Abstract
AIM: To test the effect of the dephytinization of three different commercial infant cereals on iron, calcium, and zinc bioavailability by estimating the uptake, retention, and transport by Caco-2 cells.
METHODS: Both dephytinized (by adding an exogenous phytase) and non-dephytinized infant cereals were digested using an in vitro digestion protocol adapted to the gastrointestinal conditions of infants younger than 6 mo. Mineral cell retention, transport, and uptake from infant cereals were measured using the soluble fraction of the simulated digestion and the Caco-2 cells.
RESULTS: Dephytinization of infant cereals significantly increased (P < 0.05) the cell uptake efficiency (from 0.66%-6.05% to 3.93%-13%), retention (from 6.04%-16.68% to 14.75%-20.14%) and transport efficiency (from 0.14%-2.21% to 1.47%-6.02%), of iron, and the uptake efficiency (from 5.0%-35.4% to 7.3%-41.6%) and retention (from 4.05%-20.53% to 14.45%-61.3%) of zinc, whereas calcium only cell uptake showed a significant increase (P < 0.05) after removing phytate from most of the samples analyzed. A positive relationship (P < 0.05) between mineral solubility and the cell uptake and transport efficiencies was observed.
CONCLUSION: Removing phytate from infant cereals had a beneficial effect on iron and zinc bioavailability when infant cereals were reconstituted with water. Since in developing countries cereal-based complementary foods for infants are usually consumed mixed with water, exogenous phytase additions could improve the nutritional value of this weaning food.
Keywords: Infant cereals, Phytate, Iron, Calcium, Zinc, Caco-2 cells, Bioavailability
INTRODUCTION
Insufficient mineral intake during infancy is responsible for many diseases which can not only influence immediate health, but may also have an adverse impact on adult health. Anaemia, rickets, osteoporosis, and immune diseases are caused by a deficiency of iron, calcium and/or zinc[1]. An adequate intake of these minerals is important for meeting infant nutritional needs[2]. Cereals are considered a rich plant source of carbohydrate, proteins, vitamins, and minerals, and are therefore usually introduced to an infant’s diet between the ages of 4 and 6 mo. However, cereals are also rich in phytate, which can decrease the bioavailability of critical nutrients such as iron, calcium, and zinc because of its high ability to chelate and precipitate minerals[3,4]. Dephytinization by adding an exogenous phytase or by activating the naturally occurring plant phytases has been proposed as a sustainable strategy for reducing mineral deficiency by increasing mineral bioavailability in infant complementary cereal-based foods[5]. An estimate of mineral bioavailability from infant cereals is important because not only must the absolute amounts of minerals be increased in the edible portions of foods, but they must also be in forms bioavailable to infants. Bioavailability should preferably be determined by in vivo testing, however, these studies could also be based on preliminary in vitro methods[6]. Caco-2 cells are human intestinal adenocarcinoma cells exhibiting biochemical and morphological characteristics of small intestinal absorptive enterocytes, and together with a simulation of gastrointestinal digestion, have been used widely for mineral bioavailability studies[7–9]. Caco-2 cells grown on microporous supports, allow the measurement of mineral uptake and transport across cell monolayers, improving the estimation of bioavailability by in vitro methods used until now (solubility and dialysis)[10,11]. In western countries, infants are usually fed with infant cereals reconstituted with follow-on formula; nevertheless in developing countries, where mineral deficiencies are particularly frequent, cereal-based complementary foods destined to infants are usually consumed mixed with water[12]. Given the importance of an adequate intake of minerals during infancy, the purpose of the current investigation was to study the effect of dephytinization on iron, calcium and zinc solubility, retention, transport, and uptake by Caco-2 cells from infant cereals when reconstituted with water, with the aim of obtaining data on mineral bioavailability from infant cereals with this kind of reconstitution.
MATERIALS AND METHODS
Chemicals
Enzymes and bile salts were purchased from Sigma Chemical Co. (St. Louis, MO): pepsin (porcine, catalogue no. P-7000), pancreatin (porcine, catalogue no. P-1750), and bile extract (porcine, catalogue no. B-8756). Pepsin solution was prepared by dissolving 1.6 g of pepsin in 10 mL of 0.1 mol/L HCl. Pancreatin-bile extract solution was prepared by dissolving 0.2 g of pancreatin and 1.25 g of bile extract in 50 mL of 0.1 mol/L NaHCO3. Millipore Milli-Q distilled-deionized water (Millipore Ibérica S.A., Barcelona, Spain) was used throughout the experiments. Cell culture media, antibiotics (penicillin and streptomycin), glucose, 4-(2-hydroxyethyl)-1-piperazineethanosulfonic acid (HEPES), 2-(N-morpholino) ethanesulfonic acid (MES), and Hank´s Balanced Salt Solution (HBSS) were obtained from Gibco BRL Life Technologies (Paisley, Scotland).
Inositol phosphate content
Inositol phosphates were determined by HPLC using a Merck Hitachi chromatograph [pump L-7100, refraction index (RI)-detector L-7490, and L-7350 column oven] according to the method of Lehrfeld[13]. Inositol phosphates were extracted from the different samples with 0.5 mol/L HCl at room temperature for 2 h. Because of the high binding capacity of inositol pentaphosphate (IP5) and inositol hexaphosphate (IP6) to minerals, we consider the sum of IP5 and IP6 to determine phytate content[4,13]. The molar ratios of phytate to iron, calcium, and zinc were calculated as the millimoles of phytate present in the sample divided by the millimoles of iron, calcium, and zinc present in the sample, respectively. To find the phytate × (Ca/Zn) molar ratio, the total amount of Ca (mmol) in 100 g of infant cereal was multiplied by the phytate/Zn molar ratio.
