Abstract
Spinal cord injury (SCI) has been regarded clinically as an irreversible damage caused by tissue contusion due to a blunt external force. Past research had focused on the analysis of the pathogenesis of secondary injury that extends from the injury epicenter to the periphery, as well as tissue damage and neural cell death associated with secondary injury. Recent studies, however, have proven that neural stem (progenitor) cells are also present in the brain and spinal cord of adult mammals including humans. Analyses using spinal cord injury models have also demonstrated active dynamics of cells expressing several stem cell markers, and methods aiming at functional reconstruction by promoting the potential self-regeneration capacity of the spinal cord are being explored. Furthermore, reconstruction of the neural circuit requires not only replenishment or regeneration of neural cells but also regeneration of axons. Analysis of the tissue microenvironment after spinal cord injury and research aiming to remove axonal regeneration inhibitors have also made progress. SCI is one of the simplest central nervous injuries, but its pathogenesis is associated with diverse factors, and further studies are required to elucidate these complex interactions in order to achieve spinal cord regeneration and functional reconstruction.
Key words: glia, regeneration, spinal cord, injury, axon
Animal Models of Incomplete Spinal Cord Injury
Producing spinal cord injury models.
Spinal cord injury (SCI) resulting from an external mechanical force varies depending on the area of the exerted force, degree of acceleration, and the direction of the force. To reproduce complete injury, a spinal cord transaction model is the simplest model with the highest reproducibility. Study using a spinal cord transaction in young rats has reported axonal regeneration and motor functional recovery after injury.1 Spinal cord traumas in humans almost always cause mechanical contusive injuries, and reproduction of the same pathological condition is demanded in animal models. Various SCI models in rat have been reported. A representative model is the weight drop method reported by Allen et al.2,3 Modifications of this method have been attempted to improve the reproducibility of the model, and the New York University (NYU) weight-drop device is widely used.4,5 By quantifying the impact exerted on the spinal cord and the resulting injury, experiments for different pathological states from complete injury to incomplete injury can be conducted.
Other models include the gradual compression method by inflating a balloon inserted into the peridural space,6 and the epidural clip method reported by Rivlin and Tator.7 By adjusting the compression force of the clip and the compression time, it is possible to produce models of different severity relatively easily.
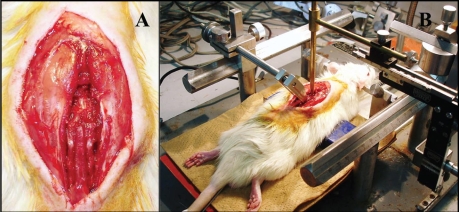
We have used a model manifesting mainly lower limb palsy produced by the following methods (Fig. 1A and B). First laminectomy is performed at the lower thoracic vertebrae (Th8 to 11), then the spinal cord is exposed and a clamp is used to immobilize the spinal cord. Using a compression rod of 2-mm diameter, a force of 20 to 40 g is applied slowly by hand in a direction perpendicular to the spinal cord. By varying the compression time from 10 to 30 min, it is possible to produce models of different severity from mild incomplete paralysis to complete paralysis.8
Figure 1.
(A). The lamina of lower thoracic vertebrae were removed using microrongeurs. After laminectomy, the dura mater was exposed and confirmed to be intact. (B) A 2 mm diameter metal rod weighting 20∼40 g was placed onto the dorsal dura of spinal cord.
Process of functional recovery.
After complete spinal cord transection in cats and rats, the occurrence of bilaterally symmetrical locomotive activities in the hindlimbs (walking-like neural activity) is a well known observation. This phenomenon suggests the existence in the spinal cord of a mechanism that voluntarily controls movement and this neural network is conceptually called the central pattern generator (CPG). Attempts have been made to regain walking function by activating the CPG through walk training. Dimitrijevic et al.9 have shown that walking-like movements can be induced in complete paraplegic patients by conducting epidural electrical stimulation at a given frequency. Their studies suggest that not only in lower vertebrates, the human spinal cord may also contain neural elements that may elicit rhythmic walking movement independent of the brain.
Following traumatic spinal cord injuries including those occurring in humans, initial loss of all neural functions below the injured site is often observed, a phenomenon called “spinal shock.” This results in not only motor paralysis, but also complete loss of other reflexes including vesicorectal reflex, visceral motor reflex and cutaneous reflex. In the case of incomplete spinal cord injury, gradually motor function may recovery partially in due course. To compare the degree of injury and to assess the therapeutic effects of interventions in animal models of spinal cord injury, methods of evaluating motor function are important.
Tarlov score is a well known neurological evaluation method in experimental animals.10 This scale is a relatively simple evaluation method that categorizes complete paralysis as grade 0, and progressive increase in movement until normal as grades 1 to 5 or 6. For small animals such as rats, the inclined plane method is used.11 In this method, the animal is placed on an inclined plane, and the angle of the inclination at which the animal can maintain itself without falling over is noted. This test can be set up easily and is totally non-invasive. Recently, the Basso Beattie Bresnahan (BBB) score is popularly used as a highly objective measure of locomotor recovery in a rat spinal cord injury model. This scoring system has 22 ratings on a scale of 0 to 21, which evaluates movement of the hindlimbs as well as forelimb-hindlimb coordination.12
Other evaluation methods include electrophysiological methods.13,14 Some methods measure spinal cord evoked potential upon stimulation of the spinal cord, while others measure motor evoked potential (MEP) upon transcranial or direct stimulation of the cerebral cortex. MEP can be recorded stably even under anesthesia and is not susceptible to influence of changes in blood pressure. Furthermore, MEP is characterized by good reflection of the severity of spinal cord injury, and is therefore a useful method.
Pathophysiology of spinal cord injury.
Primary injury refers to the mechanical contusion and necrosis of neural elements, while secondary injury refers to the stepwise and continuous tissue destructive changes. Past studies of spinal cord injury had focused on how the secondary injury can be controlled.
In experimental SCI, studies from the past have demonstrated lowered perfusion, ischemia and congestion caused by damage to the intraspinal blood vessels.15,16 Injection of colloidal carbon into injured animals has been used as a method to evaluate the architecture of blood vessels in the spinal cord.17 Obstruction of micro-vessels in the spinal cord, infiltration of inflammatory cells around blood vessels and damage to vascular endothelial cells have been demonstrated.
After injury, Wallerian degeneration occurs in ascending and descending white matter fiber tracts, centered on the site of injury. Wallerian degeneration is known to cause following of myelin sheath and demyelination. Therefore quantification of Wallerian degeneration has been tried to evaluate the degree of SCI.18 After the acute phase has elapsed and the injury enters the subacute and chronic phase, cystic formation occurs at the necrotic part of the injury epicenter, and proliferation of glial scar tissue in the surrounding is observed. Thereafter, even though regeneration and elongation of axon may occur, they are blocked by the glial scar and do not lead to significant neural function improvement.19
Astrocyte in Spinal Cord Injury
Overview of the roles of astrocyte.
The central nervous system (CNS) is composed of neurons that are responsible for various glial cells surrounding neurons. Astrocytes are the largest and most abundant glial cells. They occupy a greater proportion of the human brain compared with the brains of other animals, and the proportion has been shown to increase in higher animals. The internal structure of astrocyte is composed of dense glial filaments (cytoskeletal protein). The glial filaments confer mechanical strength to the cells and the astrocytes together make up the supporting structure of the neurons in the brain and spinal cord. The astrocytes are present in close proximity to neurons, and they function to maintain the blood-brain barrier (BBB) and replenish the neural network. When neurons are lost due to injury to the central nerves, the astrocytes are known to proliferate resulting in the onset of a process called “gliosis.”
