Abstract
F3/Contactin is an immunoglobulin superfamily component expressed in the nervous tissue of several species. Here we focus on the structural and functional properties of its mouse relative, on the mechanisms driving its regulated expression and on its developmental role. F3/Contactin is differentially expressed in distinct populations of central and peripheral neurons and in some non-neuronal cells. Accordingly, the regulatory region of the underlying gene includes promoter elements undergoing differential activation, associated with an intricate splicing profile, indicating that transcriptional and posttranscriptional mechanisms contribute to its expression. Transgenic models allowed to follow F3/Contactin promoter activation in vivo and to modify F3/Contactin gene expression under a heterologous promoter, which resulted in morphological and functional phenotypes. Besides axonal growth and pathfinding, these concerned earlier events, including precursor proliferation and commitment. This wide role in neural ontogenesis is consistent with the recognized interaction of F3/Contactin with developmental control genes belonging to the Notch pathway.
Key words: F3/contactin, TAG-1, axonal glycoproteins, notch pathway, gene regulation, cerebellum, neural development
Introduction
Over the past several years a large body of evidences supported the original assumption that interactions mediated by neuronal surface glycoproteins exert a complex control in different aspects of neural development, including precursor commitment and differentiation, axonal growth and pathfinding,1–3 establishment/remodelling of synaptic connections and synaptic function.4–7 Most of these events imply the activation of signalling pathways8–10 and in addition several reports indicate a direct involvement of adhesive glycoproteins in neuronal function.11–17
Neural adhesive glycoproteins belonging to different gene families may be involved in the above events, including cadherins, integrins, netrins, slits, ephrins, semaphorins and immunoglobulin superfamily (IgSF) components.1–3,9,10,18,19 In particular, axonal Ig superfamily glycoproteins display peculiar structural features in that they are built of both immunoglobulin type C2 and Fibronectin type III domains (Ig/FNIII molecules).20 Association of such domains is justified by their similar overall structure, which includes two beta sheets stabilized, in IgC2, by an intra-chain disulphide bridge.21,22
Depending upon their mode of membrane association, Ig/FNIII molecules may be classified into two groups, including either transmembrane or glycosylphosphatidylinositol (GPI)-anchored molecules. Members of the former include well-known adhesion receptors as L1/NgCAM,23 NrCAM24 and Neurofascin,25 while a GPI anchor was demonstrated for F3/Contactin26–28 and for the Transient Axonal Glycoprotein TAG-1.29
Functionally, the evidence that axonal adhesive glycoproteins play a complex role in axonal growth was originally obtained through the use of in vitro models, demonstrating their ability to modulate axonal elongation in primary cultures.28,30–33 This was deduced by the inhibitory effects on axonal elongation of antibodies directed against specific functional domains,34–37 but also by the positive effects on the same event exerted by the purified molecules or by monolayers of transfected cells, used as a primary cultures substrates.28,38–42
The extensive use of the above in vitro approaches, together with in vivo transgenic models, demonstrated that structurally different adhesive glycoproteins may exert similar regulatory roles on neurite elongation and regeneration.9,10,18,43,44 Such a redundancy may be justified by the evidence of complex multimolecular cis-associations or trans-interactions among these molecules, which may be necessary for their activity to be exerted.45–52
Formation of complexes among adhesive glycoproteins requires that expression of their genes is coordinated at the cellular and tissue levels during specific developmental steps, indicating that the underlying regulatory mechanisms are themselves provided with relevant developmental significance. Coordinated regulation of axonal glycoprotein expression may therefore represent a prerequisite for their involvement in morphogenetic events typical of distinct developmental steps. In this respect, while the primary role of such molecules was supposed to be the control of neurite elongation and pathfinding, several reports indicate that in fact some of them may mediate earlier events, related to neuronal commitment/early differentiation53–55 or later ones as development of glia,56 regeneration,57,58 myelination59–61 and synaptogenesis.4–6,62 The arising corollary is therefore that the articulated role of these molecules may be closely dependent upon the specific times and sites of activation of the underlying genes.
In this context, an emerging evidence is that the complex ontogenetic function of these molecules may imply interaction with developmental control genes, a situation recently demonstrated for the F3/Contactin and NB3 axonal glycoproteins, whose ability to activate the Notch pathway has been reported.63,64
In this review the above topics will be addressed by using the F3/Contactin glycoprotein as a molecular model.
The F3/Contactin Glycoprotein
Originally described as an immunoglobulin superfamily component expressed at the neuronal and in particular at the axonal levels,26,27 F3/Contactin is known to mediate distinct functions of neuronal or glial cells, including cell adhesion and axonal growth both in the peripheral and central nervous tissues,28,30,65,66 but also oligodendrocyte differentiation and myelination,63 indicating that F3/Contactin-dependent adhesion is provided with a wide developmental function.
Structural properties.
F3/Contactin was originally identified as a main protein component expressed at the surface of primary neurons.26 At the same time, the demonstration was achieved that the molecule was also released in the culture medium, which allowed its isolation and the generation of rabbit antibodies. The focus on this protein was originally justified by the demonstration that its sugar moiety included the L2/HNK-1 carbohydrate epitope, shared by several neural adhesive glycoproteins, of which it was supposed to represent a marker.67 Indeed, several evidences indicate that a quite important component of F3/Contactin is represented by its carbohydrate chains, as N-glycosidase treatment induced an about 15% shift in molecular weight, consistent with the evidence of several consensus for N-linked glycosylation along its sequence.27
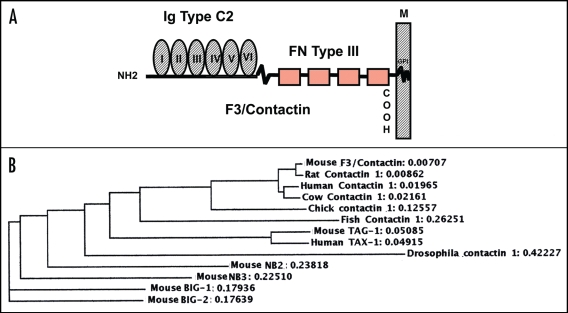
cDNA cloning revealed that F3/Contactin belongs to the immunoglobulin supergene family as its N-terminal half includes 6 immunoglobulin domains of the C2 type (IgC2), displaying a high level of internal homology. In addition, 4 Fibronectin type III repeats (FNIII) map to the pre-membrane region and hydrophobic sequences are located at both the N- and C-termini.27 Of these, the former corresponds to a typical signal peptide, shared by membrane and secreted glycoproteins, while the latter is a typical feature of GPI-anchored molecules as it plays a relevant role in the membrane-anchoring mechanism (Fig. 1A).
Figure 1.
Structure of mouse F3/Contactin and relationships to further Ig superfamily components. (A) F3/Contactin molecule is built by the association of Immunoglobulin type C2 domains, located in its N-terminal half (Ig Type C2), with Fibronectin type III domains (FN Type III), which map to the premembrane region. Attachment to the neuronal membrane is mediated by a glycosylphosphatidylinositol-containing lipid tail (GPI). (B) Phylogram comparing different GPI-anchored components of the IgC2/FNIII family.149 Note that all mammalian members of the F3/Contactin family are close to each other, lower score being oberved with chick and fish and, mostly, with Drosophila orthologs. As for the other components of the same family, highest scores were found with both mouse and human TAG-1, while Big-1 and Big-2, NB2 and NB3 were more evolutionary distant.
The use of cDNA probes revealed that F3/Contactin is encoded by a single-copy gene, mapping to band F of chromosome 15 in the mouse27 and to chromosome 12 in the human genome.68 The F3/Contactin gene is transcribed into a unique mRNA, whose size accounts to 6.3 Kbp,27 which, however, undergoes some variability in its 5′ untranslated region, depending upon the existence of a complex splicing mechanism within the 5′ flanking region.69–71
The above overall organization is shared by further axonal Ig superfamily components, which display a relatively high level of homology to F3/Contactin. Indeed, an about 30% similarity in the aminoacid sequence was found with the transmembrane glycoprotein L1,23 while a 50% similarity was found with the GPI-anchored Transient Axonal Glycoprotein TAG-1.29 In addition, F3/Contactin belongs to a cluster of structurally related axonal glycoproteins, which may be co-expressed at the neuronal and, in particular, at the axonal surfaces which, besides TAG-1 (CNTN-2), also include BIG-1 (CNTN3),72 BIG-2 (CNTN4),73 NB-2 (CNTN5)74 and NB3 (CNTN6)75 (Fig. 1B). These molecules, undergo differential expression, suggesting that the corresponding profiles may be strictly related to their functional and developmental roles.76
Some of these molecules are also highly conserved across species, as shown for F3/Contactin, found in highly related forms in mouse,26–28 chick,77 human,68 Cow,78 but also in lower vertebrates as in fishes79,80 and in invertebrates as in Drosophila81,82 (Fig. 1B). In addition related molecular species have been recently demonstrated in the nervous tissue of the terrestrial snail Helix Pomatia.83,84 Together, these data support a highly conserved role for these molecules along phylogenesis.
Expression profile.
A complex profile of F3/Contactin expression occurs during nervous tissue development at both the tissue and cellular levels. Originally, F3/Contactin was considered as a neurospecific glycoprotein, no expression being demonstrated outside the nervous tissue. Although this molecule is widely distributed over the neuronal surface, its subcellular localization is sharply modified upon differentiation, with a progressive loss from the cell bodies, concomitant with an axonal accumulation27 so that, in the differentiated nervous tissue, F3/Contactin may be considered as a fibre tract component.
This peculiar subcellular distribution may be of functional relevance as it allows the molecule to be targeted to specific axonal domains as the nodal/paranodal regions at the times when contacts between axons and myelinating cells are formed and stabilized. On the other hand, given the inhibitory effects of F3/Contactin on axonal growth from central neurons,65,66 its downregulation on the cell bodies may be significant for allowing extensions of axonal trajectories in critical developmental periods. In this context, a relevant feature is the concomitant expression on differentiating neurons of the transient axonal glycoprotein TAG-1,85 whose role in counteracting the inhibiting effects of F3/Contactin on axonal growth86 and whose positive effects on axonal and growth cone development have been demonstrated.87,88
Together, the above data on F3/Contactin/TAG-1 expression profiles should be considered of pivotal functional relevance, suggesting that coordinated expression of axonal molecules bearing similar general organization and involved in similar functions during definite developmental steps may be relevant for neural plasticity. Accordingly, this lends supports to the general hypothesis that the mechanisms which drive regulated expression of the underlying genes are themselves provided with functional and developmental relevance.66
The above aspects of F3/Contactin functional role will be discussed, by taking as a reference its expression profile within different neural structures.
