Abstract
Antipsychotic drugs are divided into two groups: typical and atypical. Recent clinical studies show atypical antipsychotics have advantages over typical antipsychotics in a wide variety of neuropsychiatric conditions, in terms of greater efficacy for positive and negative symptoms, beneficial effects on cognitive functioning, and fewer extra pyramidal side effects in treating schizophrenia. As such, atypical antipsychotics may be effective in the treatment of depressive symptoms associated with psychotic and mood disorders, posttraumatic stress disorder and psychosis in Alzheimer disease. In this paper, we describe the effects and potential neurochemical mechanisms of action of atypical antipsychotics in several animal models showing memory impairments and/or non-cognitive behavioral changes. The data provide new insights into the mechanisms of action of atypical antipsychotics that may broaden their clinical applications.
Key words: atypical antipsychotics, neuroprotective effect, memory, anxiety-like behavior, neurotoxicity
Introduction
Schizophrenia is a severe and chronic mental illness that affects about 1% of the world's population. Antipsychotic drugs having therapeutic efficacy in treating schizophrenia are divided into two groups: typical (conventional) and atypical (novel). Typical antipsychotics, represented by chlorpromazine and haloperidol, ameliorate only the positive symptoms. Atypical antipsychotics, including clozapine, olanzapine, quetiapine and risperidone, are effective in treating the positive, negative and cognitive symptoms, and have a low association with dyskinesia or Parkinsonism.1–5 In clinical studies, atypical antipsychotics have shown their efficacy in a wide variety of neuropsychiatric conditions and, as such, may be effective in the treatment of depressive symptoms associated with psychotic and mood disorders,6 in treating posttraumatic stress disorder,7 psychosis in Alzheimer's disease8 as well as cognition. Olanzapine, quetiapine and risperidone have beneficial effects on neurocognitive function in patients with early psychosis;9 quetiapine also improves psychotic symptoms and cognition in Parkinson disease.10
Both typical and atypical antipsychotics can bind to dopamine receptors, and the blockade of dopamine D2 receptors in the mesolimbic region is thought to be the mechanism responsible for the reversal of positive symptoms by antipsychotics.11 Atypical antipsychotics can also bind to serotonin (5-HT) receptors. The different affinities of antipsychotics for brain dopamine D2 and 5-HT2A receptors may be helpful in understanding some of the different therapeutic effects of atypical antipsychotics;12,13 however, the mechanisms underlying their therapeutic effects on negative and cognitive symptoms of schizophrenia may be beyond the dopamine and serotonin receptor blockade effects and therefore require further investigation.
Neuroanatomical and clinical studies of schizophrenia suggest progressive neuropathological changes (such as neuronal atrophy and/or cell death) occur over the course of the disease.14–17 Cognitive deficits tend to occur early in the course of schizophrenia, and the severity of deficits is predictive of the long-term treatment outlook for patients.18 Neural injury or neurodegeneration may cause cognitive deficits in schizophrenia.19 Therefore, the beneficial effects of atypical antipsychotics on behavior may also relate to their possible effects on neuroprotection and/or neurogenesis beyond the dopamine and serotonin receptor blockade effects. In in vivo studies using rats, atypical antipsychotics attenuated the methamphetamine-induced memory impairment and neurotoxicity,20,21 alleviated the amphetamine-induced anxiety-like behavioral changes,22 counteracted the phencyclidine-induced reference memory impairment and decrease of Bcl-XL/Bax ratio in the cortex,23 and reversed the suppression of hippocampal neurogenesis caused by repeated restraint stress.24 In in vitro studies, atypical antipsychotics were effective in reducing PC12 cell death induced by serum withdrawal or by addition of hydrogen peroxide, β-amyloid peptide, or N-methyl-4-phenylpyridinium ion (MPP+).25–28
This paper reviews the behavioral effects of atypical antipsychotics on a number of animal models relevant to schizophrenia and other neurodegenerative disorders, and explores the possible working mechanisms of atypical antipsychotics behind their beneficial behavioral effects (Table 1). To investigate the possible neuroprotective effects of atypical antipsychotics, we review animal models induced by a variety of possible neurotoxic consequences.
