Graf G, Mencke T, Boulaadass S, Molter GP. Effect of a 0.5% dilution of propofol on pain on injection during induction of anesthesia in children. Anesthesiology. 2007;106:80–4. doi: 10.1097/00000542-200701000-00015. [DOI] [PubMed] [Google Scholar]
- 12.Brazeau GA, Cooper B, Svetic KA, Smith CL, Gupta P. Current perspectives on pain upon injection of drugs. J Pharm Sci. 1998;87:667–77. doi: 10.1021/js970315l. [DOI] [PubMed] [Google Scholar]
- 13.Agarwal A, Ansari MF, Gupta D, Pandey R, Raza M, Singh PK, Shiopriye, Dhiraj S, Singh U. Pretreatment with thiopental for prevention of pain associated with propofol injection. Anesth Analg. 2004;98:683–6. doi: 10.1213/01.ane.0000103266.73568.18. [DOI] [PubMed] [Google Scholar]
- 14.Koo SW, Cho SJ, Kim YK, Ham KD, Hwang JH. Small-dose ketamine reduces the pain of propofol injection. Anesth Analg. 2006;103:1444–7. doi: 10.1213/01.ane.0000243334.83816.53. [DOI] [PubMed] [Google Scholar]
- 15.Apiliogullari S, Keles B, Apiliogullari B, Balasar M, Yilmaz H, Duman A. Comparison of diphenhydramine and lidocaine for prevention of pain after injection of propofol: a double-blind, placebo-controlled, randomized study. Eur J Anaesthesiol. 2007;24:235–8. doi: 10.1017/S026502150600202X. [DOI] [PubMed] [Google Scholar]
- 16.Fujii Y, Uemura A. Effect of metoclopramide on pain on injection of propofol. Anaesth Intensive Care. 2004;32:653–6. [PubMed] [Google Scholar]
- 17.Ambesh SP, Dubey PK, Sinha PK. Ondansetron pretreatment to alleviate pain on propofol injection: a randomized, controlled, double-blinded study. Anesth Analg. 1999;89:197–9. doi: 10.1097/00000539-199907000-00035. [DOI] [PubMed] [Google Scholar]
- 18.Cheong MA, Kim KS, Choi WJ. Ephedrine reduces the pain from propofol injection. Anesth Analg. 2002;95:1293–6. doi: 10.1097/00000539-200211000-00035. [DOI] [PubMed] [Google Scholar]
- 19.Larsen B, Beerhalter U, Biedler A, Brandt A, Doege F, Brun K, Erdkonig R, Larsen R. Less pain on injection by a new formulation of propofol? A comparison with propofol LCT. Anaesthesist. 2001;50:842–5. doi: 10.1007/s00101-001-0234-0. [DOI] [PubMed] [Google Scholar]
- 20.Collins SL, Moore RA, McQuay HJ. The visual analogue pain intensity scale: what is moderate pain in millimetres? Pain. 1997;72:95–7. doi: 10.1016/s0304-3959(97)00005-5. [DOI] [PubMed] [Google Scholar]
- 21.Yamakage M, Iwasaki S, Satoh J, Namiki A. Changes in concentrations of free propofol by modification of the solution. Anesth Analg. 2005;101:385–8. doi: 10.1213/01.ANE.0000154191.86608.AC. [DOI] [PubMed] [Google Scholar]
- 22.Tan LH, Hwang NC. The effect of mixing lidocaine with propofol on the dose of propofol required for induction of anesthesia. Anesth Analg. 2003;97:461–4. doi: 10.1213/01.ANE.0000066357.63011.75. [DOI] [PubMed] [Google Scholar]
- 23.Ruzilawati AB, Wahab MS, Imran A, Ismail Z, Gan SH. Method development and validation of repaglinide in human plasma by HPLC and its application in pharmacokinetic studies. J Pharm Biomed Anal. 2007;43:1831–5. doi: 10.1016/j.jpba.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 24.Shimamoto K, Iimura O. Measurement of circulating kinins, their changes by inhibition of kininase II and their possible blood pressure lowering effect. Agents Actions. 1987;22:297–307. doi: 10.1007/978-3-0348-9299-5_31. [DOI] [PubMed] [Google Scholar]
- 25.Ohmizo H, Obara S, Iwama H. Mechanism of injection pain with long and long-medium chain triglyceride emulsive propofol. Can J Anaesth. 2005;52:595–9. doi: 10.1007/BF03015768. [DOI] [PubMed] [Google Scholar]
- 26.Lee EH, Lee SH, Park DY, Ki KH, Lee EK, Lee DH, Noh GJ. Physicochemical properties, pharmacokinetics, and pharmacodynamics of a reformulated microemulsion propofol in rats. Anesthesiology. 2008;109:436–47. doi: 10.1097/ALN.0b013e318182a486. [DOI] [PubMed] [Google Scholar]
