Abstract
AIM: To clarify whether subclassification of the type VI pit pattern on the basis of magnifying colonoscopy findings is useful in determining the type and depth of invasion of colorectal neoplasms.
METHODS: We retrospectively analyzed 272 colorectal neoplasms (117 dysplasias and 155 submucosal invasive carcinomas; 228 patients) with a type V pit pattern [type VI, n = 202; type VN, n = 70 (Kudo and Tsuruta classification system)]. We divided lesions with a type VI pit pattern into two subclasses, mildly irregular lesions and severely irregular lesions, according to the prominent and detailed magnifying colonoscopy findings. We examined the relation between these two subclasses and histology/invasion depth.
RESULTS: One hundred and four lesions (51.5%) were judged to be mildly irregular, and 98 lesions (48.5%) were judged to be severely irregular. Ninety-seven (93.3%) mildly irregular lesions showed dysplasias or submucosal invasion of less than 1000 μm (SM < 1000 μm). Fifty-five (56.1%) severely irregular lesions showed submucosal invasion equal to or deeper than 1000 μm (SM ≥ 1000 μm). Mild irregularity was found significantly more often in dysplasias or lesions with SM < 1000 μm than in lesions with SM ≥ 1000 μm (P < 0.01).
CONCLUSION: Subclassification of the type VI pit pattern is useful for identifying dysplasias or lesions with SM < 1000 μm.
Keywords: Colorectal neoplasm, Magnification, Type VI pit pattern, Depth of invasion
INTRODUCTION
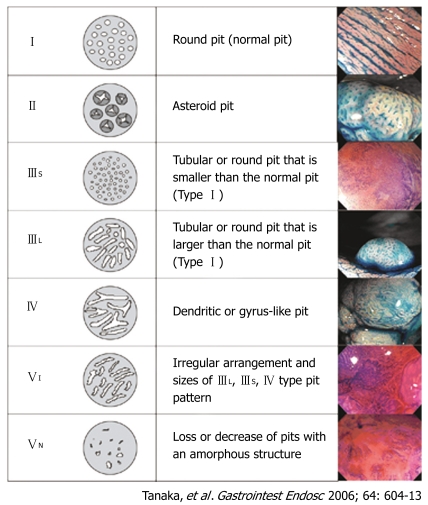
Pit pattern classification (Figure 1) of colorectal lesions, initially proposed by Kudo[1] and modified by Kudo and Tsuruta[2], is reported to be related to the histologic characteristics of the lesions[3–9]. Magnifying colonoscopy is used for differential diagnosis between non-neoplastic and neoplastic lesions[5,9–16] and for assessing the depth of invasion of early colorectal carcinoma[9,17–21].
Figure 1.
Classification of pit patterns of colorectal lesions.
Several studies have suggested that there is little risk of lymph node metastasis from early colorectal carcinoma that involves the superficial layer of the submucosa, less than 1000 μm from the muscularis mucosae[9,17,22–24]. Recently, the Japanese Society for Cancer of the Colon and Rectum proposed the following new criteria for curative histopathologic conditions after complete endoscopic mucosal resection (EMR) of submucosal carcinoma: (1) a submucosal invasion depth of less than 1000 μm (SM < 1000 μm), (2) well to moderately differentiated adenocarcinoma including the invasive portion, and (3) no vessel involvement[22,24]. In accordance with these proposed criteria, it has become important to distinguish submucosal invasion equal to or deeper than 1000 μm (SM ≥ 1000 μm) from SM < 1000 μm prior to treatment of submucosal carcinoma, to reduce the number of needless surgical resections.
Many studies have shown that the type VN pit pattern is an indicator of massive submucosal invasion of colorectal neoplasm[9]. However, colorectal neoplasms with a type VI pit pattern include various lesions, such as dysplasia and submucosal carcinoma, with either SM < 1000 μm or SM ≥ 1000 μm[9]. Thus, it is difficult to decide upon a therapeutic strategy for colorectal neoplasm on the basis of the current type VI pit pattern classification. In this study, we assessed the clinical usefulness of type VI pit pattern subclassification in determining the histology/invasion depth of colorectal neoplasms.
MATERIALS AND METHODS
We analyzed 272 colorectal neoplasms with a type V pit pattern (type VI, n = 202; type VN, n = 70). The colorectal neoplasms comprised 117 dysplasias and 155 submucosal invasive carcinomas, 129 of which were deeper than 1000 μm, resected endoscopically or surgically from 228 patients at Hiroshima University Hospital during the period January 1999 through March 2005. All lesions in this study were analyzed by five colonoscopists who were well trained in magnifying colonoscopy and blinded to the pathology findings, retrospectively.
