Abstract
AIM: To validate gastric anti-ulcer properties of Rocket “Eruca sativa” on experimentally-induced gastric secretion and ulceration in albino rats.
METHODS: Gastric acid secretion studies were undertaken using pylorus-ligated rats. Gastric lesions in the rats were induced by noxious chemicals including ethanol, strong alkalis, indomethacin and hypothermic restraint stress. The levels of gastric wall mucus (GWM), nonprotein sulfhydryls (NP-SH) and malondialdehyde (MDA) were also measured in the glandular stomach of rats following ethanol administration. The gastric tissue was also examined histologically. The extract was used in two doses (250 and 500 mg/kg body weight) in all experiments.
RESULTS: In pylorus-ligated Shay rats, the ethanolic extract of Rocket “Eruca sativa L.” (EER) significantly and dose-dependently reduced the basal gastric acid secretion, titratable acidity and ruminal ulceration. Rocket extract significantly attenuated gastric ulceration induced by necrotizing agents (80% ethanol, 0.2 mol/L NaOH, 25% NaCl), indomethacin and hypothermic restraint stress. The anti-ulcer effect was further confirmed histologically. On the other hand, the extract significantly replenished GWM and NP-SH levels, as well as the MDA level significantly reduced by extract pretreatment.
CONCLUSION: Rocket extract possesses anti-secretory, cytoprotective, and anti-ulcer activities against experimentally-induced gastric lesions. The anti-ulcer effect is possibly through prostaglandin-mediated activity and/or through its anti-secretory and antioxidant properties.
Keywords: Cytoprotection, Eruca sativa, Gastric ulcer and secretion, Malondialdehyde, Rocket, Sulfhydryls
INTRODUCTION
Gastric ulcer is an illness that affects a considerable number of people worldwide. The etiological factors of this disorder include: stress, smoking, nutritional deficiencies, infections, frequent and indiscriminate use of nonsteroidal anti-inflammatory drugs (NSAIDs)[1]. The pathogenesis of gastroduodenal ulcers are influenced by various aggressive and defensive factors, such as mucus secretion, mucosal barrier, acid-pepsin secretion, blood flow, cellular regeneration and endogenous protective agents (prostaglandins and epidermal growth factor)[2]. Although the introduction of proton-pump inhibitors to the classic anti-ulcer therapy had revolutionized treatment of peptic ulcers and other gastrointestinal disorders, but there is still no complete cure for this disease. It has been shown that long term use of these drugs leads to various adverse and side effects. Relapses of the malady, ineffectiveness of different drug regimens and even resistance to drugs are emerging[3]. Thus, there is an urgent requirement to identify more effective and safe anti-ulcer agents. During the past few decades, a widespread search has been launched to identify new anti-ulcer therapies from natural sources. Herbs, medicinal plants, spices, vegetables and crude drug substances are considered to be a potential source to combat various diseases including gastric ulcer. In the scientific literature, a large number of medicinal plants with gastric anti-ulcer potential have been reported[4–8]. In recent years, Rocket “Eruca sativa L.” (EER), a member of the Brassicacae family, has gained greater importance as a salad vegetable and spice, especially among Middle Eastern populations and Europeans[9]. It is believed that plants belonging to the Brassicacae family possess diversified medicinal and therapeutic properties including inhibition of tumorigenesis[10], anti-ulcer[11], and hepatoprotective[12] activities. Rocket, locally known as Jarjeer, is used in salads, by local herbal practitioners and in Unani medicine, and is used as a diuretic, stimulant, and in the treatment of stomach disorders and scurvy[13]. The seeds and tender leaves are known in Arabian countries to increase sexual desire and are considered to be an aphrodisiac. It is also used as a carminative and to alleviate abdominal discomfort and improve digestion. It has been reported that the rocket seed ethanolic extract possesses potent antioxidant and renal protective and diuretic activities[14–16]. Phytochemical studies of rocket leaves and seeds have revealed the presence of glucosinolates[17,18]. Weckerle et al[19] isolated and identified three new quercetins from Eruca sativa leaves. In view of the acclaimed medicinal value of rocket in Unani, Ayurvedic and Arab traditional medicine as well as its diversified therapeutic uses, we have undertaken the present study to evaluate the anti-ulcerogenic property of EER in different ulcer models in rats.
MATERIALS AND METHODS
Plant material and preparation of extract
Fresh Eruca sativa leaves were purchased from a local vegetable market in Riyadh, and the identity of these leaves was confirmed by an expert taxonomist of the Department of Pharmacognosy, where a voucher specimen (No. 8208) of the plant has been kept in the Herbarium of the College of Pharmacy, KSU, Riyadh. Shade-dried, coarsely pulverized rocket leaves were placed in a glass percolator with ethanol and were allowed to stand at room temperature for about 72 h. The percolate was collected and dried under reduced pressure in vacuo. The extract obtained was later used and dissolved in distilled water for evaluation of anti-ulcer activity.
Animals and dosing
Albino Wistar rats of either sex, approximately the same age, weighing 150 to 200 g and fed on a diet of standard chow were used in this study. They were randomly divided into experimental groups of 6 rats each. Aqueous solutions of ulcerogens and EER were freshly prepared before administration. EER at doses of 250 and 500 mg/kg were given orally in the anti-ulcer studies and intraperitoneally for gastric secretion evaluation. The rats were sacrificed, and the stomachs removed and opened along the greater curvature. After washing with saline, the gastric lesions were quantified by a person unaware of the treatments. The animal study protocol was approved by the Research and Ethics Committee of the Experimental Animal Care Society, College of Pharmacy, King Saud University, Riyadh, Saudi Arabia.
Pylorus-ligated rats
Rats were fasted for 36 h with access to water ad libitum before pylorus ligation under ether anesthesia was carried out. Care was taken not to cause bleeding or to occlude blood vessels[20]. EER was administered intraperitoneally immediately after pylorus ligation (Shay). The rats were sacrificed at 6 h after pylorus ligation. The stomachs were removed, the contents were collected, volumes measured, centrifuged and analyzed for titratable acidity against 0.01 mol/L NaOH at pH 7.
