Abstract
Cell-cell adhesion molecules play key roles at the intercellular junctions of a wide variety of cells, including interneuronal synapses and neuron-glia contacts. Functional studies suggest that adhesion molecules are implicated in many aspects of neural network formation, such as axon-guidance, synapse formation, regulation of synaptic structure and astrocyte-synapse contacts. Some basic cell biological aspects of the assembly of junctional complexes of neurons and glial cells resemble those of epithelial cells. However, the neuron specific junctional machineries are required to exert neuronal functions, such as synaptic transmission and plasticity. In this review, we describe the distribution and function of cell adhesion molecules at synapses and at contacts between synapses and astrocytes.
Key words: synapses, cell adhesion molecules, cadherin superfamily, immunoglobulin superfamily, nerve tissue proteins, axons
Introduction
The cell-cell adhesion system is involved in many aspects of neuronal development, including neuronal cell migration, axon-bundle formation, synapse formation and formation of complex of glial networks which surround axons and synapses. These adhesion systems are important for brain morphology and highly coordinated brain functions, such as memory and learning.1–3 Like epithelial junctions, cell-cell junctions in the nervous systems contain a variety of transmembrane proteins, cytoskeletal elements and signaling complexes.
During early development of the nervous systems, differentiated neurons migrate to their proper positions and elongate their axons towards their targets. Growing axons are guided by various attractive or repulsive target-derived cues.4 After reaching their target areas, axonal growth cones still need to recognize their appropriate target cells for the formation of synapses. Then, initial contacts are formed between axons and dendrites, and signaling through homophilic and heterophilic receptors induces differentiation of the synaptic specialization.5 Most of these interactions and recognitions have been shown to be mediated by cell surface proteins. Various cell surface proteins have been identified and characterized as important regulators of axon-guidance and synapse formation (Table 1). These molecules interact with each other between the cells to activate various signaling pathways and bring the apposed cell membranes into contact. Some of these molecules are defined as adhesion molecules and others as signaling molecules. However, as certain adhesion molecules are known to have signaling functions and the signaling molecules often promote cell-cell adhesion, it might not be so easy to distinguish these membrane proteins specifically involved in adhesion or signaling.
Table 1.
Lists of the neuron-neuron and neuron-glia interactions in the nervous systems
| Classification | Adhesion molecules | Localization |
| Cadherin Super Family | Classic cadherins | Synapse (PAJs), Neuron-Glia |
| Proto-cadherins | Synapse (?) | |
| Ig-like Molecules | Nectins | Synapse (PAJs), Neuron-Glia |
| Nectin-like molecules (Necls) | Neuron-Glia | |
| NCAM | Synapse | |
| Syg-1, Syg-2 | Synapse | |
| Sidekicks | Synapse | |
| Integrins | Synapse, Neuron-Glia | |
| Others | Neuroligins, neurexins | Synapse (SJs) |
| Eph receptors, ephrins | Synapse (SJs) |
In addition to neuron-neuron interactions, astrocyte-synapse interactions are also known to play important roles in the formation of neural networks. Astrocyte-synapse communication participates in synapse formation, synaptic transmission and axonal conduction, and perhaps modulates the activity of neuronal networks during development and throughout adult life.6 Recent advances have clarified the molecular compositions of astrocyte-synapse interfaces, and have provided new insight into astrocyte-synapse communication. To date, a few adhesion molecules have been identified at the astrocyte-synapse contacts. Here, we briefly summarize the key cell adhesion molecules involved in the synapse formation and in the astrocyte-synapse interactions.
Synapses
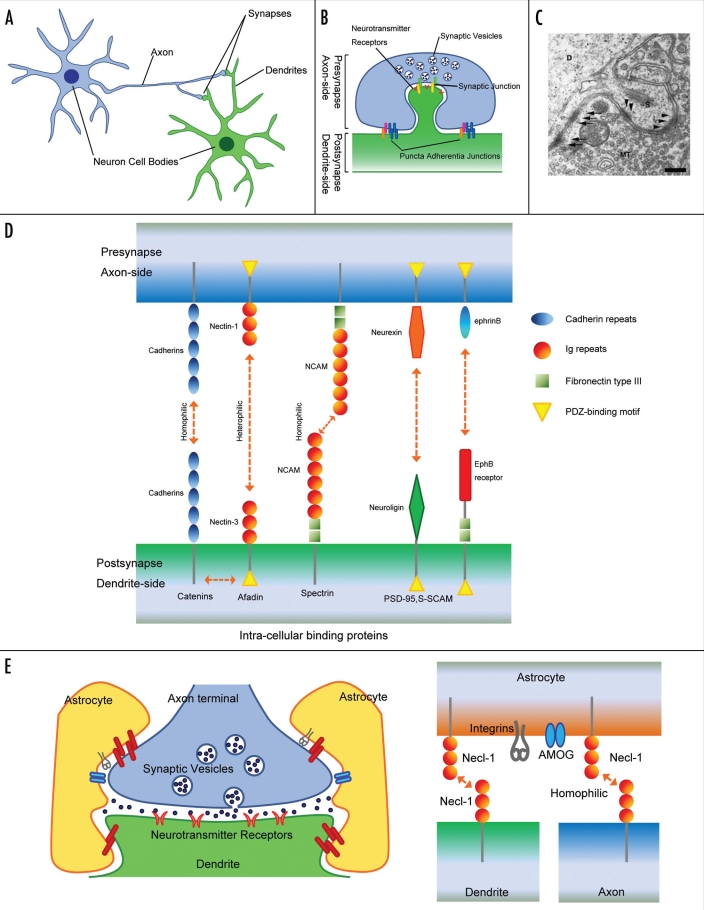
Synapses are a specialized form of intercellular junctions where the axon terminal of a neuron comes into functional contact with a target cell (Fig. 1A and B). Specificity and plasticity of synapses provide neurons with a structural and functional basis for the formation of the neuronal network system. Synapses are highly asymmetrical junctions formed between two different neurons, and early ultrastructural studies showed that the synaptic junctional areas contain at least two types of adhesion structure (Fig. 1C).7,8 One type of adhesion structure is the transmitter release zone associated with synaptic vesicles, termed synaptic junctions (SJs), and the other is a symmetrical junction, termed puncta adherentia junctions (PAJs), defined by the two criteria of symmetric paramembranous dense materials and the lack of association with synaptic vesicles (Fig. 1B). SJs are regarded as sites for neurotransmission. They are associated with presynaptic active zones containing Ca2+ channels and numerous neurotransmitter-filled synaptic vesicles which are docked on the presynaptic membrane by a complex of proteins, and postsynaptic densities where the specific neurotransmitter receptors and structural scaffolding and signaling proteins are localized. PAJs are regarded as mechanical adhesion sites between axon terminals and their targets, although their exact functions remain unknown. However, PAJs are morphologically similar to adherens junctions (AJs) formed in epithelia, and several important molecular constituents of neuronal synapses are common to both neurons and epithelial cells. Thus, some basic cell biological aspects of the assembly of junctional complexes may be shared between these two cell types.9,10 During development, specific neuronal circuits are generated by synapse formation between the appropriate pre- and postsynaptic partners. Initial contacts between synaptic partners are frequently established between axonal growth cones and dendritic filopodia extending from dendrites in vitro.11,12 Once initial axon-target interactions develop, various molecules can engage in bidirectional signaling to coordinate the differentiation of synaptic membrane specializations and stabilize the synaptic contact. Several factors that may be involved in these processes are summarized below. Although electrical synapses are formed at narrow gaps between the pre- and postsynaptic neurons known as gap junctions, we describe chemical synapses.
