Abstract
Increased protein synthesis is regulated, in part, by two eukaryotic translation initiation factors (eIFs): eIF4E and eIF2α. One or both of these factors are often overexpressed in several types of cancer cells; however, no data are available at present regarding eIF4E and eIF2α levels in brain tumors. In this study, we analyzed the expression, subcellular localization and phosphorylation states of eIF4E and eIF2α in 64 brain tumors (26 meningiomas, 16 oligodendroglial tumors, and 22 astrocytomas) and investigated the correlation with the expression of MIB-1, p53, and cyclin D1 proteins as well. There are significant differences in the phosphorylated eIF4E levels between the tumors studied, being the highest in meningiomas and the lowest in the oligodendroglial tumors. Relative to subcellular localization, eIF4E is frequently found in the nucleus of the oligodendroglial tumors and rarely in the same compartment of the meningiomas, whereas eIF2α showed an inverse pattern. Finally, cyclin D1 levels directly correlate with the phosphorylation status of both factors. The different expression, phosphorylation, or/and subcellular distribution of eIF2α and eIF4E within the brain types of tumors studied could indicate that different pathways are activated for promoting cell cycle proliferation, for instance, leading to increased cyclin D1 expression. (J Histochem Cytochem 57:503–512, 2009)
Keywords: eukaryotic translation initiation factor 2α, eukaryotic translation initiation factor 4E, cyclin D1, brain tumors, translation
Increased protein synthesis is necessary for the transition of cells from quiescence to proliferation. Most translational control occurs at the initiation step, which is usually the rate-limiting step in translation, and involves the reversible phosphorylation of key initiation factors, such as the eukaryotic initiation factors (eIFs) 2 and 4E (Dever 2002; Gebauer and Hentze 2004). Changes in the rates of two stages in the initiation of protein synthesis are believed to be involved in cell transformation: (a) the binding of GTP to eIF2, which is a prerequisite for assembly of the 43S initiation complex, and (b) the association of the cap-binding factor eIF4E with the large scaffold protein eIF4G, which is necessary for the subsequent recruitment of mRNA to the ribosome. Moreover, the translation of individual mRNA species, which differ in structure, can also be differentially regulated at these two levels (Clemens and Bommer 1999; Willis 1999; Dever 2002; Gebauer and Hentze 2004).
The possibility that eIF2- and eIF4E-dependent stages in protein synthesis are important in the regulation of cell growth or cell death is supported by the fact that one or both of these initiation factors are often overexpressed in tumors such as bronchio-alveolar carcinoma of the lung (Rosenwald et al. 2001), thyroid carcinoma (Wang et al. 2001), gastrointestinal carcinoma (Lobo et al. 2000), and non-Hodgkin's lymphoma (Wang et al. 1999), as well as in several other types of cancer cells (De Benedetti and Graff 2004). Overexpression and/or increased phosphorylation of eIF4E, now considered to be a proto-oncogene, leads to overexpression of certain proto-oncogenes, growth factors, and other cell cycle–related protein transcripts, such as nuclear factor of activated T cells, ornithine decarboxylase, c-myc, ras, basic fibroblast growth factor, vascular permeability factor, and cyclin D1 (De Benedetti and Graff 2004). Upregulation of the α subunit (38 kDa) of eIF2 (eIF2α) expression correlates with neoplastic transformation of mammary epithelial cells (Raught et al. 1996). Moreover, inhibition of the eIF2α kinase PKR transforms cells to a malignant phenotype (Koromilas et al. 1992), although paradoxically, the expression and activity of this kinase is often increased in gastrointestinal neoplasms and melanomas (Kim et al. 2002). These previous studies have mainly focused on the role of these factors and their overexpression in the mechanisms of cell transformation in vitro, and the phosphorylation patterns and subcellular distributions of these factors have rarely been evaluated in cancer tissues (Lobo et al. 2000; Martin et al. 2000b).
To date, no studies have addressed the role of eIF2α and eIF4E in brain tumors. Herein, we evaluated the expression, subcellular localization, and phosphorylation states of eIF2α and eIF4E in brain tumors. Moreover, we carried out IHC studies to determine the expression of MIB-1 (as a proliferate marker), p53, and cyclin D1 proteins. In addition, attempts were made to determine the potential correlation between the expression of these markers and the eIFs.
Materials and Methods
Patients
We analyzed 73 brain tumors from patients operated in the Department of Neurosurgery from January 2004 to January 2006. The tumors included 22 astrocytic tumors, 16 oligodendroglial tumors, 26 meningiomas, 1 ganglioglioma, 2 neurinoma, 1 hypophysis adenoma, and 5 metastatic tumors classified according to the WHO classification (Louis et al. 2007). Because of the small number of some subgroups, only the three major groups were used in the study (astrocytic tumors, oligodendroglial tumors, and meningiomas). The age of patients at the time of the surgery ranged from 19 to 82 years (mean age, 52 years; Table 1). The samples were retrieved from the Department of Pathology (Hospital Ramón y Cajal) after surgical resection. Part of a tumor sample, obtained immediately after surgical resection, was frozen in isopentane cooled in liquid nitrogen for subsequent Western blot studies, and the adjacent tissue was formalin fixed and embedded in paraffin for IHC studies. Moreover, normal brain tissue from three autopsies performed on adult patients without central nervous system (CNS) disease was used as controls for IHC studies. Relative to the Western blot studies, we used two normal (non-tumoral) samples from tissue obtained immediately after surgical resection and processed as described for the tumor samples.
Table 1.
