Abstract
Ischemia-induced ionic imbalance leads to the activation of numerous events including mitochondrial dysfunction and eventual cell death. Dysregulation of mitochondrial Ca2+ (Ca2+m) plays a critical role in cell damage under pathological conditions including traumatic brain injury and stroke. High Ca2+m levels can induce the persistent opening of the mitochondrial permeability transition pore and trigger mitochondrial membrane depolarization, Ca2+ release, cessation of oxidative phosphorylation, matrix swelling and eventually outer membrane rupture with release of cytochrome c and other apoptogenic proteins. Thus, the dysregulation of mitochondrial Ca2+ homeostasis is now recognized to play a crucial role in triggering mitochondrial dysfunction and subsequent apoptosis. Recent studies show that some secondary active transport proteins, such as Na+-dependent chloride transporter and Na+/Ca2+ exchanger, contribute to ischemia-induced dissipation of ion homeostasis including Ca2+m.
Key words: ischemia, intracellular Ca2+ dysregulation, changes of mitochondrial Ca2+, cytochrome c, apoptosis
Introduction
Ionic imbalance plays a critical role in pathogenesis of ischemic cell damage. Ischemia-induced perturbation of ion homeostasis leads to intracellular accumulation of Ca2+ and Na+ which results in the subsequent activation of numerous events including mitochondrial dysfunction and eventual cell death. Structural, biochemical and functional abnormalities of mitochondria are widely believed to contribute to ischemic or hypoxic cell injury.1 Many lines of evidences suggest that Ca2+ is a key regulator of mitochondrial function.2,3 Mitochondrial Ca2+ overload triggers the mitochondrial death pathway, which features the loss of mitochondrial membrane potential (Ψm), the opening of the mitochondrial permeability transition pore (PTP), release of Cytochrome c (Cyt. C), and enhanced generation of reactive oxygen species.4–6
This review will highlight recent studies reflecting the role of secondary active transport proteins in ischemia-induced dissipation of ion homeostasis and subsequent mitochondrial dysfunction. Secondary active ion transport proteins such as the Na+-dependent chloride transporter (NKCC) and the Na+/Ca2+ exchanger (NCX) do not use energy stored in ATP directly, but derive their energy from the combined electrochemical gradients generated by Na+/K+-ATPase and Ca2+-ATPase. Secondary active ion transport proteins are important in maintaining steady-state intracellular ionic concentrations. Results from both in vitro and in vivo experimental studies suggest that these ion transport proteins are involved in ischemia-mediated loss of ion homeostasis, leading to mitochondrial dysfunction.7–11 Therefore, they may be important targets for therapeutic intervention.
Mitochondrial Ca2+ Imbalance and Ischemic Mitochondrial Dysfunction
Mitochondrial Ca2+ imbalance.
Calcium is one of the most prominent intracellular messenger molecules, and plays a vital role in the physiology and biochemistry of cells, particularly in signal transduction pathways.12 Cytosolic free Ca2+ (Ca2+cyt) is maintained at roughly 100 nM while the extracellular milieu generally has a [Ca2+] of over 1 mM under physiological conditions. Loss of Ca2+ homeostasis, often in the form of an increase in cytoplasmic Ca2+, leads to multiple destructive processes such as the activation of proteases, lipases, nucleases, nitric oxide synthases, protein kinases and eventual cell death.4,12
Mitochondria play a central role in cellular metabolism and are responsible for cellular respiration that generates ATP from ADP and inorganic phosphate. Under physiological conditions, Ca2+m increases to buffer the amplitude of the Ca2+cyt rise. Ca2+m is typically in the range of 0.2–3 mM, which is ideal for the activation of Ca2+-dependent enzymes of the Krebs cycle. Increases of Ca2+m can be compensated by mitochondrial Ca2+ efflux mechanisms such as NCX and matrix Ca2+ buffering.3 Thus mitochondria accumulate Ca2+ and efficiently control the spatial and temporal shape of cellular Ca2+ signals, yet this situation exposes them to the hazards of Ca2+ overload.3,13 During anoxia/ischemia, oxidative phosphorylation is inhibited, triggering a rapid decline in ATP production and initiating multiple destructive processes.14 These changes include the compromising of ionic homeostasis, activation of glycolysis and intracellular acidosis, degradation of phospholipids and an increase in the plasma membrane permeability to Na+ and Ca2+.14,15 Elevated cytoplasmic Na+ (Na+cyt) during ischemia will favor the reverse-mode operation of NCX (NCXrev), causing Ca2+cyt levels to further increase.16 In the presence of ATP, Mg2+ and inorganic phosphate, respiring mitochondria are able to accumulate large amounts of Ca2+ via the mitochondrial Ca2+ uniporter and/or the rapid uptake mode.17 However, during ischemia when ATP is decreased, mitochondria would not be able to accumulate Ca2+. Upon reperfusion, increases in Ca2+cyt or the release of Ca2+ from the endoplasmic reticulum invariably induce Ca2+m uptake which helps in reestablishing physiological Ca2+cyt levels.2,15
Sustained increases in Ca2+m will initiate several death factors. The rapid uptake of Ca2+ by mitochondria stimulates the Ca2+-sensitive matrix dehydrogenases, which are key sites of NADH production for the respiratory chain and thereby for stimulation of mitochondrial energy metabolism.2 A high concentration of mitochondria Ca2+ can also induce the opening of the PTP, a high conductance inner membrane channel which consists of the voltage-dependent anion channel, the adenine nucleotide translocator and the cyclophilin D, as well as several other proteins.18–20 Activation of PTP triggers a cascade of events during cellular damage.