Table 1.
Effects of atypical antipsychotics on animal models relevant to schizophrenia and other neurodegenerative disorders
| Damage factors | Impairments (Animals) | Clinical relevance | Atypical antipsychotics | Drug effects (Reference) |
| Amphetamine | Anxiety-like behavior (Rats) | Schizophrenia | Quetiapine | Attenuation (22) |
| Methamphetamine | Memory, Tyrosine hydroxylase in caudate putamen (Rats) | Schizophrenia | Quetiapine Olanzapine | Attenuation (20, 21) |
| Phencyclidine | Memory, Cortex Bcl-XL/Bax ratio (Rats) | Schizophrenia | Quetiapine | Attenuation (23) |
| Okadaic acid | Memory, Hippocampal cell death (Rats) | Neurodegeneration | Olanzapine | Attenuation (99) |
| Cerebral ischemia | Memory, Depressive and anxiety-like behaviors, Hippocampal neurodegeneration (Mice) | Stroke | Quetiapine | Attenuation (122, 123) |
Effect of Quetiapine on a Neurotoxic Regimen of Amphetamine-Induced Anxiety-Like Behavioral Change
The most widely studied class of drug-induced models of schizophrenia is based on the behavioral effects of psychostimulant drugs, such as amphetamine. One aspect of the psychostimulant model that has generated considerable interest involves the dosage regimens required for amphetamine to produce psychotic-like behavior. Most preclinical studies show anxiogenic-like effects at low doses of amphetamine (0.5–5 mg/kg).29–32 Conversely, studies by Dawson et al. show anxiolytic-like effects of d-amphetamine (0.75, 1.5 mg/kg) in rats,33 while Lister reports no effects of d-amphetamine (1, 2 and 4 mg/kg) on anxiety-like behavior in mice.34 The long-term neurotoxic consequences of dl-amphetamine (20 mg/kg/day, 5 days) induces the decrease of striatal tyrosine hydroxylase (TH) immunostaining, and significantly reduces anxiety-like behaviors in both the light/dark box and open field tests in rats.22 Striatal TH immunoreactivity is one of the neuronal markers used to assess the integrity of dopaminergic terminals, and is decreased by toxic doses of amphetamine and amphetamine-like compounds;35,36 structural changes, pathognomonic of neuronal damage, have been noted using histofluorescent techniques in striatal dopaminergic terminals following continuous amphetamine administration.37
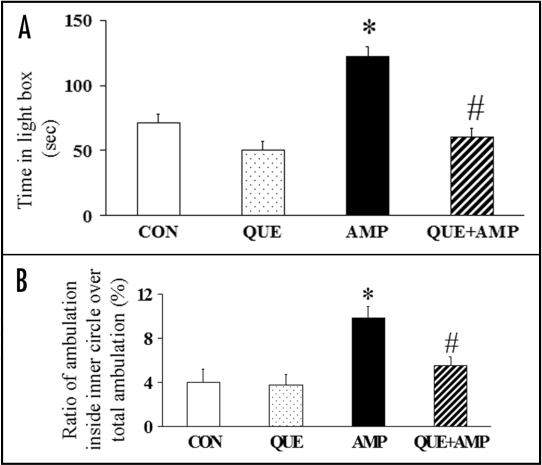
Chronic administration of quetiapine normalizes both the amphetamine-induced increase in the time spent in the light box in the light/dark box test as well as the ratio of ambulation inside the inner circle to total ambulation in the open field test in rats (Fig. 1).22 This suggests therapeutic effects of quetiapine on amphetamine-induced anxiety-like behavioral changes. Clearly, this finding has clinical relevance, recognizing the abuse potential of amphetamine and its capacity for exacerbating or inducing mood and psychiatric disturbances in humans.38,39 Quetiapine's mechanism of effect on amphetamine-induced anxiety-like behavioral changes may be related to its effect on dopaminergic and/or 5-HT receptors and its neuroprotective effects.