Gastric lesions induced by necrotizing agents (cytoprotection)
Each rat was administered 1 mL of a necrotizing agent (80% ethanol, 0.2 mol/L NaOH or 25% NaCl). Rocket extract was given 30 min before the administration of necrotizing agents. One hour after the administration of ethanol and the alkalis, the rats were sacrificed and examined for stomach lesions. The scoring of stomach lesions was as follows: Patchy lesions of the stomach induced by ethanol were scored according to the method described by Robert et al[21] using the following scale: 0 = normal mucosa; 1 = hyperemic mucosa or up to 3 small patches; 2 = from 4 to 10 small patches; 3 = more than 10 small or up to 3 medium-sized patches; 4 = from 4 to 6 medium-sized patches; 5 = more than 6 medium-sized or up to 3 large patches; 6 = from 4 to 6 large patches; 7 = from 7 to 10 large patches; 8 = more than 10 large patches or extensive necrotic zones. “small” was defined as up to 2 mm across (max. diameter), “medium-sized” between 2 and 4 mm across and “large” more than 4 mm across.
Gastric lesions induced by indomethacin
Indomethacin was suspended in 1.0% carboxy-methylcellulose (CMC) in water (6 mg/mL) and administered orally to the 36 h fasted rats at a dose of 30 mg/kg body weight. Control rats were treated similarly with an equivalent amount of vehicle[22]. The rocket extract was given 30 min prior to indomethacin administration at a dose of 250 and 500 mg/kg. The animals were sacrificed 6 h after treatment. The stomachs were excised, rinsed with normal saline and examined for ulceration.
Hypothermic restraint stress-induced ulcers
The method described by Senay et al[23] was adopted with slight modifications. Animals were fasted for 36 h but had access to water ad libitum. Thirty minutes after the oral administration of EER (250 and 500 mg/kg), the rats were immobilized in restraint cages and placed inside a ventilated refrigerator maintained at 3 ± 1°C for 3 h. The animals were then sacrificed and the stomachs were excised. They were examined for ulceration and the severity of intraluminal bleeding according to the following arbitrary scale described by Chiu et al[24]. 0 = no blood detectable; 1 = thin blood follows the rugae; 2 = thick blood follows the rugae; 3 = thick blood follows the rugae with blood clots in certain areas and 4 = extensive covering of the whole gastric mucosal surface with thick blood.
Determination of gastric wall mucus (GWM)
Gastric wall mucus was determined according to the modified procedure of Crone et al[25]. The glandular segment of the stomach was separated from the rumen of the stomach, weighed, and transferred immediately to 10 mL of 0.1% w/v Alcian blue solution (in 0.16 mmol/L sucrose solution buffered with 0.05 mL sodium acetate at pH 5). Tissue was stained for 2 h in Alcian blue, and excess dye was removed by two successive rinses with 10 mL of 0.25 mmol/L sucrose, firstly after 15 min and then after 45 min. Dye complexed with the gastric wall mucus was extracted with 10 mL of 0.5 mmol/L magnesium chloride which was intermittently shaken for 1 min at 30 min intervals for 2 h. Four milliliters of blue extract were then vigorously shaken with an equal volume of diethyl ether. The resulting emulsion was centrifuged at 4000 r/min for 10 min and the absorbance of the aqueous layer was recorded at 580 nm. The quantity of Alcian blue extracted per gram of wet glandular tissue was then calculated.
Estimation of non-protein sulfhydryls (NP-SH)
Gastric mucosal non-protein sulfhydryls were measured according to the method of Sedlak and Lindsay[26]. The glandular part of the stomach was homogenized in ice-cold 0.02 mmol/L ethylenediaminetetraacetic acid (EDTA). Aliquots of 5 mL of the homogenates were mixed in 15 mL test tubes with 4 mL of distilled water and 1 mL of 50% trichloroacetic acid (TCA). The tubes were shaken intermittently for 10 min and centrifuged at 3000 r/min. Two milliliters of supernatant were mixed with 4 mL of 0.4 mol/L Tris buffer at pH 8.9. 0.1 mL of 5,5'-dithio-bis(2-nitrobenzoic acid) (DTNB) was added and the sample was shaken. The absorbance was measured within 5 min of DTNB addition at 412 nm against a reagent blank.
Determination of malondialdehyde (MDA)
The method reported by Utley et al[27] was followed. The animals were killed 1 h after ethanol administration. The stomachs were removed and each was homogenized in 0.15 mol/L KCl (at 4°C) in a Potter-Elvehjem type C homogenizer to give a 10% w/v homogenate. Aliquots of homogenate 1 mL in volume were incubated at 37°C for 3 h in a metabolic shaker. Then 1 mL of 10% aqueous TCA was added and mixed. The mixture was then centrifuged at 800 g for 10 min. One milliliter of the supernatant was removed and mixed with 1 mL of 0.67% w-thiobarbituric acid in water and placed in a boiling water bath for 10 min. The mixture was cooled and diluted with 1 mL distilled water. The absorbance of the solution was then read at 535 nm. The content of malondialdehyde (nmol/g wet tissue) (index of the magnitude of lipid peroxidation) was then calculated, by reference to a standard curve of malondialdehyde solution.
Histopathological evaluation
Gastric tissue samples were fixed in neutral buffered formalin for 24 h. Sections of gastric tissue were histopathologically examined to study the ulcerogenic and/or anti-ulcerogenic activity of EER. The tissues were fixed in 10% buffered formalin and processed using a VIP tissue processor. The processed tissues were embedded in paraffin blocks and sections about 5 μm thick were cut using an American optical rotary microtome. These sections were stained with haematoxylin and eosin using routine procedures[28]. The slides were examined microscopically for pathomorphological changes such as congestion, hemorrhage, edema, and erosions using an arbitrary scale for severity assessment of these changes.
Statistical analysis
Values in tables and figures are given as mean ± SE. Data were analyzed by using one-way analysis of variance (ANOVA) followed by Student’s t-test.
