Abstract
The onset of menopause marks a pivotal time in which the incidence of hypertension and cardiovascular disease begins to increase dramatically in women. Prior to menopause, the incidence of these diseases is significantly lower than in similarly aged men, but following menopause the rates rise rapidly until paralleling that in men. The loss of endogenous estrogen at menopause has traditionally been thought to be the primary factor involved in these changes and resulted in the widespread use of hormone replacement therapy (HRT) to reduce cardiovascular risk factors and decrease the affective symptoms of menopause. However, the adverse effects of HRT reported in recent large-scale trials (e.g., the Women’s Health Initiative) have greatly decreased the use of HRT by postmenopausal women.
Many women are seeking alternatives to HRT, including the use of dietary supplements that have a long history of use in traditional medicine, particularly in Asia. Examples of frequently used botanicals are soy, black cohosh, red clover, grape derivatives, St. John’s wort, Ginko biloba and Echinacea. While many of these botanicals appear to ameliorate some postmenopausal symptoms (i.e., bone loss, hot flushes/flashes and night sweats), none of the tested botanicals has proven as effective as HRT in decreasing the affective disorders of menopause. Further, despite the increasing usage of botanical supplements, their efficacy and safety have not been well documented by critical research studies. This review summarizes recent findings related to the utility of botanicals for menopause-related cardiovascular and metabolic disorders, specifically hypertension, diabetes, progressive cognitive decline and hyperlipidemia. While great caution should be exercised in the translation of animal findings to the human, these studies, along with those of others, suggest that some commonly used botanical supplements may be useful adjuvants for providing protection to women (and men) against cardiovascular risk.
Keywords: estrogen, menopause, blood pressure, diabetes, lipids, hypertension
Hypertension is a major health risk that significantly contributes to cardiovascular disease and stroke. Further, the incidence of hypertension increases with age, affecting approximately 30% of all adults and more than 60% of adults over 65 years of age (1). Persistent hypertension triples the incidence of heart disease and stroke and magnifies the adverse effects of other cardiovascular risk factors, e.g., smoking, type 2 diabetes, etc. (2;3). While most studies examining the mechanisms of hypertension have focused on men, it is clear that women also experience significant morbidity and mortality from effects related to hypertension, but there is a significant gender disparity in the incidence of hypertension and cardiovascular disease. Prior to menopause, blood pressure and cardiovascular disease is significantly lower in women than age-matched men. However, following menopause, the incidence of hypertension and cardiovascular disease increases dramatically in women, eventually approximating the incidence in men (4;5). While the mechanism underlying this increase is unknown, the loss of estrogen traditionally has been considered the primary factor.
The close correlation between estrogen loss and hypertension, cardiovascular disease, bone density loss and hot flashes made the use of hormone replacement therapy (HRT) commonplace throughout the last half of the 20th Century. The cardioprotective effects of HRT were supported by numerous basic research reports and clinical observations demonstrating a reduction of hypertension, atherosclerosis and cardiovascular disease in postmenopausal women on HRT. However, an increase in breast cancer rates in woman receiving HRT led some researchers to question the safety of the treatment. The Women’s Health Initiative and other contemporary studies further questioned the safety of HRT by demonstrating increased risk in thrombolytic events and heart attacks in HRT recipients. As a result, HRT has been strongly discouraged by most physicians and medical societies. The reported adverse effects of HRT have led many clinicians and users to seek alternative methods including the use of dietary supplements to provide the health benefits of HRT without the unfavorable effects.
While reports vary, most surveys suggest that over 50% of adults in the United States regularly take a dietary supplement (most frequently multivitamins), and about 33% of women of perimenopausal/postmenopausal age (45–60 years of age) regularly take some form of botanical supplement (6–9). The widespread use of supplements extends worldwide, where the majority of people rely on traditional medication (predominantly the use of plant extracts) for primary prevention/treatment of disease (10). The significant increase in US consumption of these supplements reflects increased desire for effective, safe, non-pharmaceutical therapies. Further, compliance with these treatments is better than that for most common pharmaceutical treatments, increasing their potential effectiveness when compared to drug therapies. While many of these supplements are taken for their presumed estrogenic actions, most of them appear to be only weakly or non-estrogenic.
Some of the most common botanicals used are soy, Ginko biloba, Kava kava, grape derivatives, Echinacea and black cohosh (7). However, the efficacy of botanicals as primary treatments for most diseases lacks basic and clinical research documentation. Botanical supplements appear to be useful as adjutants to pharmaceutical therapy and as preventative treatments, but the increasingly widespread usage of botanicals presents significant safety concerns. Botanical supplements are not as closely regulated as pharmaceutical products, and therefore, many may be adulterated with unexpected metabolites that could cause adverse reactions. Also, the concentration of active ingredients in botanical extracts can be very high compared to their concentration in whole plants, and therefore, toxicity may result from their ingestion. Further, the lack of basic and clinical research translates into an absence of reliable dosage guidelines. This problem is exacerbated by the belief that “if a little helps, a little more helps more” (11). Dietary intake of soy isoflavones chronically taken in excess (150mg versus 50 mg in Western diet or 100 mg in Japanese diet) can stimulate endometrial hyperplasia (12). A second more publicized example of adverse effects of botanicals relates to ephedra, which is a very effective weight loss dietary supplement for middle age and older adults. But it can have catastrophic results when younger adults take in it in large excess (13;14). St. John’s wort decreases depression in many users, but it also stimulates the pregnane X receptor and, thereby, alters hepatic metabolism of other pharmacological therapies (15;16). Similarly, Kava kava appeared to be an effective anxiolytic and to possibly exert a wide range of other heath benefits. However, hepatotoxicity linked to kava ingestion led to a ban on the botanical and careful consideration of its mechanisms of adverse action (17). Clearly, Kava kava is not hepatotoxic on its own, but increasing evidence suggests that its ability to alter drug metabolism in the body can lead to the serious side effects. Thus, supplements cannot be assumed to be safe just because they are “natural.” Even for the “safe” botanicals, one must be vigilant to both the beneficial and the adverse interactions between the botanical and diet.
Many of the botanicals display estrogenic-like binding and appeared to be promising for the alleviation of affective postmenopausal symptoms. Thus, studies have explored the potential of botanicals as alternatives to HRT for ameliorating hot flushes/flashes and night sweats. However, none of the tested botanicals has proven as effective as HRT. Black cohosh is the best candidate for alleviating these primary symptoms (18), but it remains very controversial since several large scale trials have not demonstrated any beneficial, postmenopausal effects, e.g., (19;20).
This review summarizes recent findings in relation to the utility of botanicals in other menopausal and aging symptoms, i.e., rise in arterial pressure, cognitive decline, insulin resistance and hyperlipidemia. While considerable caution should be exercised in the translation of animal findings to humans, several studies suggest that some commonly used botanical supplements may be useful adjuvants in reducing these symptoms.
Dietary Soy
Probably the most often studied and most widely used botanical in this category is soy. Due to the ability of its major isoflavones to bind estrogen receptors, it has often been considered a likely alternative to estrogen replacement therapy in postmenopausal women. Perhaps the best-documented effect of soy is its ability to lower plasma lipids. Dietary soy lowers LDL levels (21–28), triglycerides (24–27), and apolipoprotein B plasma concentrations (28). Soy may also increase HDL levels, although this increase appears to be negligible (29). The effects of soy on plasma lipids profiles do not appear to be gender specific (30). However, at least one study indicates a beneficial lipid effect only in hypertensive patients, versus normotensive patients, suggesting that its beneficial effects on lipids may be most active in compromised individuals (29).