Ischemic mitochondrial dysfunction.
PTP opening contributes significantly to mitochondrial dysfunction. In particularly it can dramatically increase the mitochondrial membrane permeability, allowing passage of solutes with molecular masses up to 1,500 Da. Persistent PTP opening is followed by depolarization of mitochondria leading to Ca2+ release, cessation of oxidative phosphorylation, matrix swelling with inner membrane remodeling and eventual outer membrane rupture with release of Cyt. C and other apoptogenic proteins.3,21,22 Released Cyt. C then binds to apoptotic protease activating factor-1, which recruits and activates caspase-9 to form the apoptosome. Caspase-9 activation results in the cleavage and activation of caspases-3 and -7, initiating a proteolytic cascade that ultimately results in cell death.23 There are a number of factors that lower the threshold for Ca2+ induced mitochondrial permeability transition, including inorganic phosphate, prooxidants that oxidize membrane SH-groups, oxidation of NAD(P)H and GSH.15 On the other hand, a protective effect is exerted by Mg2+, ADP (and ATP), some antioxidants, carnitine, decrease in pH and cyclosporin A that binds to cyclophilin.15
Members of the Bcl-2 family of proteins, notably Bax and Bak, can also trigger the permeabilization of the outer mitochondrial membrane by integrating into it as oligomers. This process is stimulated by t-Bid and other BH3-only proteins,24 while inhibited by anti-apoptotic family members, Bcl-2 and Bcl-XL.25 Mice deficient in both Bax and Bak are resistant to most apoptotic stimuli, providing a strong support for the role of the mitochondrial pathway in apoptosis signaling.2,26
The release of Cyt. C to cytoplasm is one of the key events of mitochondrial dysfunction. In healthy cells, Cyt. C is normally bound to the inner mitochondrial membrane by its association with the anionic phospholipid cardiolipin, where it is predominantly located. The dissociation of Cyt. C from cardiolipin leads to the peroxidation of cardiolipin27,28 and decreases its binding affinity to haemoprotein. This is a crucial first step for Cyt. C release into the cytosol and for the induction of apoptosis.2,27 In addition, Ca2+ can bind to cardiolipin in the inner mitochondrial membrane, leading to decreased lipid mobility, formation of cardiolipin-enriched domains and protein aggregation.29 In turn, this rearrangement leads to increased production of reactive oxygen species by the respiratory chain, which promotes the oxidation of membrane phospholipids and proteins resulting in an increase in membrane permeability. In fact, the Ca2+-cardiolipin interaction might be an early and crucial step in the sequence of events by which Ca2+ triggers mitochondrial membrane permeabilization.2
Recent data suggests that Cyt. C release follows a biphasic kinetics rather than in an all-or-nothing manner as previously believed. An in vitro study identifies Cyt. C as a messenger that coordinates mitochondrial-endoplasmatic reticulum interactions that drive apoptosis.30 Early in apoptosis, Cyt. C translocates to the endoplasmic reticulum where it selectively binds to inositol 1,4,5-triphosphate receptor (InsP(3)R), resulting in sustained, oscillatory cytosolic Ca2+ increases.30 These Ca2+ signaling events are linked to the coordinate release of Cyt. C from all mitochondria. These findings identify a feed-forward mechanism whereby early Cyt. C release increases InsP(3)R function, resulting in augmented Cyt. C release then amplifies the apoptotic signal.30,31 Cyt. C also interacts with InsP(3) R type 1 and ryanodine receptor type 2 in gerbil hippocampus subjected to transient brain ischemia with a short reperfusion, and contributes to postischemic neuronal death.32 These findings illustrate the importance of intracellular Ca2+ stores in apoptosis, and ischemic cell damage.
Secondary Active Transport Proteins and Ca2+ Overload
Loss of Ca2+ homeostasis in the cell plays a critical role in pathogenesis of ischemic mitochondrial dysfunction and subsequent cell damage. Recent studies show that the important secondary active transport proteins, NKCC and NCX, contribute to ischemia-induced dissipation of ion homeostasis.
NKCC.
The electroneutral NKCC protein belongs to the cation-chloride cotransporter family, which transports Na+, K+ and Cl− into cells under physiological conditions with a stoichiometry of 1Na+:1K+:2Cl−.33,34 The transmembrane chemical gradients for Na+, K+ and Cl− determine net inward ion movement via NKCC.34 In most cells, the driving force for ion influx is in part supplied by the inward Na+ gradient, maintained by Na+/K+-ATPase. However, in Cl− secretory epithelial cells, the Cl− gradient is also an important driving force for ion influx.34 NKCC is characteristically inhibited by the sulfamoybenzoic acid loop diuretics, such as bumetanide and furosemide.35 To date, only two distinct isoforms, NKCC1 and NKCC2, have been identified. NKCC1 has a broad tissue distribution, while the NKCC2 isoform is only found in vertebrate kidney.33,36
NKCC serves multiple functions, including ion and fluid movements in secreting or reabsorbing epithelia and cell volume regulation.33,34 However, understanding of the role of NKCC1 in the central nervous system just began. NKCC1 is important in the maintenance of intracellular Cl− in neurons and contributes to GABA-mediated depolarization in immature neurons. Thus, NKCC1 may affect neuronal excitability through regulation of intracellular Cl− concentration.37,38 NKCC1 may also contribute to K+ clearance and maintenance of intracellular Na+ in astrocytes and oligodendrocytes. Recent studies suggest that high [K+]o activation of NKCC1 leads to astrocyte swelling and glutamate release, as well as neuronal Na+, and Cl− influx during acute excitotoxicity.39 In addition, inhibition of NKCC1 activity significantly reduces infarct volume and cerebral edema following cerebral focal ischemia.39
NCX.