Several recent studies have examined the kinetics of astrocytes after spinal cord injury, using immunohistochemical methods.20,21 From the early phase after injury, immunoreactivity of glial fibrillary acidic protein (GFAP), a marker of mature astrocyte, is increased at sites several mm away from the compressive injury center, which appears morphologically normal. Intensified immunoreactivity is observed not only for GFAP, but also other molecules including vimentin. Studies that observed axon regeneration and the local environment after a sharp spinal cord resection reported interesting findings that while astrocytes are absent in the white matter of the injury site in animals showing no regeneration GFAP- and vimentin-positive immature reactive astrocytes are present at the injury site and astrocytes also exist in the white matter in animals showing axon regeneration. These reports suggest that the absence of astrocytes in the white matter is a cause of unsuccessful regeneration, and that the presence of immature astrocytes in the white matter of the injury site in the early phase is necessary for regeneration.1,22
Relationship between nestin-positive cells and astrocytes.
Historically, it was thought that during the embryonic neurogenesis stage, the stem cells that finally differentiate into neurons and the stem cells that differentiate into glial cells (astrocytes, oligodendrocytes and microglia) exist independently. However, from the 1960's, advances in neurobiological research have elucidated that neurons and glial cells are not differentiated from different stem cells, but are derived from common stem cells called multipotent neural stem cells.23,24
Nestin is the marker protein for these stem cells. Nestin is a cytoskeletal protein classified as type IV intermediate filament, and nestin expression has been demonstrated in neuroepithelial precursor cells of the embryonal neural canal.25,26 Nestin as well as vimentin are known to be expressed in most of the progenitor cells of the central and peripheral nervous systems. As the neural cells mature, nestin expression has been reported to be downregulated.27,28 The intermediate filament nestin has been shown to be expressed during development of the human CNS and glial fibers during the stage of neuronal migration.29
Recent studies have proven the existence of neural stem cells in the CNS of adult mammals including humans.30 Ependymal cells and subependymal cells located in the cerebral ventricles are proposed to be candidates of these cells31,32 and GFAP-positive cells in the subventricular zone are also reported to be neural stem cells.33 In the above studies, nestin is used as the marker of neural stem cells. In mouse or rat models of CNS injury, nestin has been observed in the reactive astrocytes of the glial scar.34
We have also studied nestin expression in a spinal cord injury model.35 Similar to the report of Frisen, we also observed many hypertrophic reactive astrocytes surrounding the hemorrhagic and necrotic lesion of the primary injury from an early stage 24–48 hours after injury. These reactive astrocytes are nestin-positive. As time elapsed, nestin-positive cells can be seen throughout the white matter in spinal cord tissue at a distance from the injury epicenter at four weeks after injury. These nestin-positive cells show projection-like and dendritic morphologies and extend from the pia mater at the periphery of spinal cord toward the center of the spinal cord; most of the cells are GFAP-positive (Fig. 2A).35
Figure 2.
(A) Nestin immunoreaction extending from the pia mater becomes very long and expands to the whole white matter. (B) Nestin expression extending in processes from ependymal cells of central canal is observed. (C) In the white matter of the ventral spinal cord, 3CB2 (radial glia) expression extending in arborizing processes is confirmed.
Relationship between nestin-positive cell and ependymal cell.
From the developmental point of view the spinal cord, like the brain, is originated from the ectodermal neural crest and is developed from proliferation of neural epithelial cell of the neural tube formed after closure of the neural crest. In the spinal cord, the neural tube remains as the central canal even after maturation, and the central canal is lined by ependymal cells. In the embryonic stage, the ependymal cells divide and proliferate, and during this process they synthesize various growth factors and express thyroid hormone receptors necessary for the normal functions of the CNS.36 Although ependymal cells are believed to be incapable of regeneration in humans, ependymal cells in the spinal cord central canal have been shown to proliferate to regenerate neural cells in fish and lower animals when the tail was amputated.37
In animal models of spinal cord injury also, active proliferative changes of the ependymal cells in the central canal are evident. These changes are not only limited to regions close to the injury site, but proliferative changes and nestin expression are clearly observed in widespread areas at a distance from the injury epicenter.38 Furthermore, these nestin-positive ependymal cells are capable of proliferation, differentiation and migration, and the possibility that they are neural stem cells or progenitor cells has been suggested.39 Kojima et al.40 have reported that intrathecal injection of epidermal growth factor and fibroblast growth factor 2 results in proliferative changes of ependymal cells accompanied by improvement of motor function.
In our SCI model, we have also observed marked nestin expression in ependymal cells of the central canal, consistent with other reports (Fig. 2B). These reactions peak at a relatively early stage 24 hours to 1 week after injury. These cells differ from the nestin-positive cells in the white matter in that they are GFAP-negative. From these findings, we propose two origins for the nestin-positive cells that appear after SCI: (1) ependymal cells at the outermost (subpial) region of the peripheral white matter and (2) ependymal cells of the neural canal. These cells probably function and appear independent of each other.35
Relationship between radial glia, astrocyte and neuron.
In the early stage of neurogenesis, the neuroepithelial cells of the neural tube from projections that extend to the outer surface. These projections are recognized as radial fibers, and are considered to serve as a guide during migration and differentiation of the precursor cells including neuroblasts. In particular, since these radial fibers are GFAP-positive, Levitt and Lakic et al.41 named the cells of radial fibers the “radial glial cells”, to distinguish them from the neuroepithelium. Since then, many studies have been conducted on the mechanism by which glial fibers guide neuronal migration, and the role of glial fiber as a guide for cell migration during the neurogenesis process has been highlighted. Moreover, it was proposed that after neuronal migration is completed, radial glia disappear or differentiate into astrocyte.42
However, recent research has provided evidence that radial glia are not simply associated with neural cell migration, but radial glia possess properties of neural stem cell.43 In an experiment that injected a recombinant adenovirus expressing green fluorescent protein (GFP) into the cerebral ventricle in embryonic stage and observed the composition of GFP-positive cells with time, the number of radial glia decreased gradually, and were replaced by neurons. Among the cells in the ventricular zone initially labeled with GFP, 75% were bipolar, 20% were radial glia, and the remaining were spherical cells. These morphologies are identical to the morphological changes of the same neural stem cells during the cell division cycle.44 Miyata et al.45 also observed that the spherical cells produced during division of radial glia soon give rise to projections and become bipolar cells. Nestin, RC2 and vimentin are the representative specific molecular markers for radial glia, but these molecules are used also as markers for all multipotent neural stem cell. In retrospection, despite different morphologies, the radial glia and neuroepithelium are the same cells, and therefore it comes as no surprise that they share the same markers. Radial glia should be regarded as neural stem cells that retain the properties of the neuroepithelium throughout the neurogenesis process of the CNS.
Radial glia in tissues of spinal cord injury.
We have mentioned that in the spinal cord injury model, widespread distribution of nestin-positive cells was observed in the white matter of the structurally normal spinal cord at a distance from the injury site. From the cell morphology, these cells were suspected to be of a cell type different from the reactive astrocytes associated with the glial scar. To further examine this issue, analyses were conducted on tissue samples obtained from the same model, using specific markers for radial glia. Prada et al.46 reported the expression of 3CB2, a specific marker different from the conventional markers such as nestin and vimentin. Expression of 3CB2 antigen has been demonstrated in chick embryo, rat and chameleon.