Samples
Both commercial and dephytinized infant cereals were dried in an oven at 120°C overnight to obtain the dry weight, and were then milled. Infant cereals were reconstituted according to the recommendations of the manufacturer: 200 mL of water was mixed with 35 g of infant cereal. The infant cereals were dephytinized using an exogenous phytase from Aspergillus oryzae (EC 3.1.3.26 from Stern-Enzym GmbH & Co. KG, Ahrensburg, Germany, 2500 PU/g). The phytase was added to the aqueous slurry at a concentration of 3.2 U/g of sample and incubated at pH 5.5 with stirring at 55°C for 20 min. The dephytinized samples were dried in an oven at 120°C overnight to obtain the dry weight, and then ground in an electrical mill to a fine powder similar to that of commercial infant cereals. Dephytinization was checked by HPLC[13].
Caco-2 cells
Caco-2 cells were obtained from the European Collection of Cell Cultures (ECACC; number 86 010 202, Salisbury, UK) and used in assays at passages 28-55. For iron, calcium, and zinc uptake assays, cells were seeded onto polycarbonate membrane chamber inserts (24 mm diameter, 0.4 μm pore size; Transwell, Costar Corp.) at a density of 50 000 cells/cm2 and allowed to differentiate on filters for 21 d. During this period, cells were maintained in minimum essential medium (MEM) with 10% v/v heat-inactivated fetal bovine serum (FBS), 1% v/v nonessential amino acids, 1% v/v L-glutamine and 1% antibiotic solution (penicillin-streptomycin) at 37°C in an incubator with 5% CO2, 95% air atmosphere and 95% relative humidity. The medium was changed every 2 d. During the cell differentiation period, monolayer formation and tight junction maturation and sealing were assessed by measuring the passage of phenol red across the monolayer according to Ferruzza et al[14]. Briefly, following three washes of cell monolayers with phosphate-buffered saline (PBS), 0.5 mL of 1 mmol/L phenol red was added in the apical compartment, whereas 1 mL of PBS was added in the basolateral compartment. After 1 h of incubation at 37°C, 0.9 mL of basolateral medium was collected, treated with 0.1 mL of 0.1 mol/L NaOH, and read at 560 nm using a molecular absorption spectrophotometer (UV-Vis, U-200, Hitachi Ltd. Tokyo, Japan) to determine the phenol red concentration. The passage of phenol red was expressed as apparent permeability (Papp) and obtained from the following formula: Papp = Ct × VBL/∆t × C0 × A, where VBL is the volume of the basolateral compartment (cm3), A is the filter area (cm2), ∆t is the time interval (s), Ct is the phenol red concentration in the basolateral compartment at the end of time interval, and C0 is the phenol red concentration in the apical compartment at time zero. Apparent permeability of monolayers at the end of differentiation period was 1.71 × 10-5 cm/s, indicating that tight junctions were functionally mature[14]. The experiments were conducted on day 21 from seeding. Microscopic examination of the cultures revealed that confluence was reached after 3-4 d of growth. Cell viability 3 h after the addition of the soluble fraction was assessed by trypan blue exclusion and was typically 85%-95%.
In vitro gastrointestinal digestion
Gastrointestinal digestion was applied to infant cereals, whether or not dephytinized, and reconstituted with deionized distilled water using the in vitro method described by Miller et al[15] with modifications aimed at reducing the amounts of the enzymes used because the gastrointestinal tract in the early stages of life is not yet fully developed[16,17]. The method consisted of two phases: gastric and intestinal. Prior to the gastric stage, the pH of 17.5 g of each infant cereal homogenized with 100 mL of deionized-distilled water was lowered to pH 4 with 6 mol/L HCl. Then, 3 g of pepsin solution was added, and the sample was incubated in a shaking water bath at 37°C and 120 strokes/min for 2 h to allow pepsin digestion. The digest was then maintained in ice for 10 min to stop pepsin digestion. For intestinal digestion, the pH of the gastric digests was raised to 5.0 by dropwise addition of NaHCO3 1 mol/L. Then a freshly prepared pancreatin-bile solution sufficient to provide 0.005 g of pancreatin and 0.03 g of bile salts/g of sample was added, and incubation was continued for 2 h. To stop intestinal digestion, the sample was kept for 10 min in an ice bath. Then the pH was adjusted to 7.2 by dropwise addition of 0.5 mol/L NaOH. The intestinal digest was heated for 4 min at 100°C to inhibit the sample proteases and then cooled by an ice bath. The gastrointestinal digest were centrifuged at 9187 × g for 30 min at 4°C. The supernatant fraction was filtered through a centrifugal filter devices with a 30 000 MW cut-off (Millipore Corporation Bedford, MA 01730, USA) and then centrifuged at 4000 × g for 90 min at 4°C using a Sorvall centrifuge (Model RC5C, with SS-34 rotor; Sorvall instruments, DuPont, Mississauga, ON, Canada). Prior to addition of the soluble fraction to the cells, glucose (5 mmol/L final concentration), HEPES (50 mmol/L final concentration), and MES (30 mmol/L final concentration) (pH 6.5-6.9) were added to make the soluble fraction similar to the culture media; and finally, water was added to adjust the osmolarity to 310 ± 10 mOsm/kg (Freezing point osmometer 030, Berlin, Germany) according to Ekmekcioglu[18]. Then the supernatants (soluble fraction) were analyzed for mineral content and used in cell uptake assays.
Uptake, retention and transport experiments
The soluble fraction obtained from gastrointestinal digestion was used to carry out uptake, retention, and transport experiments with Caco-2 cells because it is more similar to the in vivo digests[19]. Before each experiment growth medium was removed and apical and basolateral cell surfaces of the monolayers were washed three times with phosphate-buffered saline (PBS) at 37°C. One millilitre of soluble fractions was added to the apical chamber and 1.5 mL of HBSS (pH 7.4) was added to the basal chamber of each cell monolayer. After incubation, the apical samples were collected, and the monolayers were carefully washed three times with 1 mL ice-cold HBSS to remove any nonspecific-bound mineral and residual soluble fractions. Cells on filters were lysed by the addition of 0.5 mL of deionized water to each well, and then harvested; HBSS in basal chamber also was removed. Total mineral content was measured in the apical solutions, cell monolayer, and basal solutions.