Clinical pathological data of 64 patients
| Tumor type | Total no. | Mean age (years) (mean ± SD) | Male:female ratio | WHO grading (no.) |
|---|---|---|---|---|
| Astrocytic tumors | 22 | 48.5 ± 18.1 | 13:9 | Diffuse astrocytoma (6) |
| Anaplastic astrocytoma (1) | ||||
| Glioblastoma (15) | ||||
| Oligodendroglial tumors | 16 | 47.3 ± 12.1 | 11:5 | Oligodendroglioma (11) |
| Anaplastic oligodendroglioma (1) | ||||
| Oligoastrocytoma (2) | ||||
| Anaplastic oligoastrocytoma (2) | ||||
| Meningioma | 26 | 57.5 ± 13.0 | 12:14 | Benign (19) |
| Atypical (6) | ||||
| Malignant (1) |
Antibodies
Monoclonal eIF4E was obtained from BD Biosciences (Plymouth, UK), and monoclonal anti-eIF2α was generously provided by the late Dr. E.C. Henshaw (Cancer Center, University of Rochester, Rochester, NY). Goat anti-eIF2α was purchased from Santa Cruz Biotechnology (Santa Cruz, CA), monoclonal anti-β-actin was purchased from Sigma (St. Louis, MO), and monoclonal cyclin D1, MIB-1, and p53 antibodies were purchased from Dako (Glostrup, Denmark).
Sample Preparation for Western Blot and Isoelectric Focusing (IEF) Analysis
Tissue samples were thawed, weighed, and homogenized in a microcentrifuge tube with a Pellet Pestle (Kontes; Vineland, NJ) in 1:4 (w:v) 20 mM Tris-HCl (pH 7.6), 1 mM DTT, 1 mM EDTA, 1 mM PMSF, 1 mM benzamidine, 2 mM sodium molybdate, 2 mM sodium β-glycerophosphate, 0.2 mM sodium orthovanadate, 120 mM KCl, 1 μg/ml leupeptin and pepstatin A, 10 μg/ml antipain, 0.5% NP-40, and 0.1% Triton X-100. Cell lysates were centrifuged at 12,000 × g for 10 min, and the supernatants were kept at −80C until used. Protein determination was performed by the Bradford method (Bradford 1976). To determine the eIF4E and eIF2α phosphorylation states, lysates were adjusted to 75 μg of protein per sample, resolved in horizontal isoelectric focusing slab gels, and analyzed by protein immunoblotting as described previously (Martin et al. 2000a). Proteins were stained on immunoblots that were treated with monoclonal anti-eIF4E and anti-eIF2α. To determine the levels of eIF4E and eIF2α, cell lysates (75 μg of protein) were resolved by 15% SDS-PAGE and analyzed using monoclonal anti-eIF4E or anti-eIF2α antibodies, and β-actin levels were used as a control to monitor homogeneity of loading. Three different Western blot analyses were carried out per sample. Stained bands were scanned and quantified with an analyzer equipped with the ImageQuant software package (GE Healthcare; Buckinghamshire, UK).
IHC
IHC was performed as previously described (Lobo et al. 2004). Tissue samples were sectioned with a thickness of 3 μm and dried for 16 hr at 56C before being dewaxed in xylene, rehydrated by a graded ethanol series, and washed with PBS. Antigen retrieval was achieved by heat treatment in a pressure cooker for 2 min in 10 mM citrate buffer (pH 6.5). Before staining the sections, endogenous peroxidase was blocked. IHC staining was performed on the sections using different antibodies: the anti-Ki-67 antibody (MIB-1), p53, cyclin D1, monoclonal anti-eIF4E, and goat anti-eIF2α. After incubation, immunodetection was performed using the labeled streptavidin–biotin (LSAB) visualization system (Dako) using DAB chromogen as substrate. Sections were counterstained with hematoxylin.
Nuclear staining of MIB-1, p53, and cyclin D1 was analyzed using the Computerized Analyzer System (Cas 200; BD Biosciences). To allow comparisons between results, the boundary optical density at the antibody threshold was adjusted for each sample examined. For each sample, areas with the highest density of stained nuclei were selected. Inflammatory cells, vascular components, and necrotic areas were excluded. All positive tumor cell nuclei were automatically counted. The labeling index (LI) for each sample was determined by counting at least 1000 tumor cell nuclei. Labeling of eIF4E and eIF2α was evaluated by three investigators (MEM, STS, and MVTL) using uniform criteria, and cytoplasmic and nuclear staining was scored separately. The pattern of staining was recorded as 0 when there was no detectable labeling, and values of 1, 2, and 3 were used to indicate weak, moderate, and intense labeling, respectively.
Statistics
The results are expressed as mean values ± SD unless stated otherwise. Most of the samples were analyzed in triplicate. Non-parametric testing was used for those parameters with abnormal distributions as suggested by Kolmogorov–Smirnov normality tests. For each parameter determined, the statistical significance between the different tumor types was performed by ANOVA, followed by the Tukey test or Kruskal-Wallis followed by Dunn's multiple comparison test. Comparisons of values between two groups were analyzed with the Mann-Whitney U test. Spearman's correlation coefficients (r) were calculated. Significance was assumed at p<0.05.
