Abstract
Contactins are a subgroup of molecules belonging to the immunoglobulin superfamily that are expressed exclusively in the nervous system. The subgroup consists of six members: contactin, TAG-1, BIG-1, BIG-2, NB-2 and NB-3. Since their identification in the late 1980s, contactin and TAG-1 have been studied extensively. Axonal expression and the neurite extension activity of contactin and TAG-1 attracted researchers to study the function of these molecules in axon guidance during development. After the exciting discovery of the molecular function of contactin and TAG-1 in myelination earlier this decade, these two molecules have come to be known as the principal molecules in the function and maintenance of myelinated neurons. In contrast, the function of the other four members of this subgroup remained unknown until recently. Here, we will give an overview of contactin function, including recent progress on BIG-2, NB-2 and NB-3.
Key words: contactin, GPI-anchor, nervous system, development, cerebellum, myelin, synapse, psychiatric disorder
Introduction
It has been well documented that cell adhesion/recognition molecules of the immunoglobulin (Ig) superfamily play a crucial role in the formation and maintenance of the nervous system. They comprise a large number of members and are classified into subfamilies according to numbers of Ig-like and fibronectin III-like domains. Among them, contactin and TAG-1 have been extensively studied for the last two decades and implicated in key developmental events, including neural cell adhesion and migration, neurite outgrowth and fasciculation, axon guidance and myelination (reviewed in refs. 1 and 2). Contactin and TAG-1 show high sequence similarity and share structural features. Based on their homology, BIG-1, BIG-2, NB-2 and NB-3 were identified and classified in a subfamily with contactin and TAG-1. All the members consist of six Ig-like and four fibronectin III-like domains that are anchored to the membrane by glycosylphosphatidylinositol (GPI) (Fig. 1). Then, six members of the contactin subfamily are now referred to as contactin-1 to 6. Over the recent years, further analyses of expression and function of molecules belonging to the contactin subfamily, including BIG-2, NB-2 and NB-3, have been performed using individually gene-deficient mice. Here, we summarize the expression and function of members of the contactin subfamily including recent progress.
Figure 1.
Contactins consist of six immunoglobulin-like domains and four fibronectin type III-like domains that are linked to the plasma membrane through a GPI-anchor.
Contactin-1 and TAG-1/Contactin-2
Contactin-1 was purified and identified in the late 1980's by three groups, as contactin or F11 from chicken3,4 and as F3 from mouse.5 Rat TAG-1 and its chick orthologue axonin-1 were identified during the same time period.6,7 Later, a human orthologue of TAG-1 was termed TAX1.8 Now we also refer to TAG-1 as contactin-2.
Expression of contactin-1 and TAG-1/contactin-2 during neural development.
TAG-1 expression starts early in development, while expressions of other contactins become apparent after birth. During the embryonic development of the nervous system, TAG-1 expression is regulated in a spatio-temporal pattern in a subpopulation of neurons. TAG-1 is transiently expressed in commissural fibers and subsets of neurons in the spinal cord, the dorsal root ganglia and the retinal ganglion cells (RGCs), as well as in corticofugal fibers and tangentially migrating neurons that form several precerebellar nuclei.9–17 TAG-1 has been implicated in the tangential migration of caudal medulla neurons and cortical interneurons.14,15 In the cortex, GABA-containing interneurons originate primarily in the medial ganglionic eminence of the ventral telencephalon and follow tangential migratory routes to reach the dorsal telencephalon. Migration of these neurons occurs along the TAG-1-expressing axons of the developing corticofugal system. Blocking TAG-1 function with anti-TAG-1 antibodies or soluble TAG-1 protein markedly reduces GABAergic neurons in the cortex, suggesting that TAG-1 is involved in neuronal migration.14 However, migration of GABAergic interneurons is normal in TAG-1-deficient mice.16 These results suggest that TAG-1 may act as a migration cue but is dispensable for cortical interneuron migration, where other molecules may compensate for the absence of TAG-1. In the caudal medulla, neuronal populations destined to form several precerebellar nuclei are generated by the rhombic lip. They gather into the olivary and superficial migratory streams and migrate tangentially around the hindbrain to reach their final position. These migrating cells express TAG-1. Blocking TAG-1 function alters superficial migration.15 Though superficial migration is observed in TAG-1-deficient mice, a significant proportion of the cells in the superficial stream die during migration, which reduces the size of the lateral reticular nuclei.16 Therefore, TAG-1 function is required for survival of neurons in some precerebellar nuclei. In the optic nerve, TAG-1-deficient mice displays anomalies in the axonal caliber of RGCs, associated with an abnormal organization of the astroglial network.17 This result indicates that TAG-1 is essential for the normal structure of RGC axons and their surrounding glial cells.
In the postnatal cerebellum, TAG-1/contactin-2 is transiently expressed on premigratory granule cells in the inner part of the external granule cell layer,9 whereas contactin-1 is expressed on migrating granule cells.18–20 During granule cells migration, contactin-1 changes its cellular distribution, as it is downregulated on the cell bodies and remains expressed on axonal extensions within the molecular layer.19 Contactin-1 is also expressed in the axons and cell bodies of Golgi cells and mossy fibers.18 Granule cell axon guidance and dendritic projections from granule and Golgi cells are defective in contactin-1-deficient mice, demonstrating that contactin-1 controls axonal and dendritic interactions between cerebellar interneurons.20 In contrast, no gross morphological abnormalities are detected in the cerebellum of TAG-1-deficient mice.21 On the other hand, transgenic mice in which contactin-1 expression is driven by TAG-1 gene regulatory sequences, display a drastic phenotype in which the size of the cerebellum is markedly reduced during the first two postnatal weeks but subsequently recovers.22 These observations indicate that proper expression of contactin-1 and TAG-1 is essential for normal cerebellar morphogenesis.
Roles of contactin-1 and TAG-1/contactin-2 in myelinated fibers.
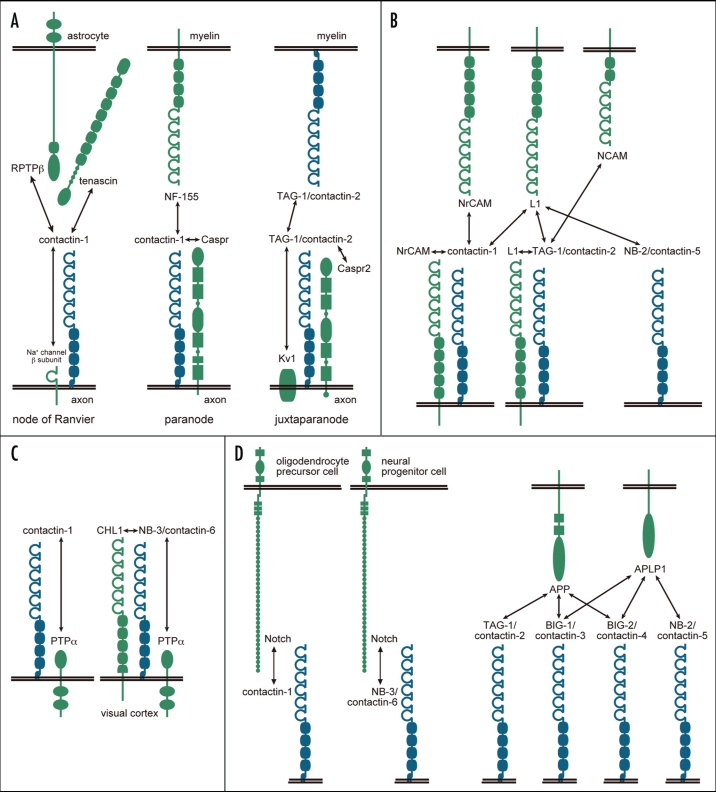
Contactin-1 and TAG-1/contactin-2 have been identified as components of specialized domains of myelinated fibers (Fig. 2A).23–27 Myelinated axons can be differentiated into distinct structural, molecular and functional domains. These domains include the nodes of Ranvier, the paranodes, the juxtaparanodes and the internodal regions.