Soy also has an ability to lower arterial pressure in postmenopausal women and in age-matched men, e.g., (31;32), similar to antihypertensive effects in animals, e.g., (33–35). Clinical data suggesting such a protective effect includes observations that dietary soy directly reduces arterial pressure in normotensive and hypertensive individuals. For instance in a randomized study of postmenopausal women, Welty, et al., (28) observed that dietary soy lowered systolic and diastolic arterial pressure in both hypertensive and normotensive individuals (28). Similarly, a clinical study by Teede et al. (30) demonstrated that three months of soy supplementation in normotensive male and female subjects significantly reduced systolic, diastolic and mean arterial pressure. Interestingly, in their follow up study they investigated the effect of soy supplementation on the same parameters in patients with established hypertension (36). In contrast to their previous findings, soy supplementation had no effect on any of the blood pressure parameters in hypertensive subjects, nor was there any beneficial effect on arterial function. This led them to propose that soy supplementation may be beneficial during the developmental but not the established phases of hypertension.
Other noted effects of soy include protective effects in heart disease (37;38), atherosclerosis of the carotid and coronary circulation in aged subjects (39–42) and stroke induced apoptosis (43). Dietary soy may also benefit diabetic individuals, as demonstrated by research showing that soy lowers fasting insulin levels, HbA1c levels insulin resistance, total cholesterol and cholesterol/HDL ratio, (44).
Soy Isoflavones and Arterial Pressure
The most studied isoflavone constituents of soy are genistein and daidzein, both of which are structurally similar to estrogen and demonstrate binding affinities for the estrogen receptors (ER), primarily ER β receptors (11;45–47). This may account for their ability to specifically stimulate positive estrogenic actions while minimizing negative aspects related to estrogen (e.g,. enhanced cellular proliferation). The cardiovascular protective effects of soy isoflavones have largely mirrored those of soy, including improvement of blood lipid profiles (48–51). Soy isoflavone intake is correlated with a reduction in arterial pressure, both in clinical trials (52–54) and rat studies (55;56) and is reported to have a positive effect on bone density (57–59), endothelial function, cognitive performance and mood (60). Increased soy isoflavones have also been reported to potentiate protective effects of exercise on body weight and body mass index (51;57;58;61). The interpretation of these studies is complicated by the significant variability in isoflavone administration, e.g., use of different extraction procedures and different dosages. Thus, the intake of genistein and daidzein may vary significantly across studies (see[11]).
Research in our laboratory has focused on the effects of soy isoflavones on blood pressure control in spontaneously hypertensive rats (SHR). SHR are a commonly used genetic model of hypertension, and exhibit a gender disparity in the degree of hypertension and salt-sensitivity exhibited. Male SHR display a pronounced elevation in hypertension as they age, and hypertension is exacerbated in response to dietary sodium intake (62;63). In contrast, arterial pressure is significantly lower in female compared to age-matched male SHR, and blood pressure is relatively resistant to dietary salt in female SHR (63). This suggests that estrogen exerts a protective effect on baseline arterial pressure and salt-sensitivity in SHR.
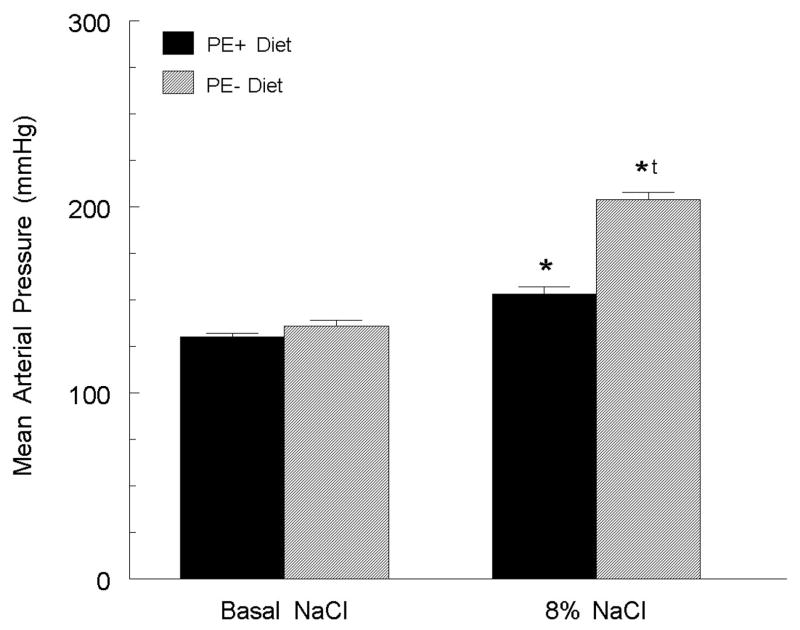
Based on evidence that dietary phytoestrogens (especially those from soy) could decrease cardiovascular and neuronal damage in animal models of disease (e.g., [64–67]), we explored whether phytoestrogens in normal commercial rodent diets (approximately 0.06% phytoestrogen, primarily from soy) played a protective role in blood pressure control. To test this, young female SHR were ovariectomized at 3 weeks of age and placed on one of four diets: 0.6% or 8.0% NaCl in the presence or absence of phytoestrogens (34). In SHR fed the phytoestrogen-free diet, the 8% NaCl diet caused a 68 ± 8 mm Hg increase in mean arterial pressure (MAP; figure 1). In contrast, in the phytoestrogen-replete diet, the 8% NaCl raised MAP by only 23 ± 4 mm Hg. In the ovariectomized SHR on the basal NaCl diet, elimination of phytoestrogen from the diet only slightly increased arterial pressure. Histological examination following 14 weeks on the diets demonstrated significant damage (frequent protein casts) in the kidneys of the high NaCl, phytoestrogen-free diet group but no appreciable damage in any other group. These data indicate that the elimination of all dietary phytoestrogens dramatically exacerbates the hypertensive effect of a high NaCl diet in young ovariectomized SHR (34).
Figure 1.
The loss of dietary soy phytoestrogens (PE- diet) increased the response of mean arterial pressure to a high (8%) NaCl diet. * p < 0.05 versus same group on basal diet; tp < 0.05 versus PE- group on 8% diet. Figure adapted from (34).
Some studies have suggested that the beneficial effects of soy are dependent on the protein contained in soy or an interaction between the soy proteins and the isoflavones (68). To better define the role of soy isoflavones in female SHR, ovariectomized SHR were maintained on a phytoestrogen-free diet that contained 0.06% genistein which is approximately equal to the genistein content in soy-based rat chow and results in circulating genistein concentrations similar to that in Japanese women on a Japanese diet and in rats on normal chow diets. We observed that genistein supplementation significantly lowered the arterial pressure response to a high salt diet by about 50 mmHg, thus confirming the ability of specific soy phytoestrogens to blunt salt-sensitive hypertension (69).