Results
eIF2α and eIF4E Levels
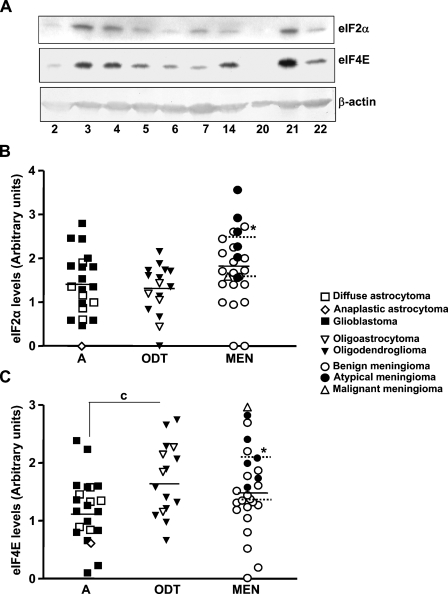
The levels of the initiation factors eIF2α and eIF4E were analyzed by SDS-PAGE and Western blot analysis. As shown in Figure 1, no differences were found in eIF2α levels between the three types of tumors studied, astrocytic tumors (A), oligodendroglial tumors (ODT), and meningiomas (MEN), or those found in the two normal control samples (1.13 ± 0.27 AU). Moreover, we compared eIF2α levels between the different grades of the tumors in the astrocytic tumors (diffuse astrocytoma vs glioblastoma) and the meningiomas (atypical vs benign). The results obtained showed that eIF2α levels were significantly higher in atypical than in benign meningiomas (p=0.0239), whereas no differences were found between the two astrocytomas grades that were compared. When eIF4E levels were analyzed, meningiomas and oligodendroglial tumors showed higher levels than astrocytomas, reaching significance between ODT and A types. eIF4E levels in normal samples (0.48 ± 0.07AU) were significantly lower compared with the total average of the all tumor types studied (1.45 ± 0.67 AU). Again, atypical meningiomas had higher levels of the factor than benign tumors (p=0.0069). No differences between astrocytoma Grades IV and II were found within these two factors.
Figure 1.
Eukaryotic translation initiation factors (eIF)2α and eIF4E levels. (A) Western blot analysis of eIF2α and eIF4E in tumor samples. The amount of actin was used as a control for the homogeneity of loading. Numbers at bottom of figure indicate sample number: A (samples 2, 5, 22), ODT (sample 14), and MEN (samples 3, 4, 20, and 21). Although two neurinomas (samples 6 and 7) are included in the Western blot analysis, they were not further studied. Scatter plots represent the quantification of eIF2α (B) and eIF4E (C) levels normalized with respect to actin levels and expressed in arbitrary units. A single point represents one sample analyzed. Horizontal bar indicates the average in each tumor type, and dotted horizontal bar represents the average expression in WHO Grade I and II meningiomas. Statistical differences between the different tumor types (ANOVA followed by Tukey's multiple comparison test): cp<0.05. Statistical differences between benign and atypical meningiomas (Mann-Whitney U test): *p<0.05 and **p<0.01. A, astrocytic tumors; ODT, oligodendroglial tumors; MEN, meningiomas.
eIF2α and eIF4E Subcellular Expression Patterns
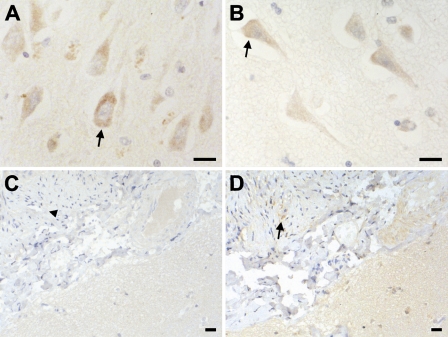
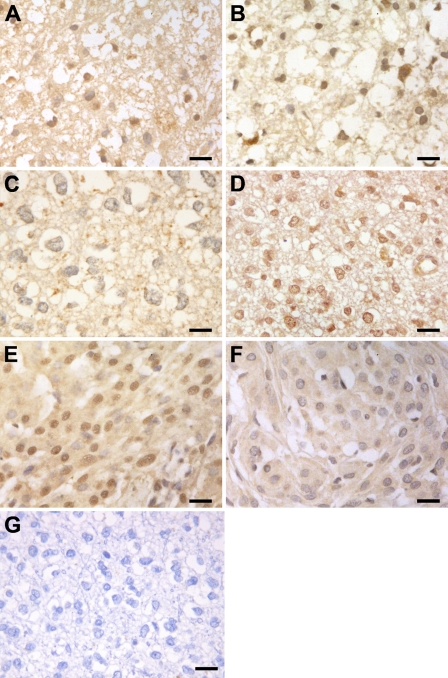
Localization of eIF2α and eIF4E was studied by IHC and evaluated as described in Materials and Methods; the cytoplasm and the nucleus were scored separately (Table 2). Human cerebral brain tissue obtained from autopsies of patients without CNS disease was used as a control. In normal tissue, most of the cells did not shown eIF2α or eIF4E immunoreactivity, with the exception of a prominent staining of both factors in the cytoplasm of pyramidal neurons in the cortex and the hippocampus (Figures 2A and 2B, arrows). Zones of non-tumoral tissue (meninges with reactive changes and normal meninges) showed a very low staining for eIF2α (Figure 2C, arrowheads), whereas eIF4E only showed focal staining in some zones (Figure 2D, arrows). We found notable differences in the eIF2α and eIF4E subcellular distribution among the three types of tumors (Table 2; Figure 3). In fact, although cytoplasmic eIF2α showed a similar expression pattern than eIF4E in all tumors studied, the nuclear localization of eIF2α and eIF4E was different depending on the type of tumor (Table 2; Figure 3). Nuclear eIF2α immunostaining was very low in the oligodendroglial tumors (Table 2; Figures 3A and 3C), and only 19% (3 of 16) of the oligodendroglial tumors showed a strong nuclear immunostaining (2/3 value). A similar nuclear eIF2α staining was found in meningiomas and astrocytic tumors within this compartment (Table 2). Regarding eIF4E, meningiomas presented the lowest nuclear eIF4E staining (Table 2; Figure 3F), and only 11.5% (3 of 26) of the meningiomas evaluated showed a strong eIF4E nuclear localization (2/3 value). On the contrary, nuclear eIF4E immunostaining was very high in the oligodendroglial tumors evaluated (Table 2), being predominantly nuclear in 69% (11 of 16) of the cases (Figures 3B and 3D).