Assessment of cell monolayer integrity during experiments
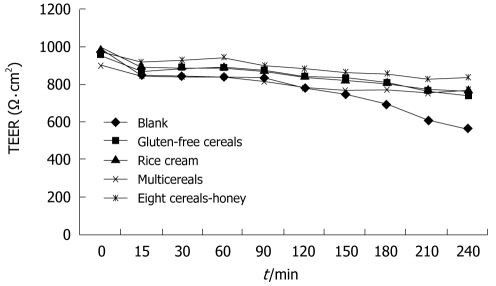
To investigate possible adverse effects of infant cereal components or digestive enzymes from the supernatants on Caco-2 cells, the integrity of the monolayer was assessed by measuring the transepithelial electrical resistance (TEER) according to the method of Okada et al[20]. At the end of differentiation period control, monolayers used for experiments had a resistance higher than 900 Ω.cm2 (Figure 1). During the uptake, retention, and transport experiments measurements of TEER were taken every 30 min. Background resistance was determined by measuring across a filter without cells in Hank’s Balanced Salt Solution (HBSS). Monolayers with resistances < 500 Ω . cm2 were discarded.
Figure 1.
TEER values of Caco-2 monolayers incubated in the presence of digested infant cereals.
Iron, calcium, and zinc determination
To estimate mineral bioavailability, iron, calcium, and zinc contents in the sample (before digestion), soluble fraction (apical solution), blank (HBSS), basal solution and in cell homogenates were determined by flame atomic absorption spectrophotometry (AAS; Perkin-Elmer, mod. 3100 Norwalk, U.S.A) according Perales et al[10] with slight modifications; dry organic matter destruction (450°C) was applied prior to analysis. An amount of lanthanum chloride sufficient to obtain a final content of 0.1% was added to eliminate phosphate interferences in the calcium determination. To dissolve the ashes, 2 mL of concentrated HCl (sp gr = 1.19) was added, and the vessel was covered with a watch glass and gently warmed (70-75°C) for 4 h, leaving about 1 mL of liquid at the end of heating. The solution was then transferred to a 10 mL volumetric flask, and the volume was completed with water. Solubility percentages were determined as follows: solubility % = 100 × S/C, where S = soluble mineral content (μg of mineral/g of sample), and C = total mineral content of the sample (μg of mineral/g of sample). Differences between the mineral content of the monolayer incubated with soluble mineral fraction and the content of monolayer not exposed (retention blank) yielded an estimation of the cellular retention (micrograms) of minerals. Transport (T) was evaluated by the difference between the mineral amount in basal chamber solutions of treated samples and HBSS. The following calculation was used for retention percentages: retention % = 100 × R/C, where R = mineral retention (μg of mineral/well), and C = mineral soluble added (μg). Transport percentages were calculated as follows: transport % = 100 × T/C, where T = cellular transport (μg of mineral/well), and C = mineral soluble added (μg)/well. The differences between the mineral content of cells cultures incubated with samples or HBSS (blank) gave an estimation of the cellular uptake (cell retention plus transport) of these mineral elements. Uptake percentage values were calculated as the percentage of the mineral applied to the Caco-2 cell monolayer which was taken up by the cells and results were used as a measure of mineral availability. Due to the differences among samples in terms of the solubility of minerals after in vitro digestion, mineral transport and uptake were normalized for solubility as follows: % transport efficiency = (% solubility × % transport)/100, % uptake efficiency = (% solubility × % uptake)/100.
Quality control of the iron, calcium, and zinc analyses
The absence of matrix interferences in AAS determi-nation of Fe and Ca in the samples was checked by the addition’s method. Community Bureau of reference material CRM-189 (wholemeal flour) (Brussels, Belgium) was used as a control to test the method for accuracy. For Fe, Ca and Zn, the measured mean values were 66.9 μg/g, 519 μg/g and 54.9 μg/g, respectively, which were in accordance with the certified range of 68.3 ± 1.9 μg/g for Fe, 520 μg/g (standard deviation non-certified) for Ca and 56.5 ± 1.7 μg/g for Zn. The detection limit was determined to be 0.6 mg/L. The method was shown to be linear (r ≥ 0.998) over the range 1-5 mg/L for both Fe (y = 2.86 × 10-3 + 4.76 × 10-2 X) and Ca (y = 2.67 × 10-3 + 5.24 × 10-2 X).
Statistical analysis
Results are reported as mean ± SD of five experiments. After testing for normality and equal variances, the mean solubility, retention, transport efficiency, and uptake efficiency percentages of Fe, Ca, and Zn from infant cereals, whether or not dephytinized, were compared by one-way analysis of variance (ANOVA) including the Tukey post-test in the data treatment to determine significant differences among means (P < 0.05). A Pearson correlation analysis was performed to investigate the possible correlation between phytate content; Fe, Ca, and Zn contents; mineral solubility (%); retention (%); uptake (%); and transport (%) by Caco-2 cells. Values of P < 0.05 were considered significant. All statistical analyses were performed with the Statistical Package for the Social Sciences (version 14.0; SPSS).
RESULTS
Inositol phosphate content
Molar ratios of phytate (IP5 + IP6) to iron, phytate to calcium and phytate to zinc, as well as the phytate × (Ca/Zn) molar ratio, are shown in Table 1. For iron, values ranged from 1.4 to 3.8; for calcium, from 0.07 to 0.18; and for zinc, 42.7 to 182.3.
Table 1.