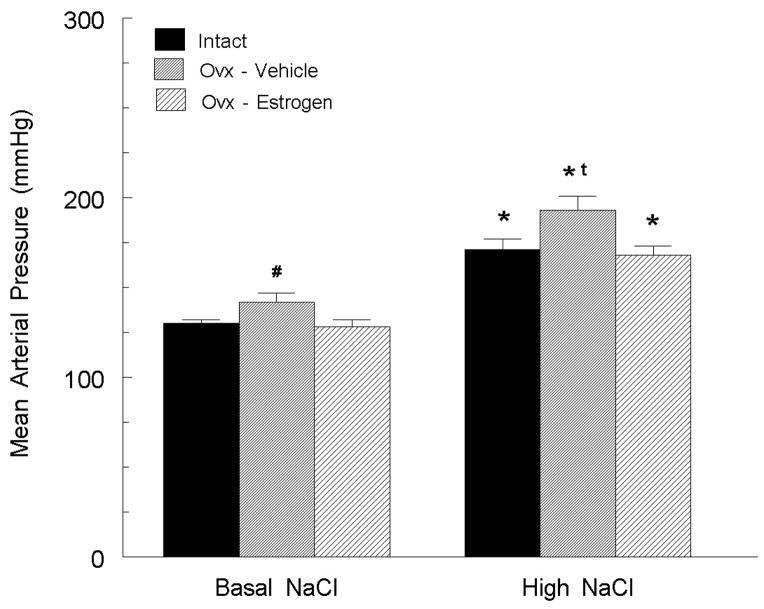
While studies in young animals are informative, experiments in middle-aged animals are likely more instructive concerning mechanisms that underlie beneficial effects of isoflavones in postmenopausal women. We therefore examined whether soy isoflavones exerted similar protective effects in older animals. SHR were ovariectomized at 10 months of age (a stage in which their estrogen cycles are becoming irregular) and placed on a phytoestrogen-free diet, containing either basal or high NaCl diet. In the basal NaCl group, estrogen-depletion increased arterial pressure (12 mm Hg) and decreased norepinephrine release in the anterior hypothalamic nucleus, which is a sympathoinhibitory site (figure 2; [70]). The high NaCl diet increased arterial pressure by over 35 mm Hg, and this effect was reversed by estrogen replacement therapy, suggesting that both dietary NaCl excess and estrogen depletion raise arterial pressure in middle-aged female SHR by decreasing hypothalamic norepinephrine release.
Figure 2.
In 10-month old SHR fed a soy phytoestrogen free diet, ovariectomy significantly increased mean arterial pressure versus sham operated rats, and estrogen replacement abolished this effect. Exposure to a high salt diet significantly elevated arterial pressure in intact rats, and this increase was exacerbated by ovariectomy and estrogen replacement eliminated this effect. # p < 0.05 versus other groups on basal NaCl diet; * p < 0.05 versus same group on basal NaCl diet; t p < 0.05 versus other groups on high NaCl diet. Figure adapted from (70).
We also examined whether phytoestrogens are similarly protective in stroke prone SHR (SHR-SP). Four-week-old female SHR-SP were ovariectomized or left intact and placed on a basal phytoestrogen-free or 0.06% genistein containing diet. Six weeks later, blood pressure and heart rate were recorded and norepinephrine (NE) was measured in the AHN. Intact and ovariectomized SHR-SP on a soy-based diet, did not display significantly different arterial pressures (70). In contrast, ovariectomized SHR-SP on a phytoestrogen-free (compared to soy-based) diet displayed a 34 mm Hg increase in arterial pressure and 90% reduction in AHN NE. The addition of phytoestrogens to the diet significantly blunted this decrease. Together, these data demonstrate that dietary soy isoflavones protect SHR-SP on a basal NaCl diet from hypertension and suggest that this effect is related to a isoflavone-induced increase in AHN NE release.
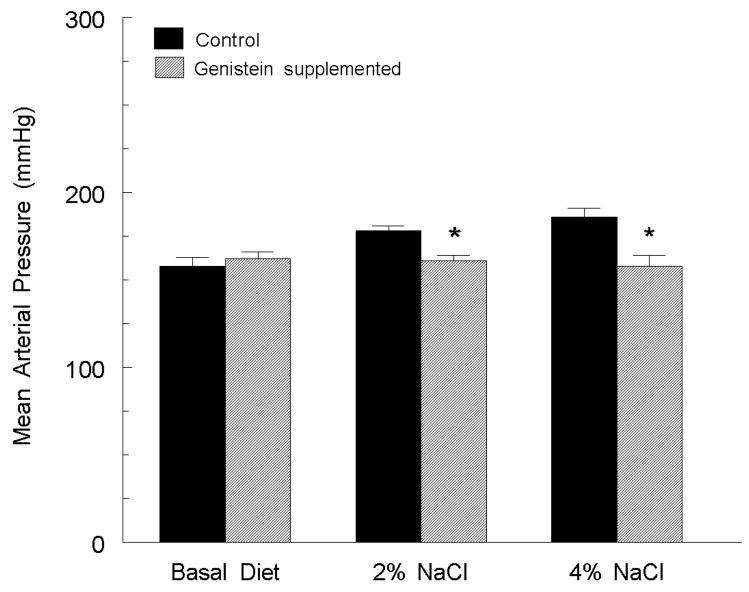
While our studies demonstrated that dietary soy isoflavones could blunt hypertension in female rats, it remained unknown if the dietary polyphenols could similarly protect male rats. Therefore, male SHR-SP were fed a basal (0.7%), medium (2%) or high (4%) NaCl diet with or without dietary phytoestrogens and blood pressure was monitored. The results demonstrated a positive correlation between salt intake and arterial pressure (figure 3, [71]) in male SHR on the polyphenol-free diet. However, in rats supplemented with genistein, the salt-induced changes in arterial pressure were eliminated. These data demonstrate that at least in this model of hypertension, soy isoflavones protect against salt-sensitive hypertension in both male and female rats.
Figure 3.
Mean arterial pressures of male SHR-SP fed basal (0.7%), medium (2.0%) or high (8.0%) NaCl diets with or without genistein supplementation. * p > 0.05 versus control diet. Figure adapted from (71).
Genistein blunts arteriolar responsiveness
To further probe the mechanism(s) underlying the beneficial effects of soy isoflavones, we investigated whether soy isoflavones reduce adrenergic receptor-mediated vasoconstriction in arteries of SHR, Wistar and Sprague-Dawley rats, using phenylephrine (PE; an α1 adrenergic receptor agonist) and acetylcholine (ACh; a potent activator of the NO-mediated vasodilation). In seven-week-old SHR on a basal NaCl diet, chronic estrogen depletion sensitized the vascular response to PE and desensitized the response to ACh. Acute (10 min) administration of genistein (<1 uM; equal to normal concentration of genistein in the plasma of rats fed a soy based diet) reversed these effects, decreasing the vasoconstrictor responses to PE and increasing the responsiveness to ACh. In SHR fed a high NaCl diet, responses to PE were sensitized by nearly a full log unit, and acute infusion of genistein returned PE responsiveness to control levels. The high NaCl diet also desensitized the vasodilator responses to ACh, and acute genistein infusion returned responses to basal conditions. Similarly, in both WKY and SD either the loss of estrogen or dietary NaCl excess favored vasoconstriction versus vasodilation and acute genistein pretreatment returned these responses to baseline (72).
Other Polyphenols
While soy isoflavones have been a primary focus of clinical and basic research, they represent only a small portion of dietary polyphenols that are consumed. It is increasingly clear that less studied polyphenolic botanicals also have significant health-related benefits. The dietary sources of these are tremendously widespread (see [73]), and include polyphenols found in grapes and numerous other fruits, teas, chocolate/cocoa, and a significant number of vegetables and nuts. Of these, grape extracts and related red wine are some of the most studied polyphenolics (e.g., see [74–78]). Although the mechanism of grape seed polyphenols remains unclear, it appears that actions are mediated via non-estrogenic pathways. Grape seed extracts provide significant antioxidants and may reduce the amount of reactive oxygen species, which are elevated in cardiovascular and metabolic diseases.