RESULTS
Effect of EER on gastric secretions in 6 h pylorus-ligated rats
When the rats were subjected to pylorus ligation for 6 h, a considerable amount of basal gastric acid secretion was noted (10.83 ± 1.16 mL) in the control group. In the same control group, the titratable acidity was found to be 196.57 ± 15.50 mEq/L and the ulcer index was recorded as 2.33 ± 1.5. EER at both doses (250 and 500 mg/kg) significantly reduced gastric acid secretion, titratable acidity and ulcer formation (6.33 ± 1.63 mL, 4.16 ± 1.16 mL P < 0.05, P < 0.01; 132.77 ± 17.43, 55.55 ± 10.46 mEq/L P < 0.05, P < 0.001, 0.66 ± 0.51, 0.50 ± 0.54, P < 0.05, P < 0.05), respectively (Table 1).
Table 1.
Effects of EER on gastric secretion, acidity and gastric lesion index in pylorus-ligated shay rats (mean ± SE)
| Group serial | Treatment | Dose (mg/kg, i.g.) | Volume of gastric content (mL) | Titratable acidity (mEq/L) | Ulcer index |
| 1 | Control (distilled water) | - | 10.83 ± 1.16 | 196.57 ± 15.50 | 2.33 ± 1.50 |
| 2 | EER | 250 | 6.33 ± 1.63a | 132.77 ± 17.43a | 0.66 ± 0.51a |
| 3 | EER | 500 | 4.16 ± 1.16b | 55.55 ± 10.46d | 0.50 ± 0.54a |
Six rats were used in each group.
P < 0.05,
P < 0.01,
P < 0.001 vs control (distilled water) group, Student’s t-test.
Effect of EER on necrotizing agents-induced gastric lesions
The treatment of rats with 80% ethanol, 0.2 mol/L NaOH and 25% NaCl produced extensive gastric lesions in the glandular mucosa of the stomach in all the control rats. The ulcer index was 6.00 ± 0.89, 6.66 ± 1.36 and 6.66 ± 0.51, respectively in control rats 1 h after administration of the necrotizing agents. Pretreatment of rats with EER at doses of 250 mg/kg (ulcer index in 80% ethanol, 0.2 mol/L NaOH and 25% NaCl = 2.00 ± 0.89, P < 0.01, 2.66 ± 1.21, P < 0.05 and 2.83 ± 0.98, P < 0.001); 500 mg/kg (ulcer index = 1.66 ± 1.03, P < 0.01, 1.50 ± 0.54, P < 0.01 and 2.16 ± 0.75, P < 0.001), respectively, significantly inhibited the formation of gastric lesions as shown in Table 2.
Table 2.
Effect of EER on gastric lesions induced by necrotizing agents (mean ± SE)
| Group serial | Treatment | Dose (mg/kg, i.g.) |
Ulcer index |
||
| 80% EtOH | 0.2 mol/L NaOH | 25% NaCl | |||
| 1 | Control (distilled water) | - | 6.00 ± 0.89 | 6.66 ± 1.36 | 6.66 ± 0.51 |
| 2 | EER | 250 | 2.00 ± 0.89b | 2.66 ± 1.21a | 2.83 ± 0.98b |
| 3 | EER | 500 | 1.66 ± 1.03b | 1.50 ± 0.54b | 2.16 ± 0.75d |
Six rats were used in each group.
P < 0.05,
P < 0.01,
P < 0.001 vs control (distilled water) group, Student’s t-test.
Effect of EER on gastric lesions induced by indomethacin
The oral administration of indomethacin induced marked damage in the rat glandular stomach. EER at the 500 mg/kg dose significantly prevented the development of gastric lesions in the rat stomach (P < 0.01), however, no significant preventive effect of EER at the 250 mg/kg dose, in indomethacin-treated rats was observed (Table 3).
Table 3.
Effect of EER on indomethacin-induced gastric mucosal lesions (mean ± SE)
| Group serial | Treatment | Animals (n) | Dose (mg/kg, i.g.) | Ulcer Index |
| 1 | Control (indomethacin only) | 6 | - | 44.50 ± 5.82 |
| 2 | EER | 6 | 250 | 25.50 ± 6.88 |
| 3 | EER | 6 | 500 | 13.50 ± 4.84b |
Six rats were used in each group.
P < 0.01 vs control (indomethacin only) group, Student’s t-test.
Effect of EER on hypothermic restraint stress-induced gastric mucosal lesions
Table 4 shows that EER at a dose of 500 mg/kg body weight significantly inhibited intraluminal bleeding and ulcer formation induced by hypothermic restraint stress (P < 0.05). Although the intraluminal bleeding and ulcer index was reduced at a dose of 250 mg/kg body weight, this reduction was not found to be statistically significant.
Table 4.
Effect of EER on hypothermic restraint stress-induced intraluminal bleeding and gastric lesions in rats (mean ± SE)
| Group serial | Treatment | Dose (mg/kg, i.g.) | Intraluminal bleeding score | Gastric lesion ulcer index |
| 1 | Control (distilled water) | - | 2.60 ± 0.50 | 24.00 ± 3.24 |
| 2 | EER | 250 | 1.20 ± 0.83 | 15.20 ± 3.96 |
| 3 | EER | 500 | 0.83 ± 0.40a | 7.66 ± 6.12a |
Six rats were used in each group.
P < 0.05 vs control (distilled water) group, Student’s t-test.
Effect of EER on ethanol-induced changes in gastric wall mucus (GWM)
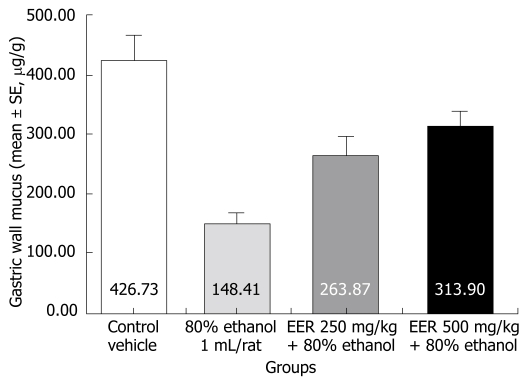
Rats treated with ethanol showed a significant decrease in the Alcian blue binding capacity of gastric wall mucus (148.41 ± 18.81 μg/g of tissue, P < 0.001) as compared to control (normal) rats (426.73 ± 39.15 µg/g). Pretreatment of rats with EER at doses of 250 mg/kg (263.87 ± 32.65 μg/g, P < 0.05) and 500 mg/kg (313.90 ± 24.30 μg/g, P < 0.001) significantly enhanced the Alcian blue binding capacity of gastric mucosa (Figure 1).