Mineral content (per 100 g), phytate (IP5 + IP6) content (per 100 g) and molar ratios of phytate to iron, calcium and zinc, and phytate × calcium/zinc of commercial infant cereals
| Infant cereal | Fe (mg) | Ca (mg) | Zn (mg) | Phytate (mg) | Phytate/Fe | Phytate/Ca | Phytate/Zn | Phytate × Ca/Zn |
| Eight cereals-honey | 8.3 ± 0.4 | 137.3 ± 5.6 | 0.6 ± 0.3 | 319.6 ± 3.1 | 3.8 | 0.16 | 53.1 | 182.3 |
| Rice cream | 8.8 ± 0.1 | 283.1 ± 27.7 | 1.2 ± 0.2 | 167.1 ± 27.5 | 1.6 | 0.11 | 14.4 | 101.9 |
| Multicereals | 8.7 ± 0.2 | 174.4 ± 21.0 | 1.5 ± 0.4 | 143.5 ± 10.6 | 1.4 | 0.07 | 9.8 | 42.7 |
| Gluten-free cereals | 7.5 ± 1.0 | 154.4 ± 38.9 | 1.0 ± 0.3 | 299.8 ± 16.9 | 3.5 | 0.18 | 31.8 | 122.7 |
Uptake, retention and transport experiments
The results obtained in the iron retention, transport, and uptake assays by Caco-2 from infant cereals are summarized in Table 2. The iron retention percentage and transport and uptake efficiencies of eight cereals-honey, Multicereals and Gluten-free cereals after dephytinization were significantly higher (P < 0.05) than that of commercial infant cereals. The iron solubility percentage was higher from eight cereals-honey and Multicereals after dephytinization; conversely the solubility percentage of commercial rice cream was higher than that of the same sample after phytase treatment. Total iron content of each infant cereal before and after phytase treatment was equal.
Table 2.
Iron retention, transport and uptake from infant cereals by Caco-2 cells
| Infant cereal | Iron added (μg) | Solubility (%) | Retention (μg) | Retention (%) | Transport (μg) | Transport efficiency (%) | Uptake (μg) | Uptake efficiency (%) | |
| - phytase | A | 12.51 | 17.42 ± 6.1a | 1 ± 0.1 | 7.99 ± 2.2b | 1.59 ± 0.6 | 2.21 ± 1a | 2.59 ± 0.8 | 3.6 ± 0.9b |
| B | 13.08 | 8.72 ± 2.7b | 0.79 ± 0.4 | 6.04 ± 0.6b | 0.21 ± 0 | 0.14 ± 0.03c | 1 ± 0.2 | 0.66 ± 0.2d | |
| C | 13.13 | 24.21 ± 1.9ª | 2.19 ± 0.1 | 16.68 ± 3.8ª | 1.09 ± 0.7 | 2.01 ± 0.8ª | 3.28 ± 1.1 | 6.05 ± 2.2ª | |
| D | 11.18 | 20.93 ± 4.8ª | 0.78 ± 0.2 | 6.98 ± 0.8b | 0.18 ± 0.04 | 0.33 ± 0.1b | 0.96 ± 0.2 | 1.8 ± 0.3c | |
| + phytase | A | 12.51 | 34.53 ± 8.61 | 2.52 ± 0.3 | 20.14 ± 3.91 | 2.18 ± 0.9 | 6.02 ± 1.31 | 4.7 ± 0.9 | 13 ± 2.51 |
| B | 13.08 | 21.1 ± 1.81 | 2.1 ± 0.2 | 16.28 ± 2.11 | 2.49 ± 0.9 | 4.01 ± 0.81 | 4.62 ± 1.6 | 7.45 ± 3.91 | |
| C | 13.13 | 19.19 ± 31 | 2.41 ± 0.3 | 18.35 ± 3.4 | 1.66 ± 0.6 | 2.43 ± 0.6 | 4.0 ± 1.2 | 5.95 ± 1.1 | |
| D | 11.18 | 16.64 ± 6.2 | 1.65 ± 0.2 | 14.75 ± 1.31 | 0.99 ± 0.2 | 1.47 ± 0.31 | 2.64 ± 0.8 | 3.93 ± 0.71 |
A: Eight cereals honey; B: Multicereals; C: Rice cream; D: Gluten-free cereals. mean ± SD, n = 5. Different letter (a-d) denotes significant differences (P < 0.05) between commercial infant cereals (without phytase treatment) to assess the effect of different phytate content.
P vs the same infant cereal dephytinized or not.
Table 3 shows the results obtained for calcium cell retention, transport, and uptake assays by Caco-2 cells. Calcium uptake efficiency percentage was higher from infant cereals analyzed after phytase treatment with the exception of eight cerals honey; however, results were significant (P < 0.05) only for Gluten-free cereals. After phytase treatment, this infant cereal showed that solubility and transport efficiency percentages of calcium increased significantly as well. From samples not dephytinized, significant highest solubility and retention percentages of calcium were observed for eight cereals-honey, and highest solubility and transport efficiency percentages for multicereals. Total calcium content of each infant cereal before and after phytase treatment was equal.
Table 3.
Calcium retention, transport and uptake from infant cereals by Caco-2 cells
| Infant cereal | Calcium added (μg) | Solubility (%) | Retention (μg) | Retention (%) | Transport (μg) | Transport efficiency (%) | Uptake (μg) | Uptake efficiency (%) | |
| - phytase | A | 206 | 38.9 ± 11.1a,1 | 5.72 ± 0.3 | 2.78 ± 1a,1 | 25.99 ± 8 | 4.9 ± 1.2a | 31.71 ± 4.9 | 5.99 ± 2a |
| B | 261.6 | 15.2 ± 1.8b1 | 7.33 ± 0.2 | 2.8 ± 0.6ª | 16.1 ± 3.9 | 0.94 ± 0.1b,1 | 23.43 ± 3.6 | 0.66 ± 0.2b | |
| C | 424.7 | 2.8 ± 1c | 2.18 ± 0.3 | 0.51 ± 0.2b | 33.1 ± 6.1 | 0.22 ± 0.02d | 35.28 ± 7.2 | 0.23 ± 0.2c | |
| D | 231.6 | 4.53 ± 1.3c | 4.45 ± 0.2 | 1.92 ± 0.3ª | 15.64 ± 4.4 | 0.31 ± 0.08c | 20.09 ± 1.8 | 0.39 ± 0.1c | |
| + phytase | A | 206 | 22.5 ± 2.3 | 0.7 ± 0.2 | 0.34 ± 0.09 | 29.59 ± 7.2 | 3.24 ± 1 | 30.29 ± 6.8 | 3.31 ± 0.9 |
| B | 261.6 | 8.03 ± 2.7 | 7.33 ± 1.4 | 2.8 ± 0.6 | 23.08 ± 2.1 | 0.71 ± 0.04 | 30.41 ± 2.2 | 0.93 ± 0.2 | |
| C | 424.7 | 3.72 ± 1.9 | 5.5 ± 0.1 | 1.3 ± 0.7 | 28.1 ± 4.4 | 0.25 ± 0.07 | 33.6 ± 7.1 | 0.43 ± 0.09 | |
| D | 231.6 | 8.31 ± 1.21 | 2.98 ± 0.7 | 1.29 ± 0.9 | 66.16 ± 10.8 | 2.38 ± 0.11 | 69.14 ± 8.2 | 2.48 ± 0.31 |
As shown in Table 4, the effect of dephytinization caused an increase (P < 0.05) in retention and uptake efficiency percentages of zinc in most of samples analyzed. Rice cream showed an increase in solubility, transport, and uptake efficiencies percentages after phytase treatment. Significant differences on the solubility percentage of zinc were observed for Gluten-free cereals and rice cream between samples, whether or not dephytinized. Total zinc content of each infant cereal before and after phytase treatment was equal.