Grape Seed Polyphenols and Arterial Pressure
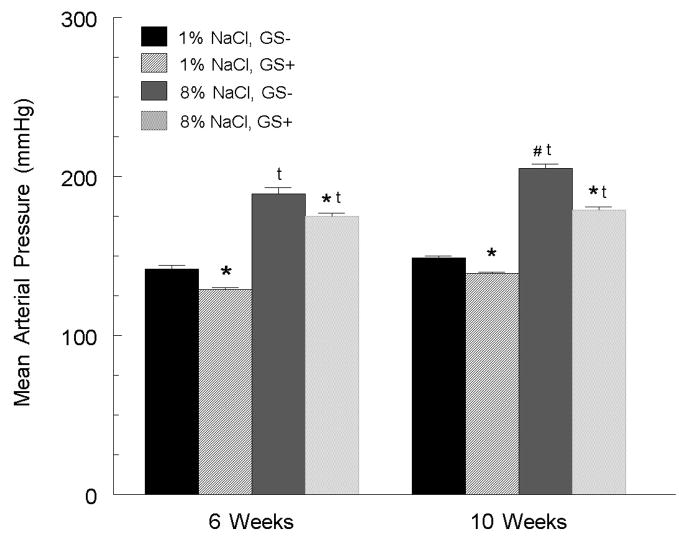
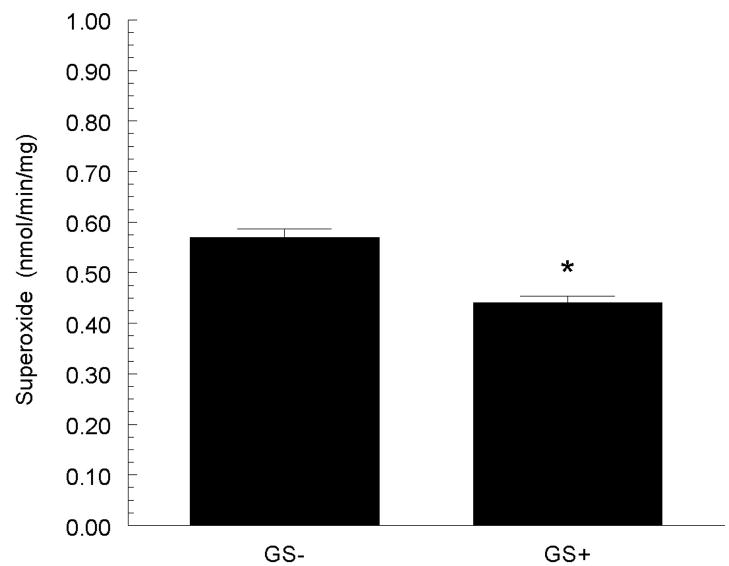
Grape seed extract contains a high concentration of polyphenols, containing a rich mixture of monomeric flavan-3-ols (catechin and epicatechin), and oligomeric proanthocyanidins that display little to no estrogenic actions. Yet they appear to confer cardiovascular protective effects. Thus, we investigated the ability of grape seed polyphenols to reduce arterial pressure and salt-sensitive hypertension in estrogen-depleted SHR (79). Female SHR were ovariectomized at 3 weeks of age and placed on diets that were either devoid of polyphenols or were supplemented with grape seed extract (0.5%) and contained either a basal or high NaCl. After 6 weeks on the basal salt diet, grape seed supplemented SHR had a significantly lower arterial pressure than the SHR fed a non-supplemented diet (figure 4; [79]). On a high NaCl diet, grape seed supplementation (compared to non-supplementation) significantly blunted the rise in arterial pressure. Further, superoxide formation in the aorta of SHR fed a high salt diet was significantly reduced in the grape seed-supplemented group (figure 5). These results indicate that like soy phytoestrogens, dietary grape polyphenols are protective in estrogen-depleted SHR, but the effects of grape polyphenols appear to have different underlying mechanisms, e.g., a greater participation of superoxide scavenging.
Figure 4.
6-weeks of grape seed extract (GS+) supplementation reduced mean arterial pressure in SHR fed either a basal (1%) or high (8%) NaCl diet. When the grape seed extract regimen was extended to 10 weeks, the arterial pressure response to the high NaCl diet was further reduced. * p < 0.05 versus rats fed a non-supplemented, same NaCl diet; t p < 0.05 versus same group on basal (1%) diet; # p < 0.05 versus same group at 6 weeks. Figure adapted from (79).
Figure 5.
Grape seed extract supplementation (GS+) reduced aortic superoxide formation in SHR fed a high (8%) NaCl diet. * p < 0.05 versus non-supplemented group (GS−). Figure adapted from (79).
Cognitive effects of polyphenols
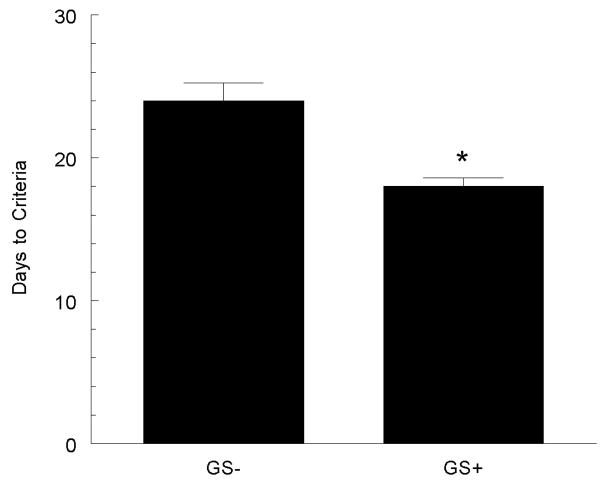
There is clear evidence that hypertension impairs cognitive function, and that this relationship is positively correlated. Male SHR display an age-related decline in cognitive performance, beginning in the 10th–12th month of life. Although a similar fall in cognitive ability occurs in normotensive male rats, the onset is around 18 months of age. Female SHR also develop similar age-related deficits, albeit several months later than their male counterparts. Additionally, antihypertensive therapy attenuates the observed declines in cognition (80). We were interested in whether antihypertensive botanical supplementation would ameliorate cognitive decline in hypertensive rats. Female ovariectomized SHR-SP were fed a basal NaCl that contained 0.0% or 0.5% grape seed extract for 8 weeks, at which time cognitive abilities were assessed using an eight-arm-radial maze task. Although the grape seed extract did not lower arterial pressure in these rats, it did result in a significant improvement in the number of trials it took the rats to reach criterion performance (79). This indicates that grape seed polyphenols can positively impact cognitive function, although the mechanism may be independent of an effect on blood pressure, since the correlation between blood pressure and cognitive function was not significant.
Kudzu Extracts
In addition to soy isoflavones and grape seed extract, research from our laboratory is interested in the protective properties of extracts derived from the root of perennial leguminous vine Kudzu, or Pueraria lobota. Kuzdu was imported to the U.S. from Japan in 1876, and its high nutritional value, rapid growth and elaborate root system resulted in widespread usage in the southern United States for animal feed, soil enrichment and erosion prevention (81). While these uses eventually became supplanted by more efficient substances and techniques, Kudzu proliferated extensively and is now a highly invasive nuisance and threatens native plants. Kuzdu has an exceptionally long tradition in traditional Chinese herbal medicine, dating back to 200 B.C. (81). There are two components of Kudzu that are utilized in traditional medicine, including the flower, Flos puerariae, and root, Radix puerariae. While the most prominent use has been as amethystic (anti-alcohol intoxication) and antidipsotropic (anti-alcoholabuse) agents, uses also include cardiovascular and stroke protection along with antipyresis, antidiarrhetic and anti-emetic agents (81). While soy and Kudzu are both primary sources of polyphenols in Chinese medicine, the isoflavone compositions are markedly different. The principle isoflavanoid of Kudzu root is puerarin (daidzein-8-C-glucoside) and daidzein and genistein, are contained resent in low (1%–3%) concentrations (82). While daidzein and genistein act via estrogenic receptors, as discussed above, research has only started deciphering how puerarin exerts its effects. Reported actions of puerarin include stimulation of bone formation (83), is cytoprotective in rodent diabetic models (84), acts as a nerve growth factor (85), exerts a vasodilatory influence (86;87), and improves lipid profiles (88;89).