Figure 1.
Chronic administration of quetiapine (QUE, 10 mg/kg/day, for 33 days) normalized the increased time spent in the light box (A) in the light/dark box test and attenuated the increased ratio of ambulation distance inside the inner circle over the total ambulation distance (%) (B) in the open field test induced by chronic administration of dl-amphetamine (AMP, 20 mg/kg/day, five days) in rats. Results are expressed as means ± S.E.M. (n = 5 in the CON group, n = 6 in each of the other three groups). *p < 0.05 vs CON, #p < 0.05 vs AMP.
The modulation effects of quetiapine on dopaminergic and/or 5-HT receptors may be involved in its therapeutic effects on the amphetamine-induced changes of anxiety-like behavior. Behavioral pharmacology experiments suggest atypical antipsychotic drugs, which are mixed dopamine D2 and 5-HT2 antagonists effective in the treatment of schizophrenia, can attenuate some behavioral effects induced by amphetamine.40–43 Animal studies show a dopaminergic mechanism is involved in the change of anxiety-like consequences of amphetamine, and that an increase in dopaminergic transmission may be responsible for its anxiogenic effect.29,32 Therefore, the effects of quetiapine on dopaminergic receptors may be involved in its therapeutic effects on amphetamine-induced changes in anxiety-like behavior. On the other hand, the lower affinity and faster dissociation of quetiapine for dopamine D2 receptor44 suggests the involvement of neurotransmitter systems other than the dopaminergic system. Among possible candidates, the 5-HT system is the most likely to be involved, as quetiapine has a high affinity for 5-HT receptors.45 Reports show decreased 5-HT function results in an apparent anxiolytic effect in rodents.46–48 Therefore, the effects of quetiapine on 5-HT receptors may also be involved in its therapeutic effects on amphetamine-induced changes in anxiety-like behavior.
The neuroprotective effects of quetiapine affecting dopaminergic and/or 5-HT system damage may also be involved in its therapeutic action, as evident in changes of anxiety-like behavior induced by a neurotoxic regimen of amphetamine. Chronic pre-treatment and/or post-treatment of atypical antipsychotic drugs upregulates neuroprotective proteins [such as B cell lymphoma protein-2 (Bcl-2) and brain-derived neurotrophic factor (BDNF)] in the brain, normalizes the stress-induced decrease of Bcl-2 and BDNF in the hippocampus, and exerts neuroprotective effects on methamphetamine-induced neurotoxicity.20,49–51 In particular, quetiapine attenuates the amphetamine-induced hyperthermia22 shown to accompany neuronal damage produced by various amphetamine-like compounds.52–56
Effect of Quetiapine on Methamphetamine-Induced Memory Impairment and Neurotoxicity
Methamphetamine (METH) is a psychomotor stimulant that can cause neuropsychiatric complications.57 In addition to acute neurochemical and behavioral effects, repeated moderate dose administration of this stimulant produces long-term neurotoxicity to dopaminergic and serotonergic nerve terminals, hyperthermia and high mortality.58–60 Hyperthermia accompanies the neuronal damage produced by METH.52,53 Tyrosine hydroxylase (TH) immunoreactivity in striatum, one of neuronal markers used to assess the integrity of dopaminergic terminals, is decreased by toxic doses of METH.35,36 The administration of METH also caused cognitive impairment in clinical study,61 and induced recognition memory impairment in rats.62,63 The METH-induced disruption of the striatal dopaminergic terminals may contribute to the object recognition impairment.63