Table 4.
Zinc retention, transport and uptake from infant cereals by Caco-2 cells
| Infant cereal | Zinc added (μg) | Solubility (%) | Retention (μg) | Retention (%) | Transport (μg) | Transport efficiency (%) | Uptake (μg) | Uptake efficiency (%) | |
| - phytase | A | 2.12 | 36.4 ± 7.1a | 0.18 ± 0.08 | 8.5 ± 2b | 1.88 ± 0.6 | 32.3 ± 3a,1 | 2.06 ± 0.8 | 35.4 ± 4.1a |
| B | 2.22 | 18.9 ± 3.8b | 0.09 ± 0.01 | 4.05 ± 2c | 0.5 ± 0.08 | 4.25 ± 1.1c | 0.59 ± 0.6 | 5 ± 0.9d | |
| C | 2.63 | 17 ± 1.9b | 0.54 ± 0.1 | 20.53 ± 3.8a | 0.62 ± 0.1 | 4 ± 0.8c | 1.16 ± 0.4 | 7.5 ± 0.2c | |
| D | 1.63 | 37.8 ± 6.2a,1 | 0.09 ± 0.01 | 5.5 ± 1.6c | 0.71 ± 0.2 | 16.5 ± 0.3b | 0.8 ± 0.4 | 18.6 ± 1.2b | |
| + phytase | A | 2.12 | 46.9 ± 6.6 | 0.84 ± 0.2 | 22.2 ± 2.71 | 1.04 ± 0.5 | 23 ± 3.6 | 1.88 ± 0.9 | 41.6 ± 6.5 |
| B | 2.22 | 18.92 ± 2.7 | 0.42 ± 0.1 | 18.92 ± 2.81 | 0.44 ± 0.2 | 3.74 ± 0.3 | 0.86 ± 0.2 | 7.3 ± 0.21 | |
| C | 2.63 | 27.3 ± 61 | 0.38 ± 0.1 | 14.45 ± 3.4 | 1.59 ± 0.6 | 16.5 ± 2.61 | 1.97 ± 0.8 | 20.4 ± 2.11 | |
| D | 1.63 | 21 ± 4.3 | 0.18 ± 0.03 | 61.3 ± 9.91 | 1.43 ± 0.4 | 18.4 ± 4.1 | 1.61 ± 0.2 | 20.7 ± 0.61 |
With significant values at P < 0.05, a negative correlation between phytate content and retention percentages of iron and zinc (r = -0.730 and r = -0.538) and iron transport (r = -0.507), and uptake efficiency (r = -0.519) percentages were found. Calcium content showed a negative correlation with transport efficiency percentage of iron (r = -0.426) and with uptake efficiency percentage of zinc (r = -0.855). Mineral solubility percentage showed for each mineral analyzed (Fe, Ca, and Zn) a positive correlation with transport efficiency percentage (r = 0.735, r = 0.912, r = 0.732, respectively) and with uptake efficiency percentage (r = 0.794, r = 0.923, r = 0.838, respectively).
DISCUSSION
Fe, Ca, and Zn contents of infant cereals analyzed were in accordance with recommendations of the European Economic Community (Directive 2006/125)[21]. In the present study, the values found for the phytate (IP5 + IP6) to iron molar ratios were higher than 1.4. Although the critical ratio (capable of compromising bioavailability) has not yet been well established, according to Hurrell[5,12], values obtained in infant cereals of this study have the potential to compromise iron bioavailability. It is interesting to note the very low phytate to calcium molar ratio for all infant cereals analyzed; apparently phytate could not compromise Ca availability since the critical value for which the absorption of calcium is compromised has been reported to be > 0.24[4]. Three of the four infant cereals analyzed showed a phytate to zinc molar ratio above 12, a value implicated in interference with zinc bioavailability in humans[22,23]. The infant cereal named eight cereals-honey showed phytate × calcium/zinc molar ratios above the critical value within the range of 150-200, which have been associated with a decrease in zinc bioavailability.
It has been reported that removing phytate increases iron bioavailability in the Caco-2 cell in vitro model[24,25]. Since iron solubility, iron retention, transport efficiency, and uptake efficiency percentages can be used as bioavailability predictors[8,26,27], our results showed that three of the four infant cereals analyzed after phytase treatment increased iron bioavailability. The source of iron used for the enrichment of infant cereals (elemental iron) plays an important role in iron solubility[28]; in this regard, values found in our study were lower than those reported by other authors[29,30] for the same element, probably due to the phytate content or fiber components from cereals, the different pH conditions applied for the assays[29], or the previously reported the inhibitory effect of calcium on iron availability[10] since infant cereals used in this study were calcium-enriched. Differences in iron bioavailability parameters between dephytinized infant cereals could indicate that other components of infant cereals can also decrease iron bioavailability; in this regard, it has been reported that some dietary fiber components can bind mineral ions decreasing their bioavailability[30,31]. Commercial infant cereal (Multicereals) showed the highest values of iron bioavailability parameter measures which can be justified because of its lower molar ratio of phytate to iron, compared to eight cereals-honey, rice cream and Gluten-free cereals.