Probably the best studied effect of puerarin relates to glycemic control in diabetes. Hsu et al. (90) demonstrated that puerarin supplementation lowered baseline plasma glucose levels in Wistar Kyoto rats and had greater effect in streptozotocin models of diabetes. They also demonstrated that puerarin injections increased glucose uptake in skeletal muscle. These results suggest that puerarin improves glucose uptake in diabetic rats, and these actions may be extended into non-diabetic animals. A subsequent study by our group examined the role of puerarin on glucose control in ob/ob mice (91). Puerarin improved glucose tolerance in ob/ob mice. Interestingly, the effect of puerarin on glucose tolerance contrasted with that of daidzin, the next most concentrated isoflavanoid in kudzu. Daidzin had worsened glucose tolerance, i.e, compared to controls, plasma glucose concentrations reached higher peaks and took longer to return to baseline. These contrasting observations reflect the issue of potential conflicting components found in many botanical supplements. Similarly, we have also found that glycemic control is improved by both acute and long-term puerarin supplementation in both SHR-SP and mice (unpublished observations).
We have also investigated whether dietary kudzu supplementation protects against NaCl-induced hypertension in SHR, similar to that seen with other botanicals studied in our laboratory. Male SHR were maintained on a high NaCl, polyphenol-free diet containing either 0.3% or 0.0% kudzu extract. After 8 weeks on the diets, the arterial pressures were significantly lower in rats receiving kudzu root powder, suggesting that kudzu polyphenols blunt NaCl-sensitive hypertension in young, male SHR.
More recently we tested the ability of kudzu root extract to protect cognitive abilities. A previous report indicated that dietary puerarin decreased memory impairment in a mouse model of aging induced by D-galactose (92) and in ovariectomized female mice (93). Using a water maze task, we tested the hypothesis that dietary kudzu protects male SHR from a NaCl-induced memory impairment. Our data demonstrate that the kudzu root-supplemented rats displayed improved learning and memory abilities, relative to the non-supplemented group.
Summary
Research from our laboratory and others indicate that several botanical compounds have beneficial effects in humans and animal models of disease. While these compounds have had fairly limited success in relation to some of the more prominent affective symptoms of menopause, they appear more effective in relation to cardiovascular, metabolic and cognitive function. Thus, the health benefits of these compounds appear significant, but the safety and mechanisms of action of each should be carefully tested in relation to the disease status of potential users.
Figure 6.
SHR fed a diet supplemented with grape seed extract (GS+) demonstrated a faster time in learning an 8-arm radial maze task. * p < 0.05 versus non-supplemented rats (GS−). Figure adapted from (79).
Acknowledgments
This work was supported by National Institutes of Health grants, AT 00477 (JMW) from the National Center for Complementary and Alternative Medicine and the Office of Dietary Supplements and NS 041071, NS 047466, the UAB Neuroscience Core Center NS47466 and the Alabama Neuroscience Blueprint Core Center NS57098 from the National Institute of Neurological Disorders and Stroke. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Center for Complementary and Alternative Medicine, the Office of Dietary Supplements, or the National Institutes of Health.
Footnotes
The authors have nothing to disclose.
Reference List
- 1.Cohen E, Wheat ME, Swiderski DM, Charney P. Hypertension: Pathophysiology, Diagnosis, and Management. 2. Vol. 1. Raven Press, Ltd; New York: 1995. Hypertension in women; pp. 159–169. [Google Scholar]
- 2.Ledingham JM. Hypertension: Pathophysiology, Diagnosis, and Management. 2. Vol. 1. Raven Press, Ltd; New York: 1995. The vascular fault in hypertension: Byrom’s work revisited; pp. 37–53. [Google Scholar]
- 3.Wilson PWF, Kannel WB. Hypertension: Pathophysiology, Diagnosis, and Management. 2. Vol. 1. Raven Press, Ltd; New York: 1995. Hypertension, other risk factors, and the risk of cardiovascular disease; pp. 99–114. [Google Scholar]
- 4.Kotchen JM, McKean HE, Kotchen TA. Blood pressure trends with aging. Hypertension. 1982;4(5 Pt 2):III128–III134. doi: 10.1161/01.hyp.4.5_pt_2.iii128. [DOI] [PubMed] [Google Scholar]
- 5.Spence JD. Cerebral consequences of hypertension: where do they lead? J Hypertens Suppl. 1996;14(5):S139–S145. [PubMed] [Google Scholar]
- 6.Radimer K, Bindewald B, Hughes J, Ervin B, Swanson C, Picciano MF. Dietary supplement use by US adults: data from the National Health and Nutrition Examination Survey, 1999–2000. Am J Epidemiol. 2004;160(4):339–349. doi: 10.1093/aje/kwh207. [DOI] [PubMed] [Google Scholar]
- 7.Radimer KL. National nutrition data: contributions and challenges to monitoring dietary supplement use in women. J Nutr. 2003;133(6):2003S–2007S. doi: 10.1093/jn/133.6.2003S. [DOI] [PubMed] [Google Scholar]
- 8.Gordon NP, Schaffer DM. Use of dietary supplements by female seniors in a large Northern California health plan. BMC Geriatr. 2005;5:4. doi: 10.1186/1471-2318-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schaffer DM, Gordon NP, Jensen CD, Avins AL. Nonvitamin, nonmineral supplement use over a 12-month period by adult members of a large health maintenance organization. J Am Diet Assoc. 2003;103(11):1500–1505. doi: 10.1016/j.jada.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 10.Farnsworth NR, Akerele O, Bingel AS, Soejarto DD, Guo Z. Medicinal plants in therapy. Bull World Health Organ. 1985;63(6):965–981. [PMC free article] [PubMed] [Google Scholar]
- 11.Wuttke W, Jarry H, Seidlova-Wuttke D. Isoflavones--safe food additives or dangerous drugs? Ageing Res Rev. 2007;6(2):150–188. doi: 10.1016/j.arr.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Unfer V, Casini ML, Costabile L, Mignosa M, Gerli S, Di Renzo GC. Endometrial effects of long-term treatment with phytoestrogens: a randomized, double-blind, placebo-controlled study. Fertil Steril. 2004;82(1):145–8. doi: 10.1016/j.fertnstert.2003.11.041. quiz. [DOI] [PubMed] [Google Scholar]
- 13.Fleming RM. The effect of ephedra and high fat dieting: A cause for concern! A case report. Angiology. 2007;58(1):102–105. doi: 10.1177/0003319706297965. [DOI] [PubMed] [Google Scholar]
- 14.Libman RB, Menna BL, Gulati S. Case report - Consequences of ephedra use in an athlete. Lancet. 2005;366:S22. doi: 10.1016/S0140-6736(05)67832-4. [DOI] [PubMed] [Google Scholar]