The development of radial glia after SCI expands with time from the pia mater at the periphery of the spinal cord, and assume typical radial and dendritic morphologies (Fig. 2C). Around the hemorrhagic and necrotic lesion, usually many nestin- and GFAP-positive reactive astrocytes are recruited, but 3CB2-positive radial glia is completely absent. Peaking at 4 weeks post-injury, radial glia are observed throughout the white matter. Then at 8–12 weeks postinjury, radial glia appear in abundance in the gray matter. These responses tend to be stronger at sites further away from the injury epicenter, where the spinal cord appears to have a normal structure. In addition, the radial glia in the gray matter are found in a restricted zone around the ependymal cells of the central canal (subventricular zone) as well as in blood vessels of the gray matter.47 In a study of neurogenesis of the hippocampus in mature rodents, Palmar et al.48 observed active proliferative changes of vascular endothelial cells accompanying proliferative changes of neural stem cells, and advocated the concept of “vascular niches.” These findings suggest the existence of mechanisms of neural repair or regeneration originating from vascular endothelial cells and radial glia even following SCI.
Microglia in Spinal Cord Injury
Origin of microglia and relationship with macrophage.
Embryologically, microglia are considered to be derived from the glioblast, which is the common progenitor for glial cells. Between the perinatal and early neonatal period in mice and rats and during the late fetal life in humans, microglia are generated following the generation of astrocytes and oligodendrocytes and they distribute in the CNS as the third glial cell.49
Unrelated to the differentiation and development of microglia, monocytes derived from the circulating blood have been shown to infiltrate the brain as inflammatory cells and penetrate the parenchyma of the spinal cord.50 The production of monoclonal antibodies has advanced rapidly from the 1980's. Monoclonal antibodies raised against blood cells, especially macrophages, specifically recognize some cells in the CNS. They cells are consistent with microglia. Microglia isolated and maintained in culture clearly exhibit chemotactic and phagocytic capabilities, and they possess markers common to macrophages. Therefore some investigators equate intracerebral microglia to macrophages.51
It is common knowledge that when pathological changes such as infection, tumor, inflammation, trauma and ischemia occur in the CNS, microglia acting as monitoring cells instantaneously become activated. The morphology of resting (ramified form) microglia changes drastically to so-called activated (ameboid form) microglia. The activated microglia proliferate and migrate toward the lesion, exhibiting bactericidal, cytotoxic and phagocytotic activities to eliminate the lesion, and playing an important role in the body defense mechanism.52 During this process, microglia secrete various biologically active substances and may display both neuroprotective and neurotoxic properties.
Modes of microglia activation in spinal cord injury and their roles.
Based on the origin and developmental process described above, an obvious issue that arises in the study of activated microglia in spinal cord injury or traumatic brain injury model would be the mixed presence of macrophage. In the case of contusive injury of the spinal cord, the BBB is always broken down, and macrophages from the circulating blood infiltrate the spinal cord parenchyma. However, the endogenous activated microglia and blood-derived macrophages share similar properties, and there are no antibodies that reliably differentiate between the two. For this reason, observations would invariably be on a mixture of the two.
Usually, infiltration of microglia or macrophages into the injury site or its surrounding is observed at the early stage after SCI (day 1 to 2). Within several days, a large number of cells accumulate and become activated, phagocytosing the debris from destroyed tissues. The mechanism of this cell infiltration include the expression of cellular adhesion factors such as intercellular adhesion molecule (ICAM)-1 and p-selectin in vascular endothelial cells after injury, causing penetration of macrophages into tissue.53 In addition, the proliferated and activated microglia and macrophages secret free radical such as O2− and NO, as well as the strongly cytotoxic cytokine TNFα, exhibiting neurotoxic effects.54–56
We investigated microglia not from the neurotoxic viewpoint, but analyzed the hypothesis that microglia are related to neuronal repair or regeneration. Inside the injured spinal cord tissue, the ED-1- and/or Iba-1-positive microglia and macrophages increase with time and peak at week 4 after injury. While cells positive for both ED1 and Iba-1 constitute the majority of the microglia and macrophages, cells positive for ED1 alone or Iba-1 alone are also found in some areas. The differences in immunoreactivity is suspected to be related to the antigen specificity of the anti-ED-1 and anti-Iba-1 antibodies, indicating a high possibility that the ED1-positive cells are macrophages derived from the blood, while the Ib1-1-positive cells are microglia.57 Further, when double immunostaining is conducted using a marker (3CB2) for radial glia that possesses properties of neural stem cells, activated (ameboid form) microglia are positive for ED1 alone, while resting (ramified form) microglia show a merge of ED1 and 3CB2 immunostaining. These findings provide evidence that at a certain time after SCI, microglia and radial glia can be demonstrated to share the same cell lineage.58 Studies conducted so far have shown that microglia activation after SCI does not follow a fixed pattern with respect to the sites of detection and the time course.59 Microglia activation associated with breakdown of the BBB at the injury site may have to be distinguished and analyzed separately from microglia activation associated with Wallerian degeneration further from the injury site. A study comparing mature SCI and fetal SCI (associated with an environment permissive to regeneration after injury) revealed differences in the pattern of microglia activation.60 Under in vitro conditions, microglia exhibit properties of neural stem cells,61 suggesting the possibility that microglia are not simply immunocompetent cells or inflammatory cells.
Oligodendrocyte in Spinal Cord Injury
Generation of oligodendrocyte and marker expression.
Oligodendrocytes are cells that form the myelin sheath in the CNS. The oligodendrocyte sends out a thin sheet of myelin and the sheet wraps around the axon several times, partially insulating the axon. By partial insulation with this myelin sheath, the electrical activity transmitted within the axon is drastically accelerated.
Oligodendrocytes are generated from glioblasts, following the differentiation to astrocytes. Studies on the differentiation of oligodendrocytes using specific markers include that of Raff et al.62 who used cultured cells. The precursor cells of oligodendrocyte are A2B5-positive but GalCer- and GFAP-negative. These precursor cells have been shown to differentiate to oligodendrocytes and also to astrocytes depending on the culture condition. These precursor cells are called O-2A progenitor cells. When committed to differentiation to oligodendrocytes, the cells first differentiate to oligodendroblasts that react with O4 monoclonal antibody, and then begin to show reactivity with bFGF. Further differentiation to immature oligodendrocyte is accompanied by the expression of galactocerebroside on the cell surface and loss of dividing capability. Finally mature oligodendrocytes are formed, which are positive for myelin basic protein (MBP).
The optic nerve oligodendrocyte progenitor cells are thought to migrate from the cerebral parenchyma. In the spinal cord, however, oligodendrocytes are generated and differentiated in the anterior half of the neural tube, and migrate to the whole of the spinal cord.63 Recently, PDGFα receptor and NG2 proteoglycan are used as markers for oligodendrocyte progenitor cells. In mouse and rat spinal cord, the oligodendrocyte progenitors appear in the ventricular zone of the spinal cord at a relatively early stage. The PDGFα receptor-positive cells first appear in the ventral ventricular zone, and migrate laterally expanding toward the direction of the dorsal side, differentiating into cyclic nucleotide phoshodiesterase (CNP)-positive and O4-positive oligodendrocytes.64,65 The generation of these ventral-specific oligodendrocyte progenitors has been reported to be induced by the sonic hedgehog (Shh) morphogen, as in floor plate cells and motor neurons.66
Oligodendrocytes related to NG2 expression.