- 27.Macario A, Weinger M, Truong P, Lee M. Which clinical anesthesia outcomes are both common and important to avoid? The perspective of a panel of expert anesthesiologists. Anesth Analg. 1999;88:1085–91. doi: 10.1097/00000539-199905000-00023. [DOI] [PubMed] [Google Scholar]
- 28.Briggs LP, Clarke RS, Dundee JW, Moore J, Bahar M, Wright PJ. Use of di-isopropyl phenol as main agent for short procedures. Br J Anaesth. 1981;53:1197–202. doi: 10.1093/bja/53.11.1197. [DOI] [PubMed] [Google Scholar]
- 29.Nakane M, Iwama H. A potential mechanism of propofol-induced pain on injection based on studies using nafamostat mesilate. Br J Anaesth. 1999;83:397–404. doi: 10.1093/bja/83.3.397. [DOI] [PubMed] [Google Scholar]
- 30.Tan CH, Onsiong MK. Pain on injection of propofol. Anaesthesia. 1998;53:468–76. doi: 10.1046/j.1365-2044.1998.00405.x. [DOI] [PubMed] [Google Scholar]
- 31.Wang H, Cork R, Rao A. Development of a new generation of propofol. Curr Opin Anaesthesiol. 2007;20:311–5. doi: 10.1097/ACO.0b013e3281667777. [DOI] [PubMed] [Google Scholar]
- 32.Moreau ME, Garbacki N, Molinaro G, Brown NJ, Marceau F, Adam A. The kallikrein–kinin system: current and future pharmacological targets. J Pharmacol Sci. 2005;99:6–38. doi: 10.1254/jphs.srj05001x. [DOI] [PubMed] [Google Scholar]
- 33.Kaplan AP, Joseph K, Shibayama Y, Reddigari S, Ghebrehiwet B, Silverberg M. The intrinsic coagulation/kinin-forming cascade: assembly in plasma and cell surfaces in inflammation. Adv Immunol. 1997;66:225–72. doi: 10.1016/s0065-2776(08)60599-4. [DOI] [PubMed] [Google Scholar]
- 34.Kaplan AP, Joseph K, Silverberg M. Pathways for bradykinin formation and inflammatory disease. J Allergy Clin Immunol. 2002;109:195–209. doi: 10.1067/mai.2002.121316. [DOI] [PubMed] [Google Scholar]
- 35.Zhao Y, Qiu Q, Mahdi F, Shariat-Madar Z, Rojkjaer R, Schmaier AH. Assembly and activation of HK–PK complex on endothelial cells results in bradykinin liberation and NO formation. Am J Physiol Heart Circ Physiol. 2001;280:H1821–9. doi: 10.1152/ajpheart.2001.280.4.H1821. [DOI] [PubMed] [Google Scholar]
- 36.Eriksson M, Englesson S, Niklasson F, Hartvig P. Effect of lignocaine and pH on propofol-induced pain. Br J Anaesth. 1997;78:502–6. doi: 10.1093/bja/78.5.502. [DOI] [PubMed] [Google Scholar]
- 37.Knibbe CA, Voortman HJ, Aarts LP, Kuks PF, Lange R, Langemeijer HJ, Danhof M. Pharmacokinetics, induction of anaesthesia and safety characteristics of propofol 6% SAZN vs propofol 1% SAZN and Diprivan-10 after bolus injection. Br J Clin Pharmacol. 1999;47:653–60. doi: 10.1046/j.1365-2125.1999.00942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCrirrick A, Hunter S. Pain on injection of propofol: the effect of injectate temperature. Anaesthesia. 1990;45:443–4. doi: 10.1111/j.1365-2044.1990.tb14329.x. [DOI] [PubMed] [Google Scholar]
- 39.Fletcher GC, Gillespie JA, Davidson JA. The effect of temperature upon pain during injection of propofol. Anaesthesia. 1996;51:498–9. doi: 10.1111/j.1365-2044.1996.tb07802.x. [DOI] [PubMed] [Google Scholar]
- 40.McCulloch MJ, Lees NW. Assessment and modification of pain on induction with propofol (Diprivan) Anaesthesia. 1985;40:1117–20. doi: 10.1111/j.1365-2044.1985.tb10615.x. [DOI] [PubMed] [Google Scholar]
- 41.Suzuki H, Miyazaki H, Andoh T, Yamada Y. Propofol formulated with long-/medium-chain triglycerides reduces the pain of injection by target controlled infusion. Acta Anaesthesiol Scand. 2006;50:568–71. doi: 10.1111/j.1399-6576.2006.00986.x. [DOI] [PubMed] [Google Scholar]
- 42.Klement W, Arndt JO. Pain on i. Br J Anaesth. 1991;66:189–95. doi: 10.1093/bja/66.2.189. [DOI] [PubMed] [Google Scholar]


