Abstract
G protein-coupled receptor kinase 2 (GRK2) was initially identified as a key player, together with β-arrestins, in the regulation of multiple G protein-coupled receptors (GPCR). Further research has revealed a complex GRK2 interactome, that includes a variety of proteins related to cell motility, and a role for GRK2 kinase activity in inhibiting chemokine-induced immune cell migration. In addition, we have recently reported that GRK2 positively regulates integrin and sphingosine-1-phosphate-dependent motility in epithelial cell types and fibroblasts, acting as a scaffold molecule. We suggest that the positive or negative correlation of GRK2 levels with cell migration would depend on the cell type, specific stimuli acting through plasma membrane receptors, or on the signalling context, leading to differential networks of interaction of GRK2 with cell migration-related signalosomes.
Key words: GRK2, GPCR, GIT1, cell migration, integrins, chemokines
Cell migration requires the spatio-temporal integration of information arising from mechanical cues and from diffusible molecules acting either trough G protein-coupled receptors (GPCR), such as chemokines or sphingosine-1-phosphate (S1P), or tyrosine-kinase growth factor receptors. Aberrant cell migration takes place in chronic inflammatory disorders, tumor invasion and metastasis, impaired wound healing or other diseases.1
G protein-coupled receptor kinases (GRKs) participate together with arrestins in the regulation of multiple GPCR.2 The GRKs constitute a group of serine/threonine protein kinases that specifically recognize and phosphorylate agonist-activated GPCRs.3,4 Regulatory proteins termed β-arrestins then bind to the phosphorylated receptor, leading first to uncoupling of the receptor from the G protein (a process known as desensitization), and later engaging the machinery required for the transient internalization of GPCRs.5 β-arrestins can also act as scaffold proteins that bring different signalling molecules into the receptor complex, such as c-Src, different components of MAPK cascades, the cAMP phosphodiesterase PDE4, the Ral-GDS regulator of the cytoskeleton, components of the PI3K-AKT and NFκB signaling pathways or the Mdm2 ubiquitin ligase, among others.6,7 Therefore, GRK-mediated arrestin recruitment is critical for triggering the modulation of important intracellular signaling cascades by GPCR.
In addition, a growing number of non-GPCR substrates and interacting proteins are being identified for GRKs, particularly for the ubiquitous GRK2 isoform. Interestingly, many of these proteins are potentially related to cell migration processes. Novel substrates include tubulin, the ezrin-radexin-moesin (ERM) family protein ezrin, or the p38 MAPK.8,9 On top of such phosphorylation-dependent processes, GRK2 may also contribute to modulate cellular responses in a phosphorylation-independent manner thanks to its ability to interact with a variety of proteins involved in signalling and trafficking, such as Gαq and Gβγ subunits, PI3K, clathrin, GIT, caveolin, MEK or AKT (reviewed in refs. 7 and 9). Therefore, both arrestins and GRKs may terminate certain signals while triggering the propagation of others by helping assemble macromolecular signalosomes in the receptor environment, thus acting as agonist-regulated adaptor scaffolds.3,7,9
A relationship between GRK2 levels and migration was first reported in immune cells. Several members of the GRK family are highly expressed in different cellular types of the immune system, and many reports have shown the involvement of GRKs in the desensitization and internalization of chemokine receptors such as CCR5, CCR2, CXCR4, CXCR2 and chemotactic receptors for substance P or formyl-peptides, (reviewed in refs. 10 and 11). Consistent with the “classical” role of GRKs in GPCR desensitization (i.e., as negative regulators of GPCR signalling) it has been shown that the 50% reduction of GRK2 levels observed in T cells isolated from GRK2 hemizygous mice correlates with a significant increase in the “in vitro” chemotactic response of these cells to CCR5 agonists, as well as in the agonist-induced calcium mobilization and in signalling to the AKT and ERK1/2 cascades triggered through CCR5 or CXCR4 receptors.10,12,13 Interestingly, neutrophils from GRK6-deficient mice show an enhanced chemotactic response to leukotriene agonists14 or to CCL12,15 although these cells display an impaired mobilization in response to growth factors such as G-colony-stimulating factor (CSF), suggesting a stimuli-dependent chemotaxis regulation by GRK6.15 It has also been described that GRK2 and GRK5 transcription is downregulated upon LPS-mediated activation of the Toll-like receptor (TLR)-4 pathway, leading to reduced chemokine receptor desensitization and enhanced migratory response of polymorphonuclear leukocytes.16 Taken together, these observations suggest an important role of GRKs in the motility of cells of the immune system. Interestingly, a significant decrease (∼55%) in GRK2 and GRK6 protein expression levels and kinase activity as compared to healthy subjects has been reported in peripheral blood mononuclear cells of patients with rheumatoid arthritis and multiple sclerosis (reviewed in refs. 10 and 11). what suggests that altered GRK levels may play an important role in the development and progression of inflammatory diseases.