NCX belongs to the Ca2+/cation antiporter superfamily. It is a ubiquitous transporter that plays an important role in regulating and maintaining cellular Ca2+ balance in various tissues. At present, three isoforms in the NCX family have been identified: NCX1, NCX2 and NCX3.40 They share biophysical and biochemical properties but exhibit differences in expression during development and in various adult tissues. NCX1 is present in most tissues and is highly expressed in heart, brain and kidney, while NCX2 and NCX3 are expressed mainly in the brain and skeletal muscles.40–42 Immunogold EM studies reveal that neuronal NCXs are preferentially located in dendrites and dendritic spines, while glial NCXs are prominently expressed in astrocytic processes ensheathing excitatory synapses.43 The specific localization indicates that NCXs are favourably located for buffering Ca2+cyt in excitatory postsynaptic sites and may participate in shaping astrocytic Ca2+cyt transients evoked by ongoing synaptic activity.43
NCX works bidirectionally depending on cytosolic Na+ concentration ([Na+]cyt), cytosolic Ca2+ concentration ([Ca2+]cyt) and plasma membrane potential. The stoichiometry of NCX1 is generally accepted to be 3 Na+ ions/1 Ca2+ ion. Thus, NCX1 transport is electrogenic, and dependent on plasma membrane potential.44 Under physiological conditions, NCX is thought to primarily pump Ca2+ to the outside of the cell using the Na+ concentration gradient across the cell membrane. In contrast, under conditions in which Na+ accumulates inside the cell such as during ischemia, NCX may work in reverse mode-operation and conduct Ca2+ influx.
Roles of NKCC1 and NCX in ischemic mitochondrial dysfunction.
Loss of Na+ homeostasis in cells following in vitro hypoxia/ischemia. A steep inwardly directed Na+ gradient is essential for cell functions, such as glutamate reuptake and regulation of intracellular ion concentrations by other secondary ion transporters.46,47 Dissipation of the Na+ gradient is one of the key elements in promoting cellular damage in cells during energy failure.47,48
A recent study shows that one hour oxygen-glucose deprivation (OGD) triggers an approximately four-fold increase in [Na+]cyt in cultured cortical neurons.11 Additionally, 60 min reoxygenation (REOX) following two hour OGD can induce a seven-fold increase in [Na+]cyt in neurons.48 An ∼3.6-fold increase in [Na+]cyt after OGD is also reported in cultured astrocytes.7 Additionally, Kintner DB, et al. reported that the onset of REOX following either of two hypoxic conditions, OGD and HAIR (a hypoxic, acidic, ion-shifted ringer buffer), triggers a rapid Na+cyt overload in astrocytes.8 This finding is consistent with reports on [Na+]cyt accumulation in rat spinal cord astrocytes,49 rat cortical astrocytes47 and mouse cortical astrocytes,50 when these cells are exposed to glucose deprivation, NaN3-mediated chemical hypoxia and simulated ischemia, respectively.
Lenart B, et al. reported that NKCC1 activity in cortical astrocytes is increased during reoxygenation (REOX) via protein phosphorylation, which is a major regulatory mechanism for stimulation of NKCC1 activity.7,51 It also has been reported that loss of NKCC1 activity either by treatment with its inhibitor bumetanide or by genetic ablation blocks ∼64% of the rise in Na+cyt in astrocytes,8 and results in ∼50% less Na+cyt accumulation in neurons.11 The remaining Na+cyt accumulation could be the result of other Na+ influx pathways (such as voltage-gated Na+ channels, Na+/H+ exchanger, etc.) or a decrease in Na+ extrusion via Na+/K+ ATPase during ischemia.7,11 In addition, NKCC1 also plays a role during glutamate-mediated excitotoxicity. [Na+]cyt accumulation is found in cultured oligodendrocytes exposed to alphaamino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and cyclothiazide (CTZ),10 and in cortical neurons exposure to 60 µM N-methyl-D-aspartic acid (NMDA).38 Blocking NKCC1 activity with bumetanide significantly reduces the AMPA/CTZ-induced Na+ influx in oligodendrocytes10 as well as the accumulation of Na+cyt content after NMDA receptor activation in neurons.38 Thus, these results imply that stimulation of NKCC1 activity evokes excessive Na+ entry and disrupts Na+ homeostasis following hypoxia/ischemia.