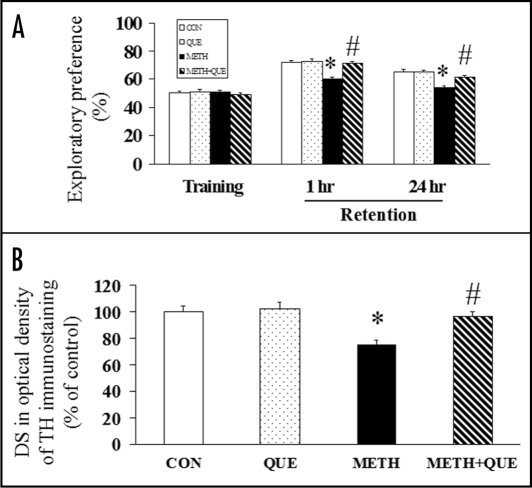
Chronic administration of quetiapine after METH injections reverses the METH-induced recognition memory impairment in an object recognition task21 (Fig. 2A). The object recognition task measures non-spatial memory in the rat, takes advantage of the rat's unprompted nature to explore its surroundings, and requires the rats to recall to which of two small objects they have had prior exposure.21 In addition, quetiapine (Fig. 2B) and olanzapine attenuate the METH-induced dopaminergic terminal neurotoxicity, shown as a decrease of TH immunostaining in the caudate putamen (CPu) of striatum in rats.20,21 The memory improvement is parallel to the attenuating effect of quetiapine on the METH-induced neurotoxicity, suggesting an association between both the neuroprotective and memory improving effects exerted by quetiapine. The ability of quetiapine to reverse METH-induced object recognition impairment may be associated with therapeutic effects of quetiapine on METH-induced striatal neurotoxicity.21
Figure 2.
(A) METH (5 mg/kg × 4, 2 hr intervals) and chronic administration of quetiapine (QUE, 10 mg/kg/day, 28 days) had no effect on the exploratory preference during the training session; chronic administration of quetiapine reversed the METH-induced decrease of exploratory preference in rats during the retention session (1 hr and 24 hr) of the object recognition task (n = 8 in CON and QUE, n = 11 in METH and METH + QUE). (B) Chronic administration of quetiapine (10 mg/kg/day, 28 days) reversed the METH (5 mg/kg × 4, 2 hr intervals)-induced decrease of DS (difference score) in optical density of TH immunostaining in the caudate putamen of rats (n = 4 in CON and QUE, n = 5 in METH and METH + QUE). Rats were sacrificed 24 hr after the object recognition task. Results are expressed as means ± S.E.M. *p < 0.05 vs CON, #p < 0.05 vs METH.
The ability of chronic administration of quetiapine to counteract METH-induced dopaminergic terminal neurotoxicity in the CPu suggests a neuroprotective action of quetiapine. METH can induce significant increases in the pro-death Bcl-2 gene family (Bad, Bax and Bid), and decreases in the anti-death genes, Bcl-2 and Bcl-XL.64 Bcl-2 protects METH-induced dose-dependent apoptosis in immortalized neural cells.65 In addition, a possible mechanism of METH neurotoxicity is the formation of reactive oxygen species and oxidative stress.66 The elevation of oxidizable dopamine concentrations may be primarily responsible for METH-induced dopaminergic terminal injury.60 Bcl-2 protects against generators of reactive oxygen species, increases antioxidant defenses, and decreases levels of reactive oxygen species and oxidative damage.67 Therefore, the neuroprotective effects of chronic administration of quetiapine on METH-induced neurotoxicity may involve the modulation of the Bcl-2 family,23,28 the upregulation of the neuroprotective protein, Bcl-2,20,49,50 and the prevention of oxidative stress and stress-related damages.68 Furthermore, the attenuating effect of quetiapine on METH-induced hyperthermia may be responsible for the neuroprotective effects of quetiapine.20,22 Correlating with the METH-induced decrease of striatal dopamine content and the striatal terminal degeneration, hyperthermia may play an important role in METH neurotoxicity.52,69 The critical determinant of METH-induced neurotoxicity is METH-induced hyperthermia;70 attenuation of the hyperthermia induced by METH affords a protective role against neurochemical depletions and striatal TH activity.53
Effect of Quetiapine on Phencyclidine-Induced Memory Impairment and Neurotoxicity