Figure 1.
(A) Synapses are formed at the contact points between axons and dendrites of their target neurons. (B) At synapses, at least two types of intercellular junctions, synaptic and puncta adherentia junctions, have been recognized. Synaptic junctions are regarded as sites of neurotransmission, associated with synaptic vesicles at the presynaptic active zone where Ca2+ channels localize, and postsynaptic densities (PSDs), where neurotransmitter receptors localize. Puncta adherentia junctions, which are not associated with synaptic vesicles or PSDs, appear to be ultrastructurally similar to adherence junctions of epithelial cells. (C) Electron microscopic morphology of the synapses between the mossy fiber terminals and the dendrites of pyramidal cells in the CA3 area of the hippocampus. Arrows indicate PAJs. Arrow heads indicate SJs. D: dendrite. S: dendritic spine. MT: mossy fiber terminal. Scale bar, 200 nm. (D) Molecular composition of the synapse. Many of these adhesion molecules possess a binding motif that binds to PDZ proteins. These interactions associate with each other and lead to the formation of a multi-molecular scaffold beneath both the pre- and post-synaptic membranes. (E) Astrocytes have many characteristic processes and ensheath synaptic junctions in the brain, but do not form myelin. Necl-1 localizes at the contact sites between axon terminals and glia cell processes and interacts homophilically.
Cadherins
Cadherins are Ca2+-dependent cell-cell adhesion molecules that constitute a superfamily comprised of more than 100 members in vertebrates, and are grouped into subfamilies that are designated as classic cadherins and protocadherins.13 Classic cadherins are single-pass transmembrane proteins and have five extracellular cadherin repeat (EC) domains (EC1 to EC5). All classic cadherins are homophilic adhesion molecules that function with their cytoplasmic (CP) partners, catenins (Fig. 1D).14 Catenins are cadherin-binding proteins that connect cadherins to the actin cytoskeleton. These include α-catenin, β-catenin and p120 catenin. The cadherin-catenin complexes are known to regulate actin polymerization, a property important for maintaining the cell-cell adhesion. Cadherins and their associated catenins have been observed in many neuronal populations in the central nervous systems (CNS). At the ultrastructural level, these proteins were found in synaptic junctions of most regions of the nervous systems, forming a symmetrical adhesion structure in the PAJs.9 During development, the cadherin-catenin complexes accumulate at early axo-dendritic filopodial contacts, and are retained in many of the mature synapses.9,15–18 A fragment of N-cadherin lacking its extracellular region serves as a dominant negative mutant of cadherins and inhibits their cell-cell adhesion activity. Expression of this mutant results in the appearance of filopodia-like spines, an increase in the spine length, and a decrease in the spine head width, and affects the organization of synapses in the cultured hippocampal neurons.18,19 Despite the evidence that cadherins are involved in the formation of synapses, they are not sufficient to form them in vitro, because expression of N-cadherin in non-neuronal cells fails to induce pre-synaptic differentiation in axons at the sites of contact.20 Recent studies also implicate catenins in the control of spine structure and synaptic organization in cultured hippocampal neurons. Deletion of β-catenin affects localization of synaptic vesicles along the axon,21 and loss of p120 catenin affects Rho-family small G-protein signaling, which results in a reduced spine density.22 A remarkable feature of classic cadherins is its binding specificity and region-specific distribution. In the brain, many subtypes of classic cadherins are expressed by restricted groups of functionally connected nuclei and laminas.23 Whether cadherin-mediated adhesion contributes to the formation of selective inter-neuronal connections during neural network formation remains unknown.
Protocadherins
Protocadherins are a group of transmembrane proteins that belong to the cadherin superfamily, and have varying numbers of the EC domains but divergent cytoplasmic domains that do not appear to signal through catenins.24,25 Various protocadherins are expressed in the nervous systems, and some of them are localized at synapses. Multiple α- and γ-protocadherin isoforms are highly expressed in distinct, although partially overlapping, sets of neurons and concentrated at synapses. The complex genomic organization and alternative splicing of protocadherins have led to the speculation that their diversity underlies synaptic specificity.26 γ-protocadherins are required for survival of specific neuronal types27 and arcadlin is required for activity-dependent synaptic morphogenesis.28 However, the biological functions of most protocadherins are unknown.
