Abstract
AIMS
We aimed to assess the clinical effectiveness of oral vs. intravenous (i.v.) regular-dose proton pump inhibitor (PPI) after endoscopic injection of epinephrine in patients with peptic ulcer bleeding.
METHODS
Peptic ulcer patients with active bleeding, nonbleeding visible vessels, or adherent clots were enrolled after successful endoscopic haemostasis achieved by epinephrine injection. They were randomized to receive either oral rabeprazole (RAB group, 20 mg twice daily for 3 days) or i.v. omeprazole (OME group, 40 mg i.v. infusion every 12 h for 3 days). Subsequently, the enrolled patients receive oral PPI for 2 months (rabeprazole 20 mg or esomeprazole 40 mg once daily). The primary end-point was recurrent bleeding up to 14 days. The hospital stay, blood transfusion, surgery and mortality within 14 days were compared as well.
RESULTS
A total of 156 patients were enrolled, with 78 patients randomly allocated in each group. The two groups were well matched for factors affecting the clinical outcomes. Primary end-points (recurrent bleeding up to 14 days) were reached in 12 patients (15.4%) in the OME group and 13 patients (16.7%) in the RAB group [95% confidence interval (CI) of difference −12.82, 10.22]. All the rebleeding events occurred within 3 days of enrolment. The two groups were not different in hospital stay, volume of blood transfusion, surgery or mortality rate (1.3% of the OME group and 2.6% of the RAB group died, 95% CI of difference −5.6, 3.0).
CONCLUSIONS
Oral rabeprazole and i.v. regular-dose omeprazole are equally effective in preventing rebleeding in patients with high-risk bleeding peptic ulcers after successful endoscopic injection with epinephrine.
Keywords: epinephrine, omeprazole, peptic ulcer bleeding, rabeprazole, rebleeding
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT?
Endoscopic therapy significantly reduces recurrent bleeding, surgery and mortality in patients with bleeding peptic ulcers.
Intravenous (i.v.) proton pump inhibitors (PPIs) have been found to be effective as adjuvant pharmacotherapy in preventing rebleeding in these patients.
It remains undetermined whether oral and i.v. regular-dose PPIs are equally effective.
WHAT THIS STUDY ADDS?
Oral rabeprazole and i.v. regular-dose omeprazole are comparable in preventing rebleeding in patients with high-risk bleeding peptic ulcers after successful endoscopic injection with epinephrine.
Introduction
A bleeding peptic ulcer remains a serious medical problem with significant morbidity and mortality. Endoscopic therapy significantly reduces recurrent bleeding, surgery and mortality in patients with bleeding peptic ulcers [1] and is now recommended as the first-line haemostatic modality for these patients [1, 2].
Epinephrine injection has become the most popular endoscopic therapy for peptic ulcer bleeding because of its safety, low cost, and easy application [3]. Although a high initial haemostatic rate can be achieved with endoscopic injection of epinephrine, rebleeding occurs in 14–36% of these patients [4–6]. If the rebleeding rate can be lowered, epinephrine injection will be the ideal choice of therapy.
In the past few years, several controlled trials and meta-analysis studies have established the efficacy of the adjuvant use of proton pump inhibitor (PPI) after endoscopic therapy in high-risk bleeding ulcers [7–12]. However, optimal dosing of PPI in preventing rebleeding remains controversial [11–16].
Oral PPI has been found to be effective in preventing rebleeding in many studies [17–22]. For cost effectiveness, it is worth evaluating the benefits of oral PPI and intravenous (i.v.) regular-dose PPI in patients with peptic ulcer bleeding [23]. Recently, with 24-h intragastric pH monitoring, Laine et al. concluded that frequent oral PPI may be able to replace i.v. PPI therapy in patients with bleeding ulcers [24]. However, it remains uninvestigated whether oral and i.v. PPI are equally effective in clinical outcomes.
The aim of this study was to assess the clinical effectiveness of oral vs. i.v. regular-dose PPI after endoscopic injection of epinephrine in patients with peptic ulcer bleeding.