Figure 1.
Effect of EER on the changes in gastric wall mucus induced by 80% ethanol.
Effect of EER on ethanol-induced depletion of gastric mucosal NP-SH
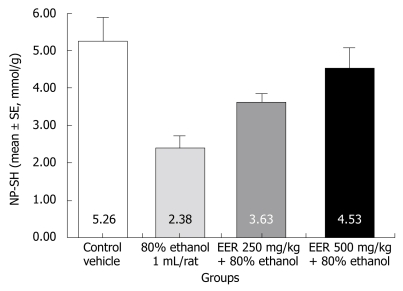
The level of NP-SH in the gastric mucosa of control rats was 5.26 ± 0.63 mmol/g of tissue, which was significantly decreased to 2.38 ± 0.33 mmol/g (P < 0.001) following the administration of 80% ethanol. Pretreatment of rats with EER at both doses (250 and 500 mg/kg) significantly replenished the ethanol-induced depletion of NP-SH (3.63 ± 0.22, P < 0.001; 4.53 ± 0.56, P < 0.01), respectively (Figure 2).
Figure 2.
Effect of EER on NP-SH concentration in gastric ulcer induced by 80% ethanol.
Effect of EER on ethanol-induced increase in MDA
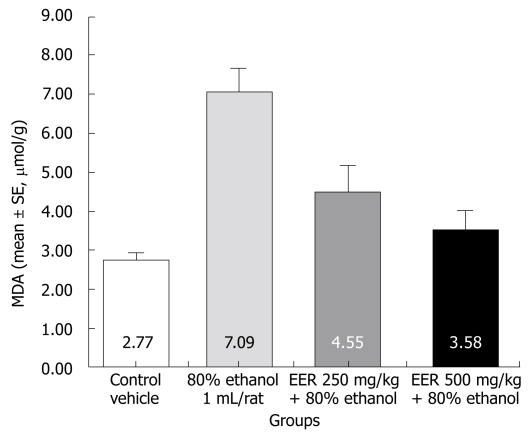
As depicted in Figure 3, MDA levels in the gastric mucosa used as an index of lipid peroxidation were significantly higher in the ethanol only treated group than in the untreated control group (7.09 ± 0.60 μmol/g of tissue; 2.77 ± 0.19 μmol/g of tissue), respectively. EER at both doses (250 and 500 mg/kg) significantly decreased the MDA content (4.55 ± 0.66 μmol/g and 3.58 ± 0.49 μmol/g), respectively.
Figure 3.
Effect of EER on MDA concentration in gastric ulcer induced by 80% ethanol.
Effect of EER on histopathological evaluation
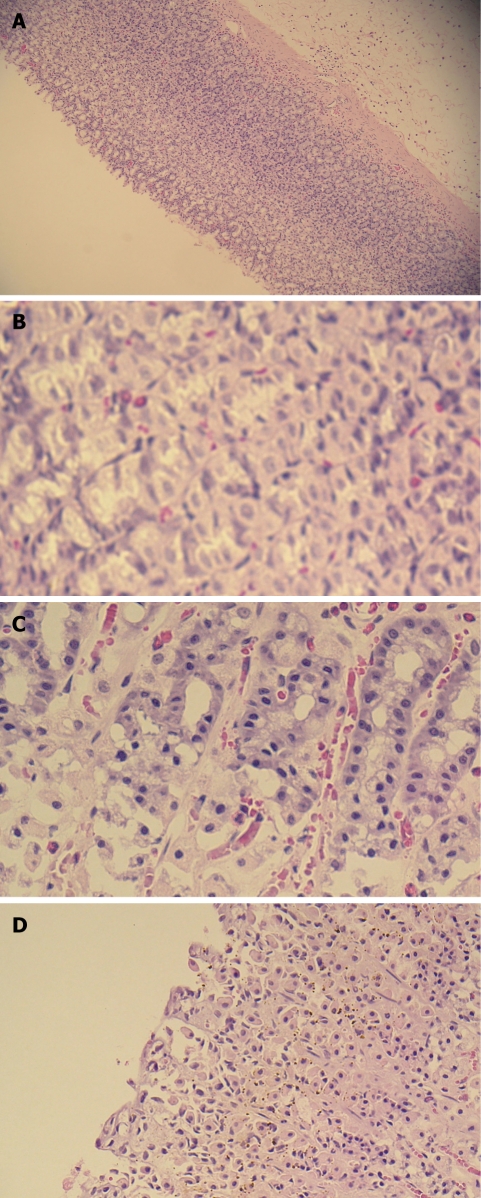
Histopathological studies (Figure 4) further confirmed that pretreatment with rocket extract prevented ethanol-induced necrosis in the superficial layers of the gastric mucosa with congestion.
Figure 4.
Light micrographs showing the effect of EER on ethanol-induced gastric lesions of rats. A: Normal mucosa; B: Ethanol-induced gastric mucosal congestion and necrosis; C: Pretreatment of rats with EER 250 mg/kg; D: Pretreatment of rats with EER 500 mg/kg.