Nectins
Nectins represent a family of Ca2+-independent immunoglobulin (Ig)-like cell-cell adhesion molecules, which consist of four members (Fig. 1D).29 At the CA3 region of hippocampus, nectin-1 and nectin-3 asymmetrically localize at the pre-and post-synaptic sides, respectively, of the PAJs, but not at SJs.10 Nectins form homo- or hetero-trans-dimers in a Ca2+-independent manner, where heterotypic binding leads to stronger adhesion than homotypic binding.30–32 In epithelial cells in culture, nectins first form cell-cell adhesion and then recruit cadherin to the nectin-based cell-cell adhesion sites to cooperatively form AJs.33,34 Afadin, an actin-filament binding protein that connects nectins to the actin cytoskeleton, is also present at PAJs. Disruption of nectin-based cell-cell adhesion in cultured hippocampal neurons decreases the size of synapses but increases their number,10 and a nectin-1 mutant causes human cleft lip/palateectodermal dysplasia, Margarita island ectodermal dysplasia, and Zlotogora-Ogür syndrome, characterized by mental retardation, cleft lip/palate, syndactyly and ectodermal dysplasia.35 In both nectin-1 and -3-deficient mice, the number of PAJs at the synapses between the mossy fiber terminals and the dendrites of the CA3 pyramidal cells in the hippocampus is reduced. In addition, the abnormal mossy fiber trajectory is observed, suggesting that nectins are involved in the formation of PAJs, which maintain the proper mossy fiber trajectory in the CA3 region of the hippocampus.36 In afadin-deficient mice, perforated synapses in the hippocampus are observed. Reduction in the number of PAJs is likely to be further enhanced in afadin-deficient mice than in nectin-1 or -3-deficient mice. The observation of loss of PAJs in the nectin and afadin-deficient mice suggests the possibility that the localization of the cadherin/catenin complex is regulated by the nectin/afadin system, as for epithelial adherens junctions. The recruitment of afadin (AF-6) to postsynapse is regulated by small G-protein Rap1, and is involved in spine formation.37
The axon-biased localization of nectin-1 and its trans-interaction with nectin-3 in cooperation with the cadherin machinery is critical for the ordered association of axons and dendrites.38 However, the sorting signal of nectin-1 to axons has not been identified.39 The genetic deletion of nectin-1 loosens the contacts between axons and dendritic spines, while the overexpression of nectin-1, causing mislocalization of nectin-1 to dendrites, induces atypical dendro-dendritic as well as excessive axo-dendritic contacts. These actions of nectins require cadherin-catenin complexes suggesting that the two adhesion systems cooperate.38 These data suggest that localized cadherin activity may be achieved by cooperative heterophilic nectin interactions. It is also likely that mechanisms work for restricting adhesion activity at specific cell-cell contact sites. These data are consistent with those obtained in epithelial cells, suggesting that nectins form initial cell-cell adhesion and recruit cadherins to the nectin-based cell-cell adhesion sites to form AJs, and suggest that nectins play similar roles in the formation of PAJs.
Other Ig Superfamily CAMs
Other Ig superfamily CAMs, which have varying numbers of Ig-like domains, have been identified at synapses and have been shown to be involved in synaptic formation and plasticity. For example, neural cell adhesion molecule (NCAM), which contains five Ig-like domains and two fibronectin type III repeats, is engaged in homophilic and heterophilic interactions with a variety of ligands at synapses, such as fibroblast growth factor receptor (FGFR), L1, TAG-1/axonin-1 and heparan sulfate proteoglycans (Fig. 1D).40,41 NCAM is widely expressed in the developing and adult brains and plays crucial roles in migration, pathfinding of axons, and synaptic plasticity. It is involved in both early synaptogenesis and subsequent synaptic maturation.42,43 NCAM is unique among adhesion molecules in that it carries a large amount of the negatively charged sugar, polysialic acid (PSA) (Bonfanti et al., in this issue). Poor axonal fasciculation is observed in the hippocampus of NCAM-deficient mice, resulting in an impaired synapse formation in the CA3 region.44 Mossy fibers also appear defasciculated in mice with the NCAM-180 isoform.45 These functions of NCAM appear to be mediated by primarily by presence of the PSA moiety.46 Neurofascin 186 (NF186), an L1 family Ig-like cell adhesion molecule, is implicated in the subcellular organization of GABAergic synapses between basket interneurons and Purkinje cells in the cerebellum.47
SYG-1/SYG-2
SYG-1/SYG-2 are specific adhesion molecules that determine synaptic specificity in a lock-and-key manner. SYG-1, a four Ig-like domain-containing protein, and SYG-2, a seven Ig-like domain- and one fibronectin type III repeat-containing protein, were isolated in a genetic screen for C. elegans mutants that exhibit defective synaptic positioning.48,49 Interactions between SYG-1 and SYG-2 induce formation of synapses at appropriate synaptic targets. The Drosophila orthologues of roughest(rst) have been implicated in axon fasciculation and layer targeting in the fly visual system.50 Moreover, SYG-1 and SYG-2 share significant homology with the mice and human proteins NEPH and Nephrin, which are expressed in the CNS,51 although their roles in the CNS remain unknown.
Sidekicks
Sidekicks, which have six Ig-like domains and thirteen fibronectin type III repeats, have been implicated in selective synapse formation in the chicken retina.52 Sidekick-1 and -2 are differentially expressed among subsets of retinal ganglion cells in a non-overlapping manner. Sidekicks act as homophilic adhesion molecules in vitro, and are highly concentrated at synapses of restricted regions in vivo. Ectopic expression of Sidekick in Sidekick-negative cells induces mistargeting. These data suggest that sidekick interactions may promote lamina-specific connectivity.