Phencyclidine (PCP), an N-methyl-D-aspartate (NMDA) receptor antagonist, can cause psychoses and negative symptoms, and is used as a pharmacological model of schizophrenia.71 PCP impairs learning and memory performance in rats.23,72–74 PCP also induces neurodegeneration75–77 and apoptosis78 in rat brain, and can decrease Bcl-XL and increase Bax in the frontal cortex of perinatal rats.79 A single dose (50 mg/kg) of PCP causes reference spatial memory impairment in the radial maze task and a decrease in the ratio of Bcl-XL (an anti-apoptotic Bcl-2 family member) to Bax (a pro-apoptotic analogue) in the posterior cingulate cortex in rats.23 The Bcl-2 protein family, which contains pro- and anti-apoptotic proteins, represents some of the most well-defined regulators of the neurodegenerative process.80 The Bcl-XL/Bax ratio is an index that can determine whether an apoptotic stimulus results in the life or death of a cell.81 The PCP-induced lower ratio of Bcl-XL/Bax indicates PCP may decrease the survival of cells in the posterior cingulate cortex. The posterior cingulate plays an important role in analyzing the significance of objects within a topographical representation and in passing this representation to the hippocampal system for memory formation.82 The posterior cingulate cortex, per se, plays a role in spatial learning in animals;83 therefore, the PCP-induced reference spatial memory impairment is likely associated, at least in part, with the neurotoxicity in the posterior cingulate cortex caused by PCP.
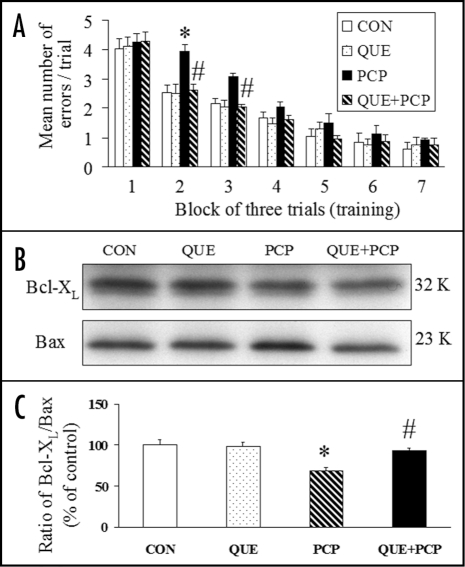
Chronic administration of quetiapine counteracts the PCP-induced reference memory impairment in an eight-arm radial maze task and attenuates the PCP-induced decrease of the Bcl-XL/Bax ratio in the posterior cingulate cortex23 (Fig. 3). In all training trials of the radial maze task, the same four arms were baited (one bait per arm), while the other four arms were never baited. Reference memory is regarded as a long-term memory for information that remains constant over repeated trials (memory for the positions of baited arms), while working memory is considered as a short-term memory in which the information to be remembered changes in every trial (memory for the positions of arms that have already been visited in each trial).23 The memory improvement due to quetiapine is parallel to the alleviating effect of quetiapine on the PCP-induced decrease of Bcl-XL/Bax ratio in the posterior cingulate cortex, suggesting an association between both the neuroprotective and the memory improving effects exerted by quetiapine. Because evidence suggests excessive dopaminergic transmission could contribute to the PCP-induced cell injury in the brain,84,85 quetiapine may attenuate PCP-induced neurotoxicity by acting on dopamine receptors as do other antipsychotic agents, such as clozapine and olanzapine.86–88 Like olanzapine,49,50,89 the neuroprotective effects of chronic administration of quetiapine may also involve the upregulation of neuroprotective proteins such as Bcl-2 and BDNF.
Figure 3.
(A) Chronic administration of quetiapine (QUE, 10 mg/kg/day, 16 days) counteracted the PCP (50 mg/kg)-induced spatial reference memory formation impairment of rats in the radial arm maze task. (B) Representative western blot bands of Bcl-XL and Bax in the posterior cingulate cortex of rats. (C) Chronic administration of quetiapine (10 mg/kg/day, 16 days) counteracted the PCP (50 mg/kg)-induced decrease of Bcl-XL/Bax ratio in the posterior cingulate cortex. Results are expressed as means ± S.E.M. (n = 6–8 in each group). *p < 0.05 vs CON, #p < 0.05 vs PCP.