Table 2.
eIF2α and eIF4E expression in cytoplasm and nucleus of control and tumor samples
| Labeling index (mean ± SD)a
|
||||
|---|---|---|---|---|
| eIF2α levels
|
eIF4E levels
|
|||
| Tumor type (no. total of patients) | Cytoplasm | Nucleus | Cytoplasm | Nucleus |
| C(3) | 1 ± 0 | 0 ± 0 | 1 ± 0 | 0 ± 0 |
| A (22) | 1.63 ± 0.73 | 1.18 ± 1.05 | 1.86 ± 0.77 | 1.68 ± 1.04 |
| ODT (16) | 1.60 ± 0.74 | 0.68 ± 0.79 | 1.64 ± 0.63 | 2.43 ± 0.75 |
| MEN (26) | 1.35 ± 0.74 | 1.31 ± 1.05 | 1.69 ± 0.97 | 0.73 ± 0.78 |
Scale = 0–3 immunoreactivity.
eIF2α, eukaryotic translation initiation factor 2α; eIF4E, eukaryotic translation initiation factor 4E; C, control; A, astrocytic tumors; ODT, oligodendroglial tumors; MEN, meningiomas.
Figure 2.
IHC staining of eIF4E and eIF2α in normal human brain and reactive meninges adjacent tumoral tissue. IHC was performed as described in Materials and Methods. Expression of eIF2α (A) and eIF4E (B) in the human brain obtained from patients without central nervous system diseases showed pale staining with the exception of pyramidal neurons of hippocampus and cortical neurons in the neocortex (arrows). Very low staining for eIF2α (C, arrowhead) and focal staining for eIF4E (D, arrow) was observed in zones of non-tumoral meninges. Bar = 50 μm.
Figure 3.
IHC staining of eIF2α and eIF4E in brain tumors. IHC was performed as described in Materials and Methods. IHC on serial sections of astrocytic tumors (A,B; sample 29), oligodendroglioma (C,D; sample 62), and meningioma (E,F; sample 19) using eIF2α (A,C,E) and eIF4E (B,D,F) antibodies. No specific staining was present when the primary antibodies were omitted (G). Bar = 50 μm.
eIF2α and eIF4E Phosphorylation Status
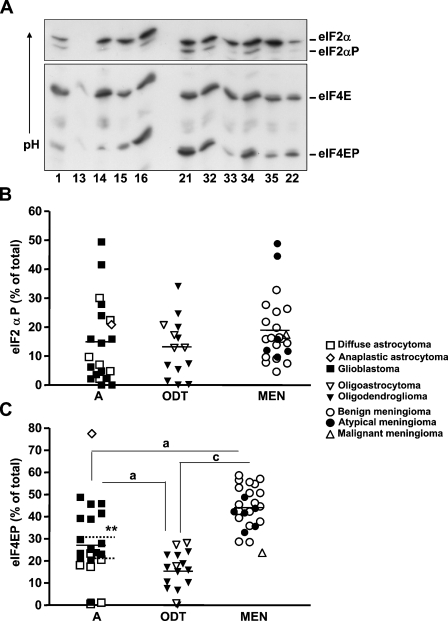
Next, we analyzed the eIF2α and eF4E phosphorylation status by IEF and Western blot (Figure 4). No differences were found in phosphorylated eIF2α (eIF2αP) between the three tumors types; the total average (15.7 ± 12.1%) was similar to that obtained in two normal brain samples (16.2 ± 2.3%). In contrast, phosphorylated eIF4E (eIF4EP) levels changed significantly among the different types of tumors studied, being higher in meningiomas than in astrocytic and oligodendroglial tumors; the latter had eIF4EP levels similar to normal brain samples (13.24 ± 10.26%). In astrocytic tumors, eIF4EP levels were significantly higher in glioblastoma than in diffuse astrocytoma (p=0.0057; Figure 4C).
Figure 4.
eIF2α and eIF4E phosphorylation status in brain tumors. (A) Samples were subjected to isoelectric focusing electrophoresis, and bands corresponding to non-phosphorylated eIF2α and eIF4E and phosphorylated eIF2α and eIF4E (eIF2αP and eIF4EP) were analyzed by Western blot analysis as described in Materials and Methods. Numbers at bottom of figure indicate number of the sample: A (samples 1, 13, 15, 22, and 35), ODT (samples 14 and 33), and MEN (samples 16, 21, 32, and 34). Scatter plots represent the quantification of eIF2αP (B) and eIF4EP (C) levels and are expressed as the percentage of the total. A single point represents one sample analyzed. Horizontal bar indicates the average in each tumor type, and dotted horizontal bar represents the average in astrocytoma diffuse and glioblastoma. Statistical differences between the different tumor types (ANOVA followed by Tukey's multiple comparison test): ap<0.001; cp<0.05. Statistical differences between diffuse astrocytoma and glioblastoma (Mann-Whitney U test); **p<0.01. A, astrocytic tumors; ODT, oligodendroglial tumors; MEN, meningiomas.
Analysis of Proliferative Activity by Ki-67 Expression
The proliferative activity in tumors is generally evaluated by the Ki-67/MIB-1 LI. The MIB-1 antibody that recognizes the Ki-67 antigen in paraffin-embedded tissue sections was used in this study as a marker of proliferation. In brain tumors, MIB-1 can be used as additional histological support to grade these tumors (Bouvier-Labit et al. 1998; Lanzafame et al. 2000). Accordingly, MIB-1 LI showed significant differences between Grade I and Grade II meningiomas (3.18 ± 2.31 vs 7.21 ± 3.84, p=0.025) and between Grade II and IV astrocytomas (4.41 ± 4.44 vs 22.42 ± 13.63, p=0.033).