Neuroligin
Neuroligin is an esterase-like domain-containing protein and localizes at the post-synaptic side of SJs, whereas β-neurexin is a laminin-globular-domain-containing protein and localizes at the pre-synaptic side of SJs. These two molecules interact with one another and this interaction induces the formation of synapses in vitro (Fig. 1D). The neurexin family was first identified as receptors for alpha-latrotoxin, which acts presynaptically to release neurotransmitters from sensory and motor neurons.53 More than 1,000 neurexin isoforms are generated by alternative splicing, which are differentially expressed in the nervous systems.54 Neuroligins is a β-neurexin binding partner.55,56 β-Neurexin binds neuroligins trans-synaptically and induces formation of glutamatergic and GABAergic presynaptic specializations in vitro.20,57,58 However, neuroligins are indispensable for synapse maturation and synaptic transmission, but not for triggering initial synapse formation from the phenotypes of knockout mice.59
Necl-2
Necl-2 was previously characterized as a tumor suppressor gene, and is also termed TSLC1/SgIGSF/RA175/IGSF4/SynCAM1. Necl-2 is a homophilic adhesion molecule, but also shows heterophilic cell-cell adhesion activity with Necl-1 and nectin-3.60 Necl-2 is widely expressed in various tissues and localizes at the basolateral plasma membrane in epithelial cells, not in the specialized cell-cell junctions such as AJs, TJs and desmosomes.61 Necl-2 localizes at synapses and induces presynaptic differentiation and stabilization, at least in vitro.62
Eph Receptor
Eph receptor tyrosine kinases and their ephrin ligands are grouped into two families: ephrinA ligands are tethered to the plasma membrane by a GPI linkage and bind to EphA receptors, whereas ephrinB ligands are transmembrane proteins that bind preferentially to EphB receptors (Fig. 1D).63 EphB receptors localize to synapses, where they can bind the NMDA-type glutamate receptor subunit NR1 via the extracellular domain.64 Stimulation of EphB receptors by ephrin ligands results in increased synaptic density and in NMDA receptor-mediated calcium influx and gene expression.65 EphBs multiple mutant mice develop abnormal spines in the hippocampus both in vitro and in vivo.66 However, the molecular mechanisms of many ephrin/Eph-related synaptic functions and their roles in the initial steps of synapse assembly are still largely unknown.
Axon-Astrocyte Contacts
Astrocytes are characteristic star-shaped glial cells, and their many processes ensheath synaptic junctions in the brain, but do not form myelin (Fig. 1E). Astrocytes are also known to regulate synaptic transmission by uptake of neurotransmitters, such as glutamate, ATP and GABA, from the synaptic cleft through membrane transporters, and release of glutamate upon reversal of the transporter.6 Other substances released by astrocytes can strengthen synaptic transmission by co-activating NMDA receptors in the postsynaptic membrane (e.g., D-serine) or can reduce it by binding to neurotransmitters.67,68 Synapse formation may also be regulated by factors produced by astrocytes.69 The co-culture of purified neurons with astrocytes can facilitate synaptogenesis.70 For example, an astrocyte-derived factor induces the maturation of retinal ganglion cells 71; this diffusible factor has been identified as cholesterol complexed with apolipoprotein E-containing lipoproteins.69 A recent study showed that thrombospondin-1 and -2, astrocyte-secreted proteins, promote CNS synaptogenesis in vitro and in vivo.72 On the other hand, integrins localize at contacts between neurons and astrocytes promote synaptogenesis.73 The interaction between neurons and astrocytes is important for synaptogenesis. However, a few adhesion molecules have been identified at contacts between neurons and astrocytes.
AMOG (Adhesion Molecule on Glia)
AMOG (Adhesion molecule on glia), the β2-subunit of the Na+/K+ ATPase, is a membrane glycoprotein and localizes at contacts between neurons and astrocytes. AMOG is implicated in neurite outgrowth and neuronal migration.74–76 AMOG associates with the catalytic α-subunit of the Na+/K+ ATPase and forms a functional ion channel.77 This unique molecule serves as a cell adhesion molecule and a subunit of ion channel. AMOG is firstly expressed in the brain shortly before granule cell migration. The expression level of AMOG increases during early postnatal development and reaches the highest expression level in adult.78 AMOG-deficient mice present motor incoordination and paralysis in early postnatal life and die shortly after birth.79 The exact functions of AMOG still remain elusive.
Integrins
Integrins are cell surface receptors that interact with the extracellular matrix (ECM) and transduce the signal from the ECM to the cell. Integrins consist of two distinct chains, α- and β-subunits. Integrins at contacts between neurons and astrocytes activates protein kinase C (PKC) signaling and promotes synaptogenesis in vitro.73 However, the involvement of the integrins-dependent PKC signaling in synaptogenesis still remains elusive in vivo.
Necl-1/TSLL1/SynCAM3
Necl-1/TSLL1/SynCAM3, which has a domain structure similar to those of nectins, localizes at axon-astrocyte contacts (Fig. 1E).80 Necl-1 shows Ca2+-independent homophilic cell-cell adhesion activity and heterophilic cell-cell adhesion activity with Necl-2, nectin-1 and nectin-3, but not Necl-5 or nectin-2. Necl-1 does not bind afadin, but binds Dlg3/MPP3, a membrane-associated guanylate kinase family member, Pals2 and CASK. Necl-1 is specifically expressed in neural tissue, and localizes to contact sites along axons, nerve terminals, glial cell processes, axon bundles and myelinated axons. However, the exact functions of Necl-1 remain unknown.