Cell type specificity of the F3/contactin gene in the cerebellum.
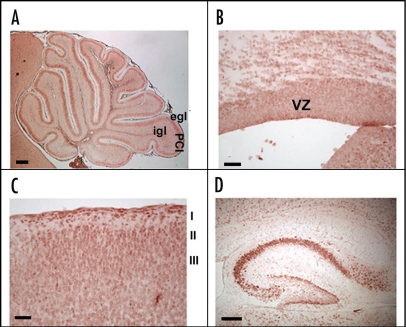
In the cerebellum, high levels of F3/Contactin expression are observed, shown at postnatal day 8 in Figure 2A. During development, the molecule undergoes sharp changes in its cellular distribution, concomitant with neuronal differentiation.27,66,89 In particular, while silent on proliferating precursors, the F3/Contactin gene is activated on precursors which leave the external granular layer, contact the flanking Bergmann glia and begin to migrate. This profile suggests that the molecule modulates events related to cell cycle exit and initial neuronal precursor migration, besides than axonal elongation.
Figure 2.
F3/Contactin expression profile. The F3/Contactin expression is shown in postnatal day 8 cerebellum (A), in newborn mice cerebral cortex, including the ventricular zone (VZ) (B) and cortical layers I–III (C). Expression in postnatal day 8 hippocampus is also reported (D). egl: external granular layer; igl: inner granular layer; PCl: Purkinje cells layer. I-II-III refer to the corresponding cortical layers. Scale bars: (A and D) = 200 µm; (B and C) = 40 µm.
In addition, during granule cell migration, F3/Contactin expression undergoes changes in its cellular distribution, as it is progressively lost from the cell bodies to become concentrated on axonal extensions within the molecular layer.89 Ultrastructural studies also provided evidence that the molecule maps at the synaptic level, where it may represent a component of the molecular machinery, which links the presynaptic to the postsynaptic compartments.90
The above timing of the F3/Contactin gene activation and in particular its differential expression within cell bodies/processes may correlate with distinct developmental events. Early expression on the perikaria may modulate the ability of neuronal precursors to interact with flanking glia, which may be relevant for their migratory movements.91 On the other hand, subsequent expression on axonal extensions may modulate axonal growth and pathfinding, as demonstrated by the changes in parallel fibres orientation, axonal maturation and myelination, which occur in F3/Contactin null mutant mice.92
During cerebellar development, F3/Contactin gene undergoes differential cell type-specific activation in granule (GC) versus Purkinje neurons (PC). In GC bodies, F3/Contactin is predominant in earlier stages, mostly at the birth and within the first postnatal week. By contrast, on PC it begins to be expressed at around postnatal day 3, a peak being attained at around postnatal day 8.89 The F3/Contactin gene is then downregulated on both neuronal populations. Sharper effects, however, are observed on PC, while on GC relatively low expression is maintained until adulthood, indicating that in these cells it may exert a functional role, which may go behind its described developmental function.
This profile suggests that F3/Contactin gene activation undergoes differential regulation on GC versus PC. Earlier expression on the former likely depends upon the endogenous developmental program,93 while activation on PC most likely implies interaction of these neurons with flanking structures, mostly migrating granule cells and ingrowing climbing fibres. Therefore, on PC, F3/Contactin gene expression is likely to imply trans-synaptic regulation at the sites of cell-to-cell contacts, as shown for further CAMs expressed at the synaptic level.94 These two mechanisms may be differentially activated in critical developmental periods, with the former being responsible for the earlier F3/Contactin developmental profile, expression in later development rather relying on cell interactions.
F3/contactin expression in developing cerebral cortex.
As in the cerebellum, F3/Contactin expression is also regulated in developing cerebral cortex with some differences, however. Indeed, in developing telencephalon, F3/Contactin is also expressed in the proliferative region of the ventricular zone (VZ) at embryonic day 16th (Fig. 2B), indicating expression on dividing precursors. At the same time, F3/Contactin gene activation occurs in layers II–III89 (see Fig. 2C), consistent with the evidence that the postmitotic neurons, which populate these layers, originate from later developing precursors, located in the upper ventricular zone.95–97
In developing cortex, the molecule is therefore expressed by differentiating postmitotic neurons undergoing radial migration. During migratory events of these precursors, in addition, the molecule undergoes changes in its cellular distribution. Indeed, in the early postnatal period, F3/Contactin expression is predominant on precursor perikarya. Subsequent expression on axon tracts occurs at the same time than overall downregulation of the corresponding gene, indicating that also on cortical precursors F3/Contactin gene downregulation is necessary to allow for growth of axonal extensions, in keeping with its demonstrated inhibitory effects on central neuron differentiation, observed in in vitro models.65,86
By contrast, on neurons undergoing tangential migration,98–101 a sustained F3/Contactin expression is observed all over the postnatal development, as for precursors originating from the anterior ventricular zone, which feed the olfactory pathway.89 Therefore, F3/Contactin gene expression undergoes a differential regulation on radially—versus tangentially-migrating precursors, with a wider time course on the latter.
F3/contactin expression in developing hippocampus.
Based on the above, it may be supposed that F3/Contactin expression undergoes persistent activation on neurons which develop postnatally, which, besides those belonging to the cerebellum and to the olfactory pathway, also include hippocampal precursors,102 in which F3/Contactin was found both on pyramidal neurons of the CA1–CA3 fields and on granule cells of the dentate gyrus89 (Fig. 2D). In the latter the F3/Contactin gene was upregulated on the outer face, which bears neurons undergoing differentiation, while it is absent from the inner face where granule cell precursors are generated.103 In pyramidal neurons, F3/Contactin expression is sharply regulated at the cellular level, with strong expression on cell bodies in earlier developmental steps, and subsequent polarization on axonal extensions. Therefore, unlike the cerebral cortex, F3/Contactin expression in the hippocampus is sustained all over the postnatal life, which is in keeping with the evidence that the molecule is unable to counteract neurite elongation in primary hippocampal neurons.65
F3/contactin expression on subcellular structures.
A special mention deserves the expression of the molecule on specific subcellular structures, and in particular in the axonal nodal, paranodal and juxtaparanodal regions, all of them playing a relevant role in myelination and in nerve impulse conduction.13,51,60,61,104–108 Indeed, the molecular architecture of these regions is of critical relevance for the saltatory conduction of the action potential, which mostly relies on the relative topography of Na+ and K+ channels and implies the regulated expression of several associated molecular components. In this respect, a special role is played by Ig/FNIII axonal glycoproteins, in particular F3/Contactin itself and the closely-related Transient Axonal Glycoprotein TAG-1. Within the node of Ranvier, F3/Contactin interaction with the B1 subunit of the Na channels15 is critical for surface expression of the latter and therefore for their functional role.105–108 Within the node, Na+ channels also undergo cis-association with the Ig superfamily glycoprotein Nf186,108–110 and complexes between F3/Contactin, Nf186 and Na+ channels then interact in trans with glial components as Tenascin-C, Tenascin R,111–115 receptor protein tyrosine phosphatase β (RPTP β) and its Phosphocan derivative.116,117 Together, these data support the view that F3/Contactin is a relevant component of the nodal region, in which it undergoes association with and modulates the topography of molecules of critical relevance for neuronal function as the Na+ channels.
F3/Contactin also participates to the complex molecular interactions occurring at the paranode, where it associates with the transmembrane neurexin family component CASPR (Contactin associated protein).104,118 As for Na+ channels, interaction with F3/Contactin allows CASPR (also called Paranodin) to be expressed at the cell surface,119 and in addition this also regulates glycosylation of F3/Contactin and its transport to the cell surface.120 At the paranode the F3/Contactin/CASPR complex mediates axonal interactions with myelinating cells, which implies the glial Ig superfamily component Neurofascin 155,121,122 as well as the Nogo-A glycoprotein.123 F3/Contactin-dependent interactions are also critical for linking the axoglial complex to the cytoskeleton as the CASPR intracellular domain binds the cytoskeletal protein 4.1b.124,125
It is worth mentioning that axon-glial contacts at the level of the paranode display special morphological features as, at both sides of the node of Ranvier, the compact myelin membrane opens, forming cytoplasm-filled glial loops connected to the axon via a series of transverse bands, reminiscent of the invertebrate septate juctions.14 Both CASPR and Contactin are essential for generating septate-like junctions in vertebrates as in both Contactin and CASPR mutant mice the latter are disrupted.13,14
Molecular interactions of Ig superfamily components with ionic channels are highly relevant for the definition of the axonal domains and in particular for separating the nodal region, containing the Na+ channels, from the juxtaparanode in which K+ channels are clustered. In the juxtaparanodal region, the Ig superfamily glycoprotein TAG-1 interacts in cis with a further component of the neurexin family, the CASPR2 protein and with delayed rectifier K+ channels so as to restrict the trimeric TAG-1/CASPR2/K+ channels complexes to this region.16 In addition, the CASPR2/TAG-1 heterodimer on the axonal membrane interacts with the TAG-1 protein located on the apposing glial counterpart,16,50 which contributes to stabilizing axoglial interactions. But the most relevant consequence of these molecular interactions is that Na+ channels, responsible for the depolarization phase of action potential, and K+ channels, responsible for membrane repolarization, are physically separated from each other, which is of critical relevance for generating the peak-shaped potential typical of the nerve impulse. Indeed, disruptions of either the TAG-1 or the CASPR-2 genes lead to mislocalization of the K+ channels in the central and peripheral nervous tissues and are responsible for the defects of nerve impulse conduction, which occur in mutant mice.16
F3/contactin expression in non-neuronal cells.
As part of its role in developmental control the expression should be considered of F3/Contactin by non neuronal cells. Indeed, although brain-specific, F3/Contactin cannot be strictly considered as a neuron-specific glycoprotein as it is also expressed on cells of glial derivation. Indeed, some expression has been also found on an astrocytic glioma,56 although the main non-neuronal cells expressing the molecules are rather oligodendrocytes, on which the protein has been found by both in vitro and in vivo approaches.126 Expression on oligodendrocytes may be in keeping with the hypothesis of the F3/Contactin involvement in early stages of myelination besides than in the abovementioned organization of the nodal/paranodal regions.