NG2 is one of the major molecules of chondroitin sulfate proteoglycans (CSPG), and a marker of oligodendrocyte progenitor cells (OPC). In mature central nervous tissue, NG2-positive glia is classified as adult OPC and they transform to mature oligodendrocyte.67 These NG2-positive glia are observed in both the white and gray matters, and they express PDGFα receptor as described above.68–70
In the embryonic spinal cord, cells expressing AN2, which is a homologue of rat NG2 protein, are oligodendrocyte progenitors and have been observed to migrate using radial glia as the scaffold.71 In demyelinated lesions of the spinal cord, before these lesions are remyelinated, rapid proliferation of endogenous NG2-positive cell occurs.72 In a contusive SCI model, proliferation of endogenous NG2 progenitors was observed for several weeks after injury, and presumed to contribute to an increase in number of oligodendrocytes.73 However, recent studies have reported that although the NG2-positive cells in adult CNS include adult OPC, the morphological characteristic and cell cycle are different from those observed in embryonic or postnatal stage.74 Some investigators even advocate that NG2-positive glia should be regarded as the fifth element distinct from neuron, astrocyte, microglia and oligodendrocytes; and propose the name polydendrocyte or synantocyte for NG2-positive glia.74–76 These cells with NG2-positive phenotype communicate with neurons or axon at synapses, and are injury responsive.
Changes of oligodendrocyte in spinal cord injury, and regeneration and repair.
Recent studies have indicated that secondary SCI is associated with apoptotic death of neural cells, and cell death extending widely to regions far from the injury epicenter has been observed.77 In addition, apoptotic cell death after SCI is now known to occur specifically in oligodendrocytes and as a result damaging a large number of axons.78,79
The cuprizon-induced experimental demyelination model has been used to study regeneration of myelin sheath in so called dymyelination lesion. Cuprizon is administered to mature CNS, and regeneration of oligodendroglia is observed even in completely demyelinated lesions. These experiments confirm the possibility of the existence of progenitor cells in the brain, which possess the capability to proliferate and differentiate into oligodendroglia in response to injury, even though these cells do not belong to oligodendroglia at other times.80,81 In SCI also, spontaneous remyelination of injured axons82 and upregulation of oligodendrocyte markers such as MBP mRNA and CNP mRNA have been reported.83,84 In addition, a definitive increase in number of A2B5-, O4- and O1-positive oligodendrocyte progenitor cells is observed in the tissue from the early stage of SCI.85 Horner et al.86 have reported that the majority of the cells that proliferate slowly but repeatedly in the parenchyma of normal spinal cord are NG2-positive glial precursor cells, and some of them differentiate into astrocytes or oligodendrocytes.
From the above evidence, one can state with reasonable confidence that neural precursor cells are present in mature spinal cord. However, very few cells continue to proliferate in the normal spinal cord, and most of the progenitor cells are at the resting phase and they probably grow at an extremely slow pace.86,87 In contrast, when injury-causing stimuli such as resection, crushing and compression are exerted, many proliferative cells appear in the spinal cord. Among them, many are inflammatory cells such as astrocytes and microglia that contribute to the formation of glial scar. However, nestin-positive proliferative cells not expressing glial markers such as GFAP and NG2 appear and spread extremely widely, some of which have been shown to be endogenous neural progenitor cells.88 Inducing the activation and differentiation of these endogenous neural progenitor cells will be an innovative therapeutic approach to realize regeneration and restoration of injured spinal cord.
Oligodendrocyte and axon regeneration after injury.
In recent years, the existence of neural stem cells also in mature CNS has been demonstrated in higher primates including humans.89 Despite the conventional notion that “since the central nervous tissue does not regenerate, repair will be very difficult once damage has set in,” attempts in the field of neuro-regenerative medicine have greatly heightened the expectation of nervous tissue regeneration and repair. While regeneration of the central nerves (spinal cord) is difficult in mammals, spinal cord regeneration is easy in lower vertebrates. These observations have led to the hypothesis that the difference in regeneration capability is due to a difference in the micro-environment; in other words, the central nerve of lower vertebrate is permissive, while the CNS of mammals is non-permissive to axonal regeneration.90,91
Several reasons have been proposed to explain the difficulty of central nerve regeneration in mature mammals, including the presence of axon regeneration inhibiting factors in oligodendrocytes, myelin and extracellular matrix. Among them Nogo-A is a well known myelin-derived factor that inhibits regeneration. By neutralization with anti-Nogo antibodies, the axons of a completely resected corticospinal tract have been shown to regenerate by 7 to 11 mm.92 Apart from Nogo, myelin associated glycoprotein (MAG)93 and oligodendrocytes myelin glycoprotein (OMgp)94 have been reported to block axonal elongation, and it becomes clear that multiple proteins act to block regeneration. Thereafter, all the three structurally different axon elongation inhibitory proteins Nogo, MAG and OMgp have been shown to be ligands of the Nogo receptor. From these results, it is now clear that the signals that inhibit regeneration are consolidated as one at receptor level. All these inhibitory proteins bind with the Nogo receptor (NgR) present in the growth cone, and they inhibit axonal elongation via a receptor with low affinity for neural growth factor (p75 NTR). For this reason, p75 NTR is considered to be a major factor that inhibits regeneration.95,96
Bergman et al.97 used fetal spinal cord transplant combined with antibody against myelin-related protein to treat SCI, and reported good regeneration of injured axons as well as motor function recovery. Cheng et al.98 resected the spinal cord mylomeres in mature rats, and tried the repair strategy of bridging the spinal cord gaps with multiple intercostal nerve grafts. It has been known for a long time that peripheral nerves show good nerve regeneration. The regenerative capability of Schwann cells had been highlighted. Axonal regeneration of Schwann cell depends to a great extent on the secretion of neurotrophic factors and expression of adhesion factors, which are related to its capability of remyelination and axon induction. Therefore, Schwann cell transplantation has been attempted in an experimental SCI model.99,100 Furthermore, since pure culture of large number of Schwann cells can be obtained from peripheral nerves, in the case of planning auto-transplantation, this strategy may be practical because of little risk of rejection. Apart from Schwann cells, the olfactory ensheathing glia are also attracting attention. Their excellent axon induction capability makes them a good candidate of cell transplantation therapy for SCI.101,102 The characteristics of olfactory ensheathing glia include a high migratory capability in spinal cord tissues and they show effective regeneration of the corticospinal tract which is the most important neural circuit for motor function.103,104
Inside the spinal cord tissue, glial scar is formed around the injury site. This glial scar not only constitutes a mechanical block for axonal regeneration, but also expresses factors inhibiting axonal elongation. These inhibitors are called “chondroitin sulfate proteoglycans (CSPG)” and they exist as the major component molecules of the extracellular matrix in the CNS. Various CSPG have been identified, including aggrecan, neurocan, phosphacan and NG2.105,106 Generally, the CSPG function to inhibit migration of various neural cells as well as elongation and induction of axons. In the glial scar, expression or upregulation of a diversity of molecules that are known to inhibit axon regeneration and elongation have been reported and they include upregulation of several CSPG, expression of NG2 in immature oligodendrocytes and macrophages surrounding the injury site,107 expression of semaphorin 3A in fibroblasts108 and increased expression of ephrin-B2 and EphB2 in reactive astrocytes and fibroblasts.109 Injection of condroitinase ABC that degrades CSPG into the site of SCI significantly promotes axonal regeneration, and is associated with improvement of motor function.110 These research data highlight the importance of overcoming the adverse local microenvironment after SCI, and the significance of creating a permissive microenvironment for neural regeneration.