Methods
Design and patients
This was a single-centre prospective, randomized, controlled trial conducted in a tertiary teaching hospital (Veterans General Hospital, Taipei) in Taiwan and was approved by the Clinical Research Committee of the Veterans General Hospital, Taipei. From January 2007 to December 2007, peptic ulcer patients with high-risk stigmata were considered eligible if they fulfilled the following inclusion criteria: (i) underwent urgent endoscopy within 24 h after presentation, (ii) had peptic ulcers in the distal oesophagus, stomach or duodenum, (iii) had high-risk stigmata including active bleeding (Forrest IA, IB), nonbleeding visible vessels (NBVV, Forrest IIA), or adherent clots (Forrest IIB), and (iv) successful haemostasis was achieved with endoscopic injection of epinephrine. Written informed consent was obtained before enrolment.
Patients were excluded from the study if they were pregnant, did not obtain initial haemostasis with endoscopic injection of epinephrine, did not give written informed consent, had bleeding tendency (platelet count <50 × 109 l−1, serum prothrombin <30% of normal, or were taking anticoagulants), had used PPI within 14 days of enrolment, had uraemia or bleeding gastric cancer.
Endoscopic procedures
For enrolled patients, an Olympus GIF-XQ240 video-endoscope and an NM-8L injector were used to perform the endoscopic injection. Active bleeding was defined as a continuous blood spurting (Forrest IA) or oozing (Forrest IB) from the ulcer base. An NBVV at endoscopy was defined as a discrete protuberance at the ulcer base (Forrest IIA). An adherent clot was resistant to forceful irrigation or suction (Forrest IIB). We injected 10 ml diluted epinephrine (at a 1 : 10 000 ratio of epinephrine to saline) around the bleeder, NBVV or clot, and then observed the lesion for 3 min. If bleeding persisted, the patient was excluded from analysis and received other endoscopic therapies. All patients underwent endoscopic biopsy at gastric antrum for rapid urease test [Camplyobacter-like organism (CLO) test]. Those who were positive for urease test received a 1-week course of esomeprazole (40 mg twice daily) or rabeprazole (20 mg twice daily), plus clarithromycin (500 mg twice daily) and amoxicillin (1 g twice daily) after discharge.
Randomization process
Enrolled patients were randomly allocated into two groups using sealed envelopes containing a therapeutic option (either i.v. omeprazole or oral rabeprazole) derived from a random number table. In the omeprazole (OME) group, 40 mg continuous infusion of omeprazole was administered every 12 h for 3 days. Thereafter, the patients received oral esomeprazole 40 mg (Nexium®; AstraZeneca, Molndal, Sweden) once daily for 2 months. In the rabeprazole (RAB) group, we gave 20 mg of oral rabeprazole (Pariet®; Eisai Co., Ltd, Tokyo, Japan) twice daily for 3 days followed by once daily for 2 months. Endoscopy was repeated 72 h after enrolment. If no blood clot or haemorrhage was observed at the ulcer base, the patients were discharged and followed in the outpatient department.
Assessments
Patients’ vital signs were checked every hour for the first 12 h, every 2 h for the second 12 h, every 4 h for the following 24 h until they became stable, and then four times daily. The haemoglobin level and haematocrit were checked at least once daily, and blood transfusion was given if the haemoglobin level decreased to lower than 90 g l−1 or if the patient's vital signs deteriorated. Shock was defined as systolic blood pressure <100 mmHg and a pulse rate of >100 min−1 accompanied by cold sweats, pallor or oligurea. Initial endoscopic haemostasis was defined as no visible haemorrhage with observation for 3 min. Ultimate haemostasis was defined as no rebleeding within 14 days after endoscopic therapy.
Rebleeding was suspected if unstable vital signs, continuous tarry, bloody stool, or a drop of haemoglobin level >20 g l−1 within 24 h were noted. For these patients, an emergent endoscopy was performed immediately. Rebleeding was concluded if active bleeding, fresh blood or blood clots were found. All patients with rebleeding were treated with rescue endoscopic therapies including heater probe thermocoagulation or haemoclip placement.
At entry to the study, the following data were recorded: age, sex, location of the ulcer (oesophagus, stomach, duodenum or stoma), ulcer size, appearance of the gastric contents (clear, coffee ground, or blood), bleeding stigmata (spurting, oozing or NBVV), volume of blood transfusion at entry, presence of shock, haemoglobin, nonsteroidal anti-inflammatory drug ingestion, cigarette smoking, alcohol drinking, and comorbid illness. The Rockall scoring system was used to assess the severity of bleeding in both groups [25].