Conclusions and Perspectives
Herein, we described the roles of the various adhesion molecules in synapse formation, and neuron-glia interactions. Functional studies of individual cell adhesion molecules have provided a wealth of information on their roles in synapse assembly, spine morphogenesis and synaptic plasticity. Although the various adhesion systems can mediate adhesive interactions, individually, they probably control specific aspects of synapse formation. Because multiple systems appear to cooperate at individual synapse, it will be of great interest to determine whether they act in a parallel or in a hierarchical manner. For most of the functions of neuron-glia contacts, we still lack sufficient information on their functions at both cellular and molecular levels. Future research on the mechanisms of neuron-glia interactions will lead to greater insight into the mechanisms underlying the formation of complex neural circuitries.
Acknowledgements
We are grateful to Dr. Yoshihisa Kudo at Tokyo University of Pharmacy and Life Sciences and colleagues at Kobe University for comments on the manuscript. This work was supported by grants-in-aid for Scientific Research and for Cancer Research from Ministry of Education, Culture, Sports, Science and Technology, Japan.
Footnotes
Previously published online as a Cell Adhesion & Migration E-publication: http://www.landesbioscience.com/journals/celladhesion/article/6773
References
- 1.Sanes JR, Yamagata M. Formation of lamina-specific synaptic connections. Curr Opin Neurobiol. 1999;9:79–87. doi: 10.1016/s0959-4388(99)80010-5. [DOI] [PubMed] [Google Scholar]
- 2.Yamagata M, Sanes JR, Weiner JA. Synaptic adhesion molecules. Curr Opin Cell Biol. 2003;15:621–632. doi: 10.1016/s0955-0674(03)00107-8. [DOI] [PubMed] [Google Scholar]
- 3.Washbourne P, Dityatev A, Scheiffele P, Biederer T, Weiner JA, Christopherson KS, et al. Cell adhesion molecules in synapse formation. J Neurosci. 2004;24:9244–9249. doi: 10.1523/JNEUROSCI.3339-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- 5.Scheiffele P. Cell-cell signaling during synapse formation in the CNS. Annu Rev Neurosci. 2003;26:485–508. doi: 10.1146/annurev.neuro.26.043002.094940. [DOI] [PubMed] [Google Scholar]
- 6.Fields RD, Stevens-Graham B. New insights into neuron-glia communication. Science. 2002;298:556–562. doi: 10.1126/science.298.5593.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spacek J, Lieberman AR. Three dimensional reconstruction in electron microscopy of the central nervous system. Sb Ved Pr Lek Fak Karlovy Univerzity Hradci Kralove. 1974;17:203–222. [PubMed] [Google Scholar]
- 8.Peters A, Palay SL, Webster HD. The fine structure of the nervous system: neurons and their supporting cells. New York: Oxford University Press; 1991. [Google Scholar]
- 9.Uchida N, Honjo Y, Johnson KR, Wheelock MJ, Takeichi M. The catenin/cadherin adhesion system is localized in synaptic junctions bordering transmitter release zones. J Cell Biol. 1996;135:767–779. doi: 10.1083/jcb.135.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mizoguchi A, Nakanishi H, Kimura K, Matsubara K, Ozaki-Kuroda K, Katata T, et al. Nectin: an adhesion molecule involved in formation of synapses. J Cell Biol. 2002;156:555–565. doi: 10.1083/jcb.200103113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jontes JD, Smith SJ. Filopodia, spines and the generation of synaptic diversity. Neuron. 2000;27:11–14. doi: 10.1016/s0896-6273(00)00003-9. [DOI] [PubMed] [Google Scholar]
- 12.Ziv NE, Garner CC. Cellular and molecular mechanisms of presynaptic assembly. Nat Rev Neurosci. 2004;5:385–399. doi: 10.1038/nrn1370. [DOI] [PubMed] [Google Scholar]
- 13.Takeichi M. The cadherin superfamily in neuronal connections and interactions. Nat Rev Neurosci. 2007;8:11–20. doi: 10.1038/nrn2043. [DOI] [PubMed] [Google Scholar]
- 14.Wheelock MJ, Johnson KR. Cadherins as modulators of cellular phenotype. Annu Rev Cell Dev Biol. 2003;19:207–235. doi: 10.1146/annurev.cellbio.19.011102.111135. [DOI] [PubMed] [Google Scholar]
- 15.Yamagata M, Herman JP, Sanes JR. Lamina-specific expression of adhesion molecules in developing chick optic tectum. J Neurosci. 1995;15:4556–4571. doi: 10.1523/JNEUROSCI.15-06-04556.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fannon AM, Colman DR. A model for central synaptic junctional complex formation based on the differential adhesive specificities of the cadherins. Neuron. 1996;17:423–434. doi: 10.1016/s0896-6273(00)80175-0. [DOI] [PubMed] [Google Scholar]
- 17.Benson DL, Tanaka H. N-cadherin redistribution during synaptogenesis in hippocampal neurons. J Neurosci. 1998;18:6892–6904. doi: 10.1523/JNEUROSCI.18-17-06892.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Togashi H, Abe K, Mizoguchi A, Takaoka K, Chisaka O, Takeichi M. Cadherin regulates dendritic spine morphogenesis. Neuron. 2002;35:77–89. doi: 10.1016/s0896-6273(02)00748-1. [DOI] [PubMed] [Google Scholar]