As for the expression on glial cells, the TAG-1 glycoprotein bears some of the F3/Contactin expression properties. Indeed, as for F3/Contactin, it is expressed at the oligodendrocyte and not at the astroglial surface.127 The functional consequences of this on myelination and in particular on the stabilization of the iuxtaparanodal regions have been discussed in the previous section. In addition, TAG-1 is provided with a functional role in modulating the astroglial morphology/function as demonstrated by using transgenic models undergoing TAG-1 gene suppression.87 This indicates that while axonal glycoproteins are mainly endowed with a functional role in controlling axonal functions, they can also modulate differentiation of flanking glial cells via heterophilic trans-interactions.
In the case of F3/Contactin its role in modulating astroglial function and morphology has not been addressed in detail, although its ability to interact with glial receptors as Receptor Protein Tyrosin Phosphatase (RPTPβ)128 suggests that this possibility should be taken into account, which represents matter for further investigation. If this will turn out to be the case, the general role in modulating the development of F3/Contactin expressing cells (mostly neurons) or of flanking cells (mostly oligodendrocytes and possibly cells of astroglial derivation) should be both considered an integrant part of F3/Contactin function, thus providing this glycoprotein with a wide role in developmental control.
Developmental significance of F3/contactin regulated expression.
From the above it may be inferred that the F3/Contactin expression may be relevant for development of both neuronal and glial precursors. This topic has been addressed by modifying it in transgenic models. Suppressing the F3/Contactin gene in vivo led to specific effects on the structural organization and function of myelinated axons, which fits with the expression and function of the molecule at the nodal/paranodal regions. These changes affect dramatically the function of myelinated axons, so that the mutant mice did not survive past the second postnatal week.13 However the most relevant phenotype was observed as a consequence of a change in the F3/Contactin expression profile from a heterologous promoter. In this respect the promoter region of the gene encoding the human relative of the Transient Axonal Glycoprotein was used.66 The expression profile of the Transient Axonal Glycoprotein TAG-1 is significantly different from the F3/Contactin one, being characterized by an earlier activation on premigratory neuronal precursors, while F3/Contactin is rather upregulated on migrating and differentiating neurons. Besides this general profile, as above indicated the two molecules are differentially expressed at the level of axonal domains involved in myelination and on myelinating cells themselves.
Our claim has then been to verify whether regulated expression of F3/Contactin may be provided with functional relevance in specific aspects of neuronal function, a hypothesis we have verified by setting up the following in vitro and in vivo approaches: (1) the F3/Contactin gene regulatory region has been characterized in detail by using both in vitro and in vivo models; (2) the significance of the F3/Contactin profile has been addressed in transgenic mice, in which its cDNA has been driven under control of a heterologous promoter. For this, a regulatory region selected of the human homologue of the Transient Axonal Glycoprotein (TAG-1) gene, the TAX-1 gene, has been chosen.66,129
Organization of the F3/contactin gene regulatory region.
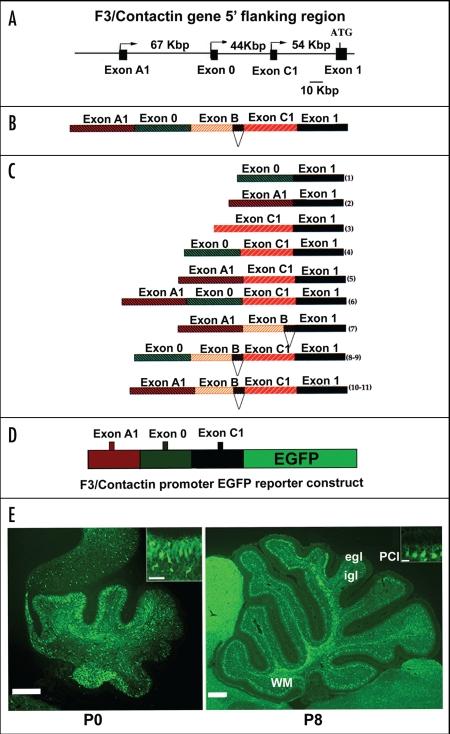
In vitro models for elucidating the F3/contactin promoter organization. The organization of the F3/Contactin gene, studied both in chick130 and in rodents,131 revealed a quite similar structure compared to other Ig superfamily components, with some specific features, however. The overall size of the rodent gene accounts to about 290 Kbp. Of these, the coding region spans more than 100 Kbp. As for other Ig superfamily axonal glycoproteins, each domain is encoded by two distinct exons; in addition different exons encode the amino- and the carboxy-terminal hydrophobic sequences, respectively.
A specific feature of the F3/Contactin gene concerns its 5′ flanking region, which spans more than 170 Kbp (Fig. 3A).69,70 The functional organization of this region has been first characterized in vitro upon transfection of putative promoter/reporter constructs in cell lines of different derivation, in order to identify the associated promoter elements and to check for their cell type-specificity. The F3/Contactin gene 5′ flanking region includes 3 alternative neurospecific promoters, associated with distinct 5′ flanking exons called A1, 0 and C1,69,131 (Fig. 3A). Within the same region a further exon, called exon B, was detected which, however, was not associated with any transcription start site. Because of the presence of internal acceptor/donor splice sites, these exons undergo complex splicing events leading to at least 11 profiles of the F3/Contactin mRNA 5′ region69 (Fig. 3B and C).
Figure 3.
Activation of the F3/Contactin promoter in developing mouse cerebellum. (A) Organization of the 5′ flanking region of the mouse F3/Contactin gene. In (B) the 5′ flanking exons are shown, which undergo complex splicing events (shown in C), resulting in a high level of complexity of the F3/Contactin mRNA (reviewed in ref. 66). (D and E) Map of the F3/Contactin promoter/EGFP reporter construct (D), and its expression in developing cerebellum (E). Transgene expression recapitulates the endogenous gene with an earlier activation on migrating granule cells (P0, see also inset) and subsequent expression on Purkinje neurons (P8, see also inset). Egl, external granular layer; Igl, inner granular layer; PCl, Purkinje cells layer; WM, white matter. Scale bars: P0, P8 = 200 µm (insets 20 µm).
Therefore, these data indicate that the complex expression of the F3/Contactin glycoprotein is the result of an articulated regulation, including transcriptional and posttranscriptional, besides than the above-reported posttranslational mechanisms.
In vivo models for studying the profile of the F3/contactin promoter activation. The reported organization of the regulatory region of the F3/Contactin gene is both necessary and sufficient for driving its articulated profile. Indeed, in mice expressing an F3/Contactin promoter-EGFP reporter construct, including the genomic regions flanking exons A1, 0 and C1 (Fig. 3D), transgene expression essentially recapitulated the endogenous gene, indicating that the selected genomic region included all the elements necessary for driving F3/Contactin in vivo.70 Indeed, in the cerebellar cortex, early transgene expression was observed at P0 on postmitotic granule neurons entering the inner granular layer, with a progressive downregulation in subsequent developmental steps, when these neurons progress in their migration (Fig. 3E, P0, see also inset). In Purkinje neurons a sharp upregulation was found at around the end of the first postnatal week at the level of both their soma and dendrite arborisation (Fig. 3E, P8, see also inset).
Past P8, a downregulation was observed on both types of cerebellar neurons. However, as for the protein, a sharper decline was observed on Purkinje neurons, compared to the more gradual decrease observed on granule cells. The downregulation of the F3/Contactin promoter on granule cells during the first postnatal week strongly suggests that the mechanism responsible for sustained F3/Contactin expression in the adult molecular layer most likely relies on axonal transport.
Besides than on neuronal cells, activation of the F3/Contactin promoter-EGFP transgene was also detected on Ng2-positive precursors and on O4-positive oligodendrocytes, in keeping with the evidence that the endogenous gene is active in these cell populations.70
These data indicate that although the F3/Contactin gene regulatory region displays a very large size and a complex organization, the relevant sequences necessary to recapitulate its overall profile at the tissue and cellular levels are restricted to the genomic regions surrounding the relevant 5′ flanking exons. However, the fine activation of the different promoters in different neuronal or non-neuronal cell populations was not studied in detail and, in addition, it is likely that the large 5′ flanking non-coding sequences, separating the identified promoters (Fig. 3A), may be necessary to account for their independent activation.
It is worth mentioning that large 5′ introns have been also described in the case of the genes encoding other neural adhesive glycoproteins, as for instance the neural cell adhesion molecule NCAM itself.132
Developmental significance of F3/contactin regulated expression.
The high complexity of the organization of the F3/Contactin gene regulatory region and its articulated activation profile suggest that modulation of F3/Contactin expression may be relevant for its developmental function.
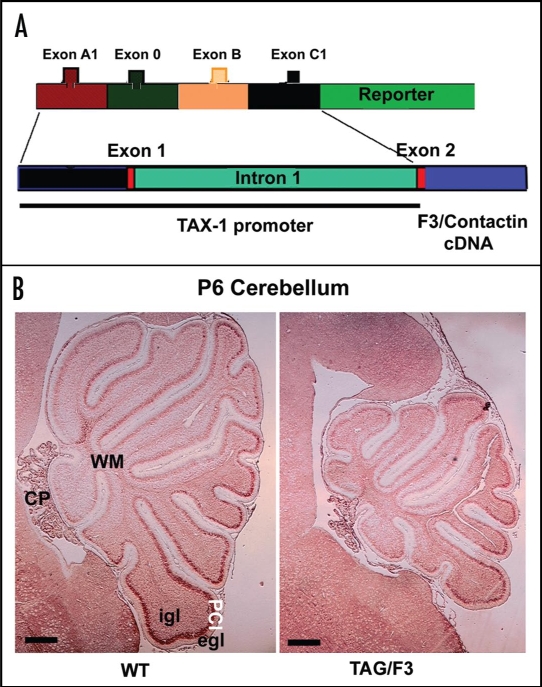
To address this possibility, a transgenic approach has been devised, aiming at driving F3/Contactin expression in vivo upon a temporal profile different from the one of the endogenous gene. In this attempt, a regulatory region from the gene encoding the human homologue of the Transient Axonal Glycoprotein TAG-1, the TAX-1 gene,129 has been selected in order to replace the F3/Contactin gene regulatory region (Fig. 4A). The rationale for this choice was that, although expressed on overlapping populations of postmitotic neurons, the TAG-1 gene is activated earlier than the F3/Contactin one on both premigratory cells and cycling precursors.66 In addition, in the cerebellar cortex, differential expression is observed on Purkinje neurons in which, unlike F3/Contactin, the TAG-1 gene is silent.133
Figure 4.