Figure 2.
The interactions of contactins in a variety of tissues/cells/compartments. Contactins are drawn in blue, and other molecules are in green. (A) The molecular complexes in the myelinated nerves. (B) Interactions of contactins with molecules of the L1 family and NCAM. (C) Interactions of contactins with PTPα. (D) Interactions of contactins with Notch and molecules of the APP family.
The nodes of Ranvier are short, regular interruptions in the myelin sheath. The voltage-gated sodium channels that are responsible for action potentials during saltatory conduction are concentrated at these nodes. Voltage-gated sodium channels are heterooligomers consisting of a pore-forming α-subunit and at least one auxiliary β-subunit. The β-subunits of sodium channels increase functional expression and modulate gating of the α-subunit, and also act as cell adhesion molecules.28 The extracellular domains of these β-subunits contain a single Ig-like domain. The β2-subunit shows homology with contactin-1,29 and the β1-subunit binds to contactin-1.30 Association with contactin-1 enhances cell surface expression of Na+ channels.30–32 The expression of Na+ channels is markedly reduced in the optic nerve of contactin-1-deficient mice.33
The nodes of Ranvier are flanked by the paranodes, which regulate junctional attachment between axonal and glial membranes. Contactin-1 is found on the axolemma at the paranode where it interacts with contactin-associated protein (Caspr; also known as paranodin)34–37 and neurofascin-155 (NF-155), a glial isoform of neurofascin.38,39 Caspr is a member of the Caspr family, a subgroup of the neurexin family, that consists of five molecules (Caspr, Caspr2, Caspr3, Caspr4 and Caspr5).34,40,41
Contactin-1 binds to Caspr in cis34 and their interaction is essential for Caspr sorting from the endoplasmic reticulum to the plasma membrane.42 Association of contactin-1 and Caspr during biosynthesis results in cell surface expression of low molecular weight, high-mannose glycoforms of contactin-1 and Caspr.43,44 A non-conventional Golgi-independent pathway may be implicated in this process. The Pro-Gly-Tyr repeats in the extracellular domain of Capsr, which are responsible for endoplasmic reticulum retention of Caspr, might govern contactin-1 chaperoning of Caspr.39 Moreover, the high-mannose glycoform of contactin-1, which is expressed in association with Caspr, strongly binds NF-155, its glial partner at paranodes.39
Contactin-1, Caspr and NF-155 are all essential for the formation of the paranodal junction. Deficiencies in any of these proteins produces disorganization of the paranodal junctions and reduces nerve conduction velocity.45–47 Intracellular transport and surface expression of Caspr are impeded and Caspr expression at the paranode is abolished in contactin-1-deficient mice.45 Likewise, contactin-1 can not be detected in the paranodes in Caspr-deficient mice.46 Although NF-155 is still detectable at the paranodes in the absence of the contactin-1-Caspr complex,45,46 absence of NF-155 at the paranodes causes the loss of both contactin-1 and Caspr from the paranodal junction.47
The juxtaparanode resides just beyond the innermost paranodal junction next to the internode. At the juxtaparanodal axolemma, Shaker-type K+ channels colocalize with Caspr2, the second member of the Caspr family,40 and TAG-1/contactin-2,48 which is also present in the glial membrane.48,49 TAG-1 can associate in cis with Caspr2 and in trans with itself such that TAG-1 and Caspr2 form a complex consisting of an axonal TAG-1/Caspr2 heterodimer and a glial TAG-1.49,50 This complex is required for the clustering of Shaker-type K+ channels at the juxtaparanode. In addition, it was shown that TAG-1 directly interacts with Shaker-type K+ channels.51 A TAG-1 or Caspr2 deficiency disrupts enrichment of Shaker-type K+ channels in this region.49,50,52
Localization of contactin-1 and TAG-1/contactin-2 at synapses.
Contactin-1 is localized at synaptic sites as investigated at the electron microscopic level.18 Long-term depression is impaired in the hippocampus of contactin-1-deficient mice.53 Molecular analyses using the contactin-1-deficient mice indicate that contactin-1 is essential for the synaptic targeting of Caspr and for the proper distribution of receptor-type protein tyrosine phosphatase β (RPTPβ)/phosphacan.53 On the other hand, deletion of the Caspr gene has no effect on synaptic transmission and plasticity.54 These results indicate that contactin-1 plays an important role in synaptic plasticity independent of its association with Caspr, on the different molecular mechanism from the paranodal junctions of myelinated nerves. Subcellular fractionation of rat brain homogenate revealed that TAG-1/contactin-2 is also present in the synaptic plasma membrane along with Caspr2, a binding partner of TAG-1 at the juxtaparanodal region of the myelinated axon.55 In addition, immunohistochemical analyses showed that both NB-2/contactin-5 and NB-3/contactin-6 are colocalized with markers for glutamatergic synapses at the superior olivary complex of the auditory system and the parallel fibers of the cerebellum, respectively (ref. 56 and our unpublished results). These observations suggest that contactins might generally play an essential role in synaptic physiology.
BIG-1/Contactin-3 and BIG-2/Contactin-4
BIG-1 and BIG-2 were identified by PCR cloning with degenerate primers based on homologous amino acid sequences in contactin-1 and TAG-1/contactin-2.57,58 BIG-1 was also described as plasmacytoma-associated neuronal glycoprotein (PANG).59 Recently, a chick orthologue of BIG-2 was identified as a binding partner of amyloid precursor protein (APP).60
BIG-1/contactin-3 is abundantly expressed in the adult brain. Expression of BIG-1 is uniquely restricted to subsets of neurons, such as Purkinje cells of the cerebellum, granule cells of the hippocampal dentate gyrus, and neurons in the superficial layers of the cerebral cortex.57 Little more about BIG-1 has been reported so far.
BIG-2/contactin-4 expression in the olfactory system.
BIG-2/contactin-4 expression in mice increases after birth and reaches a maximum in adulthood.58 BIG-2 is expressed in different subsets of neurons in various brain regions,58 including the olfactory system. Recently, BIG-2 was identified as an axon guidance molecule that mediates proper neuronal wiring in the mouse olfactory system.61 In the olfactory system, individual olfactory sensory neurons express only one odorant receptor gene.62 And olfactory sensory neurons expressing the same odorant receptor converge their axons onto a specific set of glomeruli in the olfactory bulb.63 BIG-2 is expressed in the glomerular array of the olfactory bulb with a mosaic pattern overlapping with but distinct from other axon guidance molecules, such as Kirrel2 and ephrin-A5. In BIG-2-deficient mice, olfactory sensory neurons expressing the same odorant receptor aberrantly project to multiple glomeruli. These results suggest that BIG-2 is crucial for the formation and maintenance of odor map in the olfactory bulb.