To evaluate pit patterns, we used a magnifying colonoscope (EC-410CM, EC-450ZM, EC-450ZH or EC-450ZW, Fujinon Toshiba, Omiya, Japan; or CF-240Z or CF-H260AZI, Olympus, Tokyo, Japan) with zoom functions ranging from × 17 to × 126. When a lesion was detected by standard colonoscopic observation, the surface mucus was washed away with lukewarm water, and indigo carmine dye was spread over the lesion. This dye enhances the colonoscopic appearance because it is retained within the pits and grooves of the mucosal surface. For more precise assessment, crystal violet stain was applied to the margins of the pits, rendering each pit pattern clearly visible in all cases. The type V pit pattern was classified as one of two subtypes according to the Kudo and Tsuruta classification system[1,2] (Figure 1): type VI, irregularly arranged and similar to type IIIL, IIIS, or IV patterns in size; and type VN, an area of obvious non-structure (as per the Hakone consensus meeting in April 2004)[9,25].
Each resected neoplasm was fixed routinely with 10% buffered formalin and embedded in paraffin, after which the entire tumor was cut into serial 2- to 3-mm thick slices. Microscopic examination of hematoxylin and eosin-stained sections was performed by one pathologist unaware of other features of the case. Dysplasia was defined according to the Vienna criteria[26]. According to previously proposed measuring methods[22,24], the depth of submucosal invasion was determined using a micrometer under a microscope, and taken as the distance from the muscularis mucosae to the point of the deepest invasion (tumor apex).
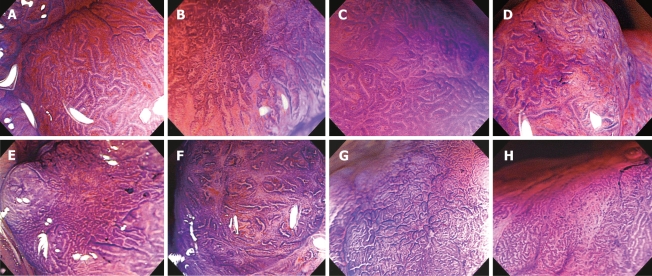
We first analyzed the relation between the type V pit pattern subtype (VI or VN) and histology/invasion depth. We then examined the relation between SM ≥ 1000 μm and histology/invasion depth and the following five detailed magnifying colonoscopy findings: (1) irregularity of the pit margins, (2) staining characteristics of the areas between pits, (3) area diameter of the type VI pit pattern (< 5 mm or ≥ 5 mm), (4) density of the pits, and (5) width of the intervening membrane between the pits (Figure 2).
Figure 2.
Magnifying features of colorectal neoplasm (crystal violet): A: Regular pit margins; B: Irregular pit margins; C: Clear staining characteristics of the areas between pits; D: Unclear staining characteristics of the areas between pits; E: High residual pit density; F: Low residual pit density; G: Narrow intervening membrane between pits; H: Wide intervening membrane between pits.
We divided the lesions with a type VI pit pattern into two subclasses (mildly irregular lesions and severely irregular lesions) according to the prominent and detailed magnifying colonoscopy findings of the first analysis. Mildly irregular lesions were defined as lesions with one or no significant magnifying colonoscopy findings, and severely irregular lesions were defined as lesions with two or more significant magnifying colonoscopy findings. This was done to diagnose SM ≥ 1000 μm on the basis of cluster analysis[27,28]. Using these data, we examined the relation between the type VI pit pattern subclassifications and histology/invasion depth, the status of the muscularis mucosae, and the presence of desmoplastic reactions at the surface of the lesion (Figure 3). The muscularis mucosae were classified as detected, partially disappeared, or disappeared, as reported previously[29]. Desmoplastic reaction of the submucosal layer was classified as absent (-), mild to moderate (+), or severe (++), as reported previously[18,30].
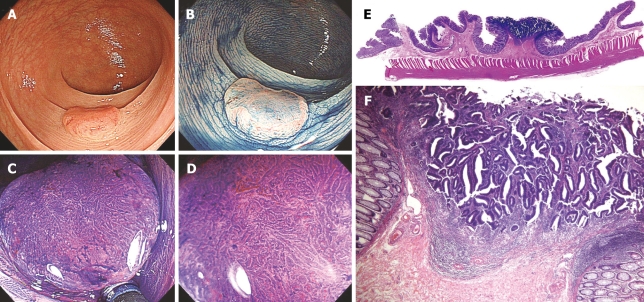
Figure 3.
Type IIa + 10IIc lesion, 12 mm in diameter. A: Standard colonoscopic view; B: Standard colonoscopic view with indigo carmine spraying; C, D: Magnifying colonoscopic picture with crystal violet staining reveals type VI pit pattern. Irregular pit margins, unclear staining characteristics of the areas between pits, > 5 mm area of type VI pit pattern, high residual pit density, and narrow intervening membrane between pits is revealed; E: Cross-section (hematoxylin-eosin, × 8) of a surgically resected specimen showing submucosal invasion (1800 μm); F: Low-power view (hematoxylin-eosin, × 40) of type VI pit pattern. Muscularis mucosae have disappeared. Desmoplastic reactions are mild to moderate. In this case, lymph node metastasis was not detected.