Intracellular Na+ overload leads to reversal of NCX. Reduction in the Na+ gradient affects Ca2+ homeostasis in cells. Ca2+ entry pathways involve L-type voltage-gated Ca2+ channels, ionotropic glutamate receptors, and NCXrev.52 Li et al. reported that elevation of [Na+]cyt increases Ca2+cyt via NCXrev in neurons and causes irreversible injury during anoxia and ischemia.53 An increase in Ca2+cyt via NCXrev occurs in rat astrocytes when [Na+]cyt is raised by inhibition of Na+/K+-ATPase activity or activation of AMPA channels.54,55 Furthermore, Ca2+ entry is detected in astrocytes after OGD. Inhibition of NCXrev with 3 µM KB-R7943 abolishes the OGD-mediated increase in releasable Ca2+ in bradykinin-sensitive Ca2+ stores.7 These findings further suggest that NCXrev plays a role in Ca2+ entry and Ca2+ signaling in cells after OGD. So far, more benzyloxyphenyl derivatives (SEA0400, SN-6 and YM-244769) have been developed as selective NCXrev inhibitors.45 They effectively prevent ischemia-reperfusion injury in several animal models.45 Thus, NCX inhibitors may have therapeutic potential as novel drugs for reperfusion injury.
In addition to a significant rise in [Ca2+]cyt during HAIR, REOX induces a secondary increase in [Ca2+]cyt.8 Approximately 70% of the REOX-triggered Ca2+ rise following 15 min HAIR is abolished with 10 µM KB-R7943. Interestingly, NKCC1-/- astrocytes exhibit a much lower level of REOX-triggered Ca2+ rise.8 The data suggest that Ca2+entry during REOX is largely via NKCC1-mediated reversal of NCX in astrocytes. This finding is consistent with thermodynamic modeling that predicts NCX will operate in the reverse mode and mediate Ca2+ influx when [Na+]cyt in astrocytes is increased ∼25 mM.56 Maximum values of [Na+]cyt reached 70–80 mM during REOX following either OGD or HAIR, a condition that favors NCXrev. This view is supported by several recent reports. For example, inhibition of NCX with 100 nM KB-R7943 significantly attenuates the rise in [Ca2+]cyt in response to severe mechanical strain injuries in rat cortical astrocytes. The strain injury leads to a rapid rise in [Na+]cyt in astrocytes that is sustained for ∼50 min.57
Moreover, a transient elevation of [Ca2+]cyt following 25–30 min HAIR is blocked by NCXrev inhibitor KB-R7943.58 The effects of KB-R7943 imply that REOX evokes Na+ entry, which subsequently triggers the reversal of NCX. The elevation of [Na+]cyt and the reversal of NCX have been suggested to play a role in spinal cord astrocyte ischemic damage during the reperfusion period.49 NCX inhibitors bepridil and KB-R7943 improve functional recovery of white matter tracts after anoxic and traumatic injury.53 In addition, Ca2+ influx via NCXrev is decreased by approximately 70% in NCX+/− neurons, which exhibit reduced NCX1 protein expression compared to NCX+/+ neurons.59 KB-R7943 treatment attenuates the transient Ca2+ influx by 60% and sustained Ca2+ influx by 70% in oligodendrocytes.10 Moreover, inhibition of NKCC1 activity with bumetanide reduced the sustained Ca2+ rise by 50%.10 These data suggest that NKCC1 and NCXrev are involved in AMPA-triggered Ca2+ signaling in oligodendrocytes. The protective effects, via inhibition of NKCC1 and NCXrev on Ca2+cyt overload, may result from reducing Na+cyt overload and inhibition of NCXrev.10
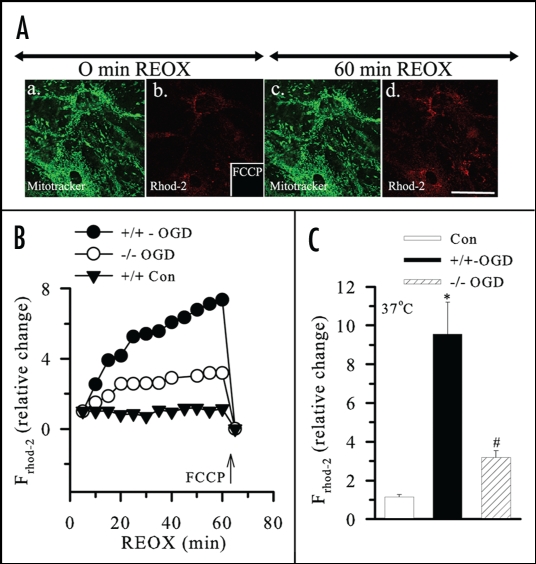
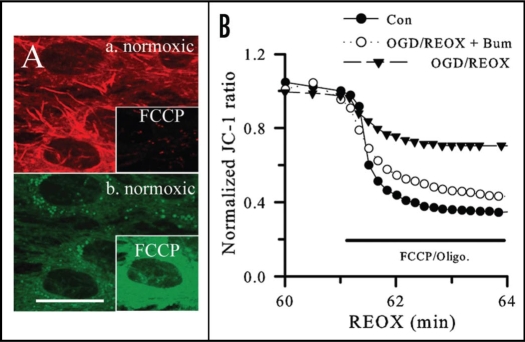
Cam2+ overload, mitochondrial dysfunction and cell death. The mechanisms of ischemic injury include Ca2+ entry and Ca2+-mediated cell death. Ca2+m overload leads to mitochondrial dysfunction via many pathways, including inhibition of oxidative phosphorylation, oxygen free radical formation or formation of the mitochondrial PTP, which subsequently releases proapoptotic molecules such as Cyt. C.5 OGD/REOX can cause a dramatic increase in Ca2+m accumulation and Cyt. C release in cultured neurons.48 An approximately five-fold increase of Ca2+m accompanies an ∼58% depolarization of Ψm and subsequent Cyt. C release in cortical astrocytes at 1 h REOX following 2 h OGD (Figs. 1 and 2).8 In addition, REOX following 15 min HAIR can cause significant dissipation of Ψm and Ca2+m increase in NKCC1+/+ astrocytes. Pharmacological inhibition or genetic ablation of NKCC1 not only attenuates REOX-induced Ca2+m overload and Ψm depolarization, but it also blocks Cyt. C release from mitochondria and results in less cell death in culture astrocytes (Figs. 1 and 2).8 Moreover, in NCX1+/−/NKCC1+/− double heterozygous neurons, with lower NCX1 and NKCC1 protein expression, NCX-mediated Ca2+ influx is nearly abolished. These neurons exhibit approximately 65% less neuronal death and increased tolerance to ischemic damage.9 Studies in glutamate-mediated excitotoxicity in oligodendrocyte damage shows the AMPA/CTZ-elicited Ca2+cyt increase, Ca2+m overload, mitochondrial damage, and cell death were significantly reduced by inhibiting NKCC1 or NCXrev.10 In summary, these findings suggest that NKCC1 in conjunction with NCX1 mediate dysregulation of cellular Na+ and Ca2+ homeostasis and can seriously impair cell function and survival.8
Figure 1.