In contrast to such negative correlation between GRK2 levels and immune cell migration in response to specific signals, in line with the established role of GRK2 as a GPCR-desensitizing molecule, we have recently shown that GRK2 positively regulates integrin-dependent motility in epithelial cell types and fibroblasts.17 Such effect is specific for the engagement of integrin receptors to fibronectin and involves the GRK2-dependent modulation of the Rac/PAK/MEK/ERK1/2 pathway. The extent and duration of activation of such signalling module is critical in cell motility, as it promotes turnover of focal adhesions by modifying paxillin, calpain or myosin-light-chain kinase (MLCK) activities and membrane protrusions by stimulating cortical actin polymerization. We find that increased GRK2 expression both weakens adhesion strength and promotes cortical actin rearrangements at the cell periphery in concert with a reduction in stress fibers. Importantly, we determined that fibronectin-induced migration involves the paracrine/autocrine activation of Gi-coupled sphingosine-1-phosphate (S1P) receptors, since this process is inhibited by pertussis toxin, an S1P1/S1P3 receptor antagonist or sphingosine kinase inhibitors that cause S1P cellular depletion. We have further shown that increased GRK2 protein levels promote enhanced ERK1/2 signalling downstream integrin receptor occupancy in a S1P receptor-dependent manner. Thus, GRK2 has a positive role in S1P receptor-dependent signalling, which is critical for the functional cooperation of integrins and S1P receptors that underlies efficient migration in epithelial cells.
Interestingly, such GRK2 effects on integrin/S1P-mediated migration do not require its catalytic activity but just its recruitment to the plasma membrane, pointing to a scaffold function for GRK2 at specific cellular locations. In keeping with this notion, a membrane-targeted kinase mutant strongly enhances cell motility, and GRK2 is present at the leading edge of polarized/migrating epithelial cells in wound-healing assays (unpublished observations). We have further characterized that the pro-migratory scaffolding role of GRK2 depends on its interaction with GIT1 (Fig. 1). This protein was initially characterized as a GRK2-interacting factor involved in the regulation of GPCR internalization by means of its GTPase-activating protein activity for the small G protein ARF6.18 Further research has shown that GIT1 plays a central role in cell motility by acting as an adaptor protein that recruits active Rac in close proximity to its downstream effector PAK, leading to PAK activation both at focal adhesions and at the cell leading edge.19 GIT1 also acts as a scaffold for MEK/ERK1/2 activation at focal adhesions, thus promoting their disassembly and cell motility.20
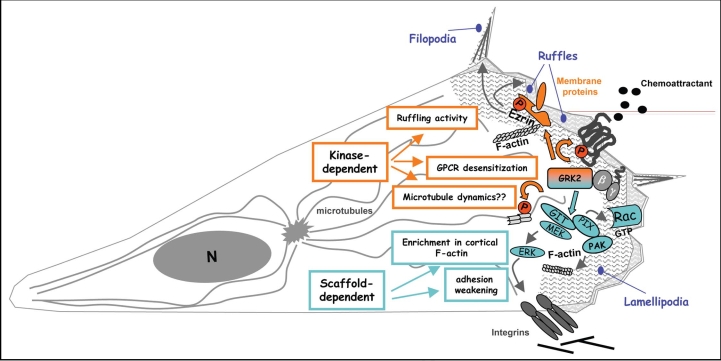
Figure 1.