The associations of dysplasia, SM < 1000 μm, and SM ≥ 1000 μm with the type V pit pattern subtypes, detailed magnifying colonoscopy findings, and the type VI lesion subclasses, were analyzed by chi-square test. P < 0.05 was accepted as statistically significant. In addition, to identify predictors of SM ≥ 1000 μm, we performed multivariate logistic regression analysis. All statistical analyses were performed using JMP statistical software, version 5.0.1 J (SAS Institute Inc, Cary, NC).
RESULTS
Histology/invasion depth of colorectal neoplasm in relation to type V pit pattern subtypes
Dysplasia, SM < 1000 μm and SM ≥ 1000 μm were found in association with 57.9% (117/202), 11.4% (23/202), and 30.7% (62/202) of the neoplasms with type VI pit patterns, respectively (Table 1). Dysplasia, SM < 1000 μm, and SM ≥ 1000 μm were found in association with 0% (0/70), 4.3% (3/70), and 95.7% (67/70) of the neoplasms with type VN pit patterns, respectively. The type VN pit pattern was found significantly more often in lesions with SM ≥ 1000 than in dysplasias or lesions with SM < 1000 μm (P < 0.01). Sensitivity and specificity of the type VN pit pattern for a diagnosis of SM ≥ 1000 were 51.9% (67/129) and 97.9% (140/143), respectively.
Table 1.
Histology/invasion depth of colorectal neoplasm in relation to type V pit pattern subtypes, n (%)
| Type V pit pattern subtypes |
Histology/invasion depth |
|||
| Dysplasia | SM < 1000 μm | 1000 μm ≤ SM | ||
| VI | 202 (100) | 117 (57.9) | 23 (11.4) | 62 (30.7) |
| VN1 | 70 (100) | 0 (0) | 3 (4.3) | 67 (95.7) |
Dysplasia. SM < 1000 μm vs SM ≥ 1000 μm, P < 0.01. SM: Submucosa.
Histology/invasion depth of colorectal neoplasm with a type VI pit pattern in relation to detailed magnifying colonoscopy findings
SM ≥ 1000 μm was found in association with 58.2% (46/79) of the lesions with irregular pit margins, 57.5% (50/87) of the lesions with unclear staining characteristics of the areas between pits, 41.3% (57/138) of the lesions with a type VI pit pattern area ≥ 5 mm in diameter, 26.6% (29/109) of the lesions with high residual pit density, and 31.9% (44/138) of the lesions with a wide intervening membrane between pits (Table 2). SM ≥ 1000 μm was found significantly more often in association with irregular pit margins, unclear staining characteristics of the areas between pits, and a type VI pit pattern area ≥ 5 mm in diameter than in association with regular pit margins, clear staining characteristics of the areas between pits, and a type VI pit pattern area < 5 mm in diameter.
Table 2.
Histology/invasion depth of colorectal neoplasm with type VI pit pattern in relation to detailed magnifying colonoscopy findings, n (%)
| Magnifying |
Histology/invasion depth |
|||
| colonoscopy | ||||
| finding | Dysplasia | SM < 1000 μm | 1000 μm ≤ SM | |
| Irregular pit | 79 (100) | 23 (29.1) | 10 (12.7) | 46 (58.2) |
| margins1 | ||||
| Unclear staining | 87 (100) | 21 (24.1) | 16 (18.4) | 50 (57.5) |
| characteristics | ||||
| of the area | ||||
| between pits1 | ||||
| Area diameter | 138 (100) | 65 (47.1) | 16 (11.6) | 57 (41.3) |
| of type VI pit | ||||
| pattern ≥ 5 mm1 | ||||
| High residual | 109 (100) | 64 (58.7) | 16 (14.7) | 29 (26.6) |
| pit density | ||||
| Wide intervening | 138 (100) | 81 (58.7) | 13 (9.4) | 44 (31.9) |
| membrane | ||||
| between pits | ||||
Dysplasia. SM < 1000 μm vs SM ≥ 1000 μm, P < 0.01. SM: Submucosa.
Results of multivariate logistic regression analysis for predictors of SM ≥ 1000 μm
In multivariate logistic regression analysis, unclear staining characteristics of the areas between pits, irregular pit margins, and a VI pit pattern area diameter of ≥ 5 mm were shown to be significant predictors of SM ≥ 1000 μm (Table 3). High residual pit density and a wide intervening membrane between pits were not significant.
Table 3.