Changes in mitochondrial Ca2+ (Ca2+m) in astrocytes following OGD/REOX. (A) Mitotracker (green in a) and Rhod-2 (red in b) images were shown in NKCC1+/+ astrocytes at the end of 2 h OGD and at 60 min REOX (c and d). Scale bar = 12.3 µm. (B) Average tracings of relative change in Rhod-2 fluorescence during 60 min REOX following 2 h OGD in NKCC1+/+ and NKCC1−/− astrocytes. Arrow: response of Rhod-2 fluorescence to FCCP. (C) Summary data. Data are means ± SEM; n = 4–5. * p < 0.05 vs. control; # p < 0.05 vs. NKCC1+/+ OGD/REOX. Reprinted with permission from Kinter et al.8
Figure 2.
Changes in membrane potential (Ψm) in astrocytes following OGD/REOX. (A) JC-1 aggregate (a.) and monomer (b.) fluorescence in NKCC1+/+ cells under normoxic control conditions. Insets in (A): changes of JC-1 fluorescence following 3 min of FCCP (10 µM). Scale bar = 12.3 µm. (B) A representative tracing of changes of the JC-1 ratios. n = 4–5. Reprinted with permission from Kinter et al.8
Conclusion
Loss of ionic homeostasis plays an important role in ischemic brain damage. Recent findings suggest that NKCC and NCXrev are involved in dissipation of ion homeostasis and mitochondrial dysfunction. The concerted activities of multiple ion transport proteins not only perturbate intracellular Na+ and Ca2+ homeostasis in response to hypoxia/ischemia, but also affect mitochondrial Ca2+ and Cyt. C release. Therefore, these ion transport proteins may be potential therapeutic targets to reduce or prevent ischemia-mediated loss of ion homeostasis.
Acknowledgements
We thank Douglas Kintner for critical reading of the manuscript and many helpful suggestions. This work was supported by National Institutes of Health Grant R01NS-38118 (D.S.), Tsinghua-Yue-Yuen Medical Sciences Fund (X.J.L), and China Scholarship Council Postgraduate Scholarship Program (Y.L).
Abbreviation
- AMPA
alphaamino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- Ca2+m
mitochondrial Ca2+
- Ca2+cyt
cytosolic free Ca2+
- [Ca2+]cyt
cytosolic Ca2+ concentration
- Cyt. C
cytochrome c
- CTZ
cyclothiazide
- HAIR
a hypoxic, acidic, ion-shifted ringer buffer
- InsP(3) R
inositol 1,4,5-triphosphate receptor
- Na+cyt
cytoplasmic Na+
- [Na+]cyt
cytosolic Na+ concentration
- NCX
Na+/Ca2+ exchanger
- NCXrev
reverse-mode operation of NCX
- NKCC
Na+-dependent chloride transporter
- NMDA
N-methyl-D-aspartic acid
- OGD
oxygen-glucose deprivation
- PTP
permeability transition pore
- REOX
reoxygenation
- Ψm
mitochondrial membrane potential
Footnotes
Previously published online as a Cell Adhesion & Migration E-publication: http://www.landesbioscience.com/journals/celladhesion/article/7516
References
- 1.McBride HM, Neuspiel M, Wasiak S. Mitochondria: more than just a powerhouse. Curr Biol. 2006;16:551–560. doi: 10.1016/j.cub.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 2.Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol. 2003;4:552–565. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- 3.Bernardi P, Rasola A. Calcium and cell death: the mitochondrial connection. Subcell Biochem. 2007;45:481–506. doi: 10.1007/978-1-4020-6191-2_18. [DOI] [PubMed] [Google Scholar]
- 4.Hoyt KR, Stout AK, Cardman JM, Reynolds IJ. The role of intracellular Na+ and mitochondria in buffering of kainate-induced intracellular free Ca2+ changes in rat forebrain neurones. J Physiol (Lond) 1998;509:103–116. doi: 10.1111/j.1469-7793.1998.103bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287:817–833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 6.Cao G, Xiao M, Sun F, Xiao X, Pei W, Li J, et al. Cloning of a novel Apaf-1-interacting protein: a potent suppressor of apoptosis and ischemic neuronal cell death. J Neurosci. 2004;24:6189–6201. doi: 10.1523/JNEUROSCI.1426-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lenart B, Kintner DB, Shull GE, Sun D. Na-K-Cl cotransporter-mediated intracellular Na+ accumulation affects Ca2+ signaling in astrocytes in an in vitro ischemic model. J Neurosci. 2004;24:9585–9597. doi: 10.1523/JNEUROSCI.2569-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kintner DB, Luo J, Gerdts J, Ballard AJ, Shull GE, Sun D. Role of Na+-K+-Cl− cotransport and Na+/Ca2+ exchange in mitochondrial dysfunction in astrocytes following in vitro ischemia. Am J Physiol Cell Physiol. 2007;292:1113–1122. doi: 10.1152/ajpcell.00412.2006. [DOI] [PubMed] [Google Scholar]