Conclusion
The basic research on SCI has taken a major step forward, from the era when the majority of research were focused on inhibiting tissue damage and cell death in secondary injury to the recent trials in activation of endogenous neural stem cells (precursor cells) or transplantation of exogenous cells. The preservation of self-regeneration capacity is a fact beyond doubt, although this is limited to the CNS. The goal of reconstructing neural circuit after SCI is the reconstruction of point-to-point projection, in which individual neural cells project restrictively precisely according to a somatotopic map. In addition, clinical SCI differs from sharp transection models in having a volume lesion, usually with a large gap at the injury site. The future challenge of treating SCI is a multifactorial strategy encompassing different approaches to overcome various factors, including suppression of secondary injury with highly effective drugs, induction of migration and differentiation of neural precursor cells (endogenous or transplanted), improvement of the adverse micro-environment for axon regeneration, and promotion of axon regeneration. This strategy is very important for the realization of spinal cord regeneration and functional reconstruction.
Footnotes
Previously published online as a Cell Adhesion & Migration E-publication: http://www.landesbioscience.com/journals/celladhesion/article/7372
References
- 1.Inoue T, Kawaguchi S, Kurisu K. Spontaneous regeneration of the pyramidal tract after transection in young rats. Neurosci Lett. 1998;247:151–154. doi: 10.1016/s0304-3940(98)00297-3. [DOI] [PubMed] [Google Scholar]
- 2.Allen AR. Surgery of experimental lesion of spinal cord equivalent to crush injury of fracture dislocation of spinal column. JAMA. 1911;57:878–880. [Google Scholar]
- 3.de la Torre JC, Boggan JE. Neurophysiological recording in rat spinal cord trauma. Exp Neurol. 1980;70:356–370. doi: 10.1016/0014-4886(80)90033-3. [DOI] [PubMed] [Google Scholar]
- 4.Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol. 1996;139:244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- 5.Cheng H, Wu JP, Tzeng SF. Neuroprotection of glial cell line-derived neurotrophic factor in damaged spinal cords following contusive injury. J Neurosci Res. 2002;69:397–405. doi: 10.1002/jnr.10303. [DOI] [PubMed] [Google Scholar]
- 6.Tator CH. Acute spinal cord injury in primates produced by an inflatable extradural cuff. Can J Surg. 1973;16:222–231. [PubMed] [Google Scholar]
- 7.Rivlin AS, Tator CH. Effect of duration of acute spinal cord compression in a new acute cord injury model in the rat. Surg Neurol. 1978;10:38–43. [PubMed] [Google Scholar]
- 8.Shibuya S, Miyamoto O, Janjua NA, Itano T, Mori S, Norimatsu H. Post-traumatic moderate systemic hypothermia reduces TUNEL positive cells following spinal cord injury in rat. Spinal Cord. 2004;42:29–34. doi: 10.1038/sj.sc.3101516. [DOI] [PubMed] [Google Scholar]
- 9.Dimitrijevic MR, Gerasimenko Y, Pinter MM. Evidence for a spinal central pattern generator in humans. Ann N Y Acad Sci. 1998;860:360–376. doi: 10.1111/j.1749-6632.1998.tb09062.x. [DOI] [PubMed] [Google Scholar]
- 10.Tarlov IM, Klinger H. Spinal cord compression studies II. Time limits for recovery after acute compression in dogs. AMA Arch Neurol Psychiatry. 1954;71:271–290. [PubMed] [Google Scholar]
- 11.Rivlin AS, Tator CH. Objective clinical assessment of motor function after experimental spinal cord injury in the rat. J Neurosurg. 1977;47:577–581. doi: 10.3171/jns.1977.47.4.0577. [DOI] [PubMed] [Google Scholar]
- 12.Basso DM, Beattie MS, Bresnahan JC, Anderson DK, Faden AI, Gruner JA, et al. MASCIS evaluation of open field locomotor scores: effects of experience and teamwork on reliability. Multicenter Animal Spinal Cord Injury Study. J Neurotrauma. 1996;13:343–359. doi: 10.1089/neu.1996.13.343. [DOI] [PubMed] [Google Scholar]
- 13.Fehlings MG, Tator CH, Linden RD, Piper IR. Motor evoked potentials recorded from normal and spinal cord-injured rats. Neurosurgery. 1987;20:125–130. doi: 10.1097/00006123-198701000-00027. [DOI] [PubMed] [Google Scholar]
- 14.Patton HD, Amassian VE. Single and multiple-unit analysis of cortical stage of pyramidal tract activation. J Neurophysiol. 1954;17:345–363. doi: 10.1152/jn.1954.17.4.345. [DOI] [PubMed] [Google Scholar]
- 15.Balentine JD. Pathology of experimental spinal cord trauma I. The necrotic lesion as a function of vascular injury. Lab Invest. 1978;39:236–253. [PubMed] [Google Scholar]
- 16.Guha A, Tator CH, Rochon J. Spinal cord blood flow and systemic blood pressure after experimental spinal cord injury in rats. Stroke. 1989;20:372–377. doi: 10.1161/01.str.20.3.372. [DOI] [PubMed] [Google Scholar]
- 17.Wallace MC, Tator CH, Frazee P. Relationship between posttraumatic ischemia and hemorrhage in the injured rat spinal cord as shown by colloidal carbon angiography. Neurosurgery. 1986;18:433–439. doi: 10.1227/00006123-198604000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Iizuka H, Yamamoto T, Iwasaki Y, Konno H, Kadoya S. Experimental spinal cord injury: quantitation of axonal damage by automated image analysis. J Neurosurg. 1986;64:304–308. doi: 10.3171/jns.1986.64.2.0304. [DOI] [PubMed] [Google Scholar]
- 19.Bovolenta P, Wandosell F, Nieto-Sampedro M. CNS glial scar tissue: a source of molecules which inhibit central neurite outgrowth. Prog Brain Res. 1992;94:367–379. doi: 10.1016/s0079-6123(08)61765-3. Review. [DOI] [PubMed] [Google Scholar]
- 20.Farooque M, Badonic T, Olsson Y, Holtz A. Astrocytic reaction after graded spinal cord compression in rats: immunohistochemical studies on glial fibrillary acidic protein and vimentin. J Neurotrauma. 1995;12:41–52. doi: 10.1089/neu.1995.12.41. [DOI] [PubMed] [Google Scholar]
- 21.Baldwin SA, Broderick R, Blades DA, Scheff SW. Alterations in temporal/spatial distribution of GFAP- and vimentin-positive astrocytes after spinal cord contusion with the New York University spinal cord injury device. J Neurotrauma. 1998;15:1015–1026. doi: 10.1089/neu.1998.15.1015. [DOI] [PubMed] [Google Scholar]
- 22.Kikukawa S, Kawaguchi S, Mizoguchi A, Ide C, Koshinaga M. Regeneration of dorsal column axons after spinal cord injury in young rats. Neurosci Lett. 1998;249:135–138. doi: 10.1016/s0304-3940(98)00406-6. [DOI] [PubMed] [Google Scholar]
- 23.Fujita S. The matrix cell and cytogenesis in the developing central nervous system. J Comp Neurol. 1963;120:37–42. doi: 10.1002/cne.901200104. [DOI] [PubMed] [Google Scholar]
- 24.Fujita S. Analysis of neuron differentiation in the central nervous system by tritiated thymidine autoradiography. J Comp Neurol. 1964;122:311–327. doi: 10.1002/cne.901220303. [DOI] [PubMed] [Google Scholar]