It has been established that wild-type p53 protein, because of its short half-life, is not detectable by IHC methods. However, positive p53 immunoreactivity may indicate stabilization of the wild-type protein through the deregulation of the p14ARF–HDM2–p53 pathway. As shown in Table 3, p53 LI was significantly higher in astrocytomas than in ODT (p<0.05) and meningiomas (p<0.001). Moreover, the protein p53 LI positively correlated with the MIB-1 LI in our series (Spearman coefficient, r = 0.527; p=0.000001).
Table 3.
p53 and cyclin D1 expression in brain tumors
| Labeling index (mean ± SD)
|
||
|---|---|---|
| Tumor type (no. total of patients) | p53 | Cyclin D1 |
| A (22) | 14.21 ± 16.02 | 3.4 ± 6.95 |
| ODT (16) | 3.51 ± 5.03 | 2.72 ± 4.47 |
| MEN (26) | 1.47 ± 3.00 | 9.3 ± 14.54 |
A, astrocytic tumors; ODT, oligodendroglial tumors; MEN, meningiomas.
Relative to cyclin D1, the staining of tumor cells showed that this protein was predominantly localized to the nucleus, although a few cases (10%) showed faint diffuse cytoplasmic staining (it was not included in the counting). As shown in Table 3, meningiomas showed the highest cyclin D1 expression. Moreover, the cyclin D1 LI was higher in glioblastomas than in diffuse astrocytomas (4.35 ± 7.93 vs 0.92 ± 2.05) and was higher in atypical than in benign meningiomas (12.60 ± 15.08 vs 3.97 ± 7.87), but the differences were not statistically significant.
MIB-1, p53, and Cyclin D1 Correlation With Initiation Factor Levels and Phosphorylation Status
Finally, we analyzed the correlation between the expression and/or phosphorylation status of eIF2α and eIF4E and the expression of MIB-1, p53, and cyclin D1 using Spearman correlation coefficients. As shown in Table 4, MIB-1 inversely correlated with eIF4EP. Certainly, this result was surprising because a positive association between eIF4EP and MIB-1 would be expected. This result, however, is probably due to the higher levels of eIF4EP in MEN, which could be a consequence of higher levels within the tissues of origin. In fact, the inverse correlation disappears when each of the different tumor types is analyzed separately. Relative to cyclin D1, a straight and significant correlation between this protein, cyclin D1, and eIF2αP or eIF4EP levels was found. When the different tumor types were analyzed separately, cyclin D1 strongly correlated with both eIF2α and phosphorylated eIF2α levels in the meningiomas (r = 0.419, p=0.033 and r = 0.559, p=0.004, respectively), whereas a significant correlation between phosphorylated eIF4E and cyclin D1 was only found in astrocytic tumors (r = 0.483, p=0.023).
Table 4.
Correlation between initiation factor phosphorylation state and levels of MIB-1, p53, and cyclin D1 expression
| eIF2α (n=64) | eIF2αP (n=59) | eIF4E (n=64) | eIF4EP (n=63) | |
|---|---|---|---|---|
| MIB-1 | ||||
| Spearman coefficient (r) | 0.126 | −0.021 | 0.054 | −0.265a |
| Significance (p) | 0.320 | 0.872 | 0.673 | 0.036 |
| p53 | ||||
| Spearman coefficient (r) | −0.058 | 0.001 | −0.112 | −0.142 |
| Significance (p) | 0.650 | 0.993 | 0.380 | 0.266 |
| Cyclin D1 | ||||
| Spearman coefficient (r) | 0.089 | 0.342b | 0.110 | 0.337b |
| Significance (p) | 0.486 | 0.008 | 0.387 | 0.007 |
p<0.05.
p<0.01.
eIF2α, eukaryotic translation initiation factor 2α; eIF2αP, phosphorylated eukaryotic translation initiation factor 2α; eIF4E, eukaryotic translation initiation factor 4E;. eIF4EP, phosphorylated eukaryotic translation initiation factor 4E.
Discussion
One or both eIF2α and eIF4E proteins are often overexpressed in tumors such as those of the breast, colon, lung, and thyroid, as well as in several other types of cancer cells (reviewed in Rosenwald 2004; Schneider and Sonenberg 2007). However, no data are available at present regarding eIF2α and eIF4E levels in brain tumors, with the exception of an IHC study in 10 biopsies from patients with astrocytic tumors (Gu et al. 2005). In this study, we analyzed the expression, localization, and phosphorylation states of both eIF2α and eIF4E in 64 brain tumors from three different tumor types, astrocytic and oligodendroglial tumors, and meningiomas using IHC studies and Western blot analysis. For this purpose, we only included in this study those tissue samples obtained fresh immediately after surgical resection (necessary for Western blot studies). It is relevant to point out that the levels of the translational machinery in the different types of solid tumors may differ depending on the tissue origins. Moreover, the mechanisms of translation regulation in tumors may differ according to the tissue of origin and the stage of transformation (Schneider and Sonenberg 2007). Herein, although no outstanding differences were found in the expression of eIF2α or eIF4E levels within the three tumor types, we showed an increased eIF4E and eIF2α expression in more aggressive meningioma tumors (atypical vs benign), indicating that these factors may contribute to tumor progression and therefore to less favorable clinical outcomes.