Results of multivariate logistic regression analysis for predictors of submucosal invasion deeper than 1000 μm (n = 202)
| Magnifying colonoscopy finding | Odds ratio (P value) | Relevant finding |
| Unclear staining characteristics of the areas between pits | 6.24 (< 0.0001) | Clear staining characteristics of the areas between pits |
| Irregular pit margins | 4.89 (< 0.0001) | Regular pit margins |
| Area diameter of type VI pit pattern ≥ 5 mm | 4.14 (0.0132) | Area diameter of type VI pit pattern < 5 mm |
| High residual pit density | 1.51 (0.3335) | Low residual pit density |
| Wide intervening membrane between pits | 1.02 (0.9740) | Narrow intervening membrane between pits |
Histology/invasion depth of colorectal neoplasm in relation to type VI pit pattern subclassifications
One hundred and four lesions (51.5%) were judged to be mildly irregular, and 98 lesions (48.5%) were judged to be severely irregular (Table 4). Ninety-seven (93.3%) mildly irregular lesions showed dysplasias or SM < 1000 μm. Fifty-five (56.1%) severely irregular lesions showed SM ≥ 1000 μm. Mild irregularity was found significantly more often in dysplasias or in lesions with SM < 1000 μm than in lesions with SM ≥ 1000 (P < 0.01). Sensitivity and specificity of mild irregularity for dysplasias or SM < 1000 μm were 69.3% (97/140) and 88.7% (55/62), respectively.
Table 4.
Histology/invasion depth of colorectal neoplasm in relation to type VI pit pattern subclassifications, n (%)
| Type VI pit pattern subclassification |
Histology/invasion depth |
|||
| Dysplasia | SM < 1000 μm | 1000 μm ≤ SM | ||
| Mildly irregular1 | 104 (100) | 89 (85.6) | 8 (7.7) | 7 (6.7) |
| Severely irregular | 98 (100) | 28 (28.6) | 15 (15.3) | 55 (56.1) |
Dysplasia. SM < 1000 μm vs SM ≥ 1000 μm, P < 0.01. SM: Submucosa.
Status of the muscularis mucosae in relation to type V pit patterns
The muscularis mucosae was detected in 97 (93.2%) mildly irregular lesions (Table 5). Partial disappearance or disappearance of the muscularis mucosae was seen in 60 (61.2%) severely irregular lesions and 67 (100%) lesions with a type VN pit pattern. Severe irregularity was found significantly more often in association with partial disappearance or disappearance of the muscularis mucosae than in association with detection of the muscularis mucosae (P < 0.05). The type VN pit pattern was found significantly more often in association with partial disappearance or disappearance of the muscularis mucosae than in association with detection of the muscularis mucosae (P < 0.01).
Table 5.
Status of muscularis mucosae in relation to type V pit patterns, n (%)
| Type V pit pattern |
Status of muscularis mucosae |
|||
| Detected | Partially disappeared | Disappeared | ||
| VI | ||||
| Mildly irregular1 | 104 (100) | 97 (93.2) | 6 (5.8) | 1 (1.0) |
| Severely irregulara | 98 (100) | 38 (38.8) | 31 (31.6) | 29 (29.6) |
| VNb | 67 (100) | 8 (11.9) | 59 (88.1) | |
Detected vs partially disappeared, disappeared,
P < 0.01,
P < 0.05.
Desmoplastic reactions at the surface of the lesion in relation to type V pit patterns
No desmoplastic reaction of the superficial layer was observed in 100 (96.2%) mildly irregular lesions (Table 6). Desmoplastic reactions (+)/(++) were observed in 50 (51.0%) severely irregular lesions and 67 (100%) lesions with a type VN pit pattern. The type VN pit pattern was found significantly more often in lesions with a desmoplastic reaction (+)/(++) than in lesions with desmoplastic reaction (-) (P < 0.01).
Table 6.
Desmoplastic reaction at the lesion surface in relation to type V pit pattern, n (%)
|
Desmoplastic reaction |
||||
| Type V pit pattern | Absent (-) | Mild to moderate (+) | Severe (++) | |
| VI | ||||
| Mildly irregular | 104 (100) | 100 (96.2) | 4 (3.8) | |
| Severely irregular | 98 (100) | 48 (49.0) | 29 (29.6) | 21 (21.4) |
| VNb | 67 (100) | 16 (23.9) | 51 (76.1) | |
P < 0.01 (-) vs (+), (++).
DISCUSSION
Endoscopic treatment, such as EMR, is both a therapeutic technique and an important diagnostic technique. Therefore, it is important to be able to identify lesions for which endoscopic resection would be curative to avoid meaningless endoscopic resection for lesions that should be treated surgically. Pit pattern classification is used clinically to help determine the best treatment for colorectal tumors[9]. Type I and II pit patterns predict nonneoplastic lesions, whereas type III, IV, and V pit patterns predict neoplastic lesions. Lesions with a type III or IV pit pattern are almost always dysplasias and are thus indications for endoscopic resection. Almost all lesions with a type VN pit pattern show SM ≥ 1000 µm. The reported accuracy of detection of massive submucosal invasion on the basis of the type VN pit pattern is 97%[23]. In our study, SM ≥ 1000 µm was found in 95.7% of lesions with a type VN pit pattern. Therefore, surgical resection is indicated for such lesions. By contrast, lesions with a type VI pit pattern include dysplasia and various submucosal carcinomas; thus, it is difficult to decide upon a therapeutic strategy on the basis of the current pit pattern classification system. It is necessary to analyze the type VI pit pattern in detail to determine the appropriate therapeutic strategy. The present study revealed that irregular pit margins, unclear staining characteristics of the areas between pits, and a type VI pit pattern area diameter ≥ 5 mm are significant predictors for submucosal invasion of colorectal neoplasms of 1000 μm or more.