- 15.Kliewer SA, Willson TM. Regulation of xenobiotic and bile acid metabolism by the nuclear pregnane X receptor. J Lipid Res. 2002;43(3):359–364. [PubMed] [Google Scholar]
- 16.Moore LB, Goodwin B, Jones SA, Wisely GB, Serabjit-Singh CJ, Willson TM, et al. St. John’s wort induces hepatic drug metabolism through activation of the pregnane X receptor. Proc Natl Acad Sci U S A. 2000;97(13):7500–7502. doi: 10.1073/pnas.130155097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clouatre DL. Kava kava: examining new reports of toxicity. Toxicol Lett. 2004;150(1):85–96. doi: 10.1016/j.toxlet.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Geller SE, Studee L. Botanical and dietary supplements for mood and anxiety in menopausal women. Menopause. 2007;14(3 Pt 1):541–549. doi: 10.1097/01.gme.0000236934.43701.c5. [DOI] [PubMed] [Google Scholar]
- 19.Newton KM, Reed SD, Lacroix AZ, Grothaus LC, Ehrlich K, Guiltinan J. Treatment of vasomotor symptoms of menopause with black cohosh, multibotanicals, soy, hormone therapy, or placebo: a randomized trial. Ann Intern Med. 2006;145(12):869–879. doi: 10.7326/0003-4819-145-12-200612190-00003. [DOI] [PubMed] [Google Scholar]
- 20.Spangler L, Newton KM, Grothaus LC, Reed SD, Ehrlich K, Lacroix AZ. The effects of black cohosh therapies on lipids, fibrinogen, glucose and insulin. Maturitas. 2007;57(2):195–204. doi: 10.1016/j.maturitas.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Anderson JW, Johnstone BM, Cook-Newell ME. Meta-analysis of the effects of soy protein intake on serum lipids. N Engl J Med. 1995;333(5):276–282. doi: 10.1056/NEJM199508033330502. [DOI] [PubMed] [Google Scholar]
- 22.Demonty I, Lamarche B, Jones PJ. Role of isoflavones in the hypocholesterolemic effect of soy. Nutr Rev. 2003;61(6 Pt 1):189–203. doi: 10.1301/nr.2003.jun.189-203. [DOI] [PubMed] [Google Scholar]
- 23.Demonty I, Lamarche B, Deshaies Y, Jacques H. Role of soy isoflavones in the hypotriglyceridemic effect of soy protein in the rat. J Nutr Biochem. 2002;13(11):671–677. doi: 10.1016/s0955-2863(02)00214-0. [DOI] [PubMed] [Google Scholar]
- 24.Merz-Demlow BE, Duncan AM, Wangen KE, Xu X, Carr TP, Phipps WR, et al. Soy isoflavones improve plasma lipids in normocholesterolemic, premenopausal women. Am J Clin Nutr. 2000;71(6):1462–1469. doi: 10.1093/ajcn/71.6.1462. [DOI] [PubMed] [Google Scholar]
- 25.Wangen KE, Duncan AM, Xu X, Kurzer MS. Soy isoflavones improve plasma lipids in normocholesterolemic and mildly hypercholesterolemic postmenopausal women. Am J Clin Nutr. 2001;73(2):225–231. doi: 10.1093/ajcn/73.2.225. [DOI] [PubMed] [Google Scholar]
- 26.Taku K, Umegaki K, Sato Y, Taki Y, Endoh K, Watanabe S. Soy isoflavones lower serum total and LDL cholesterol in humans: a meta-analysis of 11 randomized controlled trials. Am J Clin Nutr. 2007;85(4):1148–1156. doi: 10.1093/ajcn/85.4.1148. [DOI] [PubMed] [Google Scholar]
- 27.Allen JK, Becker DM, Kwiterovich PO, Lindenstruth KA, Curtis C. Effect of soy protein-containing isoflavones on lipoproteins in postmenopausal women. Menopause. 2007;14(1):106–114. doi: 10.1097/01.gme.0000229572.21635.49. [DOI] [PubMed] [Google Scholar]
- 28.Welty FK, Lee KS, Lew NS, Zhou JR. Effect of soy nuts on blood pressure and lipid levels in hypertensive, prehypertensive, and normotensive postmenopausal women. Arch Intern Med. 2007;167(10):1060–1067. doi: 10.1001/archinte.167.10.1060. [DOI] [PubMed] [Google Scholar]
- 29.Crawford P, Paden SL, Park MK. Clinical inquiries: What is the dietary treatment for low HDL cholesterol? J Fam Pract. 2006;55(12):1076–1078. [PubMed] [Google Scholar]
- 30.Teede HJ, Dalais FS, Kotsopoulos D, Liang YL, Davis S, McGrath BP. Dietary soy has both beneficial and potentially adverse cardiovascular effects: a placebo-controlled study in men and postmenopausal women. J Clin Endocrinol Metab. 2001;86(7):3053–3060. doi: 10.1210/jcem.86.7.7645. [DOI] [PubMed] [Google Scholar]
- 31.Nagata C, Shimizu H, Takami R, Hayashi M, Takeda N, Yasuda K. Association of blood pressure with intake of soy products and other food groups in Japanese men and women. Prev Med. 2003;36(6):692–697. doi: 10.1016/s0091-7435(03)00052-5. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura M, Aoki N, Yamada T, Kubo N. Feasibility and effect on blood pressure of 6-week trial of low sodium soy sauce and miso (fermented soybean paste) Circ J. 2003;67(6):530–534. doi: 10.1253/circj.67.530. [DOI] [PubMed] [Google Scholar]
- 33.Greaves KA, Parks JS, Williams JK, Wagner JD. Intact dietary soy protein, but not adding an isoflavone-rich soy extract to casein, improves plasma lipids in ovariectomized cynomolgus monkeys. J Nutr. 1999;129(8):1585–1592. doi: 10.1093/jn/129.8.1585. [DOI] [PubMed] [Google Scholar]
- 34.Fang Z, Carlson SH, Chen YF, Oparil S, Wyss JM. Estrogen depletion induces NaCl-sensitive hypertension in female spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2001;281(6):R1934–R1939. doi: 10.1152/ajpregu.2001.281.6.R1934. [DOI] [PubMed] [Google Scholar]
- 35.Martin DS, Breitkopf NP, Eyster KM, Williams JL. Dietary soy exerts an antihypertensive effect in spontaneously hypertensive female rats. Am J Physiol Regul Integr Comp Physiol. 2001;281(2):R553–R560. doi: 10.1152/ajpregu.2001.281.2.R553. [DOI] [PubMed] [Google Scholar]
- 36.Teede HJ, Giannopoulos D, Dalais FS, Hodgson J, McGrath BP. Randomised, controlled, cross-over trial of soy protein with isoflavones on blood pressure and arterial function in hypertensive subjects. J Am Coll Nutr. 2006;25(6):533–540. doi: 10.1080/07315724.2006.10719569. [DOI] [PubMed] [Google Scholar]
- 37.Hasler CM. The cardiovascular effects of soy products. J Cardiovasc Nurs. 2002;16(4):50–63. doi: 10.1097/00005082-200207000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Zhang X, Shu XO, Gao YT, Yang G, Li Q, Li H, et al. Soy food consumption is associated with lower risk of coronary heart disease in Chinese women. J Nutr. 2003;133(9):2874–2878. doi: 10.1093/jn/133.9.2874. [DOI] [PubMed] [Google Scholar]
- 39.Yamori Y, Horie R. Community-based prevention of stroke: nutritional improvement in Japan. Health Rep. 1994;6(1):181–188. [PubMed] [Google Scholar]
- 40.Yamori Y. Experimental evidence for dietary prevention of cardiovascular diseases. Clin Exp Pharmacol Physiol. 1989;16(4):303–307. doi: 10.1111/j.1440-1681.1989.tb01562.x. [DOI] [PubMed] [Google Scholar]
- 41.Clarkson TB. Soy, soy phytoestrogens and cardiovascular disease. J Nutr. 2002;132(3):566S–569S. doi: 10.1093/jn/132.3.566S. [DOI] [PubMed] [Google Scholar]