NB-2/Contactin-5 and NB-3/Contactin-6
NB-2 and NB-3 were isolated by our group64 using a strategy similar to that described for BIG-1 and BIG-2. A chick orthologue of NB-2 has been described as FAR-2.65 NB-2 and NB-3 are exclusively expressed in the central nervous system.64
NB-2/contactin-5 expression in the developing auditory system.
Expression of NB-2/contactin-5 becomes apparent after birth and reaches a maximum around postnatal day (P) 14.66 NB-2 mRNA is expressed in highly restricted brain regions, the auditory system in particular.66,67 Immunohistochemistry using an anti-NB-2 monoclonal antibody revealed that at P7, NB-2 is expressed in all areas of the auditory system.56 NB-2-deficient mice have no gross abnormalities but they are less sensitive to the audiogenic seizure susceptibility test and express less c-Fos after audiogenic seizure susceptibility induction and pure-tone stimulation than wild-type mice.67 We measured the auditory brainstem response to examine whether NB-2-deficient mice are hard of hearing. Auditory brainstem response thresholds did not differ significantly between NB-2-deficent and wild-type mice, indicating that NB-2-deficient mice are not hard of hearing. However, auditory brainstem response wave latencies tend to be delayed in NB-2-deficient mice compared to wild-type mice (our unpublished results). The number of fibers and synapses in the auditory region of the brainstem is reduced in NB-2-deficient mice (our unpublished results). Thus, NB-2 may be involved in auditory system development.
NB-3/contactin-6 expression in the developing cerebellum.
Expression of NB-3/contactin-6 mRNA in the cerebrum is evident after birth, reaches a maximum around P7 and declines thereafter to low levels.68 In contrast, NB-3 expression in the cerebellum increases until adulthood.68 However, NB-3-deficient mice show no gross abnormalities in the brain, including the cerebellum.69 Based on the strong expression of NB-3 mRNA in the cerebellum, it has been suggested that NB-3 plays a role in cerebellar control of motor coordination in adulthood. Behavioral tests that examine motor function, such as the rotorod test and wire hang test, revealed that motor coordination is impaired in NB-3-deficient mice.69 We hypothesized that this functional impairment must result from cellular and/or molecular defect(s) in the cerebellum. NB-3 immunofluorescence in neonatal cerebellum revealed that NB-3 localizes to the radially migrating granule cells located underneath the inner edge of TAG-1/contactin-2-positive zone (our unpublished results). NB-3 expression is observed as punctuated signals and overlapped with vGluT1, a presynaptic marker of glutamatergic neurons. The number of vGluT1-positive puncta in NB-3-deficient mice was less than that in the wild-type mice (our unpublished results), suggesting that NB-3 may play an important role in granule cell maturation and/or synaptic formation in the developing cerebellum.
Molecular Interactions of Contactins
As described above, contactins are involved in a variety of molecular and cellular events. The molecular mechanisms underlying these events require complex interactions between contactins and other proteins (Fig. 2). Interactions of contactin-1 or TAG-1/contactin-2 with molecules of the L1 family of the Ig superfamily have been studied extensively. The L1 family is comprised of L1, NrCAM, neurofascin and close homologue of L1 (CHL1). L1 binds to TAG-1 in cis70–74 and to contactin-1 in trans.75 NrCAM binds in trans to TAG-176,77 and both in cis and in trans to contactin-1.78–81 FAR-2, a chick ortholog of NB-2/contactin-5, binds weakly to L1 but not to NrCAM.65 Recently, it was reported that CHL1 associates with NB-3/contactin-6 in cis and enhances cell surface expression of NB-3.82 Besides the L1 family, it has been reported that contactins associate with various molecules including transmembrane proteins and extracellular matrix components. Contactin-1 associates with protein tyrosine phosphatase α (PTPα) in cis, thereby recruiting intracellular src family tyrosine kinases to receptor complexes.83,84 NB-3 also interacts with PTPα to regulate apical dendrite orientation in the visual cortex.82 TAG-1 binds to NCAM, phosphacan and tenascin-C.85 Contactin-1 associates with tenascin-C, tenascin-R and RPTPβ.86–91
It has recently been proposed that contactins play a novel role in regulation of neural precursor cell differentiation. Notch signaling pathways are controlled by regulated intramembrane proteolysis in which γ-secretase cleaves type-1 transmembrane proteins to release their intracellular domains. This cleavage is regulated by ligand binding to the receptor protein. Contactin-1 and NB-3/contactin-6 are functional ligands for Notch that are involved in oligodendrocyte differentiation.92,93 APP is cleaved by γ-secretase in a similar manner. It was reported recently that TAG-1/contactin-2 and BIG-2/contactin-4 interact with APP.60,94 TAG-1 and APP colocalize in the neural stem cell niche of the mouse fetal ventricular zone, and interaction between these two proteins negatively regulates neurogenesis.60 APP and BIG-2 are expressed in locations that permit an interaction in the developing chick retinotectal system, where their interaction is involved in axon outgrowth in vitro.94 In addition, in vitro binding assays reveal direct interactions of APP, and its homologue amyloid precursor-like protein 1 (APLP1), with BIG-1/contactin-3 and BIG-2/contactin-4.94 NB-2/contactin-5 binds to APLP1, but not to APP.94
Contactins and Neuropsychiatric Disorders
Contactin-1-deficient mice show a weight loss and die by P19,20 resembling the anorexia mouse. Contactin-1 is expressed in the hypothalamic neuropil of wild-type mice,95,96 and contactin-1-deficient mice show hypothalamic alterations similar to the anorexia mouse.96,97 Thus, the contactin-1-deficient mouse may be a good model for studying eating disorders.
Human BIG-2/contactin-4, NB-3/contactin-6 and CHL1 genes are contiguously located on chromosome 3p25-p26.98–100 The loci are deleted in 3p deletion syndrome, which is characterized by developmental delays, growth retardation and dysmorphic features. Furthermore, the BIG-2 gene is disrupted or deleted in subjects with characteristic features of the 3p deletion syndrome.101,102 BIG-2 mutations also have been found in subjects with autistic spectrum disorder,103 although none of these subjects demonstrate symptoms of the classical 3p deletion syndrome. In addition, it has been reported recently that human CNTNAP2 gene encoding Caspr2, a binding partner of TAG-1/contactin-2, is associated with a number of neuropsychiatric disorders, including seizures, mental retardation, schizophrenia and autistic spectrum disorder.55,104–107 These findings suggest that contactins might play an important role(s) in the normal development and maintenance of central nervous system function.