Lesions that were subclassified as mildly irregular lesions were mainly dysplasias or lesions that showed SM < 1000 μm (93.3%). Therefore, endoscopic resection is indicated for mildly irregular lesions. On the contrary, lesions that were classified as severely irregular lesions included not only dysplasias or lesions with SM < 1000 μm (43.9%), but also lesions with SM ≥ 1000 μm (56.1%). For severely irregular lesions, the therapeutic strategy should be determined on the basis of standard endoscopic findings in conjunction with those of other modalities, such as contrast enema radiography or endoscopic ultrasonography[29,31,32]. New diagnostic modalities, such as narrow band imaging, are expected to provide more information about the invasion depth of colorectal carcinomas[33–36].
Our results revealed that there is a significant histologic difference between mildly irregular lesions and severely irregular lesions. The degree of disappearance of the muscularis mucosae increased as the pit patterns changed from VI with mildly irregularity to VI with severely irregularity to VN. If we could determine the status of the muscularis mucosae by magnifying colonoscopy, the pit pattern would be helpful in determining the depth of submucosal invasion depth by endoscopic ultrasonography. The muscularis mucosae had disappeared in all lesions with a type VN pit pattern; thus, we can measure the invasion depth from the surface of a lesion of this type to the deepest portion[29]. It has been reported that desmoplastic reactions are related to massive submucosal invasion[18]. In the present study, the incidence of desmoplastic reactions increased as the pit patterns changed from VI with mildly irregularity to VI with severely irregularity to VN. There were no desmoplastic reactions in mildly irregular lesions. These results indicate that changes in the appearance of the pits are caused by the process of submucosal infiltration of the colorectal neoplasm. Although the mechanism underlying this process is not clear, it is possible that irregular pit margins and unclear staining characteristics of the areas between pits may involve several molecular markers. We reported previously that the proliferation, infiltration and lymph node metastasis of submucosal colorectal carcinoma are significantly related to the expression of markers such as Ki-67, E-cadherins, MUC1, cathepsin D and MMP-7 at the deepest portion[37–43]. We also reported previously that MUC1 expression at the superficial layer may be related to colorectal tumors with a type V pit pattern[42]. However, there are few reports pertaining to the relation between the expression of specific molecular markers and morphogenesis of the type VI pit pattern. There may be a relation between the expression of molecular markers and detailed magnifying colonoscopy features of the type VI pit pattern. Further investigation will clarify the relation between molecular morphogenesis at the lesion surface and type VI pit pattern subclassifications.
We conclude that type VI pit pattern subclassification is useful for identifying dysplasias or lesions with SM < 1000 μm. Subclassifications can be applied to decisions about whether endoscopic treatment is indicated for colorectal neoplasms. However, we cannot identify lesions with SM ≥ 1000 μm on the basis of type VI pit pattern subclassifications.
COMMENTS
Background
Colorectal neoplasms with a type VI pit pattern include various lesions, such as dysplasias and submucosal carcinomas, with either SM < 1000 μm or SM ≥ 1000 μm. Thus, it is difficult to decide upon a therapeutic strategy for colorectal neoplasm on the basis of the current type VI pit pattern classification.
Research frontiers
In this study, we assessed the clinical usefulness of type VI pit pattern subclassification in determining the histology/invasion depth of colorectal neoplasms. There has been little study on type VI pit pattern subclassification.
Innovations and breakthroughs
Type VI pit pattern subclassification is useful for identifying dysplasias or lesions with SM < 1000 μm.
Applications
Subclassifications can be applied to deciding whether endoscopic treatment is indicated for colorectal neoplasms.
Terminology
Type VI pit pattern subclassification: We divided the lesions with a type VI pit pattern into two subclasses (mildly irregular lesions and severely irregular lesions) according to the prominent detailed magnifying colonoscopy findings of the first analysis. Mildly irregular lesions were defined as lesions with one or no significant magnifying colonoscopy findings, and severely irregular lesions were defined as lesions with two or more significant magnifying colonoscopy findings.
Peer review
The authors retrospectively investigated whether subclassification of the type VI pit pattern on the basis of magnifying colonoscopy findings was useful in determining the type and depth of invasion of colorectal neoplasm. They concluded that subclassification of the type VI pit pattern is useful for identifying dysplasias or lesions with SM < 1000 μm.