End-points
The primary end-point was 14-day rebleeding rate. Volume of blood transfusion, surgery, mortality within 14 days, and hospital stay were considered as secondary end-points.
Statistics
The sample size estimation was based on an expected rebleeding rate of 30% in the RAB group. The trial was designed to detect a 25% difference in favor of the OME group with a type I error of 0.05 and type II error of 0.05. At least 65 patients were essential for each group. Taking into account a possible drop-out rate of 15%, 78 patients were enrolled for each group in this study. We used unpaired Student's t-test to compare the numerical variables including age, ulcer size, volume of blood transfused, haemoglobin, and length of hospital stay between the two groups. Pearson's χ2 test and Fisher's exact test were used (if expected frequency in any of the cells was <10) to compare categorical variables such as the location of the bleeders, endoscopic findings, gastric contents, number of patients with Helicobacter pylori infection, shock, comorbid illness, haemostasis, emergent surgery, and mortality between the two groups. All statistic examinations were two-tailed and a probability value of <0.05 was considered significant.
Results
Between January 2007 and December 2007, 1561 patients presented with haematemesis, tarry stool or both to the emergency room. A total of 1304 patients received an urgent endoscopic examination within 24 h of arrival. Of the 1080 patients with peptic ulcers, 180 had high-risk stigmata of active bleeding, NBVV, or adherent clot. Twenty-four patients were excluded from the study for the following reasons: lack of informed consent (n= 3), bleeding tendency (n= 3), lack of cooperation (n= 2), gastric malignancy (n= 3), prior use of PPI (n= 11) and failure to obtain initial haemostasis (n= 2) (Figure 1). Finally, 156 patients were enrolled in this study (78 in the OME group and 78 in the RAB group). The two groups were well matched for the factors affecting outcome (Table 1).
Figure 1.
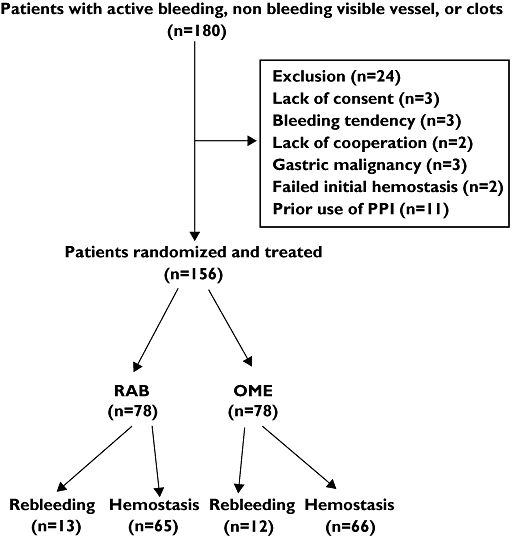
Flow chart illustrating the study progress from initial enrolment, through randomization, to primary end-point assessment
Table 1.
Clinical variables of patients at entry to the study
| OME (n= 78) | RAB (n= 78) | |
|---|---|---|
| Age (years) | 69.4 (20.3, 80.4) | 67.9 (21.2, 81.9) |
| Sex (%) | ||
| Male | 55 (70.5%) | 58 (74.4%) |
| Female | 23 (29.5%) | 20 (25.6%) |
| Locations of ulcer (%) | ||
| Stomach | 42 (53.8%) | 39 (50%) |
| Duodenum | 32 (41.0%) | 37 (47.4%) |
| Oesophagus | 4 (5.2%) | 2 (2.6%) |
| Endoscopic findings (%) | ||
| Spurting | 3 (3.8%) | 0 |
| Oozing | 28 (35.9%) | 33 (42.3%) |
| NBVV | 24 (30.8%) | 18 (23.1%) |
| Clot | 23 (29.5%) | 26 (33.3%) |
| Gastric contents (%) | ||
| Blood | 25 (32.1%) | 20 (25.6%) |
| Coffee grounds | 33 (42.3%) | 35 (44.9%) |
| Clear | 20 (25.6%) | 23 (29.5%) |
| Shock (%) | 21 (26.9%) | 16 (20.5%) |
| Medical comorbidity (%) | 50 (64.1%) | 51 (65.4%) |
| Ulcer size (cm) | 1.06 (0.4, 2.0) | 1.12 (0.5, 2.1) |
| Helicobacter pylori infection (%) | 48 (61.5%) | 51 (65.4%) |
| Haemoglobin (g l−1) | 9.81 (9.32, 10.48) | 10.31 (9.83, 10.85) |
| Rockall score | 5.4 (3.8, 7.0) | 5.3 (3.5, 7.1) |
Numerical variables expressed as mean with 95% confidence interval of distribution. No statistically significant difference between the two groups. OME, omeprazole; RAB, rabeprazole; NBVV, nonbleeding visible vessels.