- 25.Hockfield S, McKay RD. Identification of major cell classes in the developing mammalian nervous system. J Neurosci. 1985;5:3310–3328. doi: 10.1523/JNEUROSCI.05-12-03310.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- 27.Dahlstrand J, Zimmerman LB, McKay RD, Lendahl U. Characterization of the human nestin gene reveals a close evolutionary relationship to neurofilaments. J Cell Sci. 1992;103:589–597. doi: 10.1242/jcs.103.2.589. [DOI] [PubMed] [Google Scholar]
- 28.Dahlstrand J, Lardelli M, Lendahl U. Nestin mRNA expression correlates with the central nervous system progenitor cell state in many, but not all, regions of developing central nervous system. Brain Res Dev Brain Res. 1995;84:109–129. doi: 10.1016/0165-3806(94)00162-s. [DOI] [PubMed] [Google Scholar]
- 29.Tohyama T, Lee VM, Rorke LB, Marvin M, McKay RD, Trojanowski JQ. Nestin expression in embryonic human neuroepithelium and in human neuroepithelial tumor cells. Lab Invest. 1992;66:303–313. [PubMed] [Google Scholar]
- 30.Gage FH, Coates PW, Palmer TD, Kuhn HG, Fisher LJ, Suhonen JO, et al. Survival and differentiation of adult neuronal progenitor cells transplanted to the adult brain. Proc Natl Acad Sci USA. 1995;92:11879–11883. doi: 10.1073/pnas.92.25.11879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johansson CB, Momma S, Clarke DL, Risling M, Lendahl U, Frisén J. Identification of a neural stem cell in the adult mammalian central nervous system. Cell. 1999;96:25–34. doi: 10.1016/s0092-8674(00)80956-3. [DOI] [PubMed] [Google Scholar]
- 32.Chiasson BJ, Tropepe V, Morshead CM, van der Kooy D. Adult mammalian forebrain ependymal and subependymal cells demonstrate proliferative potential, but only subependymal cells have neural stem cell characteristics. J Neurosci. 1999;19:4462–4471. doi: 10.1523/JNEUROSCI.19-11-04462.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doetsch F, Caillé I, Lim DA, García-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 34.Frisén J, Johansson CB, Török C, Risling M, Lendahl U. Rapid, widespread, and longlasting induction of nestin contributes to the generation of glial scar tissue after CNS injury. J Cell Biol. 1995;131:453–464. doi: 10.1083/jcb.131.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shibuya S, Miyamoto O, Auer RN, Itano T, Mori S, Norimatsu H. Embryonic intermediate filament, nestin, expression following traumatic spinal cord injury in adult rats. Neuroscience. 2002;114:905–916. doi: 10.1016/s0306-4522(02)00323-8. [DOI] [PubMed] [Google Scholar]
- 36.Graff MN, Baas D, Puymirat J, Sarlieve LL, Delaunoy JP. The alpha and beta thyroid receptors are expressed by cultured ependymal cells. Correlation with the effect of L-3,5,3′-triiodothyronine on glutamine synthetase mRNAs. Neurosci Lett. 1993;150:174–178. doi: 10.1016/0304-3940(93)90529-t. [DOI] [PubMed] [Google Scholar]
- 37.Anderson MJ, Waxman SG. Caudal spinal cord of the teleost Sternarchus albifrons resembles regenerating cord. Anat Rec. 1983;205:85–92. doi: 10.1002/ar.1092050111. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi M, Arai Y, Kurosawa H, Sueyoshi N, Shirai S. Ependymal cell reactions in spinal cord segments after compression injury in adult rat. J Neuropathol Exp Neurol. 2003;62:185–194. doi: 10.1093/jnen/62.2.185. [DOI] [PubMed] [Google Scholar]
- 39.Mothe AJ, Tator CH. Proliferation, migration and differentiation of endogenous ependymal region stem/progenitor cells following minimal spinal cord injury in the adult rat. Neuroscience. 2005;131:177–187. doi: 10.1016/j.neuroscience.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 40.Kojima A, Tator CH. Intrathecal administration of epidermal growth factor and fibroblast growth factor 2 promotes ependymal proliferation and functional recovery after spinal cord injury in adult rats. J Neurotrauma. 2002;19:223–238. doi: 10.1089/08977150252806974. [DOI] [PubMed] [Google Scholar]
- 41.Levitt P, Rakic P. Immunoperoxidase localization of glial fibrillary acidic protein in radial glial cells and astrocytes of the developing rhesus monkey brain. J Comp Neurol. 1980;193:815–840. doi: 10.1002/cne.901930316. [DOI] [PubMed] [Google Scholar]
- 42.Pixley SK, de Vellis J. Transition between immature radial glia and mature astrocytes studied with a monoclonal antibody to vimentin. Brain Res. 1984;317:201–209. doi: 10.1016/0165-3806(84)90097-x. [DOI] [PubMed] [Google Scholar]
- 43.Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- 44.Tamamaki N, Nakamura K, Okamoto K, Kaneko T. Radial glia is a progenitor of neocortical neurons in the developing cerebral cortex. Neurosci Res. 2001;41:51–60. doi: 10.1016/s0168-0102(01)00259-0. [DOI] [PubMed] [Google Scholar]
- 45.Miyata T, Kawaguchi A, Okano H, Ogawa M. Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron. 2001;31:727–741. doi: 10.1016/s0896-6273(01)00420-2. [DOI] [PubMed] [Google Scholar]
- 46.Prada FA, Dorado ME, Quesada A, Prada C, Schwarz U, de la Rosa EJ. Early expression of a novel radial glia antigen in the chick embryo. Glia. 1995;15:389–400. doi: 10.1002/glia.440150404. [DOI] [PubMed] [Google Scholar]
- 47.Shibuya S, Miyamoto O, Itano T, Mori S, Norimatsu H. Temporal progressive antigen expression in radial glia after contusive spinal cord injury in adult rats. Glia. 2003;42:172–183. doi: 10.1002/glia.10203. [DOI] [PubMed] [Google Scholar]
- 48.Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 49.Kitamura T, Miyake T, Fujita S. Genesis of resting microglia in the gray matter of mouse hippocampus. J Comp Neurol. 1984;226:421–433. doi: 10.1002/cne.902260310. [DOI] [PubMed] [Google Scholar]
- 50.Kitamura T, Hattori H, Fujita S. Autoradiographic studies on histogenesis of brain macrophages in the mouse. J Neuropathol Exp Neurol. 1972;31:502–518. doi: 10.1097/00005072-197207000-00008. [DOI] [PubMed] [Google Scholar]
- 51.Imamoto K, Leblond CP. Radioautographic investigation of gliogenesis in the corpus callosum of young rats II. Origin of microglial cells. J Comp Neurol. 1978;180:139–163. doi: 10.1002/cne.901800109. [DOI] [PubMed] [Google Scholar]
- 52.Nakajima K, Kohsaka S. Microglia: neuroprotective and neurotrophic cells in the central nervous system. Curr Drug Targets Cardiovasc Haematol Disord. 2004;4:65–84. doi: 10.2174/1568006043481284. [DOI] [PubMed] [Google Scholar]
- 53.Isaksson J, Farooque M, Holtz A, Hillered L, Olsson Y. Expression of ICAM-1 and CD11b after experimental spinal cord injury in rats. J Neurotrauma. 1999;16:165–173. doi: 10.1089/neu.1999.16.165. [DOI] [PubMed] [Google Scholar]
- 54.Chao CC, Hu S, Molitor TW, Shaskan EG, Peterson PK. Activated microglia mediate neuronal cell injury via a nitric oxide mechanism. J Immunol. 1992;149:2736–2741. [PubMed] [Google Scholar]