The scheme shows some of the potential kinase activity-dependent and scaffold-dependent mechanisms by which GRK2 may modulate epithelial cell migration. In response to pro-migratory stimuli acting through GPCRs, GRK2 would be recruited in a Gβγ-dependent manner to sites of the plasma membrane wherein chemotactic activation is taking place. At such specific locations, GRK2 could interact with different signalling complexes involved in cell migration in a receptor- and cell-type specific manner, what might drive different migratory responses. In response to Gi-coupled S1P receptors, GRK2 dynamically interacts with the adaptor protein GIT1 in epithelial cells and fibroblasts.17 Acting as a scaffold molecule, GRK2 facilitates the localized activation of the Rac/PAK/MEK/ERK pathway, leading to increased focal adhesion (FA) turnover and F-actin polymerization at the leading edge, events that are critical for cell motility and invasion. On the other hand, many chemokine receptors promote the phosphorylation of the receptor itself by GRK2 leading to desensitization of G-protein-dependent signalling pathways, what might be instrumental for the maintenance of motility. Finally, some chemoatractants can trigger GRK2-mediated phosphorylation of ezrin and tubulin. Phosphorylation-dependent activation of ezrin, a molecule that links the actin cytoskeleton to the plasma membrane in order to promote membrane ruffles and filopodia among other structures, is essential for locomotion of cells adopting an amoeboid-like migration mode, while microtubule dynamics might affect cell polarization or protrusive activity in different cell types. See main text for details.
We have unveiled that the transient association of GIT1 with GRK2 upon cell adhesion or S1P challenge is critical for proper ERK1/2 activation and efficient cell migration. The transient interaction of GRK2 with GIT1 relies on the dynamic phosphorylation of GRK2: c-Src-mediated tyrosine phosphorylation enhances binding to GIT1, whereas the interaction is disrupted by ERK-dependent phosphorylation at Serine670. Therefore, the sequential and/or localized stimulation of signalling pathways (c-Src and ERK1/2) with opposing effects on GRK2/GIT binding would lead to a dynamic association/dissociation of both proteins in response to S1P or during adhesion.17 The mechanism(s) by which dynamic GRK2 association facilitates GIT1-dependent Rac1 activation and downstream signalling to ERK1/2 remains to be established. Interestingly, a recent report has suggested a mechanism whereby transient inactivation of an ARF6 GTPase could influence the activity of Rac1.21 In such model, in response to pro-migratory stimuli, active-GTP-loaded ARF6 would bring inactive Rac1 in proximity to activated receptors in order to promote Rac1 activation via recruitment of its exchange factor β-PIX, which directly interacts with GIT1. It is tempting to speculate that the association of GRK2 with GIT1 would promote the efficient translocation of this ARF-GAP (GTPase-activating protein) activity to the membrane (where active ARF6 is located), thereby facilitating Rac1 activation.
Overall, we propose a model in which integrin/S1P stimulation would cause Gβγ-dependent translocation of GRK2 to the plasma membrane and GRK2-mediated recruitment of GIT1, in a phosphotyrosine-dependent manner, to sites wherein chemotactic activation is taking place. Such events would facilitate Rac1-dependent PAK1 activation and localized ERK1/2 stimulation. Active ERK at the cell leading edge would promote turnover of integrin-mediated adhesive structures, required for directional movement, by phosphorylating paxillin, Focal adhesion kinase (FAK) or calpain, among other molecules. Adequate migration would also require the disassembly of GRK2/GIT1 complexes by means of localized phosphorylation of GRK2 by ERK at Serine670. This model predicts that alterations in either GRK2 protein levels or its phosphorylation status would compromise, in a GIT1-dependent manner, ERK1/2 activation in response to both S1P and fibronectin as well as cellular migration in different pathophysiological contexts. Indeed, we have shown that cells expressing GRK2 phosphorylation mutants display altered migration, as do mouse embryonic fibroblasts (MEFs) isolated from hemizygous GRK2 mice. Moreover, these animals exhibit decreased migration of skin epithelial cells, what results in delayed in vivo re-epithelization in wound healing assays, a process where S1P is actively released by stromal cells and the fibronectin receptor α5β1 is upregulated in keratinocytes.17
It would be of interest to determine whether the interaction of GIT1 with other members of the GRK family18 or of GRK2 with other GIT1-related proteins with ARF-GAP activity, such as GIT2, have similar consequences on cell adhesion and migration. On the other hand, although GIT1 is widely expressed in tissues and cell types (except neutrophils, that only express GIT2), it would be worth adressing whether in cells expressing lower levels of GIT1, the scaffold component of the regulatory role of GRK2 in cell migration is lost or decreased, so the component based in GRK activity towards GPCR (desensitization) would prevail.