Supported by a grant from the Japanese Society of Gastro-enterological Endoscopy, Chugoku Branch
S- Editor Zhu LH L- Editor McGowan D E- Editor Lu W
References
- 1.Kudo S, Hirota S, Nakajima T, Hosobe S, Kusaka H, Kobayashi T, Himori M, Yagyuu A. Colorectal tumours and pit pattern. J Clin Pathol. 1994;47:880–885. doi: 10.1136/jcp.47.10.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Imai Y, Kudo S, Tsuruta O, Fujii T, Hayashi S, Tanaka S, Terai T. Problems and clinical significance of V type pit pattern diagnosis: report on round-table consensus meeting [in Japanese with English abstract] Early Colorectal Cancer. 2001;5:595–613. [Google Scholar]
- 3.Kudo S, Tamura S, Nakajima T, Yamano H, Kusaka H, Watanabe H. Diagnosis of colorectal tumorous lesions by magnifying endoscopy. Gastrointest Endosc. 1996;44:8–14. doi: 10.1016/s0016-5107(96)70222-5. [DOI] [PubMed] [Google Scholar]
- 4.Kudo S, Kashida H, Tamura T, Kogure E, Imai Y, Yamano H, Hart AR. Colonoscopic diagnosis and management of nonpolypoid early colorectal cancer. World J Surg. 2000;24:1081–1090. doi: 10.1007/s002680010154. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka S, Haruma K, Ito M, Nagata S, Oh-e H, Hirota Y, Kunihiro M, Kitadai Y, Yosihara M, Sumii K, et al. Detailed colonoscopy for detecting early superficial carcinoma: recent developments. J Gastroenterol. 2000;35 Suppl 12:121–125. [PubMed] [Google Scholar]
- 6.Fujii T, Hasegawa RT, Saitoh Y, Fleischer D, Saito Y, Sano Y, Kato S. Chromoscopy during colonoscopy. Endoscopy. 2001;33:1036–1041. doi: 10.1055/s-0036-1588009. [DOI] [PubMed] [Google Scholar]
- 7.Hurlstone DP, Cross SS, Slater R, Sanders DS, Brown S. Detecting diminutive colorectal lesions at colonoscopy: a randomised controlled trial of pan-colonic versus targeted chromoscopy. Gut. 2004;53:376–380. doi: 10.1136/gut.2003.029868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hurlstone DP, Fujii T. Practical uses of chromoendoscopy and magnification at colonoscopy. Gastrointest Endosc Clin N Am. 2005;15:687–702. doi: 10.1016/j.giec.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka S, Kaltenbach T, Chayama K, Soetikno R. High-magnification colonoscopy (with videos) Gastrointest Endosc. 2006;64:604–613. doi: 10.1016/j.gie.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Togashi K, Konishi F, Ishizuka T, Sato T, Senba S, Kanazawa K. Efficacy of magnifying endoscopy in the differential diagnosis of neoplastic and non-neoplastic polyps of the large bowel. Dis Colon Rectum. 1999;42:1602–1608. doi: 10.1007/BF02236215. [DOI] [PubMed] [Google Scholar]
- 11.Kiesslich R, von Bergh M, Hahn M, Hermann G, Jung M. Chromoendoscopy with indigocarmine improves the detection of adenomatous and nonadenomatous lesions in the colon. Endoscopy. 2001;33:1001–1006. doi: 10.1055/s-2001-18932. [DOI] [PubMed] [Google Scholar]
- 12.Tung SY, Wu CS, Su MY. Magnifying colonoscopy in differentiating neoplastic from nonneoplastic colorectal lesions. Am J Gastroenterol. 2001;96:2628–2632. doi: 10.1111/j.1572-0241.2001.04120.x. [DOI] [PubMed] [Google Scholar]
- 13.Kato S, Fujii T, Koba I, Sano Y, Fu KI, Parra-Blanco A, Tajiri H, Yoshida S, Rembacken B. Assessment of colorectal lesions using magnifying colonoscopy and mucosal dye spraying: can significant lesions be distinguished? Endoscopy. 2001;33:306–310. doi: 10.1055/s-2001-13700. [DOI] [PubMed] [Google Scholar]
- 14.Konishi K, Kaneko K, Kurahashi T, Yamamoto T, Kushima M, Kanda A, Tajiri H, Mitamura K. A comparison of magnifying and nonmagnifying colonoscopy for diagnosis of colorectal polyps: A prospective study. Gastrointest Endosc. 2003;57:48–53. doi: 10.1067/mge.2003.31. [DOI] [PubMed] [Google Scholar]