It should be noted that the positive correlation observed between iron solubility with cell transport and uptake efficiencies percentages found in our study is in agreement with Bergqvist et al[26], studying iron absorption from carrot juice, and with Proulx and Reddy[32] studying iron bioavailability of maize, both using Caco-2 cells. Meanwhile, it has been reported by other authors[27,33] that solubility and bioavailability by Caco-2 cells did not show parallel trends.
In our study, a lack of significant effect of dephy-tinization on calcium bioavailability parameters by Caco-2 cells was found in most of the infant cereals analyzed, although a great variability was observed. The inhibitory effect of phytate on calcium bioavailability has been reported[34–36] but only for high ratios. Our observation could be explained by the binding of calcium phytate to the membrane of Caco-2 cells. In this regard, Phyllippy[36] reported that Caco-2 cells may not be useful for studying the effects of inositol phosphates on the calcium uptake by cells. Calcium solubility from eight cereals-honey and Multicereals decreased after phytase treatment whereas Gluten-free cereals showed a higher calcium solubility after phytase treatment. Since it has been reported that calcium solubility depends on the phytate to calcium molar ratio[34] the low ratio Ca:phytate ratio (≤ 0.18) of all infant cereals analyzed could explain the lack of effect of phytase on calcium solubility. In fact, only Gluten-free cereals (the sample with the highest phytate/Ca molar ratio) showed a significant increase in transport and uptake efficiencies after dephytinization.
Percentages of soluble zinc did not show significant differences before and after dephytinization for most of the samples analyzed. Zn solubility percentages obtained (< 37.8% for commercial infant cereals; < 46.9% for dephytinized infant cereals) were lower than values obtained by Cámara et al[27] in school meals, and by Lyon[37] in cereal products. The same trend was observed in a previous analysis in our laboratory studying infant cereals[38], since a lack of phytase effect on zinc solubility was found for most of the samples studied; moreover, Kayode et al[39] observed similar results studying opaque sorghum beer. Probably, as reported Perales et al[10], the calcium added as enrichment to infant cereals that are not Zn-fortified exerted a negative effect on Zn solubility.
However, when bioavailability was evaluated in Caco-2 cells, all infant cereals analyzed showed an increase in the Zn uptake efficiency percentage after phytase treatment. Values obtained for Multicereals, rice cream and Gluten-free cereals were significant (P < 0.05). Eight cereals-honey, Multicereals and Gluten-free cereals presented a higher percentage of zinc retention after phytase treatment with respect to the same infant cereal not dephytinized. The retention and uptake efficiencies of zinc data obtained in our study clearly demonstrate that phytate impaired bioavailability, since significant differences were found between samples dephytinized and not dephytinized. The inhibitory effect of phytate on Zn bioavailability by Caco-2 cells has been previously reported[40,41].
In conclusion, dephytinization of infant cereals by an exogenous phytase resulted in increasing bioavailability parameters of iron and zinc measured in a Caco-2 cell line. However, for calcium, a lack of effect of dephytinization of infant cereals on bioavailability by Caco-2 cells was found in our study.
COMMENTS
Background
An adequate intake of minerals is particularly important for infants in the first year of life. Although breast-feeding is considered the natural and preferred method for infant feeding, after the 6th mo of age cereals are introduced to supplement breast milk. Given the importance of an adequate nutrition during infancy, a better knowledge of the gastrointestinal conditions of infants and its effects on food components affecting intestinal absorption of minerals are essential.
Research frontiers
The research hotspot is the study and improvement of mineral bioavailability from cereal foods with a different matrix under gastrointestinal conditions of infants and to obtain major knowledge about intestinal absorption of iron, calcium and zinc using the Caco-2 cell line.
Innovations and Breakthroughs
Low absorption of minerals from infant foods is considered to be a factor in the aetiology of mineral deficiencies in infants. Gastrointestinal conditions (pH and digestive enzymes) are keys in mineral absorption. Data indicate that the Caco-2 cell line is a useful tool to study iron and zinc absorption and simultaneously to characterize the effect of some food components on mineral intestinal absorption.
Applications
Better knowledge of intestinal mineral absorption process and interactions with dietary factors at a gastrointestinal level would be helpful to develop infant foods with improved mineral availability.
Terminology
Phytic acid: (my-inositol hexaphosphoric acid), a dietary factor found principally in cereals and legumes which is a potent inhibitor of mineral absorption owing to its strong ability to bind multivalent metal ions. Mineral bioavailability: the proportion of minerals that can be absorbed and used for physiological purposes.
Peer review
This is a descriptive study that shows the effect of dephytinization on the bioavailability of iron, calcium and zinc in different commercial infant cereals using the in vitro Caco-2 cell model. The manuscript is easy to understand and in general terms well written. The work described is well done.
Acknowledgments
We appreciate the technical help of Dr. Y Sambuy. We acknowledge SternEnzym for kindly providing samples of phytase for experiments. We also thank Hero España SA for providing the samples.