Whether GRK2 positively modulates migration in cancer cells through the scaffold-dependent modulation of GIT1 is also an interesting new avenue of research. Migration of many cancer cell types often involves integrin signalling, dynamic focal contact formation and activation of Rho and Rac-dependent signalling pathways. Altered expression of several GRKs has started to be reported in different tumors.22 Protein levels and/or activity of GRK2 have been found elevated in human granulosa cell tumors, thyroid cancer (including papillary, follicular and anaplastic types) as well as in malignant mammary epithelial cells23 and breast carcinomas (our unpublished observations), while a GRK2 decrease has been reported in a subgroup of patients with primary prostate tumors.24 Interestingly, high levels of GRK2 reduced cell proliferation in poorly differentiated thyroid cell lines,25 thereby suggesting that other cellular processes relevant for tumor progression, such as migration/invasion might be favored by GRK2 expression. However, a straightforward anticipation of how GRK2 levels may affect migration of transformed cells is complicated by the fact that many different environmental factors, in addition to S1P/integrins, can trigger cancer-cell motility, including growth factors (insulin-related growth factor, epidermal growth factor), cytokines, protease-activated receptors and chemokines. It is worth noting that, besides their functions in the immune system, chemokines and their receptors play a critical role in tumor initiation, promotion and progression.26 Tumor cells often overexpress a variety of chemokine receptors (such as CXCR4 or CXCR2 in melanoma and breast, ovarian and lung cancer) that control their motility and survival.26–28 It is possible that pro-migratory signalling pathways downstream such chemokine receptors could be “desensitized” by GRK2 as occurs in some immune cell types such as lymphocytes, so enhanced GRK2 levels would decrease migration and, conversely, decreased GRK2 expression would favor motility. However, it may well be that in transformed epithelial cells chemokine receptors would engage different signal transduction routes to promote motility, which will be not desensitized by GRK2 in the “classical” manner. For instance, the potential association of GRK2 with GIT1, or with other signalling partners, in response to these chemokine factors could contribute to weaken adhesion strength and to the formation of actin-rich protrusions in cooperation with activated integrins. The effect of altered GRK2 levels on cell migration in response to specific stimuli awaits future investigation.
In this regard, the notion is emerging that different cells can adopt distinct morphological and molecular strategies in order to migrate. Thus, cancer cells move mainly in either a mesenchymal or an amoeboid mode.29 Such diversity is also observed in non-transformed cells, with fibroblasts adopting a mesenchymal-like motility and lymphocytes or neutrophils showing an amoeboid migration mode. These “variants” of cell locomotion differ in their morphological features and in the repertoire of signalling molecules required. Amoeboid movement relies on Rho and Rho-kinase (ROCK) activities but not on peri-cellular proteolysis and is largely independent of integrin-mediated adhesion, while mesenchymal motility requires activation of Rac and protrusions at the leading edge, integrin-dependent adhesion and actomyosin-mediated contractility controlled by Rho/ROCK or MLCK activities.