Table 2 shows the clinical outcomes of this study. Rebleeding occurred in 12 (15.4%) patients in the OME group and 13 patients in the RAB group within 14 days (16.7%) (P= 0.83). All rebleeding episodes occurred within 3 days of enrolment. If patients with adherent clots were excluded, the rebleeding rates in the RAB (11/51, 21.6%) and OME groups (11/55, 20%) were still comparable (P= 0.87).
Table 2.
Clinical outcomes of patients according to routes of PPI
| OME (n= 78) | RAB (n= 78) | |
|---|---|---|
| Recurrent bleeding (%) | 12 (15.4%) | 13 (16.7%) |
| Hospital stay (days) | 8.5 (7.4, 9.6) | 8.9 (7.3, 9.7) |
| Volume of blood transfusion after therapy (ml) | 1231 (487, 1995) | 1156 (489, 1569) |
| Surgery (%) | 1 (1.3%) | 1 (1.3%) |
| Death (%) | 1 (1.3%) | 2 (2.6%) |
Numerical variables expressed as mean with 95% confidence interval of distribution. No statistically significant difference between the two groups. PPI, proton pump inhibitor; OME, omeprazole; RAB, rabeprazole.
Rebleeding occurred in 12 patients (15.4%) in the OME group. Of these patients, seven received heater probe therapy plus epinephrine injection and recovered uneventfully, two received a second epinephrine injection and recovered uneventfully, three received haemoclip placements, and two recovered uneventfully, while the third received surgical intervention due to continuous bleeding.
Rebleeding occurred in 13 patients (16.7%) in the RAB group. Of these patients, four received heater probe therapy plus epinephrine injection and recovered uneventfully, four received a second epinephrine injection and recovered uneventfully, three received haemoclip placements and recovered uneventfully, one received transarterial embolization and recovered uneventfully, and one received surgical intervention due to massive rebleeding.
The mean volume of blood transfusion was 1231 ml in the OME group, not significantly different from that of 1156 ml in the RAB group (P > 0.1). The mean duration of hospital stay was 8.52 days in the OME group and 8.86 days in the RAB group (P > 0.1). One patient died of unrelated illness in the OME group (pneumonia and sepsis), whereas two patients in the RAB group died of unrelated illness (necrotizing fasciitis and sepsis in one patient, terminal lung cancer in the other patient) (1.3% vs. 2.6%, P= 1.0). The mortality and surgical rates were identical at 14 days and 30 days of enrolment.
Discussion
The most important finding of our study is that oral and i.v. administrations of PPI were equally effective as adjuvant pharmacotherapy for patients with high-risk bleeding ulcers. This is the first controlled trial to demonstrate that the clinical outcomes, including rebleeding, blood transfusion, surgery, hospital stay and mortality, are comparable in patients receiving oral and i.v. PPI in the setting of peptic ulcer bleeding with high-risk stigmata.
PPIs increase intragastric pH and thereby help the formation and stabilization of the blood clots, since gastric acid impairs haemostasis by promoting platelet degradation and fibrinolysis [26]. Previous clinical trials had confirmed the effectiveness of PPI in reducing recurrent bleeding, surgery and mortality in patients with high-risk bleeding ulcers [7–12], but the optimal route and dosage of PPI administration remained controversial [11–16].
Oral PPI has been shown effective in improving clinical outcomes in patients with peptic ulcer bleeding. Khuroo and colleagues have shown that the recurrent bleeding rate was reduced from 36.4 to 10.9% (P < 0.001) in patients with NBVV who received oral omeprazole 40 mg twice daily for 5 days in a placebo-controlled trial [17]. Javid et al. gave oral omeprazole 40 mg every 12 h for 5 days in patients with high-risk peptic ulcers after endoscopic injection of epinephrine plus 1% polidocanol and found that oral PPI was superior to placebo in reducing hospital stay, rebleeding rate, and the need for blood transfusion [18]. Kaviani et al. conducted a double-blind, randomized, placebo-controlled trial to confirm the efficacy of oral omeprazole in reducing rebleeding rate [19].