The low expression of eIF2α and eIF4E detected in normal human brain and non-tumoral meninges by IHC studies is in agreement with those of Gu et al. (2005) for eIF4E expression in astrocytic tumors. It should be noted that, according to both this study and our results with human brain tissue from patients without CNS disease, the positive staining of eIF4E and eIF2α was observed only in the cytoplasm of neurons, whereas we found that eIF4E and eIF2α could be present in the nucleus and in the cytoplasm of tumoral cells in brain tumors studied. Moreover, in these tumors, the nuclear localization of both factors differs between tumors of neuroepithelial tissue and of the meninges. Thus, the presence of eIF4E in the nucleus is more common in astrocytic and oligodendroglial tumors than in meningiomas, whereas in these tumors, eIF2α was present in the nucleus in a high proportion. The role of these factors in the nucleus remains to be established, but Borden's laboratory has shown that, in the nucleus, eIF4E upregulates the mRNA export of a substantial subset of growth-promoting mRNAs (reviewed in Culjkovic et al. 2007).
We chose three parameters to study their correlation with the expression, phosphorylation status, and localization of the initiation factors: MIB-1, as a proliferation marker, p53, and cyclin D1. Relative to MIB-1 and p53 expression, our findings strongly supported those previously reported within the same tumor types (Louis et al. 2007; Takei et al. 2007).
Cyclin D1 is a cell cycle regulatory protein that binds cyclin-dependent kinase 4 (Cdk4), promoting the phosphorylation of the retinoblastoma protein that is needed for progression through the G1-S cell cycle checkpoint (Hunter and Pines 1994). Cyclin D1 is well established as an oncogene with an important pathogenetic role in several human tumors (Fu et al. 2004; Arnold and Papanikolaou 2005). Several studies investigating astrocytic tumors showed increased cyclin D1 expression that correlated with histological grade (Chakrabarty et al. 1996; Cavalla et al. 1998; Zhang et al. 2005). In our study, the cyclin D1 LI was higher in glioblastoma than in diffuse astrocytomas, but the differences did not reach statistical significance, probably because of the low number of diffuse astrocytomas within this series. Two recent studies have reported a correlation between cyclin D1 expression, MIB-1 LI, and the grade of meningioma as well (Alama et al. 2007; Milenkovic et al. 2008).
Because eIF2 and eIF4E activity is specifically regulated by changes in their phosphorylation state and/or subcellular localization (Scheper and Proud 2002; Strudwick and Borden 2002; Holcik and Sonenberg 2005), we included these two parameters in our study. Meningiomas were the tumors with higher levels of phosphorylated eIF4E and glioblastomas showed higher levels of eIF4EP than diffuse astrocytomas. In addition, we found that eIF2αP and eIF4EP levels strongly correlated with cyclin D1 expression in the brain tumors studied. When each tumor type was considered separately, the cyclin D1 LI significantly correlated with eIF4EP levels in astrocytic tumors and with eIF2α and eIF2αP levels in meningiomas. These results could suggest the activation of two different pathways in the different types of brain tumors to induce cell cycle proliferation by increasing cyclin D1 expression (a) through eIF4EP, by promoting nucleocytoplasmic export of cyclin D1 mRNA (this pathway is important in astrocytomas), and (b) through high levels of eIF2αP, by increasing the specific translation of cyclin D1 mRNA (this pathway is important in meningiomas). The role of the overexpression of eIF4E in the nuclear export of cyclin D1 mRNA has been broadly established in different models (Rousseau et al. 1996; Culjkovic et al. 2005). In fact, recent studies have shown that certain mRNAs can be organized and exported from the nucleus as functional groups, named regulons, by RNA binding proteins, such as eIF4E (Culjkovic et al. 2007; Keene 2007). Importantly, it has been reported that the phosphorylation of “nuclear” eIF4E seems to be an important step in control of the mRNA transport and thus the transforming properties of eIF4E (Topisirovic et al. 2004). This pathway therefore may play an important role in the dysregulation of HDM2 oncoprotein expression that occurs in many human tumors (Phillips and Blaydes 2008), including brain tumors (Reifenberger et al. 1993; Biernat et al. 1997; Reis et al. 2000; Matsumoto et al. 2004). Finally, the absolute requirement for Ser209 phosphorylation by the eIF4E kinases Mnk1/2 for eIF4E's oncogenic action has been reported (Wendel et al. 2007; Silva and Wendel 2008). It is important to point out that a significant fraction of eIF4E resides in the nucleus of these tumors, where it should be regulating the nuclear export of cyclin D1 mRNA among others.
With respect to eIF2αP, it has been clearly established that eIF2α phosphorylation increases the translation of selected mRNAs containing open reading frames (ORFs) in the 5′ untranslated region (5′UTR). This mechanism, which was first described for the transcription factor GCN4 mRNA in yeast (Dever 2002; Holcik and Sonenberg 2005), has also been found in mammals, for example, to upregulate genes related to endoplasmic reticulum stress such as the activating transcription factor 4 (Harding et al. 2000; Vattem and Wek 2004). Interestingly, cyclin D1 mRNA has two suspected upstream ORFs in the 5′ UTR, raising the possibility of an eIF2αP-mediated cyclin D1 translation.
Finally, we point out that, although desirable, we have not yet been able to obtain correlation between clinical data and our results due to, (a) the limited number of the tumors studied (only those samples obtained immediately after surgical resection in our hospital over a 2-year period of time have been included in this study) and (b) the period of time since the surgery is insufficient to analyze clinico-pathological features such as survival or prognosis outcome. Nevertheless, this is the first study concerning brain tumors, and our findings indicate that expression and phosphorylation status of these initiation factors are involved in cyclin D1expression, a well-established oncogene with an important pathogenetic role in several human tumors. Interestingly, the results obtained with the meningiomas clearly showed that the expression of the initiation factors are increased in Grade II tumors with respect to Grade I tumors; thus, these markers may help in grading tumors in borderline atypical cases. Whether the parameters determined may add new information to what is known clinically deserves further study.
Acknowledgments
This study was supported by Grant FMM06/180 from Fundación Mutua Madrileña (Madrid, Spain). M.E.M. is a researcher from Fundación para la Investigación Biomédica del Hospital Universitario Ramón y Cajal supported by Consejeria de Sanidad y Consumo (CAM).