Interestingly, the rounded-type cell motility mode requires the localized activity of ezrin in the direction of cell movement in a Rho- and ROCK-dependent manner.30 Ezrin, a member of the ezrin-radixin-moesin (ERM) family of proteins that link the actin cytoskeleton to the plasma membrane, is required for peripheral actin assembly leading to formation of different F-actin rich protrusions, such as membrane ruffles or filopodia involved in cell migration. It has been reported that GRK2 phosphorylates and activates ezrin in the transformed epithelial cell line Hep-2 upon stimulation of the M1-muscarinic receptor, what results in a ROCK-independent but GRK2-dependent membrane ruffle formation.31 Since several other GPCRs that promote potent chemotactic migration, such as CCR2, S1P-1, the protease activated receptor PAR-2 or CXCR4, also trigger membrane ruffling, it is feasible that the GRK2-dependent modulation of ezrin could play a major role in the dynamics of actin cystoskeleton linked to the motility of certain cell types (Fig. 1). GRK2 could also contribute to migration by means of the modulation of microtubules, as previous reports have shown that GRK2 specifically phosphorylates tubulin.32 The microtubule network is dynamically reorganized during cell migration in order to support a polarized trafficking of signalling molecules and cellular components to the leading edge. Moreover, microtubules might be involved in the generation of a protrusive activity, affect actin polymerization and actively promote focal adhesion disassembly in certain cell types.33,34 However, the functional consequences of GRK2-dependent phosphorylation of tubulin have not been yet described.
Taken together, we speculate that GRK2 could potentiate cell migration of a mesenchymal phenotype in a kinase-independent manner by means of its association with GIT1, resulting in Rac activation and focal adhesion disassembly , while amoeboid-like motility could be enhanced by the functional interaction of GRK2 with ezrin, that is critically involved in this sort of migration. However, it is also possible that tumor cells adopting an amoeboid-like invasive motility become less sensitive to changes in GRK2 expression than those cells with a mesenchymal-like one, as this type of invasive migration, but not the former, is markedly dependent on the regulated spatio-temporal activity of additional GRK2-interacting proteins, such as c-Src or PI3K.9,30
In summary, the complex GRK2 “interactome” puts forward this kinase as a relevant signalling node of the cellular transduction networks controlling migration. Altered GRK2 expression or activity may differentially affect the functionality of its interaction partners, thus affecting cell motility in distinct ways, depending on the cell type and/or stimuli involved. Further research is needed to better understand how the different networks of GRK2 functional interactions are orchestrated in a stimulus or context-specific way, as well as the functional consequences on cell migration of altering GRK2 expression in specific cellular and animal experimental models. This knowledge would help to ascertain whether altered levels of this protein in epithelial cells could play a role in cancer progression and metastasis.
Acknowledgements
Our laboratory is funded by grants from Ministerio de Educación y Ciencia (SAF2005-03053), Fundacion Mutua Madrileña, Fundación Ramón Areces, The Cardiovascular Network (RECAVA) of Ministerio Sanidad y Consumo-Instituto Carlos III (RD06-0014/0037), Comunidad de Madrid (S-SAL-0159-2006), and the MAIN European Network (LSHG-CT-2003-502935). P.P. is recipient of a “Ramón y Cajal” contract and I.A. is a “Juan de la Cierva” postdoctoral fellow.