Concluding Remarks
Each of contactins is expressed in a cell-or tissue-specific manner and distributed almost separately with each other. It is likely that contactins play distinct roles in the development and function of the nervous system. On the other hand, there may be some common features in the molecular mechanisms by which contactins function. Formation of molecular complexes, contactin-1 with Caspr and TAG-1/contactin-2 with Caspr2, on the myelinated axon suggests possibility of other combination between each of contactins and any member of the Caspr family. Because contactins are linked via GPI-anchor to the membrane and therefore lack cytoplasmic domain, if contactins are acting as a receptor, they need to associate in cis with transmembrane proteins in order to transduce extracellular signals across the membrane. Contactins can associate in cis with some of the Caspr family, some of the L1 family, PTPα and likely unidentified co-receptors. Moreover, a novel role of contactins in intercellular interaction has been raised recently. Contactins were characterized as a functional ligand of Notch or APP. Though there seems to be little common structural feature between Notch and APP, they are cleaved by γ-secretase depending on the binding of contactins. In both cases, we need to understand the mode of ligand-receptor interaction and clarify intracellular signaling pathways activated by contactins. These elucidations of extracellular and intracellular mechanisms will reveal an essential role(s) of contactins in the formation and maintenance of a variety of tissues/cells/compartments in the nervous system.
Abbreviations
- Ig
immunoglobulin
- GPI
glycosylphosphatidylinositol
- RGC
retinal ganglion cell
- RPTPβ
receptor-type protein tyrosine phosphatase β
- APP
amyloid precursor protein
- P
postnatal day
- PTPα
protein tyrosine phosphatase α
Footnotes
Previously published online as a Cell Adhesion & Migration E-publication: http://www.landesbioscience.com/journals/celladhesion/article/7764
References
- 1.Falk J, Bonnon C, Girault JA, Faivre-Sarrailh C. F3/contactin, a neuronal cell adhesion molecule implicated in axogenesis and myelination. Biol Cell. 2002;94:327–334. doi: 10.1016/s0248-4900(02)00006-0. [DOI] [PubMed] [Google Scholar]
- 2.Karagogeos D. Neural GPI-anchored cell adhesion molecules. Front Biosci. 2003;8:1304–1320. doi: 10.2741/1214. [DOI] [PubMed] [Google Scholar]
- 3.Ranscht B. Sequence of contactin, a 130-kD glycoprotein concentrated in areas of interneuronal contact, defines a new member of the immunoglobulin supergene family in the nervous system. J Cell Biol. 1988;107:1561–1573. doi: 10.1083/jcb.107.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brummendorf T, Wolff JM, Frank R, Rathjen FG. Neural cell recognition molecule F11: homology with fibronectin type III and immunoglobulin type C domains. Neuron. 1989;2:1351–1361. doi: 10.1016/0896-6273(89)90073-1. [DOI] [PubMed] [Google Scholar]
- 5.Gennarini G, Cibelli G, Rougon G, Mattei MG, Goridis C. The mouse neuronal cell surface protein F3: a phosphatidylinositol-anchored member of the immunoglobulin superfamily related to chicken contactin. J Cell Biol. 1989;109:775–788. doi: 10.1083/jcb.109.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furley AJ, Morton SB, Manalo D, Karagogeos D, Dodd J, Jessell TM. The axonal glycoprotein TAG-1 is an immunoglobulin superfamily member with neurite outgrowth-promoting activity. Cell. 1990;61:157–170. doi: 10.1016/0092-8674(90)90223-2. [DOI] [PubMed] [Google Scholar]
- 7.Zuellig RA, Rader C, Schroeder A, Kalousek MB, Von Bohlen und Halbach F, Osterwalder T, et al. The axonally secreted cell adhesion molecule, axonin-1. Primary structure, immunoglobulin-like and fibronectin-type-III-like domains and glycosyl-phosphatidylinositol anchorage. Eur J Biochem. 1992;204:453–463. doi: 10.1111/j.1432-1033.1992.tb16655.x. [DOI] [PubMed] [Google Scholar]
- 8.Tsiotra PC, Karagogeos D, Theodorakis K, Michaelidis TM, Modi WS, Furley AJ, et al. Isolation of the cDNA and chromosomal localization of the gene (TAX1) encoding the human axonal glycoprotein TAG-1. Genomics. 1993;18:562–567. doi: 10.1016/s0888-7543(05)80357-x. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto M, Boyer AM, Crandall JE, Edwards M, Tanaka H. Distribution of stage-specific neurite-associated proteins in the developing murine nervous system recognized by a monoclonal antibody. J Neurosci. 1986;6:3576–3594. doi: 10.1523/JNEUROSCI.06-12-03576.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dodd J, Morton SB, Karagogeos D, Yamamoto M, Jessell TM. Spatial regulation of axonal glycoprotein expression on subsets of embryonic spinal neurons. Neuron. 1988;1:105–116. doi: 10.1016/0896-6273(88)90194-8. [DOI] [PubMed] [Google Scholar]
- 11.Wolfer DP, Henehan-Beatty A, Stoeckli ET, Sonderegger P, Lipp HP. Distribution of TAG-1/axonin-1 in fibre tracts and migratory streams of the developing mouse nervous system. J Comp Neurol. 1994;345:1–32. doi: 10.1002/cne.903450102. [DOI] [PubMed] [Google Scholar]
- 12.Wolfer DP, Giger RJ, Stagliar M, Sonderegger P, Lipp HP. Expression of the axon growth-related neural adhesion molecule TAG-1/axonin-1 in the adult mouse brain. Anat Embryol (Berl) 1998;197:177–185. doi: 10.1007/s004290050129. [DOI] [PubMed] [Google Scholar]
- 13.Jung M, Petrausch B, Stuermer CA. Axon-regenerating retinal ganglion cells in adult rats synthesize the cell adhesion molecule L1 but not TAG-1 or SC-1. Mol Cell Neurosci. 1997;9:116–131. doi: 10.1006/mcne.1997.0611. [DOI] [PubMed] [Google Scholar]
- 14.Denaxa M, Chan CH, Schachner M, Parnavelas JG, Karagogeos D. The adhesion molecule TAG-1 mediates the migration of cortical interneurons from the ganglionic eminence along the corticofugal fiber system. Development. 2001;128:4635–4644. doi: 10.1242/dev.128.22.4635. [DOI] [PubMed] [Google Scholar]
- 15.Kyriakopoulou K, de Diego I, Wassef M, Karagogeos D. A combination of chain and neurophilic migration involving the adhesion molecule TAG-1 in the caudal medulla. Development. 2002;129:287–296. doi: 10.1242/dev.129.2.287. [DOI] [PubMed] [Google Scholar]