Supported by Fundación Séneca, 0578/PI/07, Consejería de Educación, Ciencia a Investigación de la Comunidad Autónoma de la Región de Murcia and CONSOLIDER FUN-C-FOOD. Nuevos ingredientes funcionales para mejorar la salud
Peer reviewer: Silvana Zanlungo, Professor, Departamento de Gastroenterología, Pontificia Universidad Católica de Chile, Marcoleta 367, Casilla 114-D, Santiago, Chile
S- Editor Li LF L- Editor O’Neill M E- Editor Ma WH
References
- 1.World Health Organization and Food and Agriculture Organization of the United Nations. Vitamin and mineral requirements in human nutrition. 2nd ed. (WHO/FAO), Geneve. 2004. p. 258. [Google Scholar]
- 2.Committee on Medical Aspects of Food Policy. Department of Health. Weaning and the weaning diet. Report on Health and Social Subjects Nº 45. Her Majesty’s Stationery Office: London; 1995. [Google Scholar]
- 3.Weaver CM, Kannan S. Phytate and mineral bioavailability. In: NR Reddy, SK Sathe., editors. Food Phytate. CRC Press: FL, Boca Raton; 2002. pp. 211–223. [Google Scholar]
- 4.Ma G, Li Y, Jin Y, Zhai F, Kok FJ, Yang X. Phytate intake and molar ratios of phytate to zinc, iron and calcium in the diets of people in China. Eur J Clin Nutr. 2007;61:368–374. doi: 10.1038/sj.ejcn.1602513. [DOI] [PubMed] [Google Scholar]
- 5.Hurrell RF. Phytic acid degradation as a means of improving iron absorption. Int J Vitam Nutr Res. 2004;74:445–452. doi: 10.1024/0300-9831.74.6.445. [DOI] [PubMed] [Google Scholar]
- 6.Beiseigel JM, Hunt JR, Glahn RP, Welch RM, Menkir A, Maziya-Dixon BB. Iron bioavailability from maize and beans: a comparison of human measurements with Caco-2 cell and algorithm predictions. Am J Clin Nutr. 2007;86:388–396. doi: 10.1093/ajcn/86.2.388. [DOI] [PubMed] [Google Scholar]
- 7.Etcheverry P, Wallingford JC, Miller DD, Glahn RP. Calcium, zinc, and iron bioavailabilities from a commercial human milk fortifier: a comparison study. J Dairy Sci. 2004;87:3629–3637. doi: 10.3168/jds.S0022-0302(04)73501-8. [DOI] [PubMed] [Google Scholar]
- 8.Perales S, Barbera R, Lagarda MJ, Farre R. Bioavailability of calcium from milk-based formulas and fruit juices containing milk and cereals estimated by in vitro methods (solubility, dialyzability, and uptake and transport by caco-2 cells) J Agric Food Chem. 2005;53:3721–3726. doi: 10.1021/jf047977y. [DOI] [PubMed] [Google Scholar]
- 9.Proulx AK, Reddy MB. Iron bioavailability of hemoglobin from soy root nodules using a Caco-2 cell culture model. J Agric Food Chem. 2006;54:1518–1522. doi: 10.1021/jf052268l. [DOI] [PubMed] [Google Scholar]
- 10.Perales S, Barbera R, Lagarda MJ, Farre R. Fortification of milk with calcium: effect on calcium bioavailability and interactions with iron and zinc. J Agric Food Chem. 2006;54:4901–4906. doi: 10.1021/jf0601214. [DOI] [PubMed] [Google Scholar]
- 11.Fairweather-Tait S, Phillips I, Wortley G, Harvey L, Glahn R. The use of solubility, dialyzability, and Caco-2 cell methods to predict iron bioavailability. Int J Vitam Nutr Res. 2007;77:158–165. doi: 10.1024/0300-9831.77.3.158. [DOI] [PubMed] [Google Scholar]
- 12.Hurrell RF, Reddy MB, Juillerat MA, Cook JD. Degradation of phytic acid in cereal porridges improves iron absorption by human subjects. Am J Clin Nutr. 2003;77:1213–1219. doi: 10.1093/ajcn/77.5.1213. [DOI] [PubMed] [Google Scholar]
- 13.Lehrfeld J. High-performance liquid chromatography analysis of phytic acid on a. pH-stable, macroporous polymer column. Cereal Chem. 1989;66:510–515. [Google Scholar]
- 14.Ferruzza S, Sambuy Y, Onetti-Muda A, Nobili F, Scarino ML. Copper toxicity to tight junctions in the human intestinal Caco-2 cell line. In: EJ Massaro., editor. Handbook of Copper Pharmacology and Toxicology. Humana Press: Totowa; 2002. pp. 397–416. [Google Scholar]
- 15.Miller DD, Schricker BR, Rasmussen RR, Van Campen D. An in vitro method for estimation of iron availability from meals. Am J Clin Nutr. 1981;34:2248–2256. doi: 10.1093/ajcn/34.10.2248. [DOI] [PubMed] [Google Scholar]
- 16.Bosscher D, Van Caillie-Bertrand M, Robberecht H, Van Dyck K, Van Cauwenbergh R, Deelstra H. In vitro availability of calcium, iron, and zinc from first-age infant formulae and human milk. J Pediatr Gastroenterol Nutr. 2001;32:54–58. doi: 10.1097/00005176-200101000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Jovani M, Barbera R, Farre R, Martin de Aguilera E. Calcium, iron, and zinc uptake from digests of infant formulas by Caco-2 cells. J Agric Food Chem. 2001;49:3480–3485. doi: 10.1021/jf010106t. [DOI] [PubMed] [Google Scholar]
- 18.Ekmekcioglu C. Physiological approach for preparing and conducting intestinal bioavailability studies using experimental systems. Food Chem. 2002;76:225–230. [Google Scholar]
- 19.Perales S, Barbera R, Lagarda MJ, Farre R. Bioavailability of calcium from milk-based formulas and fruit juices containing milk and cereals estimated by in vitro methods (solubility, dialyzability, and uptake and transport by caco-2 cells) J Agric Food Chem. 2005;53:3721–3726. doi: 10.1021/jf047977y. [DOI] [PubMed] [Google Scholar]