DISCUSSION
The results of this study show that the ethanolic extract of Rocket possesses significant anti-secretory, anti-ulcer and cytoprotective properties in rats. Pretreatment with EER produced a dose-dependent decrease in the volume of basal gastric secretion, titratable acidity and lesions in pylorus-ligated Shay rats. It has been reported that anti-secretory agents such as histamine H2-receptor antagonists ameliorate the decrease in gastric mucosal blood flow caused by factors that disturb the gastric mucosa such as NSAIDs and ethanol[29]. Gastric acid is an important factor in the genesis of ulceration in pylorus-ligated rats[20]. The activation of the vagus-vagal reflux by stimulation of pressure receptors in the antral gastric mucosa in the hypersecretion model of pylorus ligature is believed to increase gastric acid secretion[30]. Since EER markedly inhibited gastric acid secretion and ruminal ulcers in pylorus-ligated rats, this observed effect could be related, at least in part, to the ability of EER to reduce gastric acid secretion. It is now accepted that gastric acid secretion plays an important role in the progression from an erosive mucus layer to a gastric lesion. On the other hand, substances which have the ability to suppress gastric acid secretion, such as proton pump inhibitors and histamine H2-receptor antagonists are believed to accelerate the healing process of the gastric lesions or inhibit the formation of mucosal injury[31]. Rocket extract was found to offer the gastric mucosa, a statistically significant and dose-dependent protection against ulceration caused by various necrotizing agents including ethanol and strong alkalis. Ethanol-induced gastric ulcers have been widely used in the evaluation of gastroprotective activity. Ethanol is metabolized in the body and releases superoxide anion and hydroperoxy free radicals. It has been found that oxygen-derived free radicals are implicated in the mechanism of acute and chronic ulceration in the stomach[32]. The genesis of ethanol-induced gastric lesions is of multifactorial origin with a decrease in gastric mucus, and is associated with the significant production of free radicals leading to increased lipid peroxidation which in turn causes damage to cells and cell membranes[33]. The cytoprotective effect of EER may be related to its ability to prevent gastric acid secretion and/or enhance the mucosal defensive factors such as prostaglandins and decrease lipid peroxidation[34]. Treatment of rats with indomethacin, a non-selective cyclooxygenase inhibitor is known to induce gastric damage through multiple mechanisms which include suppression of prostaglandin generation, overproduction of leukotrienes, acting as a topical irritant and by reducing the local blood-flow[35]. Rats pretreated with EER produced significant protection in this model. It is possible that an enhanced level of gastric mucus, generating prostaglandins and inhibiting leukotriene may contribute to the gastroprotective effect of rocket extract.
Hypothermic-restraint stress ulcers have been used as an experimental model in the evaluation of anti-ulcer activity in rats due to data reproducibility[36]. Disturbances of gastric mucosal microcirculation, enhancement of acid secretion and reduction in mucus production are mediated by histamine release and abnormal gastric motility[37]. It is also reported that free radicals may play a major role in stress-induced gastric injury[38]. Stress is reported to inactivate mucosal prostaglandin syntheses by accumulating hydrogen peroxide, a prostaglandin biosynthesis inhibitor, which also causes reactive oxygen species (ROS) generation[39]. In addition, a positive correlation has been reported between the level of gastric mucosal lipid peroxidation products, a marker of oxidative stress, and stomach damage in cold restraint-stressed rats[40]. The protective efficacy against cold restraint-stress may be due to the antioxidant activities of EER, as an antioxidant activity was reported earlier in Eruca sativa[14], this together with its antisecretagogue potential, thereby strengthens the animals physiological capabilities to decrease stress ulcers.
Our results revealed that the rocket ethanol extract significantly protected gastric mucosa against the depletion of gastric wall mucus. The mucus gel adhering to the gastric mucosal surface protects the underlying epithelium against acid[41,42], pepsin[43] and necrotizing agents such as ethanol and indomethacin[11]. Gastric wall mucus however, plays a more important role in the defense of the gastric mucosa against chemical or mechanical aggression than the soluble mucus in the lumen of the stomach[44]. The gastric mucus coat is thought to be important in facilitating the repair of the damaged gastric epithelium[45]. It seems likely that the cytoprotective activity of EER could result, at least in part, from interaction with the adhering gastric mucus layer.
Sulfhydryl compounds in living organisms plays a central role in the maintenance of gastric integrity, particularly when ROS are involved in the pathogenesis of tissue damage[46]. A significant decrease in gastric NP-SH following ethanol administration indicated massive generation of oxygen derived free radicals (ODFR). Our findings are in agreement with earlier reports showing depletion of sulfhydryls in ethanol-induced gastric lesions[3,47]. Treatment of rats with glutathione depletors has been shown to significantly potentiate ulcerogen-induced gastric mucosal injury[48], whereas an increase in mucosal NP-SH exerts a gastroprotective effect[49]. Our observations clearly point towards the mediation of sulfhydryls in EER gastric mucosal protection.
Furthermore, the extract also showed significant inhibition of lipid peroxidation. The generation of MDA from lipids that react with thiobarbituric acid was found to be inhibited by the EER. Thus, it appears that the antioxidant property of the rocket extract may possibly counteract oxidative damage caused by alcohol toxicity. The observed anti-ulcerogenic activity may be due to its antioxidant effects and appears to strengthen the mucosal barrier, which is the first line of defense against endogenous and exogenous ulcerogenic agents.
The preliminary phytochemical screening of rocket revealed the presence of flavonoids, sterols and/or triterpenes. Moreover, quercetin and its derivatives were also reported in rocket leaves. Previous studies have shown that flavonoids may be related to the anti-ulcer activity[50], and play a major role in the mechanism of gastroprotection[51,52]. In addition to flavonoids, other constituents in rocket such as sterol and/or triterpenes are known for their antioxidant activities, which may contribute to some of the anti-ulcer mechanisms[53].
In conclusion, the data obtained confirm the traditional indications for this salad herb and present a new therapeutic option for the treatment of gastric ailments. The exact mechanism(s) underlying this anti-ulcerogenic effect remain unknown, but the extract contains substances, which might increase endogenous prostaglandins and mucus synthesis through its potent antioxidant activity. Furthermore, the anti-secretory mechanism can not, however, be dismissed.