- 16.Denaxa M, Kyriakopoulou K, Theodorakis K, Trichas G, Vidaki M, Takeda Y, et al. The adhesion molecule TAG-1 is required for proper migration of the superficial migratory stream in the medulla but not of cortical interneurons. Dev Biol. 2005;288:87–99. doi: 10.1016/j.ydbio.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 17.Chatzopoulou E, Miguez A, Savvaki M, Levasseur G, Muzerelle A, Muriel MP, et al. Structural requirement of TAG-1 for retinal ganglion cell axons and myelin in the mouse optic nerve. J Neurosci. 2008;28:7624–7636. doi: 10.1523/JNEUROSCI.1103-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faivre-Sarrailh C, Gennarini G, Goridis C, Rougon G. F3/F11 cell surface molecule expression in the developing mouse cerebellum is polarized at synaptic sites and within granule cells. J Neurosci. 1992;12:257–267. doi: 10.1523/JNEUROSCI.12-01-00257.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Virgintino D, Ambrosini M, D'Errico P, Bertossi M, Papadaki C, Karagogeos D, Gennarini G. Regional distribution and cell type-specific expression of the mouse F3 axonal glycoprotein: a developmental study. J Comp Neurol. 1999;413:357–372. doi: 10.1002/(sici)1096-9861(19991025)413:3<357::aid-cne1>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 20.Berglund EO, Murai KK, Fredette B, Sekerkova G, Marturano B, Weber L, et al. Ataxia and abnormal cerebellar microorganization in mice with ablated contactin gene expression. Neuron. 1999;24:739–750. doi: 10.1016/s0896-6273(00)81126-5. [DOI] [PubMed] [Google Scholar]
- 21.Fukamauchi F, Aihara O, Wang YJ, Akasaka K, Takeda Y, Horie M, et al. TAG-1-deficient mice have marked elevation of adenosine A1 receptors in the hippocampus. Biochem Biophys Res Commun. 2001;281:220–226. doi: 10.1006/bbrc.2001.4334. [DOI] [PubMed] [Google Scholar]
- 22.Bizzoca A, Virgintino D, Lorusso L, Buttiglione M, Yoshida L, Polizzi A, et al. Transgenic mice expressing F3/contactin from the TAG-1 promoter exhibit developmentally regulated changes in the differentiation of cerebellar neurons. Development. 2003;130:29–43. doi: 10.1242/dev.00183. [DOI] [PubMed] [Google Scholar]
- 23.Arroyo EJ, Scherer SS. On the molecular architecture of myelinated fibers. Histochem Cell Biol. 2000;113:1–18. doi: 10.1007/s004180050001. [DOI] [PubMed] [Google Scholar]
- 24.Peles E, Salzer JL. Molecular domains of myelinated axons. Curr Opin Neurobiol. 2000;10:558–565. doi: 10.1016/s0959-4388(00)00122-7. [DOI] [PubMed] [Google Scholar]
- 25.Girault JA, Peles E. Development of nodes of Ranvier. Curr Opin Neurobiol. 2002;12:476–485. doi: 10.1016/s0959-4388(02)00370-7. [DOI] [PubMed] [Google Scholar]
- 26.Poliak S, Peles E. The local differentiation of myelinated axons at nodes of Ranvier. Nat Rev Neurosci. 2003;4:968–980. doi: 10.1038/nrn1253. [DOI] [PubMed] [Google Scholar]
- 27.Salzer JL. Polarized domains of myelinated axons. Neuron. 2003;40:297–318. doi: 10.1016/s0896-6273(03)00628-7. [DOI] [PubMed] [Google Scholar]
- 28.Isom LL. The role of sodium channels in cell adhesion. Front Biosci. 2002;7:12–23. doi: 10.2741/isom. [DOI] [PubMed] [Google Scholar]
- 29.Isom LL, Ragsdale DS, De Jongh KS, Westenbroek RE, Reber BF, Scheuer T, et al. Structure and function of the beta 2 subunit of brain sodium channels, a transmembrane glycoprotein with a CAM motif. Cell. 1995;83:433–442. doi: 10.1016/0092-8674(95)90121-3. [DOI] [PubMed] [Google Scholar]
- 30.Kazarinova-Noyes K, Malhotra JD, McEwen DP, Mattei LN, Berglund EO, Ranscht B, et al. Contactin associates with Na+ channels and increases their functional expression. J Neurosci. 2001;21:7517–7525. doi: 10.1523/JNEUROSCI.21-19-07517.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu CJ, Dib-Hajj SD, Black JA, Greenwood J, Lian Z, Waxman SG. Direct interaction with contactin targets voltage-gated sodium channel Na(v)1.9/NaN to the cell membrane. J Biol Chem. 2001;276:46553–46561. doi: 10.1074/jbc.M108699200. [DOI] [PubMed] [Google Scholar]
- 32.Shah BS, Rush AM, Liu S, Tyrrell L, Black JA, Dib-Hajj SD, et al. Contactin associates with sodium channel Nav1.3 in native tissues and increases channel density at the cell surface. J Neurosci. 2004;24:7387–7399. doi: 10.1523/JNEUROSCI.0322-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kazarinova-Noyes K, Shrager P. Molecular constituents of the node of Ranvier. Mol Neurobiol. 2002;26:167–182. doi: 10.1385/MN:26:2-3:167. [DOI] [PubMed] [Google Scholar]
- 34.Peles E, Nativ M, Lustig M, Grumet M, Schilling J, Martinez R, et al. Identification of a novel contactin-associated transmembrane receptor with multiple domains implicated in protein-protein interactions. EMBO J. 1997;16:978–988. doi: 10.1093/emboj/16.5.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Einheber S, Zanazzi G, Ching W, Scherer S, Milner TA, Peles E, et al. The axonal membrane protein Caspr, a homologue of neurexin IV, is a component of the septate-like paranodal junctions that assemble during myelination. J Cell Biol. 1997;139:1495–1506. doi: 10.1083/jcb.139.6.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Menegoz M, Gaspar P, Le Bert M, Galvez T, Burgaya F, Palfrey C, et al. Paranodin, a glycoprotein of neuronal paranodal membranes. Neuron. 1997;19:319–331. doi: 10.1016/s0896-6273(00)80942-3. [DOI] [PubMed] [Google Scholar]
- 37.Rios JC, Melendez-Vasquez CV, Einheber S, Lustig M, Grumet M, Hemperly J, et al. Contactin-associated protein (Caspr) and contactin form a complex that is targeted to the paranodal junctions during myelination. J Neurosci. 2000;20:8354–8364. doi: 10.1523/JNEUROSCI.20-22-08354.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Charles P, Tait S, Faivre-Sarrailh C, Barbin G, Gunn-Moore F, Denisenko-Nehrbass N, et al. Neurofascin is a glial receptor for the paranodin/Caspr-contactin axonal complex at the axoglial junction. Curr Biol. 2002;12:217–220. doi: 10.1016/s0960-9822(01)00680-7. [DOI] [PubMed] [Google Scholar]
- 39.Bonnon C, Bel C, Goutebroze L, Maigret B, Girault JA, Faivre-Sarrailh C. PGY Repeats and N-Glycans Govern the Trafficking of Paranodin and Its Selective Association with Contactin and Neurofascin-155. Mol Biol Cell. 2007;18:229–241. doi: 10.1091/mbc.E06-06-0570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poliak S, Gollan L, Martinez R, Custer A, Einheber S, Salzer JL, et al. Caspr2, a new member of the neurexin superfamily, is localized at the juxtaparanodes of myelinated axons and associates with K+ channels. Neuron. 1999;24:1037–1047. doi: 10.1016/s0896-6273(00)81049-1. [DOI] [PubMed] [Google Scholar]