- 9.Luo J, Wang Y, Chen H, Kintner DB, Cramer SW, Gerdts JK, et al. A concerted role of Na(+)-K(+)-Cl(-) cotransporter and Na(+)/Ca(2+) exchanger in ischemic damage. J Cereb Blood Flow Metab. 2008;28:737–746. doi: 10.1038/sj.jcbfm.9600561. [DOI] [PubMed] [Google Scholar]
- 10.Chen H, Kintner DB, Jones M, Matsuda T, Baba A, Kiedrowski L, et al. AMPA-mediated excitotoxicity in oligodendrocytes: role for Na(+)-K(+)-Cl(-) co-transport and reversal of Na(+)/Ca(2+) exchanger. J Neurochem. 2007 doi: 10.1111/j.1471-4159.2007.04638.x. [DOI] [PubMed] [Google Scholar]
- 11.Chen H, Luo J, Kintner DB, Shull GE, Sun D. Na+-dependent chloride transporter (NKCC1)-null mice exhibit less gray and white matter damage after focal cerebral ischemia. J Cereb Blood Flow Metab. 2005;25:54–66. doi: 10.1038/sj.jcbfm.9600006. [DOI] [PubMed] [Google Scholar]
- 12.Clapham DE. Calcium signaling. Cell. 1995;80:259–268. doi: 10.1016/0092-8674(95)90408-5. [DOI] [PubMed] [Google Scholar]
- 13.Fall CP, Keizer JE. Mitochondrial modulation of intracellular Ca(2+) signaling. J Theor Biol. 2001;210:151–165. doi: 10.1006/jtbi.2000.2292. [DOI] [PubMed] [Google Scholar]
- 14.Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- 15.Saris NE, Eriksson KO. Mitochondrial dysfunction in ischaemia-reperfusion. Acta Anaesthesiol Scand Suppl. 1995;107:171–176. doi: 10.1111/j.1399-6576.1995.tb04353.x. [DOI] [PubMed] [Google Scholar]
- 16.Grinwald PM. Calcium uptake during post-ischemic reperfusion in the isolated rat heart: influence of extracellular sodium. J Mol Cell Cardiol. 1982;14:359–365. doi: 10.1016/0022-2828(82)90251-6. [DOI] [PubMed] [Google Scholar]
- 17.Gunter TE, Yule DI, Gunter KK, Eliseev RA, Salter JD. Calcium and mitochondria. FEBS Lett. 2004;567:96–102. doi: 10.1016/j.febslet.2004.03.071. [DOI] [PubMed] [Google Scholar]
- 18.Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J. 1999;341:233–249. [PMC free article] [PubMed] [Google Scholar]
- 19.Brustovetsky N, Brustovetsky T, Jemmerson R, Dubinsky JM. Calcium-induced cytochrome c release from CNS mitochondria is associated with the permeability transition and rupture of the outer membrane. J Neurochem. 2002;80:207–218. doi: 10.1046/j.0022-3042.2001.00671.x. [DOI] [PubMed] [Google Scholar]
- 20.Hunter DR, Haworth RA. The Ca2+-induced membrane transition in mitochondria I. The protective mechanisms. Arch Biochem Biophys. 1979;195:453–459. doi: 10.1016/0003-9861(79)90371-0. [DOI] [PubMed] [Google Scholar]
- 21.Haworth RA, Hunter DR. The Ca2+-induced membrane transition in mitochondria II. Nature of the Ca2+ trigger site. Arch Biochem Biophys. 1979;195:460–467. doi: 10.1016/0003-9861(79)90372-2. [DOI] [PubMed] [Google Scholar]
- 22.Rasola A, Bernardi P. The mitochondrial permeability transition pore and its involvement in cell death and in disease pathogenesis. Apoptosis. 2007;12:815–833. doi: 10.1007/s10495-007-0723-y. [DOI] [PubMed] [Google Scholar]
- 23.Strasser A, O'Connor L, Dixit VM. Apoptosis signaling. Annu Rev Biochem. 2000;69:217–245. doi: 10.1146/annurev.biochem.69.1.217. [DOI] [PubMed] [Google Scholar]
- 24.Eskes R, Desagher S, Antonsson B, Martinou JC. Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol Cell Biol. 2000;20:929–935. doi: 10.1128/mcb.20.3.929-935.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Antonsson B, Conti F, Ciavatta A, Montessuit S, Lewis S, Martinou I, et al. Inhibition of Bax channel-forming activity by Bcl-2. Science. 1997;277:370–372. doi: 10.1126/science.277.5324.370. [DOI] [PubMed] [Google Scholar]