We are grateful to the technicians of the Pathologic Anatomy Service at the Hospital Ramón y Cajal for their technical assistance and to Dr. Víctor M. Gónzalez for his comments on the manuscript.
References
- Alama A, Barbieri F, Spaziante R, Bruzzo C, Dadati P, Dorcaratto A, Ravetti JL (2007) Significance of cyclin D1 expression in meningiomas: a preliminary study. J Clin Neurosci 14:355–358 [DOI] [PubMed] [Google Scholar]
- Arnold A, Papanikolaou A (2005) Cyclin D1 in breast cancer pathogenesis. J Clin Oncol 23:4215–4224 [DOI] [PubMed] [Google Scholar]
- Biernat W, Kleihues P, Yonekawa Y, Ohgaki H (1997) Amplification and overexpression of MDM2 in primary (de novo) glioblastomas. J Neuropathol Exp Neurol 56:180–185 [DOI] [PubMed] [Google Scholar]
- Bouvier-Labit C, Chinot O, Ochi C, Gambarelli D, Dufour H, Figarella-Branger D (1998) Prognostic significance of Ki67, p53 and epidermal growth factor receptor immunostaining in human glioblastomas. Neuropathol Appl Neurobiol 24:381–388 [DOI] [PubMed] [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254 [DOI] [PubMed] [Google Scholar]
- Cavalla P, Dutto A, Piva R, Richiardi P, Grosso R, Schiffer D (1998) Cyclin D1 expression in gliomas. Acta Neuropathol 95:131–135 [DOI] [PubMed] [Google Scholar]
- Chakrabarty A, Bridges LR, Gray S (1996) Cyclin D1 in astrocytic tumours: an immunohistochemical study. Neuropathol Appl Neurobiol 22:311–316 [DOI] [PubMed] [Google Scholar]
- Clemens MJ, Bommer UA (1999) Translational control: the cancer connection. Int J Biochem Cell Biol 31:1–23 [DOI] [PubMed] [Google Scholar]
- Culjkovic B, Topisirovic I, Borden KL (2007) Controlling gene expression through RNA regulons: the role of the eukaryotic translation initiation factor eIF4E. Cell Cycle 6:65–69 [DOI] [PubMed] [Google Scholar]
- Culjkovic B, Topisirovic I, Skrabanek L, Ruiz-Gutierrez M, Borden KLB (2005) eIF4E promotes nuclear export of cyclin D1 mRNAs via an element in the 3′UTR. J Cell Biol 169:245–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Benedetti A, Graff JR (2004) eIF-4E expression and its role in malignancies and metastases. Oncogene 23:3189–3199 [DOI] [PubMed] [Google Scholar]
- Dever TE (2002) Gene-specific regulation by general translation factors. Cell 108:545–556 [DOI] [PubMed] [Google Scholar]
- Fu M, Wang C, Li Z, Sakamaki T, Pestell RG (2004) Minireview: cyclin D1: normal and abnormal functions. Endocrinology 145:5439–5447 [DOI] [PubMed] [Google Scholar]
- Gebauer F, Hentze MW (2004) Molecular mechanisms of translational control. Nat Rev Mol Cell Biol 5:827–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Jones L, Lowery-Norberg M, Fowler M (2005) Expression of eukaryotic initiation factor 4E in astrocytic tumors. Appl Immunohistochem Mol Morphol 13:178–183 [DOI] [PubMed] [Google Scholar]
- Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D (2000) Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell 6:1099–1108 [DOI] [PubMed] [Google Scholar]
- Holcik M, Sonenberg N (2005) Translational control in stress and apoptosis. Nat Rev Mol Cell Biol 6:318–327 [DOI] [PubMed] [Google Scholar]
- Hunter T, Pines J (1994) Cyclins and cancer. II: cyclin D and CDK inhibitors come of age. Cell 79:573–582 [DOI] [PubMed] [Google Scholar]
- Keene JD (2007) RNA regulons: coordination of post-transcriptional events. Nat Rev Genet 8:533–543 [DOI] [PubMed] [Google Scholar]
- Kim SH, Gunnery S, Choe JK, Mathews MB (2002) Neoplastic progression in melanoma and colon cancer is associated with increased expression and activity of the interferon-inducible protein kinase, PKR. Oncogene 21:8741–8748 [DOI] [PubMed] [Google Scholar]
- Koromilas AE, Roy S, Barber GN, Katze MG, Sonenberg N (1992) Malignant transformation by a mutant of the IFN-inducible dsRNA-dependent protein kinase. Science 257:1685–1689 [DOI] [PubMed] [Google Scholar]
- Lanzafame S, Torrisi A, Barbagallo G, Emmanuele C, Alberio N, Albanese V (2000) Correlation between histological grade, MIB-1, p53, and recurrence in 69 completely resected primary intracranial meningiomas with a 6 year mean follow-up. Pathol Res Pract 196:483–488 [DOI] [PubMed] [Google Scholar]
- Lobo MV, Arenas MI, Alonso FJ, Gomez G, Bazan E, Paino CL, Fernandez E, et al. (2004) Nestin, a neuroectodermal stem cell marker molecule, is expressed in Leydig cells of the human testis and in some specific cell types from human testicular tumours. Cell Tissue Res 316:369–376 [DOI] [PubMed] [Google Scholar]
- Lobo MV, Martin ME, Perez MI, Alonso FJ, Redondo C, Alvarez MI, Salinas M (2000) Levels, phosphorylation status and cellular localization of translational factor eIF2 in gastrointestinal carcinomas. Histochem J 32:139–150 [DOI] [PubMed] [Google Scholar]
- Louis DN, Ohgaki H, Wiestler OD, Cavenee WK (2007) WHO Classification of Tumours of the Central Nervous System. Lyon, IARC Press [DOI] [PMC free article] [PubMed]