- 41.Spiegel I, Salomon D, Erne B, Schaeren-Wiemers N, Peles E. Caspr3 and caspr4, two novel members of the caspr family are expressed in the nervous system and interact with PDZ domains. Mol Cell Neurosci. 2002;20:283–297. doi: 10.1006/mcne.2002.1110. [DOI] [PubMed] [Google Scholar]
- 42.Faivre-Sarrailh C, Gauthier F, Denisenko-Nehrbass N, Le Bivic A, Rougon G, Girault JA. The glycosylphosphatidyl inositol-anchored adhesion molecule F3/contactin is required for surface transport of paranodin/contactin-associated protein (caspr) J Cell Biol. 2000;149:491–502. doi: 10.1083/jcb.149.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bonnon C, Goutebroze L, Denisenko-Nehrbass N, Girault JA, Faivre-Sarrailh C. The paranodal complex of F3/contactin and caspr/paranodin traffics to the cell surface via a non-conventional pathway. J Biol Chem. 2003;278:48339–48347. doi: 10.1074/jbc.M309120200. [DOI] [PubMed] [Google Scholar]
- 44.Gollan L, Salomon D, Salzer JL, Peles E. Caspr regulates the processing of contactin and inhibits its binding to neurofascin. J Cell Biol. 2003;163:1213–1218. doi: 10.1083/jcb.200309147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boyle ME, Berglund EO, Murai KK, Weber L, Peles E, Ranscht B. Contactin orchestrates assembly of the septate-like junctions at the paranode in myelinated peripheral nerve. Neuron. 2001;30:385–397. doi: 10.1016/s0896-6273(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 46.Bhat MA, Rios JC, Lu Y, Garcia-Fresco GP, Ching W, St Martin M, et al. Axon-glia interactions and the domain organization of myelinated axons requires neurexin IV/Caspr/Paranodin. Neuron. 2001;30:369–383. doi: 10.1016/s0896-6273(01)00294-x. [DOI] [PubMed] [Google Scholar]
- 47.Sherman DL, Tait S, Melrose S, Johnson R, Zonta B, Court FA, et al. Neurofascins are required to establish axonal domains for saltatory conduction. Neuron. 2005;48:737–742. doi: 10.1016/j.neuron.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 48.Traka M, Dupree JL, Popko B, Karagogeos D. The neuronal adhesion protein TAG-1 is expressed by Schwann cells and oligodendrocytes and is localized to the juxtaparanodal region of myelinated fibers. J Neurosci. 2002;22:3016–3024. doi: 10.1523/JNEUROSCI.22-08-03016.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Traka M, Goutebroze L, Denisenko N, Bessa M, Nifli A, Havaki S, et al. Association of TAG-1 with Caspr2 is essential for the molecular organization of juxtaparanodal regions of myelinated fibers. J Cell Biol. 2003;162:1161–1172. doi: 10.1083/jcb.200305078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Poliak S, Salomon D, Elhanany H, Sabanay H, Kiernan B, Pevny L, et al. Juxtaparanodal clustering of Shaker-like K+ channels in myelinated axons depends on Caspr2 and TAG-1. J Cell Biol. 2003;162:1149–1160. doi: 10.1083/jcb.200305018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tzimourakas A, Giasemi S, Mouratidou M, Karagogeos D. Structure-function analysis of protein complexes involved in the molecular architecture of juxtaparanodal regions of myelinated fibers. Biotechnol J. 2007;2:577–583. doi: 10.1002/biot.200700023. [DOI] [PubMed] [Google Scholar]
- 52.Savvaki M, Panagiotaropoulos T, Stamatakis A, Sargiannidou I, Karatzioula P, Watanabe K, et al. Impairment of learning and memory in TAG-1 deficient mice associated with shorter CNS internodes and disrupted juxtaparanodes. Mol Cell Neurosci. 2008;39:478–490. doi: 10.1016/j.mcn.2008.07.025. [DOI] [PubMed] [Google Scholar]
- 53.Murai KK, Misner D, Ranscht B. Contactin supports synaptic plasticity associated with hippocampal long-term depression but not potentiation. Curr Biol. 2002;12:181–190. doi: 10.1016/s0960-9822(02)00680-2. [DOI] [PubMed] [Google Scholar]
- 54.Pillai AM, Garcia-Fresco GP, Sousa AD, Dupree JL, Philpot BD, Bhat MA. No effect of genetic deletion of contactin-associated protein (CASPR) on axonal orientation and synaptic plasticity. J Neurosci Res. 2007;85:2318–2331. doi: 10.1002/jnr.21374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bakkaloglu B, O'Roak BJ, Louvi A, Gupta AR, Abelson JF, Morgan TM, et al. Molecular cytogenetic analysis and resequencing of contactin associated protein-like 2 in autism spectrum disorders. Am J Hum Genet. 2008;82:165–173. doi: 10.1016/j.ajhg.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Toyoshima M, Sakurai K, Shimazaki K, Takeda Y, Nakamoto M, Serizawa S, Shimoda Y, Watanabe K. Preferential localization of neural cell recognition molecule NB-2 in developing glutamatergic neurons in the rat auditory brainstem. J Comp Neurol; 2009;513:349–362. doi: 10.1002/cne.21972. [DOI] [PubMed] [Google Scholar]
- 57.Yoshihara Y, Kawasaki M, Tani A, Tamada A, Nagata S, Kagamiyama H, et al. BIG-1: a new TAG-1/F3-related member of the immunoglobulin superfamily with neurite outgrowth-promoting activity. Neuron. 1994;13:415–426. doi: 10.1016/0896-6273(94)90357-3. [DOI] [PubMed] [Google Scholar]
- 58.Yoshihara Y, Kawasaki M, Tamada A, Nagata S, Kagamiyama H, Mori K. Overlapping and differential expression of BIG-2, BIG-1, TAG-1 and F3: four members of an axon-associated cell adhesion molecule subgroup of the immunoglobulin superfamily. J Neurobiol. 1995;28:51–69. doi: 10.1002/neu.480280106. [DOI] [PubMed] [Google Scholar]
- 59.Connelly MA, Grady RC, Mushinski JF, Marcu KB. PANG, a gene encoding a neuronal glycoprotein, is ectopically activated by intracisternal α-type particle long terminal repeats in murine plasmacytomas. Proc Natl Acad Sci USA. 1994;91:1337–1341. doi: 10.1073/pnas.91.4.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Osterfield M, Egelund R, Young LM, Flanagan JG. Interaction of amyloid precursor protein with contactins and NgCAM in the retinotectal system. Development. 2008;135:1189–1199. doi: 10.1242/dev.007401. [DOI] [PubMed] [Google Scholar]
- 61.Kaneko-Goto T, Yoshihara S, Miyazaki H, Yoshihara Y. BIG-2 mediates olfactory axon convergence to target glomeruli. Neuron. 2008;57:834–846. doi: 10.1016/j.neuron.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 62.Serizawa S, Miyamichi K, Sakano H. One neuron-one receptor rule in the mouse olfactory system. Trends Genet. 2004;20:648–653. doi: 10.1016/j.tig.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 63.Mori K, Takahashi YK, Igarashi KM, Yamaguchi M. Maps of odorant molecular features in the mammalian olfactory bulb. Physiol Rev. 2006;86:409–433. doi: 10.1152/physrev.00021.2005. [DOI] [PubMed] [Google Scholar]