Currently available evidence does not indicate that oral PPI is inferior to i.v. administration. Andriulli et al. evaluated 35 randomized trials that compared PPI with placebo or histamine type 2 receptor antagonist (H2RA) and concluded that the benefits of PPI appeared to be independent of the route and dose of PPI [20]. A Cochrane meta-analysis by Leontiadis and colleagues found no evidence to suggest route of PPI administration influenced the rebleeding, surgery or mortality [11]. A recent ‘head to head’ comparative trial conducted by Laine et al. investigated the ability of oral (120 mg bolus followed by 30 mg every 3 h) vs. i.v. (90 mg bolus followed by 9 mg h−1) high-dose lansoprazole to increase intragastric pH above 6. This well-designed study demonstrated that intragastric pH > 6 was maintained for 67.8% of the study period (24 h) in patients with i.v. PPI, and 64.8% in those with oral PPI (95% confidence interval of difference –9.2, 15.2). They concluded that frequent oral PPI may replace i.v. infusion PPI. Nevertheless, this study did not evaluate clinical outcomes as study end-points. Moreover, the laboriously frequent dosing schedule (every 3 h) limited clinical application of their study result.
In our randomized comparative trial, we found that recurrent bleeding, surgery, blood transfusion, and mortality were similar between the oral RAB and i.v. OME groups. The overall rebleeding rate of our study was 16% (15.4% in the i.v. PPI group and 16.7% in the oral PPI group, P= 0.83), which was lower than previous studies observed with placebo [7, 8]. Nevertheless, the rebleeding rates of our study appeared to be higher than in those receiving endoscopic and PPI therapy [6–8]. One probable reason and also a major limitation of our study is that we adopted epinephrine injection as the primary haemostatic measure, which might be considered suboptimal for high-risk bleeders [2, 6, 27]. Calvet et al. analysed 16 trials comparing epinephrine injection alone with combination therapy (epinephrine injection plus a second endoscopic therapy) and found the rebleeding rate to be 18.4% in the epinephrine alone, significantly higher than 10.6% in the combination therapy [27]. In a meta-analysis evaluating combination endoscopic therapy vs. epinephrine injection, Marmo et al. showed recurrent bleeding occurred in 15.58% (n= 193) of the pooled 1239 patients with single endoscopic therapy of epinephrine injection [6]. In fact, our results might reflect the poorer efficacy of epinephrine injection. We used epinephrine injection as standardized endoscopic therapy in this study because it is among the most popular endoscopic therapies, and therefore our result could be applied in most hospitals. We did not intend to recognize endoscopic epinephrine injection as the best available therapy. Instead, we excluded those whose haemostasis was not achieved by injection therapy alone, and used thermal or mechanical methods as rescue haemostatic procedures in rebleeding ulcers. On the other hand, our study has revealed that oral and i.v. PPI were similarly effective adjuvant pharmacotherapies even if the endoscopic therapy was limited to epinephrine injection.
Whether dosage of PPI influences clinical effectiveness is another unsettled issue in the management of patients with peptic ulcer bleeding. In a double-blind comparative trial, Udd et al. randomized 142 patients to receive i.v. omeprazole with either a regular dose (20 mg once daily) or a high-dose (80 mg bolus followed by 8 mg h−1) in patients with bleeding peptic ulcers (Forrest I–II), and found the rebleeding rates (8.2%) of the regular-dose group was equivalent to that (11.6%) of the high-dose group [13]. They concluded that a regular dose of omeprazole was as successful as a high dose. Similarly, Cheng et al. found that low-dose i.v. omeprazole (80 mg day−1) was equally effective as a high-dose (200 mg day−1) in preventing rebleeding in patients after endoscopic therapy (injection with or without thermal therapy) [14]. On the other hand, a retrospective analysis by Simon-Rudler and colleagues found continuous infusion of high-dose omeprazole (80-mg bolus followed by 8 mg h−1) was more effective than a standard dose of i.v. omeprazole (40 mg day−1) in the occurrence of rebleeding, death due to haemorrhagic shock, and need of surgery [15]. Meta-analysis studies have not resolved this highly debated issue [11, 12, 20]. At present, we consider the available evidence conflicting in determining the relative effectiveness of a high-dose PPI over a regular dose. Since it was the route rather than the dosage that we aimed to investigate, we had to control the dosage of PPI. We did not consider a third arm of high-dose infusion PPI in order not to make the results difficult to interpret. Further well-designed studies are necessary to elucidate the controversy regarding the dosage of PPI. With the knowledge derived from Laine's and our study [24], we consider a future large factorial study with four arms (high and regular dosage vs. i.v. and oral route) may be valuable to better define the dosing method of PPI.