- 42.Clarkson TB, Anthony MS, Morgan TM. Inhibition of postmenopausal atherosclerosis progression: a comparison of the effects of conjugated equine estrogens and soy phytoestrogens. J Clin Endocrinol Metab. 2001;86(1):41–47. doi: 10.1210/jcem.86.1.7151. [DOI] [PubMed] [Google Scholar]
- 43.Linford NJ, Dorsa DM. 17beta-Estradiol and the phytoestrogen genistein attenuate neuronal apoptosis induced by the endoplasmic reticulum calcium-ATPase inhibitor thapsigargin. Steroids. 2002;67(13–14):1029–1040. doi: 10.1016/s0039-128x(02)00062-4. [DOI] [PubMed] [Google Scholar]
- 44.Jayagopal V, Albertazzi P, Kilpatrick ES, Howarth EM, Jennings PE, Hepburn DA, et al. Beneficial effects of soy phytoestrogen intake in postmenopausal women with type 2 diabetes. Diabetes Care. 2002;25(10):1709–1714. doi: 10.2337/diacare.25.10.1709. [DOI] [PubMed] [Google Scholar]
- 45.Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138(3):863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 46.Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139(10):4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 47.Abbas HK, Tak H, Boyette CD, Shier WT, Jarvis BB. Macrocyclic trichothecenes are undetectable in kudzu (Pueraria montana) plants treated with a high-producing isolate of Myrothecium verrucaria. Phytochemistry. 2001;58(2):269–276. doi: 10.1016/s0031-9422(01)00214-x. [DOI] [PubMed] [Google Scholar]
- 48.Anthony MS, Clarkson TB, Hughes CL, Jr, Morgan TM, Burke GL. Soybean isoflavones improve cardiovascular risk factors without affecting the reproductive system of peripubertal rhesus monkeys. J Nutr. 1996;126(1):43–50. doi: 10.1093/jn/126.1.43. [DOI] [PubMed] [Google Scholar]
- 49.Blair RM, Appt SE, Bennetau-Pelissero C, Clarkson TB, Anthony MS, Lamothe V, et al. Dietary soy and soy isoflavones have gender-specific effects on plasma lipids and isoflavones in golden Syrian f(1)b hybrid hamsters. J Nutr. 2002;132(12):3585–3591. doi: 10.1093/jn/132.12.3585. [DOI] [PubMed] [Google Scholar]
- 50.Washburn S, Burke GL, Morgan T, Anthony M. Effect of soy protein supplementation on serum lipoproteins, blood pressure, and menopausal symptoms in perimenopausal women. Menopause. 1999;6(1):7–13. [PubMed] [Google Scholar]
- 51.Goodman-Gruen D, Kritz-Silverstein D. Usual dietary isoflavone intake is associated with cardiovascular disease risk factors in postmenopausal women. J Nutr. 2001;131(4):1202–1206. doi: 10.1093/jn/131.4.1202. [DOI] [PubMed] [Google Scholar]
- 52.Liang YL, Teede H, Dalais F, McGrath BP. [The effects of phytoestrogen on blood pressure and lipids in healthy volunteers] Zhonghua Xin Xue Guan Bing Za Zhi. 2006;34(8):726–729. [PubMed] [Google Scholar]
- 53.Bloedon LT, Jeffcoat AR, Lopaczynski W, Schell MJ, Black TM, Dix KJ, et al. Safety and pharmacokinetics of purified soy isoflavones: single-dose administration to postmenopausal women. Am J Clin Nutr. 2002;76(5):1126–1137. doi: 10.1093/ajcn/76.5.1126. [DOI] [PubMed] [Google Scholar]
- 54.Rivas M, Garay RP, Escanero JF, Cia P, Jr, Cia P, Alda JO. Soy milk lowers blood pressure in men and women with mild to moderate essential hypertension. J Nutr. 2002;132(7):1900–1902. doi: 10.1093/jn/132.7.1900. [DOI] [PubMed] [Google Scholar]
- 55.Mahn K, Borras C, Knock GA, Taylor P, Khan IY, Sugden D, et al. Dietary soy isoflavone induced increases in antioxidant and eNOS gene expression lead to improved endothelial function and reduced blood pressure in vivo. FASEB J. 2005;19(12):1755–1757. doi: 10.1096/fj.05-4008fje. [DOI] [PubMed] [Google Scholar]
- 56.Li HF, Wang LD, Qu SY. Phytoestrogen genistein decreases contractile response of aortic artery in vitro and arterial blood pressure in vivo. Acta Pharmacol Sin. 2004;25(3):313–318. [PubMed] [Google Scholar]
- 57.Wu J, Oka J, Tabata I, Higuchi M, Toda T, Fuku N, et al. Effects of isoflavone and exercise on BMD and fat mass in postmenopausal Japanese women: a 1-year randomized placebo-controlled trial. J Bone Miner Res. 2006;21(5):780–789. doi: 10.1359/jbmr.060208. [DOI] [PubMed] [Google Scholar]
- 58.Wu J, Oka J, Higuchi M, Tabata I, Toda T, Fujioka M, et al. Cooperative effects of isoflavones and exercise on bone and lipid metabolism in postmenopausal Japanese women: a randomized placebo-controlled trial. Metabolism. 2006;55(4):423–433. doi: 10.1016/j.metabol.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 59.Chanawirat A, Khemapech S, Patumraj S, Siriviriyakul P. Genistein replacement therapy on endothelial dysfunction and bone loss in bilateral ovariectomized rats. Clin Hemorheol Microcirc. 2006;34(1–2):309–314. [PubMed] [Google Scholar]
- 60.Casini ML, Marelli G, Papaleo E, Ferrari A, D’Ambrosio F, Unfer V. Psychological assessment of the effects of treatment with phytoestrogens on postmenopausal women: a randomized, double-blind, crossover, placebo-controlled study. Fertil Steril. 2006;85(4):972–978. doi: 10.1016/j.fertnstert.2005.09.048. [DOI] [PubMed] [Google Scholar]
- 61.Aubertin-Leheudre M, Lord C, Khalil A, Dionne IJ. Effect of 6 months of exercise and isoflavone supplementation on clinical cardiovascular risk factors in obese postmenopausal women: a randomized, double-blind study. Menopause. 2007;14(4):624–629. doi: 10.1097/gme.0b013e31802e426b. [DOI] [PubMed] [Google Scholar]
- 62.Wyss JM, Liumsiricharoen M, SRIPAIROJTHIKOON W, Brown D, Gist R, Oparil S. Diets high in chloride but moderate in sodium exacerbate hypertension in NaCl sensitive SHR. Hypertension. 1987;9(III):171–175. doi: 10.1161/01.hyp.9.6_pt_2.iii171. [DOI] [PubMed] [Google Scholar]
- 63.Calhoun DA, Zhu S, Wyss JM, Oparil S. Diurnal blood pressure variation and dietary NaCl in spontaneously hypertensive rats. Hypertension. 1994;24:1–8. doi: 10.1161/01.hyp.24.1.1. [DOI] [PubMed] [Google Scholar]
- 64.Williams JK, Young DK, Adams MR, Chen M-F, Myers AK, Ramwell PW. Effects of estrogen on cardiovascular responses of premenopausal monkeys. The Journal of Pharmacology and Experimental Therapeutics. 1994;271:671–676. [PubMed] [Google Scholar]
- 65.Fisher ND, Ferri C, Bellini C, Santucci A, Gleason R, Williams GH, et al. Age, gender, and non-modulation. A sexual dimorphism in essential hypertension. Hypertension. 1997;29(4):980–985. doi: 10.1161/01.hyp.29.4.980. [DOI] [PubMed] [Google Scholar]
- 66.Clarkson TB, Anthony MS. Phytoestrogens and coronary heart disease. Baillieres Clin Endocrinol Metab. 1998;12(4):589–604. doi: 10.1016/s0950-351x(98)80006-2. [DOI] [PubMed] [Google Scholar]
- 67.Clarkson TB, Anthony MS, Williams JK, Honore EK, Cline JM. The potential of soybean phytoestrogens for postmenopausal hormone replacement therapy. Proc Soc Exp Biol Med. 1998;217(3):365–368. doi: 10.3181/00379727-217-44246. [DOI] [PubMed] [Google Scholar]