COMMENTS
Background
Gastric ulcer is an illness that affects a considerable number of people worldwide. The etiological factors of this disorder include: stress, smoking, nutritional deficiencies, infections, frequent and indiscriminate use of nonsteroidal anti-inflammatory drugs (NSAIDs). Herbs, medicinal plants, spices, vegetables and crude drug substances are considered to be a potential source to combat various diseases including gastric ulcer. In the scientific literature, a large number of medicinal plants with gastric anti-ulcer potential have been reported.
Research frontiers
Although the introduction of proton-pump inhibitors to the classic anti-ulcer therapy has revolutionized treatment of peptic ulcers and other gastrointestinal disorders, there is still no complete cure for this disease. It has been shown that long term use of these drugs leads to various adverse and side effects. Relapses of the malady, ineffectiveness of different drug regimens and even resistance to drugs are emerging. Thus, there is an urgent requirement to identify more effective and safe anti-ulcer agents. During the past few decades, a widespread search has been launched to identify new anti-ulcer therapies from natural sources.
Terminology
The anti-secretory, cytoprotective and antioxidant properties of the rocket extract caused an inhibition of chemically and stress-induced gastric ulceration in rats.
Peer review
The authors examined an ethanolic extract of rocket “Eruca sativa L.” for its claimed beneficial effects on gastrointestinal disorders. The results are interesting and may provide a better understanding and give a clue for further investigations and innovations for an effective and safe phytotherapy for peptic ulcer disease.
Acknowledgments
The Authors thank Dr. Mohd Nazam Ansari and Mr. Malik Sawood Ahmed for their technical assistance.
Peer reviewer: Bronislaw L Slomiany, Professor, Research Center, C875, UMDNJ-NJ Dental School, 110 Bergen Street, Newark, NJ, 07103-2400, United States
S- Editor Li LF L- Editor Webster JR E- Editor Yin DH
References
- 1.Khazaei M, Salehi H. Protective effect of falcaria vulgaris extract on ethanol induced gastric ulcer in rat. Iran J Pharmacol Therap. 2006;5:43–46. [Google Scholar]
- 2.Mizui T, Sato H, Hirose F, Doteuchi M. Effect of antiperoxidative drugs on gastric damage induced by ethanol in rats. Life Sci. 1987;41:755–763. doi: 10.1016/0024-3205(87)90456-5. [DOI] [PubMed] [Google Scholar]
- 3.Al Mofleh IA, Alhaider AA, Mossa JS, Al-Soohaibani MO, Rafatullah S. Aqueous suspension of anise "Pimpinella anisum" protects rats against chemically induced gastric ulcers. World J Gastroenterol. 2007;13:1112–1118. doi: 10.3748/wjg.v13.i7.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rafatullah S, Galal AM, Al-Yahya MA, Al-Said MS. Gastric and duodenal antiulcer and cytoprotective effects of Aframomum melegueta in rats. Int J Pharmacogn. 1995;33:311–316. [Google Scholar]
- 5.Al-Mofleh IA, Alhaider AA, Mossa JS, Al-Sohaibani MO, Rafatullah S, Qureshi S. Protection of gastric mucosal damage by Coriandrum sativum L. pretreatment in Wistar albino rats. Environ Toxicol Pharmacol. 2006;22:64–69. doi: 10.1016/j.etap.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Al-Yahya MA, Rafatullah S, Mossa JS, Ageel AM, Parmar NS, Tariq M. Gastroprotective activity of ginger zingiber officinale rosc., in albino rats. Am J Chin Med. 1989;17:51–56. doi: 10.1142/S0192415X89000097. [DOI] [PubMed] [Google Scholar]
- 7.Rafatullah S, Tariq M, Al-Yahya MA, Mossa JS, Ageel AM. Evaluation of turmeric (Curcuma longa) for gastric and duodenal antiulcer activity in rats. J Ethnopharmacol. 1990;29:25–34. doi: 10.1016/0378-8741(90)90094-a. [DOI] [PubMed] [Google Scholar]
- 8.Al-Mofleh IA, Alhaider AA, Mossa JS, Al-Sohaibani MO, Al-Yahya MA, Rafatullah S, Shaik SA. Gastroprotective effect of an aqueous suspension of black cumin Nigella sativa on necrotizing agents-induced gastric injury in experimental animals. Saudi J Gastroenterol. 2008;14:128–134. doi: 10.4103/1319-3767.41731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lamy E, Schröder J, Paulus S, Brenk P, Stahl T, Mersch-Sundermann V. Antigenotoxic properties of Eruca sativa (rocket plant), erucin and erysolin in human hepatoma (HepG2) cells towards benzo(a)pyrene and their mode of action. Food Chem Toxicol. 2008;46:2415–2421. doi: 10.1016/j.fct.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 10.Lynn A, Collins A, Fuller Z, Hillman K, Ratcliffe B. Cruciferous vegetables and colo-rectal cancer. Proc Nutr Soc. 2006;65:135–144. doi: 10.1079/pns2005486. [DOI] [PubMed] [Google Scholar]
- 11.Alqasoumi S, Al-Howiriny TA, Al-Yahya M, Rafatullah S. Gastroprotective effects of radish "raphanus sativus" L. on experimental gastric ulcer models in Rats. FARMACIA. 2008;46:204–214. [Google Scholar]
- 12.Rafatullah S, AlSheikh A, Alqasoumi S, Al-Yahya M, El-Tahir K, Galal A. Protective effect of fresh radish juice (Raphanus sativus L.) against carbon tetrachloride induced hepatotoxicity. Int J Pharmacol. 2008;4:1–5. [Google Scholar]