- 20.Okada T, Narai A, Matsunaga S, Fusetani N, Shimizu M. Assessment of the marine toxins by monitoring the integrity of human intestinal Caco-2 cell monolayers. Toxicol In Vitro. 2000;14:219–226. doi: 10.1016/s0887-2333(00)00014-x. [DOI] [PubMed] [Google Scholar]
- 21.OJL 339, 6.12. Commission Directive 2006/125/EC. 2006. p. 16. [Google Scholar]
- 22.Kayode AP, Nout MJ, Bakker EJ, Van Boekel MA. Evaluation of the simultaneous effects of processing parameters on the iron and zinc solubility of infant sorghum porridge by response surface methodology. J Agric Food Chem. 2006;54:4253–4259. doi: 10.1021/jf0530493. [DOI] [PubMed] [Google Scholar]
- 23.Hemalatha S, Platel K, Srinivasan K. Influence of germination and fermentation on bioaccessibility of zinc and iron from food grains. Eur J Clin Nutr. 2007;61:342–348. doi: 10.1038/sj.ejcn.1602524. [DOI] [PubMed] [Google Scholar]
- 24.He WL, Feng Y, Li XL, Yang XE. Comparison of iron uptake from reduced iron powder and FeSO4 using the Caco-2 cell model: effects of ascorbic acid, phytic acid, and pH. J Agric Food Chem. 2008;56:2637–2642. doi: 10.1021/jf0730946. [DOI] [PubMed] [Google Scholar]
- 25.Glahn RP, Wortley GM, South PK, Miller DD. Inhibition of iron uptake by phytic acid, tannic acid, and ZnCl2: studies using an in vitro digestion/Caco-2 cell model. J Agric Food Chem. 2002;50:390–395. doi: 10.1021/jf011046u. [DOI] [PubMed] [Google Scholar]
- 26.Bergqvist SW, Andlid T, Sandberg AS. Lactic acid fermentation stimulated iron absorption by Caco-2 cells is associated with increased soluble iron content in carrot juice. Br J Nutr. 2006;96:705–711. [PubMed] [Google Scholar]
- 27.Cámara F, Amaro MA, Barberá R, Clemente G. Bioaccesibility of minerals in school meals: comparison between diálisis and solubility methods. Food Chem. 2005;92:481–489. [Google Scholar]
- 28.Kapsokefalou M, Alexandropoulou I, Komaitis M, Politis I. In vitro evaluation of iron solubility and dialyzability of various iron fortificants and of iron-fortified milk products targeted for infants and toddlers. Int J Food Sci Nutr. 2005;56:293–302. doi: 10.1080/09637480500146515. [DOI] [PubMed] [Google Scholar]
- 29.García-Casal MN, Layrisse M, Peña-Rosas JP, Ramírez J, Leets I, Matus P. Iron absorption from elemental iron-fortified corn falkes in humans. Role of vitamins A and C. Nutr Res. 2003;23:451–463. [Google Scholar]
- 30.Swain JH, Newman SM, Hunt JR. Bioavailability of elemental iron powders to rats is less than bakery-grade ferrous sulfate and predicted by iron solubility and particle surface area. J Nutr. 2003;133:3546–3552. doi: 10.1093/jn/133.11.3546. [DOI] [PubMed] [Google Scholar]
- 31.Bosscher D, Van Caillie-Bertrand M, Van Cauwenbergh R, Deelstra H. Availabilities of calcium, iron, and zinc from dairy infant formulas is affected by soluble dietary fibers and modified starch fractions. Nutrition. 2003;19:641–645. doi: 10.1016/s0899-9007(03)00063-7. [DOI] [PubMed] [Google Scholar]
- 32.Proulx AK, Reddy MB. Fermentation and lactic acid addition enhance iron bioavailability of maize. J Agric Food Chem. 2007;55:2749–2754. doi: 10.1021/jf0630015. [DOI] [PubMed] [Google Scholar]
- 33.Pynaert I, Armah C, Fairweather-Tait S, Kolsteren P, van Camp J, De Henauw S. Iron solubility compared with in vitro digestion-Caco-2 cell culture method for the assessment of iron bioavailability in a processed and unprocessed complementary food for Tanzanian infants (6-12 months) Br J Nutr. 2006;95:721–726. doi: 10.1079/bjn20051722. [DOI] [PubMed] [Google Scholar]
- 34.Dendougui F, Schwedt G. In vitro analysis of binding capacity of calcium to phytic acid in different food samples. Eur Food Res Tecnol. 2004;219:409–415. [Google Scholar]
- 35.Kamchan A, Puwastien P, Sirichakwal PP, Kongkachuichai R. In vitro calcium bioavailability of vegetables, legumes and seeds. J Food Comp Anal. 2004;17:311–320. [Google Scholar]
- 36.Phillippy BQ. Transport of calcium across Caco-2 cells in the presence of inositol hexakisphosphate. Nutr Res. 2006;26:146–149. [Google Scholar]
- 37.Lyon DB. Studies on the solubility of Ca, Mg, Zn, and Cu in cereal products. Am J Clin Nutr. 1984;39:190–195. doi: 10.1093/ajcn/39.2.190. [DOI] [PubMed] [Google Scholar]
- 38.Frontela C, Haro JF, Ros G, Martinez C. Effect of dephyti-nization and follow-on formula addition on in vitro iron, calcium, and zinc availability from infant cereals. J Agric Food Chem. 2008;56:3805–38011. doi: 10.1021/jf073424m. [DOI] [PubMed] [Google Scholar]
- 39.Kayode APP, Hounhouigan JD, Nout MJR. Impact of brewing process operations on phytate, phenolic compounds and in vitros solubility of iron and zinc in opaque sorghum beer. Food Sci Tech. 2007;40:834–841. [Google Scholar]
- 40.Han O, Failla ML, Hill AD, Morris ER, Smith JC Jr. Inositol phosphates inhibit uptake and transport of iron and zinc by a human intestinal cell line. J Nutr. 1994;124:580–587. doi: 10.1093/jn/124.4.580. [DOI] [PubMed] [Google Scholar]
- 41.Hansen M, Sandstrom B, Lonnerdal B. The effect of casein phosphopeptides on zinc and calcium absorption from high phytate infant diets assessed in rat pups and Caco-2 cells. Pediatr Res. 1996;40:547–552. doi: 10.1203/00006450-199610000-00006. [DOI] [PubMed] [Google Scholar]