Footnotes
Previously published online as a Cell Adhesion & Migration E-publication: http://www.landesbioscience.com/journals/celladhesion/article/7149
References
- 1.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, et al. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 2.Jacoby E, Bouhelal R, Gerspacher M, Seuwen K. The 7 TM G-protein-coupled receptor target family. Chem Med Chem. 2006;1:761–782. doi: 10.1002/cmdc.200600134. [DOI] [PubMed] [Google Scholar]
- 3.Penela P, Ribas C, Mayor F., Jr Mechanisms of regulation of the expression and function of G protein-coupled receptor kinases. Cell Signal. 2003;15:973–981. doi: 10.1016/s0898-6568(03)00099-8. [DOI] [PubMed] [Google Scholar]
- 4.Penela P, Murga C, Ribas C, Tutor AS, Peregrin S, Mayor F., Jr Mechanisms of regulation of G protein-coupled receptor kinases (GRKs) and cardiovascular disease. Cardiovasc Res. 2006;69:46–56. doi: 10.1016/j.cardiores.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 5.Moore CA, Milano SK, Benovic JL. Regulation of receptor trafficking by GRKs and arrestins. Annu Rev Physiol. 2007;69:451–482. doi: 10.1146/annurev.physiol.69.022405.154712. [DOI] [PubMed] [Google Scholar]
- 6.Premont RT, Gainetdinov RR. Physiological roles of G protein-coupled receptor kinases and arrestins. Annu Rev Physiol. 2007;69:511–534. doi: 10.1146/annurev.physiol.69.022405.154731. [DOI] [PubMed] [Google Scholar]
- 7.DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- 8.Peregrin S, Jurado-Pueyo M, Campos PM, Sanz-Moreno V, Ruiz-Gomez A, Crespo P, et al. Phosphorylation of p38 by GRK2 at the docking groove unveils a novel mechanism for inactivating p38MAPK. Curr Biol. 2006;16:2042–2047. doi: 10.1016/j.cub.2006.08.083. [DOI] [PubMed] [Google Scholar]
- 9.Ribas C, Penela P, Murga C, Salcedo A, Garcia-Hoz C, Jurado-Pueyo M, et al. The G protein-coupled receptor kinase (GRK) interactome: role of GRKs in GPCR regulation and signaling. BBA-Biomembranes. 2007;1768:913–922. doi: 10.1016/j.bbamem.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 10.Vroon A, Heijnen CJ, Kavelaars A. GRKs and arrestins: regulators of migration and inflammation. J Leukocyte Biol. 2006;80:1214–1221. doi: 10.1189/jlb.0606373. [DOI] [PubMed] [Google Scholar]
- 11.Penela P, Murga C, Ribas C, Salcedo A, Jurado-Pueyo M, Rivas V, et al. G protein-coupled receptor kinase 2 (GRK2) in migration and inflammation. Arch Physiol Biochem. 2008;114:195–200. doi: 10.1080/13813450802181039. [DOI] [PubMed] [Google Scholar]
- 12.Vroon A, Heijnen CJ, Lombardi MS, Cobelens PM, Mayor F, Jr, Caron MG, et al. Reduced GRK2 level in T cells potentiates chemotaxis and signaling in response to CCL4. J Leukocyte Biol. 2004;75:901–909. doi: 10.1189/jlb.0403136. [DOI] [PubMed] [Google Scholar]
- 13.Jimenez-Sainz MC, Murga C, Kavelaars A, Jurado-Pueyo M, Krakstad BF, Heijnen CJ, et al. G Protein-coupled Receptor Kinase 2 Negatively Regulates Chemokine Signaling at a Level Downstream from G Protein Subunits. Mol Biol Cell. 2006;17:25–31. doi: 10.1091/mbc.E05-05-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kavelaars A, Vroon A, Raatgever RP, Fong AM, Premont RT, Patel DD, et al. Increased acute inflammation, leukotriene B4-induced chemotaxis, and signaling in mice deficient for G protein-coupled receptor kinase 6. J Immunol. 2003;171:6128–6134. doi: 10.4049/jimmunol.171.11.6128. [DOI] [PubMed] [Google Scholar]
- 15.Vroon A, Heijnen CJ, Raatgever R, Touw IP, Ploemacher RE, Premont RT, et al. GRK6 deficiency is associated with enhanced CXCR4-mediated neutrophil chemotaxis in vitro and impaired responsiveness to G-CSF in vivo. J Leukocyte Biol. 2004;75:698–704. doi: 10.1189/jlb.0703320. [DOI] [PubMed] [Google Scholar]
- 16.Fan J, Malik AB. Toll-like receptor-4 (TLR4) signaling augments chemokine-induced neutrophil migration by modulating cell surface expression of chemokine receptors. Nat Med. 2003;9:315–321. doi: 10.1038/nm832. [DOI] [PubMed] [Google Scholar]