- 55.Boje KM, Arora PK. Microglial-produced nitric oxide and reactive nitrogen oxides mediate neuronal cell death. Brain Res. 1992;587:250–256. doi: 10.1016/0006-8993(92)91004-x. [DOI] [PubMed] [Google Scholar]
- 56.Xu M, Ng YK, Leong SK. Induction of microglial reaction and expression of nitric oxide synthase I in the nucleus dorsalis and red nucleus following lower thoracic spinal cord hemisection. [DOI] [PubMed]
- 57.Wu D, Miyamoto O, Shibuya S, Okada M, Igawa H, Janjua NA, et al. Different expression of macrophages and microglia in rat spinal cord contusion injury model at morphological and regional levels. Acta Med Okayama. 2005;59:121–127. doi: 10.18926/AMO/31950. [DOI] [PubMed] [Google Scholar]
- 58.Wu D, Miyamoto O, Shibuya S, Mori S, Norimatsu H, Janjua NA, Itano T. Co-expression of radial glial marker in macrophages/microglia in rat spinal cord contusion injury model. Brain Res. 2005;1051:183–188. doi: 10.1016/j.brainres.2005.05.054. [DOI] [PubMed] [Google Scholar]
- 59.Watanabe T, Yamamoto T, Abe Y, Saito N, Kumagai T, Kayama H. Differential activation of microglia after experimental spinal cord injury. J Neurotrauma. 1999;16:255–265. doi: 10.1089/neu.1999.16.255. [DOI] [PubMed] [Google Scholar]
- 60.Fujimoto Y, Yamasaki T, Tanaka N, Mochizuki Y, Kajihara H, Ikuta Y, Ochi M. Differential activation of astrocytes and microglia after spinal cord injury in the fetal rat. Eur Spine J. 2006;15:223–233. doi: 10.1007/s00586-005-0933-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yokoyama A, Sakamoto A, Kameda K, Imai Y, Tanaka J. NG2 proteoglycan-expressing microglia as multipotent neural progenitors in normal and pathologic brains. Glia. 2006;53:754–768. doi: 10.1002/glia.20332. [DOI] [PubMed] [Google Scholar]
- 62.Raff MC, Miller RH, Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature. 1983;303:390–396. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- 63.Warf BC, Fok-Seang J, Miller RH. Evidence for the ventral origin of oligodendrocyte precursors in the rat spinal cord. J Neurosci. 1991;11:2477–2488. doi: 10.1523/JNEUROSCI.11-08-02477.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hall A, Giese NA, Richardson WD. Spinal cord oligodendrocytes develop from ventrally derived progenitor cells that express PDGF alpha-receptors. Development. 1996;122:4085–4094. doi: 10.1242/dev.122.12.4085. [DOI] [PubMed] [Google Scholar]
- 65.Noll E, Miller RH. Oligodendrocyte precursors originate at the ventral ventricular zone dorsal to the ventral midline region in the embryonic rat spinal cord. Development. 1993;118:563–573. doi: 10.1242/dev.118.2.563. [DOI] [PubMed] [Google Scholar]
- 66.Orentas DM, Hayes JE, Dyer KL, Miller RH. Sonic hedgehog signaling is required during the appearance of spinal cord oligodendrocyte precursors. Development. 1999;126:2419–2429. doi: 10.1242/dev.126.11.2419. [DOI] [PubMed] [Google Scholar]
- 67.Dawson MR, Levine JM, Reynolds R. NG2-expressing cells in the central nervous system: are they oligodendroglial progenitors? J Neurosci Res. 2000;61:471–479. doi: 10.1002/1097-4547(20000901)61:5<471::AID-JNR1>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 68.Stallcup WB, Beasley L. Bipotential glial precursor cells of the optic nerve express the NG2 proteoglycan. J Neurosci. 1987;7:2737–2744. doi: 10.1523/JNEUROSCI.07-09-02737.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Levine JM, Stincone F, Lee YS. Development and differentiation of glial precursor cells in the rat cerebellum. Glia. 1993;7:307–321. doi: 10.1002/glia.440070406. [DOI] [PubMed] [Google Scholar]
- 70.Nishiyama A, Lin XH, Giese N, Heldin CH, Stallcup WB. Co-localization of NG2 proteoglycan and PDGF alpha-receptor on O2A progenitor cells in the developing rat brain. J Neurosci Res. 1996;43:299–314. doi: 10.1002/(SICI)1097-4547(19960201)43:3<299::AID-JNR5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 71.Diers-Fenger M, Kirchhoff F, Kettenmann H, Levine JM, Trotter J. AN2/NG2 protein-expressing glial progenitor cells in the murine CNS: isolation, differentiation and association with radial glia. Glia. 2001;34:213–228. doi: 10.1002/glia.1055. [DOI] [PubMed] [Google Scholar]
- 72.Keirstead HS, Levine JM, Blakemore WF. Response of the oligodendrocyte progenitor cell population (defined by NG2 labelling) to demyelination of the adult spinal cord. Glia. 1998;22:161–170. [PubMed] [Google Scholar]
- 73.McTigue DM, Wei P, Stokes BT. Proliferation of NG2-positive cells and altered oligodendrocyte numbers in the contused rat spinal cord. J Neurosci. 2001;21:3392–3400. doi: 10.1523/JNEUROSCI.21-10-03392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Berry M, Hubbard P, Butt AM. Cytology and lineage of NG2-positive glia. J Neurocytol. 2002;31:457–467. doi: 10.1023/a:1025735513560. [DOI] [PubMed] [Google Scholar]
- 75.Butt AM, Kiff J, Hubbard P, Berry M. Synantocytes: new functions for novel NG2 expressing glia. J Neurocytol. 2002;31:551–565. doi: 10.1023/a:1025751900356. [DOI] [PubMed] [Google Scholar]
- 76.Nishiyama A, Watanabe M, Yang Z, Bu J. Identity, distribution and development of polydendrocytes: NG2-expressing glial cells. J Neurocytol. 2002;31:437–455. doi: 10.1023/a:1025783412651. [DOI] [PubMed] [Google Scholar]
- 77.Li GL, Farooque M, Holtz A, Olsson Y. Apoptosis of oligodendrocytes occurs for long distances away from the primary injury after compression trauma to rat spinal cord. Acta Neuropathol. 1999;98:473–480. doi: 10.1007/s004010051112. [DOI] [PubMed] [Google Scholar]
- 78.Shuman SL, Bresnahan JC, Beattie MS. Apoptosis of microglia and oligodendrocytes after spinal cord contusion in rats. J Neurosci Res. 1997;50:798–7808. doi: 10.1002/(SICI)1097-4547(19971201)50:5<798::AID-JNR16>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 79.Abe Y, Yamamoto T, Sugiyama Y, Watanabe T, Saito N, Kayama H, et al. Apoptotic cells associated with Wallerian degeneration after experimental spinal cord injury: a possible mechanism of oligodendroglial death. J Neurotrauma. 1999;16:945–952. doi: 10.1089/neu.1999.16.945. [DOI] [PubMed] [Google Scholar]
- 80.Blakemore WF. Remyelination of the superior cerebellar peduncle in the mouse following demyelination induced by feeding cuprizone. J Neurol Sci. 1973;20:73–83. doi: 10.1016/0022-510x(73)90119-6. [DOI] [PubMed] [Google Scholar]
- 81.Ludwin SK. An autoradiographic study of cellular proliferation in remyelination of the central nervous system. Am J Pathol. 1979;95:683–696. [PMC free article] [PubMed] [Google Scholar]