- 64.Ogawa J, Kaneko H, Masuda T, Nagata S, Hosoya H, Watanabe K. Novel neural adhesion molecules in the Contactin/F3 subgroup of the immunoglobulin superfamily: isolation and characterization of cDNAs from rat brain. Neurosci Lett. 1996;218:173–176. doi: 10.1016/s0304-3940(96)13156-6. [DOI] [PubMed] [Google Scholar]
- 65.Plagge A, Sendtner-Voelderndorff L, Sirim P, Freigang J, Rader C, Sonderegger P, et al. The contactin-related protein FAR-2 defines purkinje cell clusters and labels subpopulations of climbing fibers in the developing cerebellum. Mol Cell Neurosci. 2001;18:91–107. doi: 10.1006/mcne.2001.1006. [DOI] [PubMed] [Google Scholar]
- 66.Ogawa J, Lee S, Itoh K, Nagata S, Machida T, Takeda Y, et al. Neural recognition molecule NB-2 of the contactin/F3 subgroup in rat: Specificity in neurite outgrowth-promoting activity and restricted expression in the brain regions. J Neurosci Res. 2001;65:100–110. doi: 10.1002/jnr.1133. [DOI] [PubMed] [Google Scholar]
- 67.Li H, Takeda Y, Niki H, Ogawa J, Kobayashi S, Kai N, et al. Aberrant responses to acoustic stimuli in mice deficient for neural recognition molecule NB-2. Eur J Neurosci. 2003;17:929–936. doi: 10.1046/j.1460-9568.2003.02514.x. [DOI] [PubMed] [Google Scholar]
- 68.Lee S, Takeda Y, Kawano H, Hosoya H, Nomoto M, Fujimoto D, et al. Expression and regulation of a gene encoding neural recognition molecule NB-3 of the contactin/F3 subgroup in mouse brain. Gene. 2000;245:253–266. doi: 10.1016/s0378-1119(00)00031-7. [DOI] [PubMed] [Google Scholar]
- 69.Takeda Y, Akasaka K, Lee S, Kobayashi S, Kawano H, Murayama S, et al. Impaired motor coordination in mice lacking neural recognition molecule NB-3 of the contactin/F3 subgroup. J Neurobiol. 2003;56:252–265. doi: 10.1002/neu.10222. [DOI] [PubMed] [Google Scholar]
- 70.Kuhn TB, Stoeckli ET, Condrau MA, Rathjen FG, Sonderegger P. Neurite outgrowth on immobilized axonin-1 is mediated by a heterophilic interaction with L1(G4) J Cell Biol. 1991;115:1113–1126. doi: 10.1083/jcb.115.4.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Buchstaller A, Kunz S, Berger P, Kunz B, Ziegler U, Rader C, et al. Cell adhesion molecules NgCAM and axonin-1 form heterodimers in the neuronal membrane and cooperate in neurite outgrowth promotion. J Cell Biol. 1996;135:1593–1607. doi: 10.1083/jcb.135.6.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kunz S, Spirig M, Ginsburg C, Buchstaller A, Berger P, Lanz R, et al. Neurite fasciculation mediated by complexes of axonin-1 and Ng cell adhesion molecule. J Cell Biol. 1998;143:1673–1690. doi: 10.1083/jcb.143.6.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Malhotra JD, Tsiotra P, Karagogeos D, Hortsch M. Cis-activation of L1-mediated ankyrin recruitment by TAG-1 homophilic cell adhesion. J Biol Chem. 1998;273:33354–33359. doi: 10.1074/jbc.273.50.33354. [DOI] [PubMed] [Google Scholar]
- 74.Fitzli D, Stoeckli ET, Kunz S, Siribour K, Rader C, Kunz B, et al. A direct interaction of axonin-1 with NgCAM-related cell adhesion molecule (NrCAM) results in guidance, but not growth of commissural axons. J Cell Biol. 2000;149:951–968. doi: 10.1083/jcb.149.4.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brummendorf T, Hubert M, Treubert U, Leuschner R, Tarnok A, Rathjen FG. The axonal recognition molecule F11 is a multifunctional protein: specific domains mediate interactions with Ng-CAM and restrictin. Neuron. 1993;10:711–727. doi: 10.1016/0896-6273(93)90172-n. [DOI] [PubMed] [Google Scholar]
- 76.Suter DM, Pollerberg GE, Buchstaller A, Giger RJ, Dreyer WJ, Sonderegger P. Binding between the neural cell adhesion molecules axonin-1 and Nr-CAM/Bravo is involved in neuron-glia interaction. J Cell Biol. 1995;131:1067–1081. doi: 10.1083/jcb.131.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lustig M, Erskine L, Mason CA, Grumet M, Sakurai T. Nr-CAM expression in the developing mouse nervous system: ventral midline structures, specific fiber tracts and neuropilar regions. J Comp Neurol. 2001;434:13–28. doi: 10.1002/cne.1161. [DOI] [PubMed] [Google Scholar]
- 78.Morales G, Hubert M, Brümmendorf T, Treubert U, Tárnok A, Schwarz U, Rathjen FG. Induction of axonal growth by heterophilic interactions between the cell surface recognition proteins F11 and Nr-CAM/Bravo. Neuron. 1993;11:1113–1122. doi: 10.1016/0896-6273(93)90224-f. [DOI] [PubMed] [Google Scholar]
- 79.Buttiglione M, Revest JM, Rougon G, Faivre-Sarrailh C. F3 neuronal adhesion molecule controls outgrowth and fasciculation of cerebellar granule cell neurites: a cell-type-specific effect mediated by the Ig-like domains. Mol Cell Neurosci. 1996;8:53–69. doi: 10.1006/mcne.1996.0043. [DOI] [PubMed] [Google Scholar]
- 80.Faivre-Sarrailh C, Falk J, Pollerberg E, Schachner M, Rougon G. NrCAM, cerebellar granule cell receptor for the neuronal adhesion molecule F3, displays an actin-dependent mobility in growth cones. J Cell Sci. 1999;112:3015–3027. doi: 10.1242/jcs.112.18.3015. [DOI] [PubMed] [Google Scholar]
- 81.Sakurai T, Lustig M, Nativ M, Hemperly JJ, Schlessinger J, Peles E, Grumet M. Induction of neurite outgrowth through contactin and Nr-CAM by extracellular regions of glial receptor tyrosine phosphatase beta. J Cell Biol. 1997;136:907–918. doi: 10.1083/jcb.136.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ye H, Tan YL, Ponniah S, Takeda Y, Wang SQ, Schachner M, et al. Neural recognition molecules CHL1 and NB-3 regulate apical dendrite orientation in the neocortex via PTPα. EMBO J. 2008;27:188–200. doi: 10.1038/sj.emboj.7601939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zisch AH, D'Alessandri L, Amrein K, Ranscht B, Winterhalter KH, Vaughan L. The glypiated neuronal cell adhesion molecule contactin/F11 complexes with src-family protein tyrosine kinase Fyn. Mol Cell Neurosci. 1995;6:263–279. doi: 10.1006/mcne.1995.1021. [DOI] [PubMed] [Google Scholar]
- 84.Zeng L, D'Alessandri L, Kalousek MB, Vaughan L, Pallen CJ. Protein tyrosine phosphatase α (PTPα) and contactin form a novel neuronal receptor complex linked to the intracellular tyrosine kinase fyn. J Cell Biol. 1999;147:707–714. doi: 10.1083/jcb.147.4.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Milev P, Maurel P, Haring M, Margolis RK, Margolis RU. TAG-1/axonin-1 is a high-affinity ligand of neurocan, phosphacan/protein-tyrosine phosphatase-zeta/beta, and N-CAM. J Biol Chem. 1996;271:15716–15723. doi: 10.1074/jbc.271.26.15716. [DOI] [PubMed] [Google Scholar]