- 13.Chopra RN, Nayar SL, Chopra IC. Glossary of Indian medicinal plants. Council of Scientific & Industrial Research: New Delhi; 1956. p. 110. [Google Scholar]
- 14.Sarwar Alam M, Kaur G, Jabbar Z, Javed K, Athar M. Eruca sativa seeds possess antioxidant activity and exert a protective effect on mercuric chloride induced renal toxicity. Food Chem Toxicol. 2007;45:910–920. doi: 10.1016/j.fct.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 15.Mahran GH, Kadry HA, Isaac ZG, Thabet CK, Al-Azizi MM, El-Olemy MM. Investigation of diuretic drug plants. 1. Phytochemical screening and pharmacological evaluation of Anethum graveolens L., Apium graveolens L., Daucus carota L. and Eruca sativa mill. Phytotherapy Res. 1991;5:169–172. [Google Scholar]
- 16.Yanir Z, Schaffermann D, Zmar Z. Tradition, uses and biodiversity of rocket (Eruva sativa, Brassicaceae) in Israel. Econ Bot. 1998;52:394–400. [Google Scholar]
- 17.D'Antuono LF, Elementi S, Neri R. Glucosinolates in Diplotaxis and Eruca leaves: diversity, taxonomic relations and applied aspects. Phytochemistry. 2008;69:187–199. doi: 10.1016/j.phytochem.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 18.Graser G, Schneider B, Oldham NJ, Gershenzon J. The methionine chain elongation pathway in the biosynthesis of glucosinolates in Eruca sativa (Brassicaceae) Arch Biochem Biophys. 2000;378:411–419. doi: 10.1006/abbi.2000.1812. [DOI] [PubMed] [Google Scholar]
- 19.Weckerle B, Michel K, Balázs B, Schreier P, Tóth G. Quercetin 3,3',4'-tri-O-beta-D-glucopyranosides from leaves of Eruca sativa (Mill.) Phytochemistry. 2001;57:547–551. doi: 10.1016/s0031-9422(01)00059-0. [DOI] [PubMed] [Google Scholar]
- 20.Shay H. A simple method for the uniform production of gastric ulceration in the rat. Gastroenterology. 1945;5:43–61. [Google Scholar]
- 21.Robert A, Nezamis JE, Lancaster C, Davis JP, Field SO, Hanchar AJ. Mild irritants prevent gastric necrosis through "adaptive cytoprotection" mediated by prostaglandins. Am J Physiol. 1983;245:G113–G121. doi: 10.1152/ajpgi.1983.245.1.G113. [DOI] [PubMed] [Google Scholar]
- 22.Bhargava KP, Gupta MB, Tangri KK. Mechanism of ulcerogenic activity of indomethacin and oxyphenbutazone. Eur J Pharmacol. 1973;22:191–195. doi: 10.1016/0014-2999(73)90012-5. [DOI] [PubMed] [Google Scholar]
- 23.Senay EC, Levine RL. Synergism between cold and restraint for rapid production of stress ulcer in rats. Proc Soc Exp Biol Med. 1967;124:1221–1231. doi: 10.3181/00379727-124-31970. [DOI] [PubMed] [Google Scholar]
- 24.Chiu PJS, Gerhart C, Brown AD, Barnett A. Effects of a gastric antisecretory cytoprotectant 2-methyl-8-(phenylmethoxy)imidazo (1,2 a)-pyridine-3-acetonitrile (Sch 28080) on cysteamine, reserpine and stress ulcers in rats. Arzneim Forsch. 1984;34:783. [PubMed] [Google Scholar]
- 25.Corne SJ, Morrissey SM, Woods RJ. Proceedings: A method for the quantitative estimation of gastric barrier mucus. J Physiol. 1974;242:116P–117P. [PubMed] [Google Scholar]
- 26.Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 27.Utley HG, Bernheim F, Hochstein P. Effect of sulfhydryl reagents on peroxidation in microsomes. Arch Biochem Biophys. 1967;118:29–32. [Google Scholar]
- 28.Culling CFA. Handbook of histopathological and histochemical techniques. 3rd ed. Butterwirth and Co: London; 1974. p. 37. [Google Scholar]
- 29.Murashima Y, Kotani T, Hayashi S, Komatsu Y, Nakagiri A, Amagase K, Takeuchi K. Impairment by 5-fluorouracil of the healing of gastric lesions in rats: effect of lafutidine, a histamine H2 receptor antagonist, mediated by capsaicin-sensitive afferent neurons. Dig Dis Sci. 2009;54:36–45. doi: 10.1007/s10620-008-0325-8. [DOI] [PubMed] [Google Scholar]
- 30.Baggio CH, Freitas CS, Rieck L, Marques MC. Gastroprotective effects of a crude extract of Baccharis illinita DC in rats. Pharmacol Res. 2003;47:93–98. doi: 10.1016/s1043-6618(02)00253-0. [DOI] [PubMed] [Google Scholar]
- 31.Brzozowski T, Konturek PC, Konturek SJ, Drozdowicz D, Kwiecieñ S, Pajdo R, Bielanski W, Hahn EG. Role of gastric acid secretion in progression of acute gastric erosions induced by ischemia-reperfusion into gastric ulcers. Eur J Pharmacol. 2000;398:147–158. doi: 10.1016/s0014-2999(00)00287-9. [DOI] [PubMed] [Google Scholar]
- 32.Umamaheswari M, Asokkumar K, Rathidevi R, Sivashanmugam AT, Subhadradevi V, Ravi TK. Antiulcer and in vitro antioxidant activities of Jasminum grandiflorum L. J Ethnopharmacol. 2007;110:464–470. doi: 10.1016/j.jep.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 33.Khazaei M, Salehi H. Protective effect of falcaria vulgaris extract on ethanol induced gastric ulcer in rat. Iran J Pharmacol Ther. 2006;5:43–46. [Google Scholar]
- 34.Morimoto Y, Shimohara K, Oshima S, Sukamoto T. Effects of the new anti-ulcer agent KB-5492 on experimental gastric mucosal lesions and gastric mucosal defensive factors, as compared to those of teprenone and cimetidine. Jpn J Pharmacol. 1991;57:495–505. doi: 10.1254/jjp.57.495. [DOI] [PubMed] [Google Scholar]