Changes in the cerebellar size arising from F3/Contactin developmental misexpression from the TAX-1 promoter. (A) Organization of the TAX-1 promoter/F3 cDNA construct. The F3/Contactin regulatory region, including the genomic sequences surrounding exons A1, 0, B and C1, has been replaced for by the promoter region from the human TAX-1 gene and used to drive the F3/Contactin cDNA in transgenic mice. (B) Changes in the cerebellar size arising from F3/Contactin developmental misexpression under control of the TAX-1 promoter, observed in postnatal day 6 mice. An F3/Contactin immunostaining is shown. egl, external granular layer; igl, inner granular layer; PCl, Purkinje cells layer; WM, white matter; CP, Choroid Plexus. Scale bar: 200 µm.
In the arising TAG/F3 mice, the profile of F3/Contactin expression was modified, with an earlier upregulation on premigratory granule neurons and, based on the TAG-1 profile, also on proliferating precursors of the external granular layer. This resulted in a sharp phenotype, affecting cerebellar development both morphologically and functionally. As a consequence of F3/Contactin overexpression, the cerebellar size was reduced, with sharp effects within the first postnatal week (shown at P6 in Fig. 4B). This phenotype was consistent with significant negative effects observed on cell proliferation, and with a delay in neuronal differentiation, observed for both granule and Purkinje neurons.66 However, the effects on precursor proliferation/differentiation were transient in nature and strictly related to F3/Contactin overexpression as, when the latter reverted as a consequence of the silencing of the TAX-1 promoter, a recovery from the above phenotype was observed.
The above data indicate that overexpression of F3/Contactin in the early postnatal period, when the endogenous gene undergoes only initial activation, results in inhibition of neurogenesis, suggesting that this likely represents one of the main consequences of F3/Contactin expression in neural development.66
The above morphological effects were paralleled by those on the cerebellar function. Indeed, TAG/F3 mice undergo a functional phenotype characterized by delayed sensory-motor development and impaired motor activity, motor coordination and motor learning.134 Since these effects are persistent past the fourth postnatal week, when the morphological alterations were largely reverted, this indicated that F3/Contactin overexpression may affect cerebellar function at the same time than, and independently of its structure.
Signalling pathway potentially mediating the effects of F3/contactin developmental overexpression.
Together, the above data clearly support the view that regulated expression of axonal adhesive glycoproteins like F3/Contactin may be provided with a specific significance in the control of neural development and function. The delayed development observed in different neuronal populations as a consequence of F3/Contactin overexpression suggests the involvement of a common signalling pathway. In this respect it is worth mentioning that similar effects are known to arise in different regions of the nervous tissue, including both cerebellar and cerebral cortices, as a consequence of Notch pathway activation,135–139 suggesting that the above developmental effects may be exerted via activation of this key regulatory pathway. Indeed, F3/Contactin is know to interact with Notch receptors on oligodendrocytes.63,140 However, in these cells, rather than the canonical Hes-dependent pathway, which counteracts oligodendrocyte differentiation,141 it activates the alternate pathway dependent upon the Deltex-1 transcription factor, which results in positive effects on the same event.63 Activation of Notch receptors by different ligands may therefore result in different developmental effects, depending upon the specific signalling pathway involved. F3/Contactin may then participate with different ligands to the modulation of the Notch pathway in the nervous tissue and it is worth mentioning that similar effects have been reported for the closely related Ig superfamily component NB-3.142 In this respect it has then been proposed that the balance between canonical and alternate activation of the Notch pathway may be relevant both for regulating specific aspects of neural development and, in addition, a role has been proposed in the pathogenesis of dysmyelinating disorders.143
Potential significance of changes on the expression profile of contactin and related molecules in cancer and in the pathogenesis of neurological disorders.
Distint investigations claim that a potential correlation may exist between changes in expression of axonal adhesive glycoproteins and neurological disorders,144 the best paradigms for this being represented by the L1/NgCAM glycoprotein.145
As far as F3/Contactin is concerned some correlation may be established between its expression and specific disorders of the nervous tissue. This concerns neoplastic transformation and in addition, those pathologies which affect interactions of myelinating cells with differentiating neurons.
As for the former, the molecule was found to be expressed on a glioblastoma lines.56 Such an expression, however, may represent a consequence of astrocyte dedifferentiation as normal astrocytes do not express the molecule. On these cells, F3/Contactin bears the properties of a repellent molecule demonstrated by adhesion and migration assays. Although the underlying molecular interactions and associated signalling events are largely unknown, it has been suggested that F3/Contactin may modulate the invasive properties of neoplastic cells expressing it.
On a related topic, a wider significance has been proposed for F3/Contactin on neurological disorders affecting the integrity of the neuro-glia interactions, in particular multiple sclerosis. This is largely based on the above-mentioned complex interactions the molecule undergo to at the nodal and paranodal regions, which among other, may involve modulation of the Notch pathway146 and in this respect, it is of interest to note that changes in the expression of the Contactin Associated Protein CASPr occur in the course of multiple sclerosis.147
Finally it is worth mentioning the similarity between F3/Contactin with anosmin, the product of Kal1 gene whose mutation is responsible for the Kallmann sindrome,148 characterized by hypogonadotropic hypogonadism and anosmia, which depends upon changes in the migration behavior of olfactory neurons. This would expand the range of neurological disorders potentially associated with changes in the expression level of axonally expressed adhesive glycoproteins, F3/Contactin belongs to.
Besides than the overall expression of axonal glycoproteins, also changes in their developmental profile may be relevant for neurological disorders. This may be deduced by comparing the severity of the phenotype arising from F3/Contactin developmental misexpression to the one of null mutant mice. Indeed, mice lacking F3/Contactin undergo a complex phenotype affecting myelinated axons and die as a consequence of this at around the end of the second postnatal week.13,92 However, although these animals are ataxic and undergo a cerebellar phenotype, the latter is much milder as compared to the one of TAG/F3 mice, supporting the view that rather than axonal adhesive glycoproteins expression per se, the regulated activation of the underlying genes is of most critical developmental relevance.
Concluding Remarks
From the reported data it may be inferred that F3/Contactin represents a relevant component of the nerve cell surface, which mediates the control of several functions of the developing nervous tissue, ranging from precursor proliferation to their migration and differentiation, to the directional growth of axon tracts and the specification of synaptic contacts. This pleiotropic function is likely related to its complex expression profile, which implies its differential location at the surface of the neuronal cell bodies in earlier developmental steps, mostly during their migration events and on axonal extensions in later developmental steps. Together with the evidence that the overall profile of the F3/Contactin gene is itself regulated during development of different neuronal and non neuronal cells, this strongly supports the hypothesis that regulated expression of axonal adhesive glycoproteins is itself provided with pivotal significance in neural development, and the recent demonstration of the F3/Contactin interaction with developmental control genes of the Notch family lends support to this possibility, opening the field of the pathways mediating the developmental function of these molecules, which represents an appealing topic for future investigations.
Acknowledgements
This work was supported by grants from the Italian Ministry of the University (COFIN 2007), from the Bari University and from the Fondazione Mariani, Milano, Italy.
Abbreviation
- CASPR
Contactin associated protein
- FNIII
fibronectin type III
- GC
granule cells
- GPI
glycosylphosphatidylinositol
- Ig
immunoglobulin
- IgSF
immunoglobulin superfamily
- Kbp
kilobases pair
- NCAM
neural cell adhesion molecule
- NgCAM
neuron-glia cell-adhesion molecule
- NrCAM
neuron-glia-related cell-adhesion molecule
- PC
purkinje cells
- RPTP β
receptor protein tyrosine phosphatase beta
- TAG-1
transient axonal glycoprotein
- BIG-1
brain-derived, immunoglobulin superfamily molecule-1
- BIG-2
brain-derived, immunoglobulin superfamily molecule-2
- CNTN
contactin
- VZ
ventricular zone
Footnotes