- 19.Bozdagi O, Valcin M, Poskanzer K, Tanaka H, Benson DL. Temporally distinct demands for classic cadherins in synapse formation and maturation. Mol Cell Neurosci. 2004;27:509–521. doi: 10.1016/j.mcn.2004.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scheiffele P, Fan J, Choih J, Fetter R, Serafini T. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell. 2000;101:657–669. doi: 10.1016/s0092-8674(00)80877-6. [DOI] [PubMed] [Google Scholar]
- 21.Bamji SX, Shimazu K, Kimes N, Huelsken J, Birchmeier W, Lu B, et al. Role of beta-catenin in synaptic vesicle localization and presynaptic assembly. Neuron. 2003;40:719–731. doi: 10.1016/s0896-6273(03)00718-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elia LP, Yamamoto M, Zang K, Reichardt LF. p120 catenin regulates dendritic spine and synapse development through Rho-family GTPases and cadherins. Neuron. 2006;51:43–56. doi: 10.1016/j.neuron.2006.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suzuki SC, Inoue T, Kimura Y, Tanaka T, Takeichi M. Neuronal circuits are subdivided by differential expression of type-II classic cadherins in postnatal mouse brains. Mol Cell Neurosci. 1997;9:433–447. doi: 10.1006/mcne.1997.0626. [DOI] [PubMed] [Google Scholar]
- 24.Yagi T, Takeichi M. Cadherin superfamily genes: functions, genomic organization and neurologic diversity. Genes Dev. 2000;14:1169–1180. [PubMed] [Google Scholar]
- 25.Hirano S, Suzuki ST, Redies C. The cadherin superfamily in neural development: diversity, function and interaction with other molecules. Front Biosci. 2003;8:306–355. doi: 10.2741/972. [DOI] [PubMed] [Google Scholar]
- 26.Wu Q, Maniatis T. A striking organization of a large family of human neural cadherin-like cell adhesion genes. Cell. 1999;97:779–790. doi: 10.1016/s0092-8674(00)80789-8. [DOI] [PubMed] [Google Scholar]
- 27.Weiner JA, Wang X, Tapia JC, Sanes JR. Gamma protocadherins are required for synaptic development in the spinal cord. Proc Natl Acad Sci USA. 2005;102:8–14. doi: 10.1073/pnas.0407931101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yasuda S, Tanaka H, Sugiura H, Okamura K, Sakaguchi T, Tran U, et al. Activity-induced protocadherin arcadlin regulates dendritic spine number by triggering N-cadherin endocytosis via TAO2beta and p38 MAP kinases. Neuron. 2007;56:456–471. doi: 10.1016/j.neuron.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takai Y, Nakanishi H. Nectin and afadin: novel organizers of intercellular junctions. J Cell Sci. 2003;116:17–27. doi: 10.1242/jcs.00167. [DOI] [PubMed] [Google Scholar]
- 30.Fabre S, Reymond N, Cocchi F, Menotti L, Dubreuil P, Campadelli-Fiume G, et al. Prominent role of the Ig-like V domain in trans-interactions of nectins. Nectin3 and nectin 4 bind to the predicted C-C′-C″-D beta-strands of the nectin1 V domain. J Biol Chem. 2002;277:27006–27013. doi: 10.1074/jbc.M203228200. [DOI] [PubMed] [Google Scholar]
- 31.Yasumi M, Shimizu K, Honda T, Takeuchi M, Takai Y. Role of each immunoglobulin-like loop of nectin for its cell-cell adhesion activity. Biochem Biophys Res Commun. 2003;302:61–66. doi: 10.1016/s0006-291x(03)00106-2. [DOI] [PubMed] [Google Scholar]
- 32.Martinez-Rico C, Pincet F, Perez E, Thiery JP, Shimizu K, Takai Y, et al. Separation force measurements reveal different types of modulation of E-cadherin-based adhesion by nectin-1 and -3. J Biol Chem. 2005;280:4753–4760. doi: 10.1074/jbc.M412544200. [DOI] [PubMed] [Google Scholar]
- 33.Tachibana K, Nakanishi H, Mandai K, Ozaki K, Ikeda W, Yamamoto Y, et al. Two cell adhesion molecules, nectin and cadherin, interact through their cytoplasmic domain-associated proteins. J Cell Biol. 2000;150:1161–1176. doi: 10.1083/jcb.150.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Honda T, Shimizu K, Kawakatsu T, Yasumi M, Shingai T, Fukuhara A, et al. Antagonistic and agonistic effects of an extracellular fragment of nectin on formation of E-cadherin-based cell-cell adhesion. Genes Cells. 2003;8:51–63. doi: 10.1046/j.1365-2443.2003.00616.x. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki K, Hu D, Bustos T, Zlotogora J, Richieri-Costa A, Helms JA, et al. Mutations of PVRL1, encoding a cell-cell adhesion molecule/herpesvirus receptor, in cleft lip/palate-ectodermal dysplasia. Nat Genet. 2000;25:427–430. doi: 10.1038/78119. [DOI] [PubMed] [Google Scholar]
- 36.Honda T, Sakisaka T, Yamada T, Kumazawa N, Hoshino T, Kajita M, et al. Involvement of nectins in the formation of puncta adherentia junctions and the mossy fiber trajectory in the mouse hippocampus. Mol Cell Neurosci. 2006;31:315–325. doi: 10.1016/j.mcn.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 37.Xie Z, Huganir RL, Penzes P. Activity-dependent dendritic spine structural plasticity is regulated by small GTPase Rap1 and its target AF-6. Neuron. 2005;48:605–618. doi: 10.1016/j.neuron.2005.09.027. [DOI] [PubMed] [Google Scholar]
- 38.Togashi H, Miyoshi J, Honda T, Sakisaka T, Takai Y, Takeichi M. Interneurite affinity is regulated by heterophilic nectin interactions in concert with the cadherin machinery. J Cell Biol. 2006;174:141–151. doi: 10.1083/jcb.200601089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lim ST, Lim KC, Giuliano RE, Federoff HJ. Temporal and spatial localization of nectin-1 and l-afadin during synaptogenesis in hippocampal neurons. J Comp Neurol. 2008;507:1228–1244. doi: 10.1002/cne.21608. [DOI] [PubMed] [Google Scholar]