- 15.Hurlstone DP, Cross SS, Adam I, Shorthouse AJ, Brown S, Sanders DS, Lobo AJ. Efficacy of high magnification chromoscopic colonoscopy for the diagnosis of neoplasia in flat and depressed lesions of the colorectum: a prospective analysis. Gut. 2004;53:284–290. doi: 10.1136/gut.2003.027623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu KI, Sano Y, Kato S, Fujii T, Nagashima F, Yoshino T, Okuno T, Yoshida S, Fujimori T. Chromoendoscopy using indigo carmine dye spraying with magnifying observation is the most reliable method for differential diagnosis between non-neoplastic and neoplastic colorectal lesions: a prospective study. Endoscopy. 2004;36:1089–1093. doi: 10.1055/s-2004-826039. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto S, Watanabe M, Hasegawa H, Baba H, Yoshinare K, Shiraishi J, Kitajima M. The risk of lymph node metastasis in T1 colorectal carcinoma. Hepatogastroenterology. 2004;51:998–1000. [PubMed] [Google Scholar]
- 18.Nagata S, Tanaka S, Haruma K, Yoshihara M, Sumii K, Kajiyama G, Shimamoto F. Pit pattern diagnosis of early colorectal carcinoma by magnifying colonoscopy: clinical and histological implications. Int J Oncol. 2000;16:927–934. doi: 10.3892/ijo.16.5.927. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka S, Haruma K, Oh-E H, Nagata S, Hirota Y, Furudoi A, Amioka T, Kitadai Y, Yoshihara M, Shimamoto F. Conditions of curability after endoscopic resection for colorectal carcinoma with submucosally massive invasion. Oncol Rep. 2000;7:783–788. doi: 10.3892/or.7.4.783. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka S, Haruma K, Nagata S, Shiro Oka, Kazuaki Chayama. Diagnosis of invasion depth in early colorectal carcinoma by pit pattern analysis with magnifying endoscopy. Dig Endosc. 2001;13S:s2–s5. [Google Scholar]
- 21.Tanaka S, Nagata S, Oka S, Kuwai T, Tamura T, Kitadai Y, Sumii M, Yoshihara M, Haruma K, Chayama K. Determining depth of invasion by VN pit pattern analysis in submucosal colorectal carcinoma. Oncol Rep. 2002;9:1005–1008. [PubMed] [Google Scholar]
- 22.Kitajima K, Fujimori T, Fujii S, Takeda J, Ohkura Y, Kawamata H, Kumamoto T, Ishiguro S, Kato Y, Shimoda T, et al. Correlations between lymph node metastasis and depth of submucosal invasion in submucosal invasive colorectal carcinoma: a Japanese collaborative study. J Gastroenterol. 2004;39:534–543. doi: 10.1007/s00535-004-1339-4. [DOI] [PubMed] [Google Scholar]
- 23.Oka S, Tanaka S, Kaneko I, Mouri R, Chayama K. Diagnosis of the invasion depth using magnifying videocolonoscopy in early colorectal carcinoma [in Japanese with English abstract] Early Colorectal Cancer. 2005;9:161–168. [Google Scholar]
- 24.Ueno H, Mochizuki H, Hashiguchi Y, Shimazaki H, Aida S, Hase K, Matsukuma S, Kanai T, Kurihara H, Ozawa K, et al. Risk factors for an adverse outcome in early invasive colorectal carcinoma. Gastroenterology. 2004;127:385–394. doi: 10.1053/j.gastro.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 25.Kudo S, Kurahashi T, Kashida H, Ohtsuka K, Takeuchi T, Fukami S, Tanaka J, Ishida F, Endou S, Kigure E, et al. Diagnosis of depth of invasion of colorectal lesions using magnifying colonoscopy [in Japanese with English abstract] Stomach Intestine. 2004;39:747–752. [Google Scholar]
- 26.Schlemper RJ, Riddell RH, Kato Y, Borchard F, Cooper HS, Dawsey SM, Dixon MF, Fenoglio-Preiser CM, Flejou JF, Geboes K, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251–255. doi: 10.1136/gut.47.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hermsen M, Postma C, Baak J, Weiss M, Rapallo A, Sciutto A, Roemen G, Arends JW, Williams R, Giaretti W, et al. Colorectal adenoma to carcinoma progression follows multiple pathways of chromosomal instability. Gastroenterology. 2002;123:1109–1119. doi: 10.1053/gast.2002.36051. [DOI] [PubMed] [Google Scholar]
- 28.Selaru FM, Xu Y, Yin J, Zou T, Liu TC, Mori Y, Abraham JM, Sato F, Wang S, Twigg C, et al. Artificial neural networks distinguish among subtypes of neoplastic colorectal lesions. Gastroenterology. 2002;122:606–613. doi: 10.1053/gast.2002.31904. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka S, Yoshida S, Chayama K. Clinical usefulness of high-frequency ultrasound probes for new invasion depth diagnosis in submucosal colorectal carcinoma. Dig Endosc. 2004;16:161–164. [Google Scholar]