- 68.Anthony MS, Clarkson TB, Bullock BC, Wagner JD. Soy protein versus soy phytoestrogens in the prevention of diet-induced coronary artery atherosclerosis of male cynomolgus monkeys. Arterioscler Thromb Vasc Biol. 1997;17(11):2524–2531. doi: 10.1161/01.atv.17.11.2524. [DOI] [PubMed] [Google Scholar]
- 69.Peng N, White CR, Roysommuti S, Wyss JM. Estrogen depletion decreases vasodilation responses in spontaneously hypertensive rats: differing mechanism underlie the effect in aged versus young. FASEB J. 2002;16 [Google Scholar]
- 70.Peng N, Clark JT, Wei CC, Wyss JM. Estrogen depletion increases blood pressure nd hypothalamic norepinephrine in middle-aged spontaneously hypertensive rats. Hypertension. 2003;41(5):1164–1167. doi: 10.1161/01.HYP.0000065387.09043.2E. [DOI] [PubMed] [Google Scholar]
- 71.Cho TM, Peng N, Clark JT, Novak L, Roysommuti S, Prasain J, et al. Genistein attenuates the hypertensive effects of dietary NaCl in hypertensive male rats. Endocrinology. 2007 doi: 10.1210/en.2007-0245. [DOI] [PubMed] [Google Scholar]
- 72.Peng N, Ma W, White CR, Wyss JM. Phytoestrogens Preserve Vascular Contraction Response in Spontaneously Hypertensive Rats (SHR) on a high NaCl diet. Alternative Therapies in Health and Medicine. 2001;7:107–108. [Google Scholar]
- 73.Manach C, Scalbert A, Morand C, Remesy C, Jimenez L. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79(5):727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 74.Rush JW, Quadrilatero J, Levy AS, Ford RJ. Chronic resveratrol enhances endothelium-dependent relaxation but does not alter eNOS levels in aorta of spontaneously hypertensive rats. Exp Biol Med (Maywood) 2007;232(6):814–822. [PubMed] [Google Scholar]
- 75.Klatsky AL. Alcohol, cardiovascular diseases and diabetes mellitus. Pharmacol Res. 2007;55(3):237–247. doi: 10.1016/j.phrs.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 76.Fantinelli JC, Mosca SM. Cardioprotective effects of a non-alcoholic extract of red wine during ischaemia and reperfusion in spontaneously hypertensive rats. Clin Exp Pharmacol Physiol. 2007;34(3):166–169. doi: 10.1111/j.1440-1681.2007.04567.x. [DOI] [PubMed] [Google Scholar]
- 77.Karatzi KN, Papamichael CM, Karatzis EN, Papaioannou TG, Aznaouridis KA, Katsichti PP, et al. Red wine acutely induces favorable effects on wave reflections and central pressures in coronary artery disease patients. Am J Hypertens. 2005;18(9 Pt 1):1161–1167. doi: 10.1016/j.amjhyper.2005.03.744. [DOI] [PubMed] [Google Scholar]
- 78.Pitsavos C, Makrilakis K, Panagiotakos DB, Chrysohoou C, Ioannidis I, Dimosthenopoulos C, et al. The J-shape effect of alcohol intake on the risk of developing acute coronary syndromes in diabetic subjects: the CARDIO2000 II Study. Diabet Med. 2005;22(3):243–248. doi: 10.1111/j.1464-5491.2004.01384.x. [DOI] [PubMed] [Google Scholar]
- 79.Peng N, Clark JT, Prasain J, Kim H, White CR, Wyss JM. Antihypertensive and cognitive effects of grape polyphenols in estrogen-depleted, female, spontaneously hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2005;289(3):R771–R775. doi: 10.1152/ajpregu.00147.2005. [DOI] [PubMed] [Google Scholar]
- 80.Wyss JM, Kadish I, van Groen T. Age-related decline in spatial learning and memory: Attenuation by captopril. Clinical and Experimental Hypertension. 2003;25(7):455–474. doi: 10.1081/ceh-120024988. [DOI] [PubMed] [Google Scholar]
- 81.Keung WM, Vallee BL. Kudzu root: an ancient Chinese source of modern antidipsotropic agents. Phytochemistry. 1998;47(4):499–506. doi: 10.1016/s0031-9422(97)00723-1. [DOI] [PubMed] [Google Scholar]
- 82.Fang C, Wan X, Tan H, Jiang C. Separation and determination of isoflavonoids in several kudzu samples by high-performance capillary electrophoresis (HPCE) Ann Chim. 2006;96(1–2):117–124. doi: 10.1002/adic.200690002. [DOI] [PubMed] [Google Scholar]
- 83.Zhang Y, Zeng X, Zhang L, Zheng X. Stimulatory effect of puerarin on bone formation through activation of PI3K/Akt pathway in rat calvaria osteoblasts. Planta Med. 2007;73(4):341–347. doi: 10.1055/s-2007-967168. [DOI] [PubMed] [Google Scholar]
- 84.Kang KA, Lee KH, Kim SY, Kim HS, Kim JS, Hyun JW. Cytoprotective effects of KIOM-79 on streptozotocin induced cell damage by inhibiting ERK and AP-1. Biol Pharm Bull. 2007;30(5):852–858. doi: 10.1248/bpb.30.852. [DOI] [PubMed] [Google Scholar]
- 85.Chen HT, Yao CH, Chao PD, Hou YC, Chiang HM, Hsieh CC, et al. Effect of serum metabolites of Pueraria lobata in rats on peripheral nerve regeneration: In vitro and in vivo studies. J Biomed Mater Res B Appl Biomater. 2007 doi: 10.1002/jbm.b.30868. [DOI] [PubMed] [Google Scholar]
- 86.Sun XH, Ding JP, Li H, Pan N, Gan L, Yang XL, et al. Activation of large-conductance calcium-activated potassium channels by puerarin: the underlying mechanism of puerarin-mediated vasodilation. J Pharmacol Exp Ther. 2007;323(1):391–397. doi: 10.1124/jpet.107.125567. [DOI] [PubMed] [Google Scholar]
- 87.Yeung DK, Leung SW, Xu YC, Vanhoutte PM, Man RY. Puerarin, an isoflavonoid derived from Radix puerariae, potentiates endothelium-independent relaxation via the cyclic AMP pathway in porcine coronary artery. Eur J Pharmacol. 2006;552(1–3):105–111. doi: 10.1016/j.ejphar.2006.08.078. [DOI] [PubMed] [Google Scholar]
- 88.Yan LP, Chan SW, Chan AS, Chen SL, Ma XJ, Xu HX. Puerarin decreases serum total cholesterol and enhances thoracic aorta endothelial nitric oxide synthase expression in diet-induced hypercholesterolemic rats. Life Sci. 2006;79(4):324–330. doi: 10.1016/j.lfs.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 89.Guan L, Yeung SY, Huang Y, Chen ZY. Both soybean and kudzu phytoestrogens modify favorably the blood lipoprotein profile in ovariectomized and castrated hamsters. J Agric Food Chem. 2006;54(13):4907–4912. doi: 10.1021/jf060709a. [DOI] [PubMed] [Google Scholar]
- 90.Hsu FL, Liu IM, Kuo DH, Chen WC, Su HC, Cheng JT. Antihyperglycemic effect of puerarin in streptozotocin-induced diabetic rats. J Nat Prod. 2003;66(6):788–792. doi: 10.1021/np0203887. [DOI] [PubMed] [Google Scholar]
- 91.Meezan E, Meezan EM, Jones K, Moore R, Barnes S, Prasain JK. Contrasting effects of puerarin and daidzin on glucose homeostasis in mice. J Agric Food Chem. 2005;53(22):8760–8767. doi: 10.1021/jf058105e. [DOI] [PubMed] [Google Scholar]
- 92.Xu XH, Zhao TQ. Effects of puerarin on D-galactose-induced memory deficits in mice. Acta Pharmacol Sin. 2002;23(7):587–590. [PubMed] [Google Scholar]
- 93.Xu X, Zhang Z. Effects of puerarin on synaptic structural modification in hippocampus of ovariectomized mice. Planta Med. 2007;73(10):1047–1053. doi: 10.1055/s-2007-981564. [DOI] [PubMed] [Google Scholar]