Previously published online as a Cell Adhesion & Migration E-publication: http://www.landesbioscience.com/journals/celladhesion/article/7462
References
- 1.Shapiro L, Love J, Colman DR. Adhesion molecules in the nervous system: structural insights into function and diversity. Annu Rev Neurosci. 2007;30:451–474. doi: 10.1146/annurev.neuro.29.051605.113034. [DOI] [PubMed] [Google Scholar]
- 2.Takeichi M. The cadherin superfamily in neuronal connections and interactions. Nat Rev Neurosci. 2007;8:11–20. doi: 10.1038/nrn2043. [DOI] [PubMed] [Google Scholar]
- 3.Denda S, Reichardt LF. Studies on integrins in the nervous system. Methods Enzymol. 2007;426:203–221. doi: 10.1016/S0076-6879(07)26010-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamada S, Nelson WJ. Synapses: sites of cell recognition, adhesion and functional specification. Annu Rev Biochem. 2007;76:267–294. doi: 10.1146/annurev.biochem.75.103004.142811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lardi-Studler B, Fritschy JM. Matching of pre- and postsynaptic specializations during synaptogenesis. Neuroscientist. 2007;13:115–126. doi: 10.1177/1073858406296803. [DOI] [PubMed] [Google Scholar]
- 6.Gerrow K, El-Husseini A. Cell adhesion molecules at the synapse. Front Biosci. 2006;11:2400–2419. doi: 10.2741/1978. [DOI] [PubMed] [Google Scholar]
- 7.Latefi NS, Colman DR. The CNS synapse revisited: gaps, adhesive welds and borders. Neurochem Res. 2007;32:303–310. doi: 10.1007/s11064-006-9181-0. [DOI] [PubMed] [Google Scholar]
- 8.Brusés JL. N-cadherin signaling in synapse formation and neuronal physiology. Mol Neurobiol. 2006;33:237–252. doi: 10.1385/MN:33:3:237. [DOI] [PubMed] [Google Scholar]
- 9.Maness PF, Schachner M. Neural recognition molecules of the immunoglobulin superfamily: signaling transducers of axon guidance and neuronal migration. Nat Neurosci. 2007;10:19–26. doi: 10.1038/nn1827. [DOI] [PubMed] [Google Scholar]
- 10.Mann F, Rougon G. Mechanisms of axon guidance: membrane dynamics and axonal transport in semaphorin signalling. J Neurochem. 2007;102:316–323. doi: 10.1111/j.1471-4159.2007.04578.x. [DOI] [PubMed] [Google Scholar]
- 11.Senkov O, Sun M, Weinhold B, Gerardy-Schahn R, Schachner M, Dityatev A. Polysialylated neural cell adhesion molecule is involved in induction of long-term potentiation and memory acquisition and consolidation in a fear-conditioning paradigm. J Neurosci. 2006;26:10888–10898. doi: 10.1523/JNEUROSCI.0878-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saghatelyan AK, Nikonenko AG, Sun M, Rolf B, Putthoff P, Kutsche M, Bartsch U, Dityatev A, Schachner M. Reduced GABAergic transmission and number of hippocampal perisomatic inhibitory synapses in juvenile mice deficient in the neural cell adhesion molecule L1. Mol Cell Neurosci. 2004;26:191–203. doi: 10.1016/j.mcn.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Boyle ME, Berglund EO, Murai KK, Weber L, Peles E, Ranscht B. Contactin orchestrates assembly of the septate-like junctions at the paranode in myelinated peripheral nerve. Neuron. 2001;30:385–397. doi: 10.1016/s0896-6273(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 14.Bhat MA, Rios JC, Lu Y, Garcia-Fresco GP, Ching W, St Martin M, Li J, Einheber S, Chesler M, Rosenbluth J, Salzer JL, Bellen HJ. Contactin orchestrates assembly of the septate-like junctions at the paranode in myelinated peripheral nerve. Neuron. 2001;30:369–383. doi: 10.1016/s0896-6273(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 15.Kazarinova-Noyes K, Malhotra JD, McEwen DP, Mattei LN, Berglund EO, Ranscht B, Levinson SR, Schachner M, Shrager P, Isom LL, Xiao ZC. Contactin associates with Na+ channels and increases their functional expression. J Neurosci. 2001;21:7517–7525. doi: 10.1523/JNEUROSCI.21-19-07517.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poliak S, Salomon D, Elhanany H, Sabanay H, Kiernan B, Pevny L, Stewart CL, Xu X, Chiu SY, Shrager P, Furley AJ, Peles E. Juxtaparanodal clustering of Shaker-like K+ channels in myelinated axons depends on Caspr2 and TAG-1. J Cell Biol. 2003;162:1149–1160. doi: 10.1083/jcb.200305018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Srinivasan J, Schachner M, Catterall WA. Interaction of voltage-gated sodium channels with the extracellular matrix molecules tenascin-C and tenascin-R. Proc Natl Acad Sci USA. 1998;95:15753–15757. doi: 10.1073/pnas.95.26.15753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakamoto T, Kain KH, Ginsberg MH. Neurobiology: New connections between integrins and axon guidance. Curr Biol. 2004;14:121–123. [PubMed] [Google Scholar]
- 19.Kamiguchi H, Lemmon V. IgCAMs: bidirectional signals underlying neurite growth. Curr Opin Cell Biol. 2000;12:598–605. doi: 10.1016/s0955-0674(00)00138-1. [DOI] [PubMed] [Google Scholar]
- 20.Brümmendorf T, Rathjen FG. Structure/function relationships of axon-associated adhesion receptors of the immunoglobulin superfamily. Curr Opin Neurobiol. 1996;6:584–589. doi: 10.1016/s0959-4388(96)80089-4. [DOI] [PubMed] [Google Scholar]
- 21.Cota E, Steward A, Fowler SB, Clarke J. The folding nucleus of a fibronectin type III domain is composed of core residues of the immunoglobulin-like fold. J Mol Biol. 2001;305:1185–1194. doi: 10.1006/jmbi.2000.4378. [DOI] [PubMed] [Google Scholar]
- 22.Steward A, Adhya S, Clarke J. Sequence conservation in Ig-like domains: the role of highly conserved proline residues in the fibronectin type III superfamily. J Mol Biol. 2002;318:935–940. doi: 10.1016/S0022-2836(02)00184-5. [DOI] [PubMed] [Google Scholar]
- 23.Kadmon G, Altevogt P. The cell adhesion molecule L1: species- and cell-type-dependent multiple binding mechanisms. Differentiation. 1997;61:143–150. doi: 10.1046/j.1432-0436.1997.6130143.x. [DOI] [PubMed] [Google Scholar]
- 24.Grumet M. Nr-CAM: a cell adhesion molecule with ligand and receptor functions. Cell Tissue Res. 1997;290:423–428. doi: 10.1007/s004410050949. [DOI] [PubMed] [Google Scholar]
- 25.Rathjen FG, Wolff JM, Chang S, Bonhoeffer F, Raper JA. Neurofascin: a novel chick cell-surface glycoprotein involved in neurite-neurite interactions. Cell. 1987;51:841–849. doi: 10.1016/0092-8674(87)90107-3. [DOI] [PubMed] [Google Scholar]
- 26.Gennarini G, Rougon G, Vitello F, Corsi P, Di Benedetta C, Goridis C. Identification and cDNA cloning of a new member of the L2/HNK-1 family of neural surface glycoproteins. J Neurosci Res. 1989a;22:1–12. doi: 10.1002/jnr.490220102. [DOI] [PubMed] [Google Scholar]
- 27.Gennarini G, Cibelli G, Rougon G, Mattei MG, Goridis C. The mouse neuronal cell surface protein F3: a phosphatidylinositol-anchored member of the immunoglobulin superfamily related to chicken contactin. J Cell Biol. 1989b;109:775–788. doi: 10.1083/jcb.109.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gennarini G, Durbec P, Boned A, Rougon G, Goridis C. Transfected F3/F11 neuronal cell surface protein mediates intercellular adhesion and promotes neurite outgrowth. Neuron. 1991;6:595–606. doi: 10.1016/0896-6273(91)90062-5. [DOI] [PubMed] [Google Scholar]
- 29.Furley AJ, Morton SB, Manalo D, Karagogeos D, Dodd J, Jessell TM. The axonal glycoprotein TAG-1 is an immunoglobulin superfamily member with neurite outgrowth-promoting activity. Cell. 1990;61:157–170. doi: 10.1016/0092-8674(90)90223-2. [DOI] [PubMed] [Google Scholar]
- 30.Durbec P, Gennarini G, Goridis C, Rougon G. A soluble form of the F3 neuronal cell adhesion molecule promotes neurite outgrowth. J Cell Biol. 1992;117:877–887. doi: 10.1083/jcb.117.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bixby JL. Diversity of axonal growth-promoting receptors and regulation of their function. Curr Opin Neurobiol. 1992;2:66–69. doi: 10.1016/0959-4388(92)90164-g. [DOI] [PubMed] [Google Scholar]
- 32.Doherty P, Walsh FS. Cell adhesion molecules, second messengers and axonal growth. Curr Opin Neurobiol. 1992;2:595–601. doi: 10.1016/0959-4388(92)90024-f. [DOI] [PubMed] [Google Scholar]
- 33.Walsh FS, Doherty P. Neural cell adhesion molecules of the immunoglobulin superfamily: role in axon growth and guidance. Annu Rev Cell Dev Biol. 1997;13:425–456. doi: 10.1146/annurev.cellbio.13.1.425. [DOI] [PubMed] [Google Scholar]
- 34.Bixby JL, Pratt RS, Lilien J, Reichardt LF. Neurite outgrowth on muscle cell surfaces involves extracellular matrix receptors as well as Ca2+-dependent and -independent cell adhesion molecules. Proc Natl Acad Sci USA. 1987;84:2555–2559. doi: 10.1073/pnas.84.8.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang S, Rathjen FG, Raper JA. Extension of neurites on axons is impaired by antibodies against specific neural cell surface glycoproteins. J Cell Biol. 1987;104:355–362. doi: 10.1083/jcb.104.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pesheva P, Gennarini G, Goridis C, Schachner M. The F3/11 cell adhesion molecule mediates the repulsion of neurons by the extracellular matrix glycoprotein J1-160/180. Neuron. 1993;10:69–82. doi: 10.1016/0896-6273(93)90243-k. [DOI] [PubMed] [Google Scholar]
- 37.Henke-Fahle S, Bonhoeffer F. Inhibition of axonal growth by a monoclonal antibody. Nature. 1983;303:65–67. doi: 10.1038/303065a0. [DOI] [PubMed] [Google Scholar]
- 38.Doherty P, Cohen J, Walsh FS. Neurite outgrowth in response to transfected N-CAM changes during development and is modulated by polysialic acid. Neuron. 1990;5:209–219. doi: 10.1016/0896-6273(90)90310-c. [DOI] [PubMed] [Google Scholar]