- 86.Rathjen FG, Wolff JM, Chiquet-Ehrismann R. Restrictin: a chick neural extracellular matrix protein involved in cell attachment co-purifies with the cell recognition molecule F11. Development. 1991;113:151–164. doi: 10.1242/dev.113.1.151. [DOI] [PubMed] [Google Scholar]
- 87.Zisch AH, D'Alessandri L, Ranscht B, Falchetto R, Winterhalter KH, Vaughan L. Neuronal cell adhesion molecule contactin/F11 binds to tenascin via its immunoglobulin-like domains. J Cell Biol. 1992;119:203–213. doi: 10.1083/jcb.119.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pesheva P, Gennarini G, Goridis C, Schachner M. The F3/11 cell adhesion molecule mediates the repulsion of neurons by the extracellular matrix glycoprotein J1-160/180. Neuron. 1993;10:69–82. doi: 10.1016/0896-6273(93)90243-k. [DOI] [PubMed] [Google Scholar]
- 89.Peles E, Nativ M, Campbell PL, Sakurai T, Martinez R, Lev S, et al. The carbonic anhydrase domain of receptor tyrosine phosphatase beta is a functional ligand for the axonal cell recognition molecule contactin. Cell. 1995;82:251–260. doi: 10.1016/0092-8674(95)90312-7. [DOI] [PubMed] [Google Scholar]
- 90.Xiao ZC, Revest JM, Laeng P, Rougon G, Schachner M, Montag D. Defasciculation of neurites is mediated by tenascin-R and its neuronal receptor F3/11. J Neurosci Res. 1998;52:390–404. doi: 10.1002/(SICI)1097-4547(19980515)52:4<390::AID-JNR3>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 91.Rigato F, Garwood J, Calco V, Heck N, Faivre-Sarrailh C, Faissner A. Tenascin-C promotes neurite outgrowth of embryonic hippocampal neurons through the alternatively spliced fibronectin type III BD domains via activation of the cell adhesion molecule F3/contactin. J Neurosci. 2002;22:6596–6609. doi: 10.1523/JNEUROSCI.22-15-06596.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hu QD, Ang BT, Karsak M, Hu WP, Cui XY, Duka T, et al. F3/contactin acts as a functional ligand for Notch during oligodendrocyte maturation. Cell. 2003;115:163–175. doi: 10.1016/s0092-8674(03)00810-9. [DOI] [PubMed] [Google Scholar]
- 93.Cui XY, Hu QD, Tekaya M, Shimoda Y, Ang BT, Nie DY, et al. NB-3/Notch1 pathway via Deltex1 promotes neural progenitor cell differentiation into oligodendrocytes. J Biol Chem. 2004;279:25858–25865. doi: 10.1074/jbc.M313505200. [DOI] [PubMed] [Google Scholar]
- 94.Ma QH, Futagawa T, Yang WL, Jiang XD, Zeng L, Takeda Y, et al. A TAG1-APP signalling pathway through Fe65 negatively modulates neurogenesis. Nat Cell Biol. 2008;10:283–294. doi: 10.1038/ncb1690. [DOI] [PubMed] [Google Scholar]
- 95.Olive S, Rougon G, Pierre K, Theodosis DT. Expression of a glycosyl phosphatidylinositolanchored adhesion molecule, the glycoprotein F3, in the adult rat hypothalamo-neurohypophysial system. Brain Res. 1995;689:271–280. doi: 10.1016/0006-8993(95)00555-5. [DOI] [PubMed] [Google Scholar]
- 96.Fetissov SO, Bergstrom U, Johansen JE, Hokfelt T, Schalling M, Ranscht B. Alterations of arcuate nucleus neuropeptidergic development in contactin-deficient mice: comparison with anorexia and food-deprived mice. Eur J Neurosci. 2005;22:3217–3228. doi: 10.1111/j.1460-9568.2005.04513.x. [DOI] [PubMed] [Google Scholar]
- 97.Johansen JE, Fetissov SO, Bergstrom U, Nilsson I, Fay C, Ranscht B, et al. Evidence for hypothalamic dysregulation in mouse models of anorexia as well as in humans. Physiol Behav. 2007;92:278–282. doi: 10.1016/j.physbeh.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 98.Zeng L, Zhang C, Xu J, Ye X, Wu Q, Dai J, et al. A novel splice variant of the cell adhesion molecule contactin 4 (CNTN4) is mainly expressed in human brain. J Hum Genet. 2002;47:497–499. doi: 10.1007/s100380200073. [DOI] [PubMed] [Google Scholar]
- 99.Kamei Y, Tsutsumi O, Taketani Y, Watanabe K. cDNA cloning and chromosomal localization of neural adhesion molecule NB-3 in human. J Neurosci Res. 1998;51:275–283. doi: 10.1002/(SICI)1097-4547(19980201)51:3<275::AID-JNR1>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 100.Wei MH, Karavanova I, Ivanov SV, Popescu NC, Keck CL, Pack S, et al. In silico-initiated cloning and molecular characterization of a novel human member of the L1 gene family of neural cell adhesion molecules. Hum Genet. 1998;103:355–364. doi: 10.1007/s004390050829. [DOI] [PubMed] [Google Scholar]
- 101.Fernandez T, Morgan T, Davis N, Klin A, Morris A, Farhi A, et al. Disruption of contactin 4 (CNTN4) results in developmental delay and other features of 3p deletion syndrome. Am J Hum Genet. 2004;74:1286–1293. doi: 10.1086/421474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dijkhuizen T, van Essen T, van der Vlies P, Verheij JB, Sikkema-Raddatz B, van der Veen AY, et al. FISH and array-CGH analysis of a complex chromosome 3 aberration suggests that loss of CNTN4 and CRBN contributes to mental retardation in 3pter deletions. Am J Med Genet A. 2006;140:2482–2487. doi: 10.1002/ajmg.a.31487. [DOI] [PubMed] [Google Scholar]
- 103.Roohi J, Montagna C, Tegay DH, Palmer LE, Devincent C, Pomeroy JC, et al. Disruption of Contactin 4 in 3 Subjects with Autism Spectrum Disorder. J Med Genet. 2008 doi: 10.1136/jmg.2008.057505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Strauss KA, Puffenberger EG, Huentelman MJ, Gottlieb S, Dobrin SE, Parod JM, et al. Recessive symptomatic focal epilepsy and mutant contactin-associated protein-like 2. N Engl J Med. 2006;354:1370–1377. doi: 10.1056/NEJMoa052773. [DOI] [PubMed] [Google Scholar]
- 105.Friedman JI, Vrijenhoek T, Markx S, Janssen IM, van der Vliet WA, Faas BH, et al. CNTNAP2 gene dosage variation is associated with schizophrenia and epilepsy. Mol Psychiatry. 2008;13:261–266. doi: 10.1038/sj.mp.4002049. [DOI] [PubMed] [Google Scholar]
- 106.Alarcon M, Abrahams BS, Stone JL, Duvall JA, Perederiy JV, Bomar JM, et al. Linkage, association and gene-expression analyses identify CNTNAP2 as an autism-susceptibility gene. Am J Hum Genet. 2008;82:150–159. doi: 10.1016/j.ajhg.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Arking DE, Cutler DJ, Brune CW, Teslovich TM, West K, Ikeda M, et al. A common genetic variant in the neurexin superfamily member CNTNAP2 increases familial risk of autism. Am J Hum Genet. 2008;82:160–164. doi: 10.1016/j.ajhg.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]