- 30.Oka S, Tanaka S, Nagata S, Masanori Ito, Yasuhiko Kitadai, Fumio Shimamoto, Masaharu Yosihara, Kazuaki Chayama. Relationship between histopathological features and type V pit pattern determined by magnifying videocolonoscopy in early colorectal carcinoma. Dig Endosc. 2005;17:117–122. [Google Scholar]
- 31.Waxman I, Saitoh Y, Raju GS, Watari J, Yokota K, Reeves AL, Kohgo Y. High-frequency probe EUS-assisted endoscopic mucosal resection: a therapeutic strategy for submucosal tumors of the GI tract. Gastrointest Endosc. 2002;55:44–49. doi: 10.1067/mge.2002.119871. [DOI] [PubMed] [Google Scholar]
- 32.Matsumoto T, Hizawa K, Esaki M, Kurahara K, Mizuno M, Hirakawa K, Yao T, Iida M. Comparison of EUS and magnifying colonoscopy for assessment of small colorectal cancers. Gastrointest Endosc. 2002;56:354–360. doi: 10.1016/s0016-5107(02)70038-2. [DOI] [PubMed] [Google Scholar]
- 33.Gono K, Obi T, Yamaguchi M, Ohyama N, Machida H, Sano Y, Yoshida S, Hamamoto Y, Endo T. Appearance of enhanced tissue features in narrow-band endoscopic imaging. J Biomed Opt. 2004;9:568–577. doi: 10.1117/1.1695563. [DOI] [PubMed] [Google Scholar]
- 34.Machida H, Sano Y, Hamamoto Y, Muto M, Kozu T, Tajiri H, Yoshida S. Narrow-band imaging in the diagnosis of colorectal mucosal lesions: a pilot study. Endoscopy. 2004;36:1094–1098. doi: 10.1055/s-2004-826040. [DOI] [PubMed] [Google Scholar]
- 35.Sano Y, Horimatsu T, Fu K, Katagiri A, Muto M, Ishikawa H. Magnifying observation of microvascular architecture of colorectal lesions using a narrow-band imaging system. 2006;2:168–179. [Google Scholar]
- 36.Hirata M, Tanaka S, Oka S, Kaneko I, Yoshida S, Yoshihara M, Chayama K. Magnifying endoscopy with narrow band imaging for diagnosis of colorectal tumors. Gastrointest Endosc. 2007;65:988–995. doi: 10.1016/j.gie.2006.07.046. [DOI] [PubMed] [Google Scholar]
- 37.Tanaka S, Haruma K, Tatsuta S, Hiraga Y, Teixeira CR, Shimamoto F, Yoshihara M, Sumii K, Kajiyama G. Proliferating cell nuclear antigen expression correlates with the metastatic potential of submucosal invasive colorectal carcinoma. Oncology. 1995;52:134–139. doi: 10.1159/000227444. [DOI] [PubMed] [Google Scholar]
- 38.Aoki R, Tanaka S, Haruma K, Yoshihara M, Sumii K, Kajiyama G, Shimamoto F, Kohno N. MUC-1 expression as a predictor of the curative endoscopic treatment of submucosally invasive colorectal carcinoma. Dis Colon Rectum. 1998;41:1262–1272. doi: 10.1007/BF02258227. [DOI] [PubMed] [Google Scholar]
- 39.Hiraga Y, Tanaka S, Haruma K, Yoshihara M, Sumii K, Kajiyama G, Shimamoto F, Kohno N. Immunoreactive MUC1 expression at the deepest invasive portion correlates with prognosis of colorectal cancer. Oncology. 1998;55:307–319. doi: 10.1159/000011868. [DOI] [PubMed] [Google Scholar]
- 40.Kimura T, Tanaka S, Haruma K, Sumii K, Kajiyama G, Shimamoto F, Kohno N. Clinical significance of MUC1 and E-cadherin expression, cellular proliferation, and angiogenesis at the deepest invasive portion of colorectal cancer. Int J Oncol. 2000;16:55–64. doi: 10.3892/ijo.16.1.55. [DOI] [PubMed] [Google Scholar]
- 41.Oh-e H, Tanaka S, Kitadai Y, Shimamoto F, Yoshihara M, Haruma K. Cathepsin D expression as a possible predictor of lymph node metastasis in submucosal colorectal cancer. Eur J Cancer. 2001;37:180–188. doi: 10.1016/s0959-8049(00)00348-8. [DOI] [PubMed] [Google Scholar]
- 42.Nagata S, Tanaka S, Haruma K, Kitadai Y, Yoshihara M, Shimamoto F, Kohno N, Chayama K. MUC1 and cathepsin D expression in early colorectal carcinoma showing V type pit pattern. Int J Oncol. 2001;19:665–672. doi: 10.3892/ijo.19.4.665. [DOI] [PubMed] [Google Scholar]
- 43.Kaneko I, Tanaka S, Oka S, Kawamura T, Hiyama T, Ito M, Yoshihara M, Shimamoto F, Chayama K. Lymphatic vessel density at the site of deepest penetration as a predictor of lymph node metastasis in submucosal colorectal cancer. Dis Colon Rectum. 2007;50:13–21. doi: 10.1007/s10350-006-0745-5. [DOI] [PubMed] [Google Scholar]