- 26.Lindsten T, Ross AJ, King A, Zong WX, Rathmell JC, Shiels HA, et al. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol Cell. 2000;6:1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ott M, Robertson JD, Gogvadze V, Zhivotovsky B, Orrenius S. Cytochrome c release from mitochondria proceeds by a two-step process. Proc Natl Acad Sci USA. 2002;99:1259–1263. doi: 10.1073/pnas.241655498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petrosillo G, Ruggiero FM, Pistolese M, Paradies G. Reactive oxygen species generated from the mitochondrial electron transport chain induce cytochrome c dissociation from beef-heart submitochondrial particles via cardiolipin peroxidation. Possible role in the apoptosis. FEBS Lett. 2001;509:435–438. doi: 10.1016/s0014-5793(01)03206-9. [DOI] [PubMed] [Google Scholar]
- 29.Grijalba MT, Vercesi AE, Schreier S. Ca2+-induced increased lipid packing and domain formation in submitochondrial particles. A possible early step in the mechanism of Ca2+-stimulated generation of reactive oxygen species by the respiratory chain. Biochemistry. 1999;38:13279–13287. doi: 10.1021/bi9828674. [DOI] [PubMed] [Google Scholar]
- 30.Boehning D, Patterson RL, Sedaghat L, Glebova NO, Kurosaki T, Snyder SH. Cytochrome c binds to inositol (1,4,5) trisphosphate receptors, amplifying calcium-dependent apoptosis. Nat Cell Biol. 2003;5:1051–1061. doi: 10.1038/ncb1063. [DOI] [PubMed] [Google Scholar]
- 31.Boehning D, Patterson RL, Snyder SH. Apoptosis and calcium: new roles for cytochrome c and inositol 1,4,5-trisphosphate. Cell Cycle. 2004;3:252–254. [PubMed] [Google Scholar]
- 32.Beresewicz M, Kowalczyk JE, Zablocka B. Cytochrome c binds to inositol (1,4,5) trisphosphate and ryanodine receptors in vivo after transient brain ischemia in gerbils. Neurochem Int. 2006;48:568–571. doi: 10.1016/j.neuint.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 33.Haas M, Forbush B., III The Na-K-Cl cotransporter of secretory epithelia. Annu Rev Physiol. 2000;62:515–534. doi: 10.1146/annurev.physiol.62.1.515. [DOI] [PubMed] [Google Scholar]
- 34.Russell JM. Sodium-potassium-chloride cotransport. Physiol Rev. 2000;80:211–276. doi: 10.1152/physrev.2000.80.1.211. [DOI] [PubMed] [Google Scholar]
- 35.Forbush B, III, Palfrey HC. [3H]bumetanide binding to membranes isolated from dog kidney outer medulla. Relationship to the Na, K, Cl co-transport system. J Biol Chem. 1983;258:11787–11792. [PubMed] [Google Scholar]
- 36.Xu JC, Lytle C, Zhu TT, Payne JA, Benz E, Jr, Forbush B., III Molecular cloning and functional expression of the bumetanide-sensitive Na-K-Cl cotransporter. Proc Natl Acad Sci USA. 1994;91:2201–2205. doi: 10.1073/pnas.91.6.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schomberg SL, Su G, Haworth RA, Sun D. Stimulation of Na-K-2Cl cotransporter in neurons by activation of Non-NMDA ionotropic receptor and group-I mGluRs. J Neurophysiol. 2001;85:2563–2575. doi: 10.1152/jn.2001.85.6.2563. [DOI] [PubMed] [Google Scholar]
- 38.Beck J, Lenart B, Kintner DB, Sun D. Na-K-Cl cotransporter contributes to glutamate-mediated excitotoxicity. J Neurosci. 2003;23:5061–5068. doi: 10.1523/JNEUROSCI.23-12-05061.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Su G, Kintner DB, Sun D. Contribution of Na(+)-K(+)-Cl(-) cotransporter to high-[K(+)] (o)-induced swelling and EAA release in astrocytes. Am J Physiol Cell Physiol. 2002;282:1136–1146. doi: 10.1152/ajpcell.00478.2001. [DOI] [PubMed] [Google Scholar]
- 40.Annunziato L, Pignataro G, Di Renzo GF. Pharmacology of brain Na(+)/Ca(2+) exchanger: from molecular biology to therapeutic perspectives. Pharmacol Rev. 2004;56:633–654. doi: 10.1124/pr.56.4.5. [DOI] [PubMed] [Google Scholar]
- 41.Lee SL, Yu AS, Lytton J. Tissue-specific expression of Na(+)-Ca(2+) exchanger isoforms. J Biol Chem. 1994;269:14849–14852. [PubMed] [Google Scholar]
- 42.Nicoll DA, Ottolia M, Philipson KD. Toward a topological model of the NCX1 exchanger. Ann N Y Acad Sci. 2002;976:11–18. doi: 10.1111/j.1749-6632.2002.tb04709.x. [DOI] [PubMed] [Google Scholar]
