Intravenous (i.v.) midazolam is used to phenotype hepatic cytochrome P450 (CYP) 3A [1]. Midazolam clearance (CL) is believed to correlate with the intrinsic hepatic CL (CLint) of midazolam and thus acts as a surrogate for hepatic CYP3A activity. Midazolam is a moderate extraction ratio drug [2]. Based on the well-stirred model of hepatic clearance [3], midazolam CL depends on CLintand hepatic blood flow. Subject posture alters hepatic blood flow, is often not controlled during blood sample collection [1], and impacts pharmacokinetic studies [4]. The magnitude of posture-induced blood flow changes has been seen in a study of six subjects. Plasma midazolam CL decreased 48% in an ambulant (sitting/walking) posture compared with a supine posture [5] and suggests that changes in midazolam CL may be dependent on hepatic blood flow and not exclusively CYP3A activity. This study evaluated the effect of subject posture on midazolam CL to confirm previous findings. We quantified posture-induced hepatic blood flow changes with i.v. indocyanine green as a biomarker. Results are published elsewhere [6].
The Institutional Review Board of Bassett Healthcare (Cooperstown, NY, USA) approved the study. Written informed consent was obtained. Healthy adults 18–55 years old who were not on any medications were enrolled. Subjects were randomized to a 7-h supine or ambulant posture. A minimum of 7 days passed between study phases. Subjects maintained strict horizontal bed rest during supine posture. During ambulant posture, subjects were seated for the first 3 h and then allowed to stand, walk, or sit. Sessions began between 06.00 h and 07.00 h to minimize temporal variation [5]. Subjects fasted (except water) overnight and for the duration of each posture. To stabilize hepatic blood flow, subjects were in the assigned posture for 1 h prior to midazolam 0.025 mg kg−1 i.v. (midazolam hydrochloride, 1 mg ml−1 injection, North Chicago, IL, USA) administration. Eight blood samples were obtained over 6 h. Samples were centrifuged and stored at −80°C. Plasma concentrations were analysed by liquid chromatography/mass spectrometry/mass spectrometry as described elsewhere [7]. The linear range of the assay was 0.50–100 ng ml−1, with intra- and interday coefficients of variation of ≤7.9% at 0.75, 7.5 and 75 ng ml−1.
Midazolam pharmacokinetics was determined by noncompartmental analysis (WinNonlin v3.1; Pharsight, Cary, NC, USA). Sample size calculations were based on previously published data [5, 8–10]. Using an α= 0.05 and β= 0.20, a sample size of 12 would detect a 25% difference in midazolam CL. Bioequivalence testing was used by calculating the least squares geometric mean ratios (LS-GMR; CLambulant/CLsupine) and 90% confidence intervals (CI). Bioequivalence was concluded if the 90% CIs were within the 80–125% interval.
Thirteen (seven female) subjects completed both postures. Plasma midazolam concentrations vs. time are shown in Figure 1. Bioequivalence was observed for the geometric mean midazolam CLs in the supine and ambulant phases (595.6 vs. 565.1 ml min−1; LS-GMR 0.95, 90% CI 0.88, 1.02). Intersubject variation of midazolam CL was small, varying 1.7- and two-fold in the supine and ambulant postures, respectively. The volume of distribution (Vd) decreased 18% in the ambulant posture. The difference in Vd may be due to changes in tissue binding, but for CYP3A phenotyping in healthy adults this change is unlikely to be of clinical significance.
Figure 1.
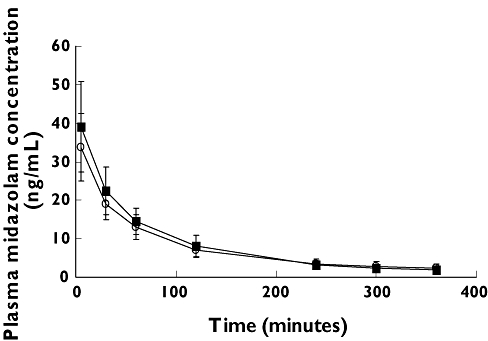
Mean ± SD midazolam plasma concentrations vs. time in the ambulant (closed squares) and supine (open circles) postures (n= 13)
Studies have observed posture-induced changes in hepatic blood flow of 17–37% for several medications [11, 12]. In the current study, subject posture did not affect midazolam CL. In contrast to the study of Klotz et al. [5], the current study used a smaller midazolam i.v. dose (0.025 vs. 0.075 mg kg−1), had a larger sample size (13 vs. 6), and had subjects remain fasting during the entire sampling period. Food intake increases hepatic blood flow and lasts up to 190 min [13]. We speculate that the small sample size and food intake may have affected midazolam CL in the Klotz et al. study [5]. Neither study reported the exact amount of time during the ambulant posture that subjects were in a sitting, walking and standing posture, and potential differences between subjects and studies may have existed in the amount of time spent in a specific ambulant position.