- 39.Kuhn TB, Stoeckli ET, Condrau MA, Rathjen FG, Sonderegger P. Neurite outgrowth on immobilized axonin-1 is mediated by a heterophilic interaction with L1(G4) J Cell Biol. 1991;115:1113–1126. doi: 10.1083/jcb.115.4.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lemmon V, Burden SM, Payne HR, Elmslie GJ, Hlavin ML. Neurite growth on different substrates: permissive versus instructive influences and the role of adhesive strength. J Neurosci. 1992;12:818–826. doi: 10.1523/JNEUROSCI.12-03-00818.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ignelzi MA, Jr, Miller DR, Soriano P, Maness PF. Impaired neurite outgrowth of src-minus cerebellar neurons on the cell adhesion molecule L1. Neuron. 1994;12:873–884. doi: 10.1016/0896-6273(94)90339-5. [DOI] [PubMed] [Google Scholar]
- 42.Webb K, Budko E, Neuberger TJ, Chen S, Schachner M, Tresco PA. Substrate-bound human recombinant L1 selectively promotes neuronal attachment and outgrowth in the presence of astrocytes and fibroblasts. Biomaterials. 2001;22:1017–1028. doi: 10.1016/s0142-9612(00)00353-7. [DOI] [PubMed] [Google Scholar]
- 43.Blackmore M, Letourneau PC. Changes within maturing neurons limit axonal regeneration in the developing spinal cord. J Neurobiol. 2006;66:1564–1583. doi: 10.1002/neu.20224. [DOI] [PubMed] [Google Scholar]
- 44.Castellani V, Rougon G. Control of semaphorin signaling. Curr Opin Neurobiol. 2002;12:532–541. doi: 10.1016/s0959-4388(02)00357-4. [DOI] [PubMed] [Google Scholar]
- 45.Lustig M, Sakurai T, Grumet M. Nr-CAM promotes neurite outgrowth from peripheral ganglia by a mechanism involving axonin-1 as a neuronal receptor. Dev Biol. 1999;209:340–351. doi: 10.1006/dbio.1999.9250. [DOI] [PubMed] [Google Scholar]
- 46.Kunz S, Spirig M, Ginsburg C, Buchstaller A, Berger P, Lanz R, Rader C, Vogt L, Kunz B, Sonderegger P. Neurite fasciculation mediated by complexes of axonin-1 and Ng cell adhesion molecule. J Cell Biol. 1998;143:1673–1690. doi: 10.1083/jcb.143.6.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buchstaller A, Kunz S, Berger P, Kunz B, Ziegler U, Rader C, Sonderegger P. Cell adhesion molecules NgCAM and axonin-1 form heterodimers in the neuronal membrane and cooperate in neurite outgrowth promotion. J Cell Biol. 1996;135:1593–1607. doi: 10.1083/jcb.135.6.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Volkmer H, Leuschner R, Zacharias U, Rathjen FG. Neurofascin induces neurites by heterophilic interactions with axonal NrCAM while NrCAM requires F11 on the axonal surface to extend neurites. J Cell Biol. 1996;135:1059–1069. doi: 10.1083/jcb.135.4.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morales G, Sanchez-Puelles JM, Schwarz U, de la Rosa EJ. Synergistic neurite-outgrowth promoting activity of two related axonal proteins, Bravo/Nr-CAM and G4/Ng-CAM in chicken retinal explants. Eur J Neurosci. 1996;8:1098–1105. doi: 10.1111/j.1460-9568.1996.tb01277.x. [DOI] [PubMed] [Google Scholar]
- 50.Traka M, Goutebroze L, Denisenko N, Bessa M, Nifli A, Havaki S, Iwakura Y, Fukamauchi F, Watanabe K, Soliven B, Girault JA, Karagogeos D. Association of TAG-1 with Caspr2 is essential for the molecular organization of juxtaparanodal regions of myelinated fibers. J Cell Biol. 2003;162:1161–1172. doi: 10.1083/jcb.200305078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bonnon C, Goutebroze L, Denisenko-Nehrbass N, Girault JA, Faivre-Sarrailh C. The paranodal complex of F3/contactin and caspr/paranodin traffics to the cell surface via a non-conventional pathway. J Biol Chem. 2003;278:48339–48347. doi: 10.1074/jbc.M309120200. [DOI] [PubMed] [Google Scholar]
- 52.Law CO, Kirby RJ, Aghamohammadzadeh S, Furley AJ. The neural adhesion molecule TAG-1 modulates responses of sensory axons to diffusible guidance signals. Development. 2008;135:2361–2371. doi: 10.1242/dev.009019. [DOI] [PubMed] [Google Scholar]
- 53.Kim BW, Son H. Neural cell adhesion molecule (NCAM) induces neuronal phenotype acquisition in dominant negative MEK1-expressing hippocampal neural progenitor cells. Exp Mol Med. 2006;38:732–738. doi: 10.1038/emm.2006.86. [DOI] [PubMed] [Google Scholar]
- 54.Tate MC, García AJ, Keselowsky BG, Schumm MA, Archer DR, LaPlaca MC. Specific beta1 integrins mediate adhesion, migration and differentiation of neural progenitors derived from the embryonic striatum. Mol Cell Neurosci. 2004;27:22–31. doi: 10.1016/j.mcn.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 55.Schmandt T, Meents E, Gossrau G, Gornik V, Okabe S, Brüstle O. High-purity lineage selection of embryonic stem cell-derived neurons. Stem Cells Dev. 2005;14:55–64. doi: 10.1089/scd.2005.14.55. [DOI] [PubMed] [Google Scholar]
- 56.Eckerich C, Zapf S, Ulbricht U, Müller S, Fillbrandt R, Westphal M, Lamszus K. Contactin is expressed in human astrocytic gliomas and mediates repulsive effects. Glia. 2006;53:1–12. doi: 10.1002/glia.20254. [DOI] [PubMed] [Google Scholar]
- 57.Soares S, Traka M, von Boxberg Y, Bouquet C, Karagogeos D, Nothias F. Neuronal and glial expression of the adhesion molecule TAG-1 is regulated after peripheral nerve lesion or central neurodegeneration of adult nervous system. Eur J Neurosci. 2005;21:1169–1180. doi: 10.1111/j.1460-9568.2005.03961.x. [DOI] [PubMed] [Google Scholar]
- 58.Skaper SD. Neuronal growth-promoting and inhibitory cues in neuroprotection and neuroregeneration. Ann N Y Acad Sci. 1053:376–385. doi: 10.1196/annals.1344.032. [DOI] [PubMed] [Google Scholar]
- 59.Falk J, Bonnon C, Girault JA, Faivre-Sarrailh C. F3/contactin, a neuronal cell adhesion molecule implicated in axogenesis and myelination. Biol Cell. 2002;94:327–334. doi: 10.1016/s0248-4900(02)00006-0. [DOI] [PubMed] [Google Scholar]
- 60.Poliak S, Peles E. The local differentiation of myelinated axons at nodes of Ranvier. Nat Rev Neurosci. 2003;4:968–980. doi: 10.1038/nrn1253. [DOI] [PubMed] [Google Scholar]
- 61.Peles E, Salzer JL. Molecular domains of myelinated axons. Curr Opin Neurobiol. 2000;10:558–565. doi: 10.1016/s0959-4388(00)00122-7. [DOI] [PubMed] [Google Scholar]
- 62.Kohsaka H, Takasu E, Nose A. In vivo induction of postsynaptic molecular assembly by the cell adhesion molecule Fasciclin2. J Cell Biol. 2007;179:1289–1300. doi: 10.1083/jcb.200705154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hu QD, Ang BT, Karsak M, Hu WP, Cui XY, Duka T, et al. F3/contactin acts as a functional ligand for Notch during oligodendrocyte maturation. Cell. 2003;115:163–175. doi: 10.1016/s0092-8674(03)00810-9. [DOI] [PubMed] [Google Scholar]
- 64.Cui XY, Hu QD, Tekaya M, Shimoda Y, Ang BT, Nie DY, et al. NB-3/Notch1 pathway via Deltex1 promotes neural progenitor cell differentiation into oligodendrocytes. J Biol Chem. 2004;279:25858–25865. doi: 10.1074/jbc.M313505200. [DOI] [PubMed] [Google Scholar]
- 65.Buttiglione M, Revest JM, Rougon G, Faivre-Sarrailh C. F3 neuronal adhesion molecule controls outgrowth and fasciculation of cerebellar granule cell neurites: a cell-type-specific effect mediated by the Ig-like domains. Mol Cell Neurosci. 1996;8:53–69. doi: 10.1006/mcne.1996.0043. [DOI] [PubMed] [Google Scholar]
- 66.Bizzoca A, Virgintino D, Lorusso L, Buttiglione M, Yoshida L, Polizzi A, et al. Transgenic mice expressing F3/contactin from the TAG-1 promoter exhibit developmentally regulated changes in the differentiation of cerebellar neurons. Development. 2003;130:29–43. doi: 10.1242/dev.00183. [DOI] [PubMed] [Google Scholar]
- 67.Liedtke S, Geyer H, Wuhrer M, Geyer R, Frank G, Gerardy-Schahn R, et al. Characterization of N-glycans from mouse brain neural cell adhesion molecule. Glycobiology. 2001;11:373–384. doi: 10.1093/glycob/11.5.373. [DOI] [PubMed] [Google Scholar]
- 68.Berglund EO, Ranscht B. Molecular cloning and in situ localization of the human contactin gene (CNTN1) on chromosome 12q11–q12. Genomics. 1994;21:571–582. doi: 10.1006/geno.1994.1316. [DOI] [PubMed] [Google Scholar]
- 69.De Benedictis L, Polizzi A, Cangiano G, Buttiglione M, Arbia S, Storlazzi CT, Rocchi M, Gennarini G. Alternative promoters drive the expression of the gene encoding the mouse axonal glycoprotein F3/contactin. Brain Res Mol Brain Res. 2001;95:55–74. doi: 10.1016/s0169-328x(01)00243-1. [DOI] [PubMed] [Google Scholar]
- 70.De Benedictis L, Bizzoca A, Corsi P, Albieri I, Consalez GG, Gennarini G. Activation profile of the F3/Contactin gene in the developing mouse cerebellum. Mol Cell Neurosci. 2006;32:403–418. doi: 10.1016/j.mcn.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 71.Rome C, Roullot V, Couillaud F. Polymorphism of the untranslated regions of the F3/contactin mRNA in the rat nervous system. Brain Res Mol Brain Res. 2005;139:184–191. doi: 10.1016/j.molbrainres.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 72.Yoshihara Y, Kawasaki M, Tani A, Tamada A, Nagata S, Kagamiyama H, Mori K. Overlapping and differential expression of BIG-2, BIG-1, TAG-1 and F3: four members of an axon-associated cell adhesion molecule subgroup of the immunoglobulin superfamily. Neuron. 1994;13:415–426. doi: 10.1002/neu.480280106. [DOI] [PubMed] [Google Scholar]
- 73.Hansford LM, Smith SA, Haber M, Norris MD, Cheung B, Marshall GM. Cloning and characterization of the human neural cell adhesion molecule, CNTN4 (alias BIG-2) Cytogenet Genome Res. 2003;101:17–23. doi: 10.1159/000073412. [DOI] [PubMed] [Google Scholar]
- 74.Kamei Y, Takeda Y, Teramoto K, Tsutsumi O, Taketani Y, Watanabe K. Human NB-2 of the contactin subgroup molecules: chromosomal localization of the gene (CNTN5) and distinct expression pattern from other subgroup members. Genomics. 2000;69:113–119. doi: 10.1006/geno.2000.6310. [DOI] [PubMed] [Google Scholar]
- 75.Kamei Y, Tsutsumi O, Taketani Y, Watanabe K. cDNA cloning and chromosomal localization of neural adhesion molecule NB-3 in human. J Neurosci Res. 1998;51:275–283. doi: 10.1002/(SICI)1097-4547(19980201)51:3<275::AID-JNR1>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 76.Yoshihara Y, Kawasaki M, Tamada A, Nagata S, Kagamiyama H, Mori K. Overlapping and differential expression of BIG-2, BIG-1, TAG-1 and F3: four members of an axon-associated cell adhesion molecule subgroup of the immunoglobulin superfamily. J Neurobiol. 1995;28:51–69. doi: 10.1002/neu.480280106. [DOI] [PubMed] [Google Scholar]