Several limitations of our study should be noted. First, the use of epinephrine injection alone is suboptimal compared with combination endoscopic therapy. In this study we adopted thermocoagulation and mechanical clipping as rescue therapy. Although this might affect the overall rebleeding rate, the impact of endoscopic therapy on clinical outcomes was minimized. Second, this study may be underpowered to detect subtle differences. Because this is the first clinical outcome research to evaluate oral vs. i.v. PPI, we assumed oral rabeprazole was comparable to H2RA when compared with i.v. PPI while estimating the sample size. The difference between the two groups turned out to be much smaller than initially expected (25% difference in rebleeding rate), and thus the predefined sample size might not be large enough for a small difference. Post hoc analysis revealed that a sample size as large as 12 515 patients in each arm was needed to detect the difference (15.4% vs. 16.7%, with an α level of 0.05 and a power of 0.8). We therefore concluded the two groups were equally effective, but recognized that the predefined sample size might not be large enough for a small difference. Third, our study enrolled Taiwanese patients only. Whether a similar result would have been found in a Western population requires further validation, inasmuch as the ethnic or environmental factors may affect the treatment response [11, 28]. Fourth, the open-label design of our study might raise some concerns as regards bias. Nevertheless, assessment bias should be negligible because the definitions of end-points were all standardized and objective.
In summary, this single-centre, prospective, randomized, controlled trial of patients with high-risk bleeding ulcers has shown that oral and i.v. regular-dose PPI were equally effective as adjuvant pharmacotherapy to endoscopic haemostasis. Oral rabeprazole (20 mg twice daily) and i.v. infusion omeprazole (40 mg every 12 h) were not different in recurrent bleeding, surgery, blood transfusion or mortality. Our results suggest that oral PPI may be able to replace i.v. infusion PPI as the treatment of choice in peptic ulcer bleeding. However, more studies, particularly validating trials in Western countries, are necessary before oral PPI can be considered as the standard treatment.
Competing interests
None to declare.
This study was supported by the Tomorrow Medical Foundation Grant no. 96-3. The authors are indebted to Miss Betty Tzu-en Lin and Mr Alex Jen-hao Lin for assistance in this study. This work will be presented at the ACG annual meeting in October, 2008, Orlando, FL, USA.
REFERENCES
- 1.Cook DJ, Guyatt GH, Salena BJ, Laine LA. Endoscopic therapy for acute nonvariceal upper gastrointestinal hemorrhage: a meta-analysis. Gastroenterology. 1992;102:139–48. doi: 10.1016/0016-5085(92)91793-4. [DOI] [PubMed] [Google Scholar]
- 2.Adler DG, Leighton JA, Davila RE, Hirota WK, Jacobson BC, Qureshi WA, Rajan E, Zuckerman MJ, Fanelli RD, Hambrick RD, Baron T, Faigel DO. ASGE guideline: the role of endoscopy in acute non-variceal upper-GI hemorrhage. Gastrointest Endosc. 2004;60:497–504. doi: 10.1016/s0016-5107(04)01568-8. [DOI] [PubMed] [Google Scholar]
- 3.Savides TJ, Jensen DM. Therapeutic endoscopy for nonvariceal gastrointestinal bleeding. Gastroenterol Clin North Am. 2000;29:465–87. doi: 10.1016/s0889-8553(05)70123-0. [DOI] [PubMed] [Google Scholar]