Phenotyping studies using i.v. midazolam as a hepatic CYP3A probe do not need to control for subject posture during blood sample collection. These findings are not applicable when using oral midazolam to phenotype intestinal and hepatic CYP3A activity, as i.v. midazolam exclusively measures hepatic CYP3A.
REFERENCES
- 1.Ma J, Nafziger A, Rhodes G, Liu S, Gartung A, Bertino J. The effect of oral pleconaril on hepatic cytochrome p450 3a activity in healthy adults using intravenous midazolam as a probe. J Clin Pharmacol. 2006;46:103–8. doi: 10.1177/0091270005283286. [DOI] [PubMed] [Google Scholar]
- 2.Rogers J, Rocci M, Haughey D, Bertino J. An evaluation of the suitability of IV midazolam as an in vivo marker for hepatic CYP3A activity. Clin Pharmacol Ther. 2003;73:153–8. doi: 10.1067/mcp.2003.23. [DOI] [PubMed] [Google Scholar]
- 3.Wilkinson G, Shand D. Commentary: a physiological approach to hepatic drug clearance. Clin Pharmacol Ther. 1975;18:377–90. doi: 10.1002/cpt1975184377. [DOI] [PubMed] [Google Scholar]
- 4.Queckenberg C, Fuhr U. Influence of posture on pharmacokinetics. Eur J Clin Pharmacol. 2008 doi: 10.1007/s00228-008-0579-2. Oct 21 [Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 5.Klotz U, Ziegler G. Physiologic and temporal variation in hepatic elimination of midazolam. Clin Pharmacol Ther. 1982;32:107–12. doi: 10.1038/clpt.1982.133. [DOI] [PubMed] [Google Scholar]
- 6.Ma J, Nafziger A, Mylott W, Haughey D, Rocci M, Bertino J. Quantitative assessment of hepatic blood flow using intravenous indocyanine green. Eur J Clin Pharmacol. 2008;64:1133–4. doi: 10.1007/s00228-008-0519-1. [DOI] [PubMed] [Google Scholar]
- 7.Fayer J, Zannikos P, Stevens J, Luo Y, Sidhu R, Kirkesseli S. Lack of correlation between in vitro inhibition of CYP3A-mediated metabolism by a PPAR-gamma agonist and its effect on the clinical pharmacokinetics of midazolam, an in vivo probe of CYP3A activity. J Clin Pharmacol. 2001;41:305–16. doi: 10.1177/00912700122010122. [DOI] [PubMed] [Google Scholar]
- 8.Hotz M, Ritz R, Linder L, Scollo-Lavizzari G, Haefeli W. Auditory and electroencephalographic effects of midazolam and a-hydroxy-midazolam in healthy subjects. Br J Clin Pharmacol. 2000;49:72–9. doi: 10.1046/j.1365-2125.2000.00104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klotz U, Reimann I. Chronopharmacokinetic study with prolonged infusion of midazolam. Clin Pharmackinetics. 1984;9:469–74. doi: 10.2165/00003088-198409050-00006. [DOI] [PubMed] [Google Scholar]
- 10.Wandel C, Witte J, Hall J, Stein C, Wood A, Wilkinson G. CYP3A activity in African American and European American men: population differences and functional effect of the CYP3A4*1B 5′-promoter regional polymorphism. Clin Pharmacol Ther. 2000;68:82–91. doi: 10.1067/mcp.2000.108506. [DOI] [PubMed] [Google Scholar]
- 11.Daneshmend T, Jackson L, Roberts C. Physiological and pharmacological variability in estimated hepatic blood flow in man. Br J Clin Pharmacol. 1981;11:491–6. doi: 10.1111/j.1365-2125.1981.tb01155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grasela D, Rocci M, Rotmensch H, Vlasses P. Lack of effect of multiple does of cimetidine on estimated hepatic blood flow. Biopharm Drug Dispos. 1987;8:63–72. doi: 10.1002/bdd.2510080108. [DOI] [PubMed] [Google Scholar]
- 13.Svensson C, Edwards D, Mauriello P, Barde S, Foster A, Lanc R, Middleton E, Lalka D. Effect of food on hepatic blood flow: implications in the ‘food effect’ phenomenon. Clin Pharmacol Ther. 1983;34:316–23. doi: 10.1038/clpt.1983.174. [DOI] [PubMed] [Google Scholar]