- 77.Ranscht B. Sequence of contactin, a 130-kD glycoprotein concentrated in areas of interneuronal contact, defines a new member of the immunoglobulin supergene family in the nervous system. J Cell Biol. 1988;107:1561–1573. doi: 10.1083/jcb.107.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Watanabe K, Shimazaki K, Hosoya H, Fukamauchi F, Takenawa T. Cloning of the cDNA encoding neural adhesion molecule F3 from bovine brain. Gene. 1995;160:245–248. doi: 10.1016/0378-1119(95)00062-b. [DOI] [PubMed] [Google Scholar]
- 79.Haenisch C, Diekmann H, Klinger M, Gennarini G, Kuwada JY, Stuermer CA. The neuronal growth and regeneration associated Cntn1 (F3/F11/Contactin) gene is duplicated in fish: expression during development and retinal axon regeneration. Mol Cell Neurosci. 2005;28:361–374. doi: 10.1016/j.mcn.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 80.Schweitzer J, Gimnopoulos D, Lieberoth BC, Pogoda HM, Feldner J, Ebert A, Schachner M, Becker T, Becker CG. Contactin 1a expression is associated with oligodendrocyte differentiation and axonal regeneration in the central nervous system of zebrafish. Mol Cell Neurosci. 2007;35:194–207. doi: 10.1016/j.mcn.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 81.Faivre-Sarrailh C, Banerjee S, Li J, Hortsch M, Laval M, Bhat MA. Drosophila contactin, a homolog of vertebrate contactin, is required for septate junction organization and paracellular barrier function. Development. 2004;131:4931–4942. doi: 10.1242/dev.01372. [DOI] [PubMed] [Google Scholar]
- 82.Banerjee S, Pillai AM, Paik R, Li J, Bhat MA. Axonal ensheathment and septate junction formation in the peripheral nervous system of Drosophila. J Neurosci. 2006;26:3319–3329. doi: 10.1523/JNEUROSCI.5383-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Milanese C, Fiumara F, Bizzoca A, Giachello C, Leitinger G, Gennarini G, Montarolo PG, Ghirardi M. F3/contactin-related proteins in Helix pomatia nervous tissue (HCRPs): distribution and function in neurite growth and neurotransmitter release. J Neurosci Res. 2008;86:821–831. doi: 10.1002/jnr.21539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Milanese C, Giachello C, Fiumara F, Bizzoca A, Gennarini G, Montarolo PG, Ghirardi M. Characterization and role of Helix contactin-related proteins in cultured Helix pomatia neurons. J Neurosci Res. 2008 doi: 10.1002/jnr.21849. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 85.Wolfer DP, Giger RJ, Stagliar M, Sonderegger P, Lipp HP. Expression of the axon growth-related neural adhesion molecule TAG-1/axonin-1 in the adult mouse brain. Anat Embryol (Berl) 1998;197:177–185. doi: 10.1007/s004290050129. [DOI] [PubMed] [Google Scholar]
- 86.Buttiglione M, Revest JM, Pavlou O, Karagogeos D, Furley A, Rougon G, Faivre-Sarrailh C. A functional interaction between the neuronal adhesion molecules TAG-1 and F3 modulates neurite outgrowth and fasciculation of cerebellar granule cells. J Neurosci. 1998;18:6853–6870. doi: 10.1523/JNEUROSCI.18-17-06853.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chatzopoulou E, Miguez A, Savvaki M, Levasseur G, Muzerelle A, Muriel MP, Goureau O, Watanabe K, Goutebroze L, Gaspar P, Zalc B, Karagogeos D, Thomas JL. Structural requirement of TAG-1 for retinal ganglion cell axons and myelin in the mouse optic nerve. J Neurosci. 2008;28:7624–7636. doi: 10.1523/JNEUROSCI.1103-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu Y, Halloran MC. Central and peripheral axon branches from one neuron are guided differentially by Semaphorin3D and transient axonal glycoprotein-1. J Neurosci. 2005;25:10556–10563. doi: 10.1523/JNEUROSCI.2710-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Virgintino D, Ambrosini M, D'Errico P, Bertossi M, Papadaki C, Karagogeos D, Gennarini G. Regional distribution and cell type-specific expression of the mouse F3 axonal glycoprotein: a developmental study. J Comp Neurol. 1999;413:357–372. doi: 10.1002/(sici)1096-9861(19991025)413:3<357::aid-cne1>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 90.Faivre-Sarrailh C, Gennarini G, Goridis C, Rougon G. F3/F11 cell surface molecule expression in the developing mouse cerebellum is polarized at synaptic sites and within granule cells. J Neurosci. 1992;12:257–267. doi: 10.1523/JNEUROSCI.12-01-00257.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sotelo C. Cellular and genetic regulation of the development of the cerebellar system. Prog Neurobiol. 2004;72:239–295. doi: 10.1016/j.pneurobio.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 92.Berglund EO, Murai KK, Fredette B, Sekerková G, Maturano B, Weber L, Mugnaini E, Ranscht B. Ataxia and abnormal cerebellar microorganization in mice with ablated contactin gene expression. Neuron. 1999;24:739–750. doi: 10.1016/s0896-6273(00)81126-5. [DOI] [PubMed] [Google Scholar]
- 93.Espinosa JS, Luo L. Timing neurogenesis and differentiation: insights from quantitative clonal analyses of cerebellar granule cells. J Neurosci. 2008;28:2301–2312. doi: 10.1523/JNEUROSCI.5157-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fogel AI, Akins MR, Krupp AJ, Stagi M, Stein V, Biederer T. SynCAMs organize synapses through heterophilic adhesion. J Neurosci. 2007;27:12516–12530. doi: 10.1523/JNEUROSCI.2739-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Desai AR, McConnel SK. Progressive restriction in fate potential by neural progenitors during cerebral cortical development. Development. 2000;127:2863–2872. doi: 10.1242/dev.127.13.2863. [DOI] [PubMed] [Google Scholar]
- 96.Frantz GD, McConnel SK. Restriction of late cerebral cortical progenitors to an upper-layer fate. Neuron. 1996;17:55–61. doi: 10.1016/s0896-6273(00)80280-9. [DOI] [PubMed] [Google Scholar]
- 97.Campbell K. Cortical neuron specification: it has its time and place. Neuron. 2005;46:273–276. doi: 10.1016/j.neuron.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 98.Casanova MF, Trippe J., 2nd Regulatory mechanisms of cortical laminar development. Brain Res Rev. 2006;51:72–84. doi: 10.1016/j.brainresrev.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 99.Marin O, Rubenstein JL. Cell migration in the forebrain. Annu Rev Neurosci. 2003;26:441–483. doi: 10.1146/annurev.neuro.26.041002.131058. [DOI] [PubMed] [Google Scholar]
- 100.Hatten ME. New directions in neuronal migration. Science. 2002;297:1660–1663. doi: 10.1126/science.1074572. [DOI] [PubMed] [Google Scholar]
- 101.Hatten ME. Central nervous system neuronal migration. Annu Rev Neurosci. 1999;22:511–539. doi: 10.1146/annurev.neuro.22.1.511. [DOI] [PubMed] [Google Scholar]
- 102.Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- 103.Ogawa J, Kaneko H, Masuda T, Nagata S, Hosoya H, Watanabe K. Novel neural adhesion molecules in the Contactin/F3 subgroup of the immunoglobulin superfamily: isolation and characterization of cDNAs from rat brain. Neurosci Lett. 1996;218:173–176. doi: 10.1016/s0304-3940(96)13156-6. [DOI] [PubMed] [Google Scholar]
- 104.Rios JC, Melendez-Vasquez CV, Einheber S, Lustig M, Grumet M, Hemperly J, Peles E, Valzer JL. Contactin-associated protein (Caspr) and contactin form a complex that is targeted to the paranodal junctions during myelination. J Neurosci. 2000;20:8354–8364. doi: 10.1523/JNEUROSCI.20-22-08354.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rios JC, Rubin M, St Martin M, Downey RT, Einheber S, Rosenbluth J, Levinson SR, Bhat M, Salzer JL. Paranodal interactions regulate expression of sodium channel subtypes and provide a diffusion barrier for the node of Ranvier. J Neurosci. 2003;23:7001–7011. doi: 10.1523/JNEUROSCI.23-18-07001.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shah BS, Rush AM, Liu S, Tyrrel L, Black JA, Dib-Hajj SD, Vaxman SG. Contactin associates with sodium channel NaV1.3 in native tissues and increases channel density at the cell surface. J Neurosci. 2004;24:7387–7399. doi: 10.1523/JNEUROSCI.0322-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu CJ, Dib-Hajj SD, Black JA, Greenwood J, Lian Z, Waxman SG. Direct interaction with contactin targets voltage-gated sodium channel Na(v)1.9/NaN to the cell membrane. J Biol Chem. 2001;276:46553–46561. doi: 10.1074/jbc.M108699200. [DOI] [PubMed] [Google Scholar]
- 108.Kazarinova-Noyes K, Shrager P. Molecular constituents of the node of Ranvier. Mol Neurobiol. 2002;26:167–182. doi: 10.1385/MN:26:2-3:167. [DOI] [PubMed] [Google Scholar]
- 109.Ratcliffe CF, Qu Y, McCormick KA, Tibbs VC, Dixon JE, Scheuer T, Catterall WA. A sodium channel signaling complex: modulation by associated receptor protein tyrosine phosphatase beta. Nat Neurosci. 2000;3:437–444. doi: 10.1038/74805. [DOI] [PubMed] [Google Scholar]
- 110.Ratcliffe CF, Westenbroek RE, Curtis R, Catterall WA. Sodium channel beta1 and beta3 subunits associate with neurofascin through their extracellular immunoglobulin-like domain. J Cell Biol. 2001;154:427–434. doi: 10.1083/jcb.200102086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xiao ZC, Revest JM, Laeng P, Rougon G, Schachner M, Montag D. Defasciculation of neurites is mediated by tenascin-R and its neuronal receptor F3/11. J Neurosci Res. 1998;52:390–404. doi: 10.1002/(SICI)1097-4547(19980515)52:4<390::AID-JNR3>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 112.Xiao ZC, Ragsdale DS, Malhotra JD, Mattei LN, Braun PE, Schachner M, Isom LL. Tenascin-R is a functional modulator of sodium channel beta subunits. J Biol Chem. 1999;274:26511–26517. doi: 10.1074/jbc.274.37.26511. [DOI] [PubMed] [Google Scholar]