- 4.Lin HJ, Perng CL, Lee SD. Is sclerosant injection mandatory after epinephrine injection for arrest of peptic ulcer haemorrhage? A prospective randomized comparative study. Gut. 1993;34:1182–5. doi: 10.1136/gut.34.9.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin HJ, Hsieh YH, Tseng GY, Perng CL, Chang FY, Lee SD. A prospective, randomized trial of large- versus small-volume endoscopic injection of epinephrine for peptic ulcer bleeding. Gastrointest Endosc. 2002;55:615–9. doi: 10.1067/mge.2002.123271. [DOI] [PubMed] [Google Scholar]
- 6.Marmo R, Rotondano G, Piscopo R, Bianco MA, D'Angella R, Cipolletta L. Dual therapy versus monotherapy in the endoscopic treatment of high-risk bleeding ulcers: a meta-analysis of controlled trials. Am J Gastroenterol. 2007;102:279–89. doi: 10.1111/j.1572-0241.2006.01023.x. [DOI] [PubMed] [Google Scholar]
- 7.Lau JY, Sung JJ, Lee KK, Yung MY, Wong SK, Wu JC, Chan FK, Ng EK, You JH, Lee CW, Chan AC, Chung SC. Effect of intravenous omeprazole on recurrent bleeding after endoscopic treatment of bleeding peptic ulcers. N Engl J Med. 2000:310–6. doi: 10.1056/NEJM200008033430501. [DOI] [PubMed] [Google Scholar]
- 8.Lin HJ, Lo WC, Lee FY, Perng CL, Tseng GY. A prospective randomized comparative trial showing that omeprazole prevents rebleeding in patients with bleeding peptic ulcer after successful endoscopic therapy. Arch Intern Med. 1998;158:54–8. doi: 10.1001/archinte.158.1.54. [DOI] [PubMed] [Google Scholar]
- 9.Palmer K. Non-variceal upper gastrointestinal haemorrhage: guideline. Gut. 2002;51(Suppl.)(4):1–6. doi: 10.1136/gut.51.suppl_4.iv1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barkun A, Bardou M, Marshall JK. Consensus recommendations for managing patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2003;139:843–57. doi: 10.7326/0003-4819-139-10-200311180-00012. [DOI] [PubMed] [Google Scholar]
- 11.Leontiadia GI, Sharma VK, Howden CW. Proton pump inhibitor treatment for acute peptic ulcer bleeding. Cochrane Database Syst Rev. 2006;1 doi: 10.1002/14651858.CD002094.pub3. CD. [DOI] [PubMed] [Google Scholar]
- 12.Bardou M, Toubouti Y, Benhaberou-Brun D, Rahme E, Barkun AN. Meta-analysis proton pump inhibition in high-risk patients with acute peptic ulcer bleeding. Aliment Pharmacol Ther. 2005;21:677–86. doi: 10.1111/j.1365-2036.2005.02391.x. [DOI] [PubMed] [Google Scholar]
- 13.Udd M, Miettinen P, Palmu A, Heikkinen M, Janatuinen E, Pasanen P, Tarvainen R, Kairaluoma MV, Lohman M, Mustonen H, Julkunen R. Regular-dose versus high-dose omeprazole in peptic ulcer bleeding. Scand J Gastroenterol. 2001;36:1332–8. doi: 10.1080/003655201317097218. [DOI] [PubMed] [Google Scholar]
- 14.Cheng HC, Kao AW, Chuang CH, Sheu BS. The efficacy of high- and low-dose intravenous omeprazole in preventing rebleeding for patients with bleeding peptic ulcers and comorbid illness. Dig Dis Sci. 2005;50:1194–201. doi: 10.1007/s10620-005-2759-6. [DOI] [PubMed] [Google Scholar]
- 15.Simon-Rudler M, Massard J, Bernard-Chabert B, Martino VD, Ratziu V, Poynard T, Thabut D. Continuous infusion of high-dose omeprazole is more effective than standard-dose omeprazole in patients with high-risk peptic ulcer bleeding: a retrospective study. Aliment Pharmacol Ther. 2007;25:949–54. doi: 10.1111/j.1365-2036.2007.03286.x. [DOI] [PubMed] [Google Scholar]
- 16.Morgan D. Intravenous proton pump inhibitors in the critical care setting. Crit Care Med. 2002;30:S369–72. doi: 10.1097/00003246-200206001-00007. [DOI] [PubMed] [Google Scholar]