- 40.Walsh FS, Doherty P. Neural cell adhesion molecules of the immunoglobulin superfamily: role in axon growth and guidance. Annu Rev Cell Dev Biol. 1997;13:425–456. doi: 10.1146/annurev.cellbio.13.1.425. [DOI] [PubMed] [Google Scholar]
- 41.Kiss JZ, Muller D. Contribution of the neural cell adhesion molecule to neuronal and synaptic plasticity. Rev Neurosci. 2001;12:297–310. doi: 10.1515/revneuro.2001.12.4.297. [DOI] [PubMed] [Google Scholar]
- 42.Dityatev A, Dityateva G, Sytnyk V, Delling M, Toni N, Nikonenko I, et al. Polysialylated neural cell adhesion molecule promotes remodeling and formation of hippocampal synapses. J Neurosci. 2004;24:9372–9382. doi: 10.1523/JNEUROSCI.1702-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Polo-Parada L, Bose CM, Landmesser LT. Alterations in transmission, vesicle dynamics and transmitter release machinery at NCAM-deficient neuromuscular junctions. Neuron. 2001;32:815–828. doi: 10.1016/s0896-6273(01)00521-9. [DOI] [PubMed] [Google Scholar]
- 44.Cremer H, Chazal G, Goridis C, Represa A. NCAM is essential for axonal growth and fasciculation in the hippocampus. Mol Cell Neurosci. 1997;8:323–335. doi: 10.1006/mcne.1996.0588. [DOI] [PubMed] [Google Scholar]
- 45.Seki T, Rutishauser U. Removal of polysialic acid-neural cell adhesion molecule induces aberrant mossy fiber innervation and ectopic synaptogenesis in the hippocampus. J Neurosci. 1998;18:3757–3766. doi: 10.1523/JNEUROSCI.18-10-03757.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Monnier PP, Beck SG, Bolz J, Henke-Fahle S. The polysialic acid moiety of the neural cell adhesion molecule is involved in intraretinal guidance of retinal ganglion cell axons. Dev Biol. 2001;229:1–14. doi: 10.1006/dbio.2000.9970. [DOI] [PubMed] [Google Scholar]
- 47.Ango F, di Cristo G, Higashiyama H, Bennett V, Wu P, Huang ZJ. Ankyrin-based subcellular gradient of neurofascin, an immunoglobulin family protein, directs GABAergic innervation at purkinje axon initial segment. Cell. 2004;119:257–272. doi: 10.1016/j.cell.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 48.Shen K, Bargmann CI. The immunoglobulin superfamily protein SYG-1 determines the location of specific synapses in C. elegans. Cell. 2003;112:619–630. doi: 10.1016/s0092-8674(03)00113-2. [DOI] [PubMed] [Google Scholar]
- 49.Shen K, Fetter RD, Bargmann CI. Synaptic specificity is generated by the synaptic guidepost protein SYG-2 and its receptor, SYG-1. Cell. 2004;116:869–881. doi: 10.1016/s0092-8674(04)00251-x. [DOI] [PubMed] [Google Scholar]
- 50.Schneider T, Reiter C, Eule E, Bader B, Lichte B, Nie Z, et al. Restricted expression of the irreC-rst protein is required for normal axonal projections of columnar visual neurons. Neuron. 1995;15:259–271. doi: 10.1016/0896-6273(95)90032-2. [DOI] [PubMed] [Google Scholar]
- 51.Donoviel DB, Freed DD, Vogel H, Potter DG, Hawkins E, Barrish JP, et al. Proteinuria and perinatal lethality in mice lacking NEPH1, a novel protein with homology to NEPHRIN. Mol Cell Biol. 2001;21:4829–4836. doi: 10.1128/MCB.21.14.4829-4836.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamagata M, Weiner JA, Sanes JR. Sidekicks: synaptic adhesion molecules that promote lamina-specific connectivity in the retina. Cell. 2002;110:649–660. doi: 10.1016/s0092-8674(02)00910-8. [DOI] [PubMed] [Google Scholar]
- 53.Ushkaryov YA, Petrenko AG, Geppert M, Sudhof TC. Neurexins: synaptic cell surface proteins related to the alpha-latrotoxin receptor and laminin. Science. 1992;257:50–56. doi: 10.1126/science.1621094. [DOI] [PubMed] [Google Scholar]
- 54.Missler M, Fernandez-Chacon R, Sudhof TC. The making of neurexins. J Neurochem. 1998;71:1339–1347. doi: 10.1046/j.1471-4159.1998.71041339.x. [DOI] [PubMed] [Google Scholar]
- 55.Ichtchenko K, Hata Y, Nguyen T, Ullrich B, Missler M, Moomaw C, et al. Neuroligin 1: a splice site-specific ligand for beta-neurexins. Cell. 1995;81:435–443. doi: 10.1016/0092-8674(95)90396-8. [DOI] [PubMed] [Google Scholar]
- 56.Nguyen T, Sudhof TC. Binding properties of neuroligin 1 and neurexin 1beta reveal function as heterophilic cell adhesion molecules. J Biol Chem. 1997;272:26032–26039. doi: 10.1074/jbc.272.41.26032. [DOI] [PubMed] [Google Scholar]
- 57.Graf ER, Zhang X, Jin SX, Linhoff MW, Craig AM. Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell. 2004;119:1013–1026. doi: 10.1016/j.cell.2004.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chih B, Engelman H, Scheiffele P. Control of excitatory and inhibitory synapse formation by neuroligins. Science. 2005;307:1324–1328. doi: 10.1126/science.1107470. [DOI] [PubMed] [Google Scholar]
- 59.Varoqueaux F, Aramuni G, Rawson RL, Mohrmann R, Missler M, Gottmann K, et al. Neuroligins determine synapse maturation and function. Neuron. 2006;51:741–754. doi: 10.1016/j.neuron.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 60.Sakisaka T, Takai Y. Biology and pathology of nectins and nectin-like molecules. Curr Opin Cell Biol. 2004;16:513–521. doi: 10.1016/j.ceb.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 61.Shingai T, Ikeda W, Kakunaga S, Morimoto K, Takekuni K, Itoh S, et al. Implications of nectin-like molecule-2/IGSF4/RA175/SgIGSF/TSLC1/SynCAM1 in cell-cell adhesion and transmembrane protein localization in epithelial cells. J Biol Chem. 2003;278:35421–35427. doi: 10.1074/jbc.M305387200. [DOI] [PubMed] [Google Scholar]