- Martin ME, Munoz FM, Salinas M, Fando JL (2000a) Ischaemia induces changes in the association of the binding protein 4E-BP1 and eukaryotic initiation factor (eIF) 4G to eIF4E in differentiated PC12 cells. Biochem J 351:327–334 [PMC free article] [PubMed] [Google Scholar]
- Martin ME, Perez MI, Redondo C, Alvarez MI, Salinas M, Fando JL (2000b) 4E binding protein 1 expression is inversely correlated to the progression of gastrointestinal cancers. Int J Biochem Cell Biol 32:633–642 [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Suzuki SO, Fukui M, Iwaki T (2004) Accumulation of MDM2 in pleomorphic xanthoastrocytomas. Pathol Int 54:387–391 [DOI] [PubMed] [Google Scholar]
- Milenkovic S, Marinkovic T, Jovanovic MB, Djuricic S, Berisavac II, Berisavac I (2008) Cyclin D1 immunoreactivity in meningiomas. Cell Mol Neurobiol 28:907–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips A, Blaydes JP (2008) MNK1 and EIF4E are downstream effectors of MEKs in the regulation of the nuclear export of HDM2 mRNA. Oncogene 27:1645–1649 [DOI] [PubMed] [Google Scholar]
- Raught B, Gingras AC, James A, Medina D, Sonenberg N, Rosen JM (1996) Expression of a translationally regulated, dominant-negative CCAAT/enhancer-binding protein beta isoform and up-regulation of the eukaryotic translation initiation factor 2alpha are correlated with neoplastic transformation of mammary epithelial cells. Cancer Res 56:4382–4386 [PubMed] [Google Scholar]
- Reifenberger G, Liu L, Ichimura K, Schmidt EE, Collins VP (1993) Amplification and overexpression of the MDM2 gene in a subset of human malignant gliomas without p53 mutations. Cancer Res 53:2736–2739 [PubMed] [Google Scholar]
- Reis RM, Konu-Lebleblicioglu D, Lopes JM, Kleihues P, Ohgaki H (2000) Genetic profile of gliosarcomas. Am J Pathol 156:425–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwald IB (2004) The role of translation in neoplastic transformation from a pathologist's point of view. Oncogene 23:3230–3247 [DOI] [PubMed] [Google Scholar]
- Rosenwald IB, Hutzler MJ, Wang S, Savas L, Fraire AE (2001) Expression of eukaryotic translation initiation factors 4E and 2alpha is increased frequently in bronchioloalveolar but not in squamous cell carcinomas of the lung. Cancer 92:2164–2171 [DOI] [PubMed] [Google Scholar]
- Rousseau D, Kaspar R, Rosenwald I, Gehrke L, Sonenberg N (1996) Translation initiation of ornithine decarboxylase and nucleocytoplasmic transport of cyclin D1 mRNA are increased in cells overexpressing eukaryotic initiation factor 4E. Proc Natl Acad Sci USA 93:1065–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheper GC, Proud CG (2002) Does phosphorylation of the cap-binding protein eIF4E play a role in translation initiation? Eur J Biochem 269:5350–5359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider R, Sonenberg N (2007) Translational control in cancer development and progression. In Matthews MB, Sonengerg N, Hershey JWB, eds. Translational Control in Biology and Medicine. New York, Cold Spring Harbor Laboratory Press, 401–431
- Silva RL, Wendel HG (2008) MNK, EIF4E and targeting translation for therapy. Cell Cycle 7:553–555 [DOI] [PubMed] [Google Scholar]
- Strudwick S, Borden KL (2002) The emerging roles of translation factor eIF4E in the nucleus. Differentiation 70:10–22 [DOI] [PubMed] [Google Scholar]
- Takei H, Bhattacharjee MB, Rivera A, Dancer Y, Powell SZ (2007) New immunohistochemical markers in the evaluation of central nervous system tumors: a review of 7 selected adult and pediatric brain tumors. Arch Pathol Lab Med 131:234–241 [DOI] [PubMed] [Google Scholar]
- Topisirovic I, Ruiz-Gutierrez M, Borden KL (2004) Phosphorylation of the eukaryotic translation initiation factor eIF4E contributes to its transformation and mRNA transport activities. Cancer Res 64:8639–8642 [DOI] [PubMed] [Google Scholar]
- Vattem KM, Wek RC (2004) Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc Natl Acad Sci USA 101:11269–11274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Lloyd RV, Hutzler MJ, Rosenwald IB, Safran MS, Patwardhan NA, Khan A (2001) Expression of eukaryotic translation initiation factors 4E and 2alpha correlates with the progression of thyroid carcinoma. Thyroid 11:1101–1107 [DOI] [PubMed] [Google Scholar]
- Wang S, Rosenwald IB, Hutzler MJ, Pihan GA, Savas L, Chen JJ, Woda BA (1999) Expression of the eukaryotic translation initiation factors 4E and 2alpha in non-Hodgkin's lymphomas. Am J Pathol 155:247–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendel HG, Silva RL, Malina A, Mills JR, Zhu H, Ueda T, Watanabe-Fukunaga R, et al. (2007) Dissecting eIF4E action in tumorigenesis. Genes Dev 21:3232–3237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis AE (1999) Translational control of growth factor and proto-oncogene expression. Int J Biochem Cell Biol 31:73–86 [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhao M, Huang AY, Fei Z, Zhang W, Wang XL (2005) The effect of cyclin D expression on cell proliferation in human gliomas. J Clin Neurosci 12:166–168 [DOI] [PubMed] [Google Scholar]