- 17.Khuroo MS, Yattoo GN, Javid G, Khan BA, Shah AA, Gulzar GM, Sodhi JS. A comparison of omeprazole and placebo for bleeding peptic ulcer. N Engl J Med. 1997;336:1054–8. doi: 10.1056/NEJM199704103361503. [DOI] [PubMed] [Google Scholar]
- 18.Javid G, Masoodi I, Zargar SA, Khan BA, Yatoo GN, Shah AH, Gulzar GM, Sodhi JS. Omeprazole as adjuvant therapy to endoscopic combination injection sclerotherapy for treating bleeding peptic ulcer. Am J Med. 2001;111:280–4. doi: 10.1016/s0002-9343(01)00812-9. [DOI] [PubMed] [Google Scholar]
- 19.Kaviani MJ, Hashemi MR, Kazemifar AR, Soozitalab S, Mostaghni AA, Merat S, Alizadeh-Naini M, Yarmohammadi H. Effect of oral omeprazole in reducing re-bleeding in bleeding peptic ulcers: a prospective, double-blind, randomized, clinical trial. Aliment Pharmacol Ther. 2003;17:211–6. doi: 10.1046/j.1365-2036.2003.01416.x. [DOI] [PubMed] [Google Scholar]
- 20.Andriulli A, Annese V, Caruso N, Pilotto A, Accadia L, Niro AG, Quitadamo M, Merla A, Fiorella S, Leandro G. Proton-pump inhibitors and outcome of endoscopic hemostasis in bleeding peptic ulcer: a series of meta-analysis. Am J Gastroenterol. 2005;100:207–19. doi: 10.1111/j.1572-0241.2005.40636.x. [DOI] [PubMed] [Google Scholar]
- 21.Jensen DM, Kovacs TOG, Jutabha R, Machicado GA, Gralnek IM, Savides TJ, Smith J, Jensen ME, Alofaituli G, Gombein J. Randomized trial of medical or endoscopic therapy to prevent recurrent ulcer hemorrhage in patients with adherent clots. Gastroenterology. 2002;123:407–13. doi: 10.1053/gast.2002.34782. [DOI] [PubMed] [Google Scholar]
- 22.Kim JII, Cheung DY, Cho SH, Park SH, Han JY, Kim JK, Han SW, Choi KY, Chung IS. Oral proton pump inhibitors are as effective as endoscopic treatment for bleeding peptic ulcer: a prospective, randomized, controlled trial. Dig Dis Sci. 2007;52:3371–6. doi: 10.1007/s10620-007-9814-4. [DOI] [PubMed] [Google Scholar]
- 23.Spiegel BM, Dulai GS, Lim BS, Mann N, Kanwal F, Gralnek IM. The cost-effectiveness and budget impact of intravenous versus oral proton pump inhibitors in peptic ulcer hemorrhage. Clin Gastroenterol Hepatol. 2006;4:988–97. doi: 10.1016/j.cgh.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 24.Laine L, Shah A, Bemanian S. Intragastric pH with oral vs intravenous bolus plus infusion proton pump inhibitor therapy in patients with bleeding ulcers. Gastroenterology. 2008;134:1836–41. doi: 10.1053/j.gastro.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 25.Rockall TA, Logan RFA, Devlin HB, Northfield TC. and the steering committee and members of Natl Audit of acute upper gastrointestinal haemorrhage. Risk assessment following acute upper gastrointestinal haemorrhage. Gut. 1996;38:316–21. doi: 10.1136/gut.38.3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barkun AN, Cockeram AW, Plourde V, Fedorak RN. Review article: acid suppression in non-variceal acute upper gastrointestinal bleeding. Aliment Pharmacol Ther. 1999;13:1565–84. doi: 10.1046/j.1365-2036.1999.00623.x. [DOI] [PubMed] [Google Scholar]
- 27.Calvet X, Vergara M, Brullet E, Gisbert JP, Campo R. Addition of a second endoscopic treatment following epinephrine injection improves outcome in high-risk bleeding ulcers. Gastroenterology. 2004;126:441–50. doi: 10.1053/j.gastro.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 28.Leontiadis GI, Sharma VK, Howden CW. Systematic review and meta-analysis: enhanced efficacy of proton-pump inhibitor therapy for peptic ulcer bleeding in Asia – a post hoc analysis from the Cochrane Collaboration. Aliment Pharmacol Ther. 2005;21:1055–61. doi: 10.1111/j.1365-2036.2005.02441.x. [DOI] [PubMed] [Google Scholar]