- 82.Salgado-Ceballos H, Guizar-Sahagun G, Feria-Velasco A, Grijalva I, Espitia L, Ibarra A, et al. Spontaneous long-term remyelination after traumatic spinal cord injury in rats. Brain Res. 1998;782:126–135. doi: 10.1016/s0006-8993(97)01252-3. [DOI] [PubMed] [Google Scholar]
- 83.Bartholdi D, Schwab ME. Oligodendroglial reaction following spinal cord injury in rat: transient upregulation of MBP mRNA. Glia. 1998;23:278–284. doi: 10.1002/(sici)1098-1136(199807)23:3<278::aid-glia10>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 84.Morin-Richaud C, Feldblum S, Privat A. Astrocytes and oligodendrocytes reactions after a total section of the rat spinal cord. Brain Res. 1998;783:85–101. doi: 10.1016/s0006-8993(97)01282-1. [DOI] [PubMed] [Google Scholar]
- 85.Ishii K, Toda M, Nakai Y, Asou H, Watanabe M, Nakamura M, et al. Increase of oligodendrocyte progenitor cells after spinal cord injury. J Neurosci Res. 2001;65:500–507. doi: 10.1002/jnr.1180. [DOI] [PubMed] [Google Scholar]
- 86.Horner PJ, Power AE, Kempermann G, Kuhn HG, Palmer TD, Winkler J, et al. Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord. J Neurosci. 2000;20:2218–2228. doi: 10.1523/JNEUROSCI.20-06-02218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Johansson CB, Momma S, Clarke DL, Risling M, Lendahl U, Frisén J. Identification of a neural stem cell in the adult mammalian central nervous system. Cell. 1999;96:25–34. doi: 10.1016/s0092-8674(00)80956-3. [DOI] [PubMed] [Google Scholar]
- 88.Yamamoto S, Yamamoto N, Kitamura T, Nakamura K, Nakafuku M. Proliferation of parenchymal neural progenitors in response to injury in the adult rat spinal cord. Exp Neurol. 2001;172:115–127. doi: 10.1006/exnr.2001.7798. [DOI] [PubMed] [Google Scholar]
- 89.Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- 90.Mukhopadhyay G, Doherty P, Walsh FS, Crocker PR, Filbin MT. A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron. 1994;13:757–767. doi: 10.1016/0896-6273(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 91.Schwab ME, Caroni P. Oligodendrocytes and CNS myelin are nonpermissive substrates for neurite growth and fibroblast spreading in vitro. J Neurosci. 1988;8:2381–2393. doi: 10.1523/JNEUROSCI.08-07-02381.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Merkler D, Metz GA, Raineteau O, Dietz V, Schwab ME, Fouad K. Locomotor recovery in spinal cord-injured rats treated with an antibody neutralizing the myelin-associated neurite growth inhibitor Nogo-A. J Neurosci. 2001;21:3665–3673. doi: 10.1523/JNEUROSCI.21-10-03665.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McKerracher L, David S, Jackson DL, Kottis V, Dunn RJ, Braun PE. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron. 1994;13:805–811. doi: 10.1016/0896-6273(94)90247-x. [DOI] [PubMed] [Google Scholar]
- 94.Wang KC, Koprivica V, Kim JA, Sivasankaran R, Guo Y, Neve RL, He Z. Oligodendrocytemyelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature. 2002;417:941–944. doi: 10.1038/nature00867. Epub 2002. [DOI] [PubMed] [Google Scholar]
- 95.Wang KC, Kim JA, Sivasankaran R, Segal R, He Z. p75 interacts with the Nogo receptor as a co-receptor for Nogo, MAG and OMgp. Nature. 2002;420:74–78. doi: 10.1038/nature01176. Epub 2002. [DOI] [PubMed] [Google Scholar]
- 96.Yamashita T, Higuchi H, Tohyama M. The p75 receptor transduces the signal from myelinassociated glycoprotein to Rho. J Cell Biol. 2002;157:565–570. doi: 10.1083/jcb.200202010. Epub 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bregman BS, Kunkel-Bagden E, Schnell L, Dai HN, Gao D, Schwab ME. Recovery from spinal cord injury mediated by antibodies to neurite growth inhibitors. Nature. 1995;378:498–501. doi: 10.1038/378498a0. [DOI] [PubMed] [Google Scholar]
- 98.Cheng H, Cao Y, Olson L. Spinal cord repair in adult paraplegic rats: partial restoration of hind limb function. Science. 1996;273:510–513. doi: 10.1126/science.273.5274.510. [DOI] [PubMed] [Google Scholar]
- 99.Xu XM, Guénard V, Kleitman N, Bunge MB. Axonal regeneration into Schwann cell-seeded guidance channels grafted into transected adult rat spinal cord. J Comp Neurol. 1995;351:145–160. doi: 10.1002/cne.903510113. [DOI] [PubMed] [Google Scholar]
- 100.Xu XM, Chen A, Guénard V, Kleitman N, Bunge MB. Bridging Schwann cell transplants promote axonal regeneration from both the rostral and caudal stumps of transected adult rat spinal cord. J Neurocytol. 1997;26:1–16. doi: 10.1023/a:1018557923309. [DOI] [PubMed] [Google Scholar]
- 101.Raisman G. Olfactory ensheathing cells—another miracle cure for spinal cord injury? Nat Rev Neurosci. 2001;2:369–375. doi: 10.1038/35072576. [DOI] [PubMed] [Google Scholar]
- 102.Ramón-Cueto A, Avila J. Olfactory ensheathing glia: properties and function. Brain Res Bull. 1998;46:175–187. doi: 10.1016/s0361-9230(97)00463-2. [DOI] [PubMed] [Google Scholar]
- 103.Ramón-Cueto A, Plant GW, Avila J, Bunge MB. Long-distance axonal regeneration in the transected adult rat spinal cord is promoted by olfactory ensheathing glia transplants. J Neurosci. 1998;18:3803–3815. doi: 10.1523/JNEUROSCI.18-10-03803.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ramón-Cueto A, Cordero MI, Santos-Benito FF, Avila J. Functional recovery of paraplegic rats and motor axon regeneration in their spinal cords by olfactory ensheathing glia. Neuron. 2000;25:425–435. doi: 10.1016/s0896-6273(00)80905-8. [DOI] [PubMed] [Google Scholar]
- 105.Margolis RK, Margolis RU. Nervous tissue proteoglycans. Experientia. 1993;49:429–446. doi: 10.1007/BF01923587. [DOI] [PubMed] [Google Scholar]
- 106.Oohira A, Matsui F, Tokita Y, Yamauchi S, Aono S. Molecular interactions of neural chondroitin sulfate proteoglycans in the brain development. Arch Biochem Biophys. 2000;374:24–34. doi: 10.1006/abbi.1999.1598. [DOI] [PubMed] [Google Scholar]
- 107.Jones LL, Yamaguchi Y, Stallcup WB, Tuszynski MH. NG2 is a major chondroitin sulfate proteoglycan produced after spinal cord injury and is expressed by macrophages and oligodendrocyte progenitors. J Neurosci. 2002;22:2792–2803. doi: 10.1523/JNEUROSCI.22-07-02792.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pasterkamp RJ, Anderson PN, Verhaagen J. Peripheral nerve injury fails to induce growth of lesioned ascending dorsal column axons into spinal cord scar tissue expressing the axon repellent Semaphorin3A. Eur J Neurosci. 2001;13:457–471. doi: 10.1046/j.0953-816x.2000.01398.x. [DOI] [PubMed] [Google Scholar]
- 109.Bundesen LQ, Scheel TA, Bregman BS, Kromer LF. Ephrin-B2 and EphB2 regulation of astrocyte-meningeal fibroblast interactions in response to spinal cord lesions in adult rats. J Neurosci. 2003;23:7789–7800. doi: 10.1523/JNEUROSCI.23-21-07789.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, et al. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]