- 62.Biederer T, Sara Y, Mozhayeva M, Atasoy D, Liu X, Kavalali ET, et al. SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science. 2002;297:1525–1531. doi: 10.1126/science.1072356. [DOI] [PubMed] [Google Scholar]
- 63.Flanagan JG, Vanderhaeghen P. The Ephrins and Eph receptors in neural development. Annu Rev of Neurosci. 1998;21:309–345. doi: 10.1146/annurev.neuro.21.1.309. [DOI] [PubMed] [Google Scholar]
- 64.Dalva MB, Takasu MA, Lin MZ, Shamah SM, Hu L, Gale NW, et al. EphB receptors interact with NMDA receptors and regulate excitatory synapse formation. Cell. 2000;103:945–956. doi: 10.1016/s0092-8674(00)00197-5. [DOI] [PubMed] [Google Scholar]
- 65.Takasu MA, Dalva MB, Zigmond RE, Greenberg ME. Modulation of NMDA receptor-dependent calcium influx and gene expression through EphB receptors. Science. 2002;295:491–495. doi: 10.1126/science.1065983. [DOI] [PubMed] [Google Scholar]
- 66.Henkemeyer M, Itkis OS, Ngo M, Hickmott PW, Ethell IM. Multiple EphB receptor tyrosine kinases shape dendritic spines in the hippocampus. J Cell Biol. 2003;163:1313–1326. doi: 10.1083/jcb.200306033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wolosker H, Blackshaw S, Snyder SH. Serine racemase: a glial enzyme synthesizing D-serine to regulate glutamate-N-methyl-D-aspartate neurotransmission. Proc Natl Acad Sci USA. 1999;96:13409–13414. doi: 10.1073/pnas.96.23.13409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Baranano DE, Ferris CD, Snyder SH. Atypical neural messengers. Trends Neurosci. 2001;24:99–106. doi: 10.1016/s0166-2236(00)01716-1. [DOI] [PubMed] [Google Scholar]
- 69.Mauch DH, Nagler K, Schumacher S, Goritz C, Muller EC, Otto A, Pfrieger FW. CNS synaptogenesis promoted by glia-derived cholesterol. Science. 2001;294:1354–1357. doi: 10.1126/science.294.5545.1354. [DOI] [PubMed] [Google Scholar]
- 70.Pfrieger FW, Barres BA. Synaptic efficacy enhanced by glial cells in vitro. Science. 1997;277:1684–1687. doi: 10.1126/science.277.5332.1684. [DOI] [PubMed] [Google Scholar]
- 71.Ullian EM, Sapperstein SK, Christopherson KS, Barres BA. Control of synapse number by glia. Science. 2001;291:657–661. doi: 10.1126/science.291.5504.657. [DOI] [PubMed] [Google Scholar]
- 72.Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, et al. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120:421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 73.Hama H, Hara C, Yamaguchi K, Miyawaki A. PKC signaling mediates global enhancement of excitatory synaptogenesis in neurons triggered by local contact with astrocytes. Neuron. 2004;41:405–415. doi: 10.1016/s0896-6273(04)00007-8. [DOI] [PubMed] [Google Scholar]
- 74.Muller-Husmann G, Gloor S, Schachner M. Functional characterization of beta isoforms of murine Na,K-ATPase. The adhesion molecule on glia (AMOG/beta2), but not beta1, promotes neurite outgrowth. J Biol Chem. 1993;268:26260–26267. [PubMed] [Google Scholar]
- 75.Antonicek H, Persohn E, Schachner M. Biochemical and functional characterization of a novel neuron-glia adhesion molecule that is involved in neuronal migration. J Cell Biol. 1987;104:1587–1595. doi: 10.1083/jcb.104.6.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lecuona E, Luquin S, Avila J, Garcia-Segura LM, Martin-Vasallo P. Expression of the beta1 and beta2(AMOG) subunits of the Na,K-ATPase in neural tissues: cellular and developmental distribution patterns. Brain Res Bull. 1996;40:167–174. doi: 10.1016/0361-9230(96)00042-1. [DOI] [PubMed] [Google Scholar]
- 77.Gloor S, Antonicek H, Sweadner KJ, Pagliusi S, Frank R, Moos M, Schachner M. The adhesion molecule on glia (AMOG) is a homologue of the beta subunit of the Na,K-ATPase. J Cell Biol. 1990;110:165–174. doi: 10.1083/jcb.110.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pagliusi SR, Schachner M, Seeburg PH, Shivers BD. The Adhesion Molecule on Glia (AMOG) Is Widely Expressed by Astrocytes in Developing and Adult Mouse Brain. Eur J Neurosci. 1990;2:471–480. doi: 10.1111/j.1460-9568.1990.tb00438.x. [DOI] [PubMed] [Google Scholar]
- 79.Magyar JP, Bartsch U, Wang ZQ, Howells N, Aguzzi A, Wagner EF, et al. Degeneration of neural cells in the central nervous system of mice deficient in the gene for the adhesion molecule on Glia, the beta2 subunit of murine Na,K-ATPase. J Cell Biol. 1994;127:835–845. doi: 10.1083/jcb.127.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kakunaga S, Ikeda W, Itoh S, Deguchi-Tawarada M, Ohtsuka T, Mizoguchi A, et al. Nectin-like molecule-1/TSLL1/SynCAM3: a neural tissue-specific immunoglobulin-like cell-cell adhesion molecule localizing at non-junctional contact sites of presynaptic nerve terminals, axons and glia cell processes. Cell Sci. 2005;118:1267–1277. doi: 10.1242/jcs.01656. [DOI] [PubMed] [Google Scholar]