- 113.Rigato F, Garwood J, Calco V, Heck N, Faivre-Sarrailh C, Faissner A. Tenascin-C promotes neurite outgrowth of embryonic hippocampal neurons through the alternatively spliced fibronectin type III BD domains via activation of the cell adhesion molecule F3/contactin. J Neurosci. 2002;22:6596–6609. doi: 10.1523/JNEUROSCI.22-15-06596.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zacharias U, Rauch U. Competition and cooperation between tenascin-R, lecticans and contactin 1 regulate neurite growth and morphology. J Cell Sci. 2006;119:3456–3466. doi: 10.1242/jcs.03094. [DOI] [PubMed] [Google Scholar]
- 115.Pesheva P, Probstmeier R, Lang DM, McBride R, Hsu NJ, Gennarini G, Spiess E, Peshev Z. Early coevolution of adhesive but not antiadhesive tenascin-R ligand-receptor pairs in vertebrates: a phylogenetic study. Mol Cell Neurosci. 2006;32:366–368. doi: 10.1016/j.mcn.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 116.Heck N, Klausmeyer A, Faissner A, Garwood J. Cortical neurons express PSI, a novel isoform of phosphacan/RPTPbeta. Cell Tissue Res. 2005;321:323–333. doi: 10.1007/s00441-005-1135-3. [DOI] [PubMed] [Google Scholar]
- 117.Revest JM, Faivre-Sarrailh C, Schachner M, Rougon G. Bidirectional signaling between neurons and glial cells via the F3 neuronal adhesion molecule. Adv Exp Med Biol. 1999;468:309–318. doi: 10.1007/978-1-4615-4685-6_25. [DOI] [PubMed] [Google Scholar]
- 118.Peles E, Nativ M, Lustig M, Grumet M, Schilling J, Martinez R, Plowman GD, Schlessinger J. Identification of a novel contactin-associated transmembrane receptor with multiple domains implicated in protein-protein interactions. EMBO J. 1997;16:978–988. doi: 10.1093/emboj/16.5.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Faivre-Sarrailh C, Gauthier F, Denisenko-Nehrbass N, Le Bivic A, Rougon G, Girault JA. The glycosylphosphatidyl inositol-anchored adhesion molecule F3/contactin is required for surface transport of paranodin/contactin-associated protein (caspr) J Cell Biol. 2000;149:491–502. doi: 10.1083/jcb.149.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gollan L, Salomon D, Valzer JL, Peles E. Caspr regulates the processing of contactin and inhibits its binding to neurofascin. J Cell Biol. 2003;163:1213–1218. doi: 10.1083/jcb.200309147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Charles P, Tait S, Faivre-Sarrailh C, Barbin G, Gunn-Moore F, Denisenko-Nehrbass N, Guennoc AM, Girault J, Brophy PJ, Lubetzki C. Neurofascin is a glial receptor for the paranodin/Caspr-contactin axonal complex at the axoglial junction. Curr Biol. 2002;12:217–220. doi: 10.1016/s0960-9822(01)00680-7. [DOI] [PubMed] [Google Scholar]
- 122.Bonnon C, Bel C, Goutebroze L, Maigret B, Girault JA, Faivre-Sarrailh C. PGY repeats and N-glycans govern the trafficking of paranodin and its selective association with contactin and neurofascin-155. Mol Biol Cell. 2007;18:229–241. doi: 10.1091/mbc.E06-06-0570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nie DY, Zhou ZH, Ang BT, Teng FY, Xu G, Xiang T, et al. Nogo-A at CNS paranodes is a ligand of Caspr: possible regulation of K(+) channel localization. EMBO J. 2003;22:5666–5678. doi: 10.1093/emboj/cdg570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Menegoz M, Gaspar P, Le Bert M, Galvez T, Burgaya F, Palfrey C, Ezan P, Arnos F, Girault JA. Paranodin, a glycoprotein of neuronal paranodal membranes. Neuron. 1997;19:319–331. doi: 10.1016/s0896-6273(00)80942-3. [DOI] [PubMed] [Google Scholar]
- 125.Gollan L, Sabanay H, Poliak S, Berglund EO, Ranscht B, Peles E. Retention of a cell adhesion complex at the paranodal junction requires the cytoplasmic region of Caspr. J Cell Biol. 2002;157:1247–1256. doi: 10.1083/jcb.200203050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Koch T, Brugger T, Bach A, Gennarini G, Trotter J. Expression of the immunoglobulin superfamily cell adhesion molecule F3 by oligodendrocyte-lineage cells. Glia. 1997;19:199–212. doi: 10.1002/(sici)1098-1136(199703)19:3<199::aid-glia3>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 127.Traka M, Dupree JL, Popko B, Karagogeos D. The neuronal adhesion protein TAG-1 is expressed by Schwann cells and oligodendrocytes and is localized to the juxtaparanodal region of myelinated fibers. J Neurosci. 2002;22:3016–3024. doi: 10.1523/JNEUROSCI.22-08-03016.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Garwood J, Heck N, Reichardt F, Faissner A. Phosphacan short isoform, a novel non-proteoglycan variant of phosphacan/receptor protein tyrosine phosphatase-beta, interacts with neuronal receptors and promotes neurite outgrowth. J Biol Chem. 2003;278:24164–24173. doi: 10.1074/jbc.M211721200. [DOI] [PubMed] [Google Scholar]
- 129.Kozlov SV, Ginger RJ, Hasler T, Korvatska E, Schorderet DF, Sonderegger P. The human TAX1 gene encoding the axon-associated cell adhesion molecule TAG-1/axonin-1: genomic structure and basic promoter. Genomics. 1995;30:141–148. doi: 10.1006/geno.1995.9892. [DOI] [PubMed] [Google Scholar]
- 130.Plagge A, Brummendorf T. The gene of the neural cell recognition molecule F11: conserved exon-intron arrangement in genes of neural members of the immunoglobulin superfamily. Gene. 1997;192:215–225. doi: 10.1016/s0378-1119(97)00066-8. [DOI] [PubMed] [Google Scholar]
- 131.Cangiano G, Ambrosini M, Patruno A, Tino A, Buttiglione M, Gennarini G. Functional organization of the promoter region of the mouse F3 axonal glycoprotein gene. Brain Res Mol Brain Res. 1997;48:279–290. doi: 10.1016/s0169-328x(97)00100-9. [DOI] [PubMed] [Google Scholar]
- 132.Goridis C, Brunet JF. NCAM: structural diversity, function and regulation of expression. Semin Cell Biol. 1992;3:189–197. doi: 10.1016/s1043-4682(10)80015-7. [DOI] [PubMed] [Google Scholar]
- 133.Stottmann RW, Rivas RJ. Distribution of TAG-1 and synaptophysin in the developing cerebellar cortex: relationship to Purkinje cell dendritic development. J Comp Neurol. 1998;395:121–135. [PubMed] [Google Scholar]
- 134.Coluccia A, Tattoli M, Bizzoca A, Arbia S, Lorusso L, De Benedictis L, Buttiglione M, Cuomo V, Furley A, Gennarini G, Cagiano R. Transgenic mice expressing F3/contactin from the transient axonal glycoprotein promoter undergo developmentally regulated deficits of the cerebellar function. Neuroscience. 2004;123:155–166. doi: 10.1016/j.neuroscience.2003.08.025. [DOI] [PubMed] [Google Scholar]
- 135.Nye JS, Kopan R, Axel R. An activated Notch suppresses neurogenesis and myogenesis but not gliogenesis in mammalian cells. Development. 1994;120:2421–2430. doi: 10.1242/dev.120.9.2421. [DOI] [PubMed] [Google Scholar]
- 136.Solecki DJ, Liu XL, Tomoda T, Fanq Y, Hatten ME. Activated Notch2 signaling inhibits differentiation of cerebellar granule neuron precursors by maintaining proliferation. Neuron. 2001;31:557–568. doi: 10.1016/s0896-6273(01)00395-6. [DOI] [PubMed] [Google Scholar]
- 137.Lutolf S, Radtke F, Aguet M, Suter U, Taylor V. Notch1 is required for neuronal and glial differentiation in the cerebellum. Development. 2002;179:373–385. doi: 10.1242/dev.129.2.373. [DOI] [PubMed] [Google Scholar]
- 138.Justice NJ, Jan YN. Variations on the Notch pathway in neural development. Curr Opin Neurobiol. 2002;12:64–70. doi: 10.1016/s0959-4388(02)00291-x. [DOI] [PubMed] [Google Scholar]
- 139.Mizutani K, Saito T. Progenitors resume generating neurons after temporary inhibition of neurogenesis by Notch activation in the mammalian cerebral cortex. Development. 2005;132:1295–1304. doi: 10.1242/dev.01693. [DOI] [PubMed] [Google Scholar]
- 140.Hu QD, Ma QH, Gennarini G, Xiao ZC. Cross-talk between F3/contactin and Notch at axoglial interface: a role in oligodendrocyte development. Dev Neurosci. 2006;28:25–33. doi: 10.1159/000090750. [DOI] [PubMed] [Google Scholar]
- 141.Wang S, Sdrulla AD, diSibio G, Bush G, Nofziger D, Hicks C, et al. Notch receptor activation inhibits oligodendrocyte differentiation. Neuron. 1998;21:63–75. doi: 10.1016/s0896-6273(00)80515-2. [DOI] [PubMed] [Google Scholar]
- 142.Cui XY, Hu QD, Tekaya M, Shimoda Y, Ang BT, Nie DY, et al. NB-3/Notch1 pathway via Deltex1 promotes neural progenitor cell differentiation into oligodendrocytes. J Biol Chem. 2004;279:25858–25865. doi: 10.1074/jbc.M313505200. [DOI] [PubMed] [Google Scholar]
- 143.Hu QD, Cui XY, Ng YK, Xiao ZC. Axoglial interaction via the notch receptor in oligodendrocyte differentiation. Ann Acad Med Singapore. 2004;35:581–588. [PubMed] [Google Scholar]
- 144.Kamiguchi H, Hlavin ML, Yamasaki M, Lemmon V. Adhesion molecules and inherited diseases of the human nervous system. Annu Rev Neurosci. 1998;21:97–125. doi: 10.1146/annurev.neuro.21.1.97. [DOI] [PubMed] [Google Scholar]
- 145.Kenwrick S, Watkins A, De Angelis E. Neural cell recognition molecule L1: relating biological complexity to human disease mutations. Hum Mol Genet. 2000;9:879–886. doi: 10.1093/hmg/9.6.879. [DOI] [PubMed] [Google Scholar]
- 146.Hu QD, Cui XY, Ng YK, Xiao ZC. Axoglial interaction via the notch receptor in oligodendrocyte differentiation. Ann Acad Med Singapore. 2004;33:581–588. [PubMed] [Google Scholar]
- 147.Wolswijk G, Balesar R. Changes in the expression and localization of the paranodal protein Caspr on axons in chronic multiple sclerosis. Brain. 2003;126:1638–1649. doi: 10.1093/brain/awg151. [DOI] [PubMed] [Google Scholar]
- 148.Cariboni A, Maggi R. Kallmann's syndrome, a neuronal migration defect. Cell Mol Life Sci. 2006;63:2512–2526. doi: 10.1007/s00018-005-5604-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Campagne F. Clustalnet: the joining of Clustal and CORBA. Bioinformatics. 2000;16:606–612. doi: 10.1093/bioinformatics/16.7.606. [DOI] [PubMed] [Google Scholar]