- 17.Penela P, Ribas C, Aymerich I, Eijkelkamp N, Barreiro O, Heijnen CJ, et al. G protein-coupled receptor kinase 2 (GRK2) positively regulates epithelial cell migration. Embo J. 2008;27:1206–1218. doi: 10.1038/emboj.2008.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Premont RT, Claing A, Vitale N, Freeman JL, Pitcher JA, Patton WA, Moss J, Vaughan M, Lefkowitz RJ. beta2-Adrenergic receptor regulation by GIT1, a G protein-coupled receptor kinase-associated ADP ribosylation factor GTPase-activating protein. P Natl Acad Sci USA. 1998;95:14082–14087. doi: 10.1073/pnas.95.24.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoefen RJ, Berk BC. The multifunctional GIT family of proteins. J Cell Sci. 2006;119:1469–1475. doi: 10.1242/jcs.02925. [DOI] [PubMed] [Google Scholar]
- 20.Yin G, Zheng Q, Yan C, Berk BC. GIT1 is a scaffold for ERK1/2 activation in focal adhesions. J Biol Chem. 2005;280:27705–27712. doi: 10.1074/jbc.M502271200. [DOI] [PubMed] [Google Scholar]
- 21.Cotton M, Boulay PL, Houndolo T, Vitale N, Pitcher JA, Claing A. Endogenous ARF6 interacts with Rac1 upon angiotensin II stimulation to regulate membrane ruffling and cell migration. Mol Biol Cell. 2007;18:501–511. doi: 10.1091/mbc.E06-06-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Metaye T, Gibelin H, Perdrisot R, Kraimps JL. Pathophysiological roles of G-protein-coupled receptor kinases. Cell Signal. 2005;17:917–928. doi: 10.1016/j.cellsig.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 23.Salcedo A, Mayor F, Penela P. Mdm2 is involved in the ubiquitination and degradation of G-protein-coupled receptor kinase 2. Embo J. 2006;25:4752–4762. doi: 10.1038/sj.emboj.7601351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prowatke I, Devens F, Benner A, Gronne EF, Mertens D, Gronne HJ, et al. Expression analysis of imbalanced genes in prostate carcinoma using tissue microarrays. Brit J Cancer. 2007;96:82–88. doi: 10.1038/sj.bjc.6603490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Metaye T, Levillain P, Kraimps JL, Perdrisot R. Immunohistochemical detection, regulation and antiproliferative function of G-protein-coupled receptor kinase 2 in thyroid carcinomas. J Endocrinol. 2008;198:101–110. doi: 10.1677/JOE-07-0562. [DOI] [PubMed] [Google Scholar]
- 26.Vandercappellen J, Van Damme J, Struyf S. The role of CXC chemokines and their receptors in cancer. Cancer Lett. 2008;267:226–244. doi: 10.1016/j.canlet.2008.04.050. [DOI] [PubMed] [Google Scholar]
- 27.Dorsam RT, Gutkind JS. G-protein-coupled receptors and cancer. Nat Rev Cancer. 2007;7:79–94. doi: 10.1038/nrc2069. [DOI] [PubMed] [Google Scholar]
- 28.Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4:540–550. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 29.Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–374. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 30.Sahai E, Marshal CJ. Differing modes of tumour cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nat Cell Biol. 2003;5:711–719. doi: 10.1038/ncb1019. [DOI] [PubMed] [Google Scholar]
- 31.Cant SH, Pitcher JA. G protein-coupled receptor kinase 2-mediated phosphorylation of ezrin is required for G protein-coupled receptor-dependent reorganization of the actin cytoskeleton. Mol Biol Cell. 2005;16:3088–3099. doi: 10.1091/mbc.E04-10-0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshida N, Haga K, Haga T. Identification of sites of phosphorylation by G-protein-coupled receptor kinase 2 in beta-tubulin. Eur J Biochem. 2003;270:1154–1163. doi: 10.1046/j.1432-1033.2003.03465.x. [DOI] [PubMed] [Google Scholar]
- 33.Etienne-Manneville S. Actin and microtubules in cell motility: which one is in control? Traffic. 2004;5:470–477. doi: 10.1111/j.1600-0854.2004.00196.x. [DOI] [PubMed] [Google Scholar]
- 34.Watanabe T, Noritake J, Kaibuchi K. Regulation of microtubules in cell migration. Trends Cell Biol. 2005;15:76–83. doi: 10.1016/j.tcb.2004.12.006. [DOI] [PubMed] [Google Scholar]