- 35.Paiva LA, Rao VS, Gramosa NV, Silveira ER. Gastroprotective effect of Copaifera langsdorffii oleo-resin on experimental gastric ulcer models in rats. J Ethnopharmacol. 1998;62:73–78. doi: 10.1016/s0378-8741(98)00058-0. [DOI] [PubMed] [Google Scholar]
- 36.Murakami M, Lam SK, Inada M, Miyake T. Pathophysiology and pathogenesis of acute gastric mucosal lesions after hypothermic restraint stress in rats. Gastroenterology. 1985;88:660–665. doi: 10.1016/0016-5085(85)90133-7. [DOI] [PubMed] [Google Scholar]
- 37.Garrick T, Leung FW, Buack S, Hirabayashi K, Guth PH. Gastric motility is stimulated but overall blood flow is unaffected during cold restraint in the rat. Gastroenterology. 1986;91:141–148. doi: 10.1016/0016-5085(86)90450-6. [DOI] [PubMed] [Google Scholar]
- 38.Bagchi M, Milnes M, Williams C, Balmoori J, Ye X, Stohs S, Bagchi D. Acute and chronic stress-induced oxidative gastrointestinal injury in rats, and the protective ability of a novel grape seed proanthocyanidin extract. Nutr Res. 1999;19:1189–1199. [Google Scholar]
- 39.Bandyopadhyay U, Das D, Bandyopadhyay D, Bhattacharjee M, Banerjee RK. Role of reactive oxygen species in mercaptomethlimidazole-induced gastric acid secretion and stress- induced gastric ulceration. Curr Sci. 1999;76:55–63. [Google Scholar]
- 40.Tandon R, Khanna HD, Dorababu M, Goel RK. Oxidative stress and antioxidants status in peptic ulcer and gastric carcinoma. Indian J Physiol Pharmacol. 2004;48:115–118. [PubMed] [Google Scholar]
- 41.Bell AE, Sellers LA, Allen A, Cunliffe WJ, Morris ER, Ross-Murphy SB. Properties of gastric and duodenal mucus: effect of proteolysis, disulfide reduction, bile, acid, ethanol, and hypertonicity on mucus gel structure. Gastroenterology. 1985;88:269–280. doi: 10.1016/s0016-5085(85)80180-3. [DOI] [PubMed] [Google Scholar]
- 42.Slomiany BL, Piasek A, Sarosiek J, Slomiany A. The role of surface and intracellular mucus in gastric mucosal protection against hydrogen ion. Compositional differences. Scand J Gastroenterol. 1985;20:1191–1196. doi: 10.3109/00365528509089275. [DOI] [PubMed] [Google Scholar]
- 43.Allen A, Sellers LA, Bennett MK. The gastric mucosal epithelial barrier: role of mucus and fibrin. Scand J Gastroenterol Suppl. 1987;128:6–13. doi: 10.3109/00365528709090963. [DOI] [PubMed] [Google Scholar]
- 44.Allen A, Hutton DA, Leonard AJ, Pearson JP, Sellers LA. The role of mucus in the protection of the gastroduodenal mucosa. Scand J Gastroenterol Suppl. 1986;125:71–78. doi: 10.3109/00365528609093820. [DOI] [PubMed] [Google Scholar]
- 45.Wallace JL, Whittle BJ. Role of mucus in the repair of gastric epithelial damage in the rat. Inhibition of epithelial recovery by mucolytic agents. Gastroenterology. 1986;91:603–611. doi: 10.1016/0016-5085(86)90629-3. [DOI] [PubMed] [Google Scholar]
- 46.Kimura M, Goto S, Ihara Y, Wada A, Yahiro K, Niidome T, Aoyagi H, Hirayama T, Kondo T. Impairment of glutathione metabolism in human gastric epithelial cells treated with vacuolating cytotoxin from Helicobacter pylori. Microb Pathog. 2001;31:29–36. doi: 10.1006/mpat.2001.0446. [DOI] [PubMed] [Google Scholar]
- 47.Miller TA, Li D, Kuo YJ, Schmidt KL, Shanbour LL. Nonprotein sulfhydryl compounds in canine gastric mucosa: effects of PGE2 and ethanol. Am J Physiol. 1985;249:G137–G144. doi: 10.1152/ajpgi.1985.249.1.G137. [DOI] [PubMed] [Google Scholar]
- 48.Hiraishi H, Terano A, Ota S, Mutoh H, Sugimoto T, Harada T, Razandi M, Ivey KJ. Protection of cultured rat gastric cells against oxidant-induced damage by exogenous glutathione. Gastroenterology. 1994;106:1199–1207. doi: 10.1016/0016-5085(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 49.Sener-Muratoğlu G, Paskaloğlu K, Arbak S, Hürdağ C, Ayanoğlu-Dülger G. Protective effect of famotidine, omeprazole, and melatonin against acetylsalicylic acid-induced gastric damage in rats. Dig Dis Sci. 2001;46:318–330. doi: 10.1023/a:1005652815921. [DOI] [PubMed] [Google Scholar]
- 50.Hiruma-Lima CA, Calvo TR, Rodrigues CM, Andrade FD, Vilegas W, Brito AR. Antiulcerogenic activity of Alchornea castaneaefolia: effects on somatostatin, gastrin and prostaglandin. J Ethnopharmacol. 2006;104:215–224. doi: 10.1016/j.jep.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 51.Havsteen BH. The biochemistry and medical significance of the flavonoids. Pharmacol Ther. 2002;96:67–202. doi: 10.1016/s0163-7258(02)00298-x. [DOI] [PubMed] [Google Scholar]
- 52.La Casa C, Villegas I, Alarcón de la Lastra C, Motilva V, Martín Calero MJ. Evidence for protective and antioxidant properties of rutin, a natural flavone, against ethanol induced gastric lesions. J Ethnopharmacol. 2000;71:45–53. doi: 10.1016/s0378-8741(99)00174-9. [DOI] [PubMed] [Google Scholar]
- 53.Al-Howiriny T, Al-Sohaibani M, Al-Said M, Al-Yahya M, El-Tahir K, Rafatullah S. Effect of Commiphora opobalsamum (L.) Engl. (Balessan) on experimental gastric ulcers and secretion in rats. J Ethnopharmacol. 2005;98:287–294. doi: 10.1016/j.jep.2005.01.034. [DOI] [PubMed] [Google Scholar]