- 43.Minelli A, Castaldo P, Gobbi P, Salucci S, Magi S, Amoroso S. Cellular and subcellular localization of Na(+)-Ca(2+) exchanger protein isoforms, NCX1, NCX2 and NCX3 in cerebral cortex and hippocampus of adult rat. Cell Calcium. 2007;41:221–234. doi: 10.1016/j.ceca.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 44.Kang TM, Hilgemann DW. Multiple transport modes of the cardiac Na+/Ca2+ exchanger. Nature. 2004;427:544–548. doi: 10.1038/nature02271. [DOI] [PubMed] [Google Scholar]
- 45.Iwamoto T. Na(+)/Ca(2+) exchange as a drug target--insights from molecular pharmacology and genetic engineering. Ann N Y Acad Sci. 2007;1099:516–528. doi: 10.1196/annals.1387.039. [DOI] [PubMed] [Google Scholar]
- 46.Walz W. Role of glial cells in the regulation of the brain ion microenvironment. Prog Neurobiol. 1989;33:309–333. doi: 10.1016/0301-0082(89)90005-1. [DOI] [PubMed] [Google Scholar]
- 47.Longuemare MC, Rose CR, Farrell K, Ransom BR, Waxman SG, Swanson RA. K+-induced reversal of astrocyte glutamate uptake is limited by compensatory changes in intracellular Na+ Neuroscience. 1999;93:285–292. doi: 10.1016/s0306-4522(99)00152-9. [DOI] [PubMed] [Google Scholar]
- 48.Luo J, Chen H, Kintner DB, Shull GE, Sun D. Decreased neuronal death in Na+/H+ exchanger isoform 1-null mice after in vitro and in vivo ischemia. J Neurosci. 2005;25:11256–11268. doi: 10.1523/JNEUROSCI.3271-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rose CR, Waxman SG, Ransom BR. Effects of glucose deprivation, chemical hypoxia and simulated ischemia on Na+ homeostasis in rat spinal cord astrocytes. J Neurosci. 1998;18:3554–3562. doi: 10.1523/JNEUROSCI.18-10-03554.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Silver IA, Deas J, Erecinska M. Ion homeostasis in brain cells: differences in intracellular ion responses to energy limitation between cultured neurons and glial cells. Neuroscience. 1997;78:589–601. doi: 10.1016/s0306-4522(96)00600-8. [DOI] [PubMed] [Google Scholar]
- 51.Flemmer AW, Gimenez I, Dowd BF, Darman RB, Forbush B. Activation of the Na-K-Cl cotransporter NKCC1 detected with a phospho-specific antibody. J Biol Chem. 2002;277:37551–37558. doi: 10.1074/jbc.M206294200. [DOI] [PubMed] [Google Scholar]
- 52.Stys PK. Anoxic and ischemic injury of myelinated axons in CNS white matter: from mechanistic concepts to therapeutics. J Cereb Blood Flow Metab. 1998;18:2–25. doi: 10.1097/00004647-199801000-00002. [DOI] [PubMed] [Google Scholar]
- 53.Li S, Jiang Q, Stys PK. Important role of reverse Na+-Ca2+ exchange in spinal cord white matter injury at physiological temperature. J Neurophysiol. 2000;84:1116–1119. doi: 10.1152/jn.2000.84.2.1116. [DOI] [PubMed] [Google Scholar]
- 54.Goldman WF, Yarowsky PJ, Juhaszova M, Krueger BK, Blaustein MP. Sodium/calcium exchange in rat cortical astrocytes. J Neurosci. 1994;14:5834–5843. doi: 10.1523/JNEUROSCI.14-10-05834.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith JP, Cunningham LA, Partridge LD. Coupling of AMPA receptors with the Na+/Ca2+ exchanger in cultured rat astrocytes. Brain Res. 2000;887:98–109. doi: 10.1016/s0006-8993(00)02973-5. [DOI] [PubMed] [Google Scholar]
- 56.Kintner DB, Look A, Shull GE, Sun D. Stimulation of astrocyte Na+/H+ exchange activity in response to in vitro ischemia in part depends on activation of extracellular signal-regulatory kinase. Am J Physiol Cell Physiol. 2005;289:934–945. doi: 10.1152/ajpcell.00092.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Floyd CL, Gorin FA, Lyeth BG. Mechanical strain injury increases intracellular sodium and reverses Na+/Ca2+ exchange in cortical astrocytes. Glia. 2005;51:35–46. doi: 10.1002/glia.20183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bondarenko A, Svichar N, Chesler M. Role of Na+-H+ and Na+-Ca2+ exchange in hypoxiarelated acute astrocyte death. Glia. 2005;49:143–152. doi: 10.1002/glia.20107. [DOI] [PubMed] [Google Scholar]
- 59.Luo J, Wang Y, Chen H, Kintner DB, Cramer SW, Gerdts JK, et al. A concerted role of Na(+)-K(+)-Cl(-) cotransporter and Na(+)/Ca(2+) exchanger in ischemic damage. J Cereb Blood Flow Metab. 2008;28:737–746. doi: 10.1038/sj.jcbfm.9600561. [DOI] [PubMed] [Google Scholar]