Effect of Olanzapine on Okadaic Acid-Induced Memory Impairment and Hippocampal Cell Death
Okadaic acid (OA), a selective and potent inhibitor of the serine/threonine phosphatases 1 and 2A,90,91 causes neuronal cell death in vitro92 and in vivo.93,94 Infusion of OA into rat brain results in severe memory impairment, accompanied by remarkable neuropathological changes including hippocampal neurodegeneration, a paired helical filament-like phosphorylation of tau protein, and the formation of β/A4-amyloid containing plaque-like structures in gray and white matter areas.94–98 A unilateral microinjection of OA (100 ng) into the dorsal hippocampus induces spatial working and reference memory impairment, decreases the number of the surviving pyramidal neurons in the CA1 region of the hippocampus, and causes hippocampal apoptosis, as revealed by terminal deoxynucleutidyl transferase-mediated biotinylated UTP nick end labeling (TUNEL) staining in rats.99 Because an intact hippocampus is required for recall, item recognition and associative recognition memory in animals,100,101 the OA-induced spatial memory impairment may partially be attributed to the hippocampal cell death it causes.
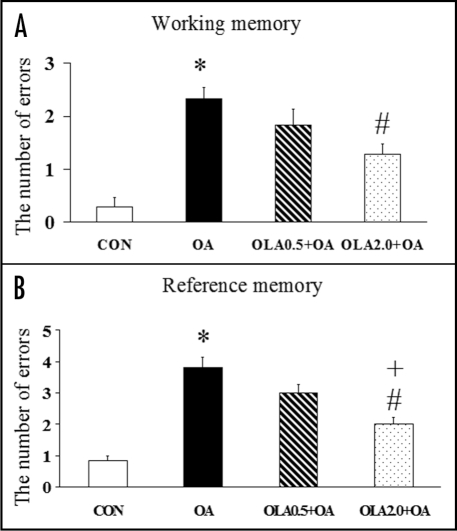
Chronic administration of olanzapine significantly attenuates the OA-induced spatial memory impairment (Fig. 4) in the radial arm maze task and cell death evaluated by TUNEL (Fig. 5) and Nissl staining in the hippocampus of rats.99 The neuroprotective effect on hippocampal cell death is associated with the memory improving effect exerted by olanzapine.99 The attenuating effect of olanzapine on the OA-induced neurodegeneration and apoptosis provides direct evidence supporting the neuroprotective action of olanzapine. Olanzapine can regulate the translocation and expression of pro- and anti-apoptotic proteins Bcl-XL and Bcl-2 in PC12 cells.28 In animal studies, olanzapine upregulates the expression of Bcl-2 and BDNF in the hippocampus49,89 and helps restore the repeated restraint stress-induced decrease in these two neuroprotective proteins in hippocampal neurons.50 OA-induced apoptosis is associated with downregulation of Bcl-2 and can be prevented by upregulation of Bcl-2.102–104 Therefore, Bcl-2 may play an important role in the neuroprotective effects of olanzapine on OA-induced neurodegeneration and apoptosis. Olanzapine may also attenuate OA-induced neurotoxicity by upregulating superoxide dismutase105–107 and perform protective effects on OA-induced apoptotic cell death by modulating the expression of pro- and anti-apoptotic proteins, such as Bax and Bcl-XL.28,103 However, further studies are necessary to elucidate whether olanzapine attenuates OA-induced neurotoxicity by directly affecting the activation of phosphatases or caspases.
Figure 4.
Olanzapine (OLA) significantly attenuated the OA-induced impairment in working (A) and reference (B) memory measured by the radial arm maze task 1 week after the microinjection of OA or saline into the right hippocampus of rats. Olanzapine did not affect the spatial working and reference memory formation before OA or saline microinjection. Results are expressed as means ± S.E.M. (n = 6–7 in each group). *p < 0.05 vs CON, #p < 0.05 vs OA and +p < 0.05 vs OLA0.5 + OA.
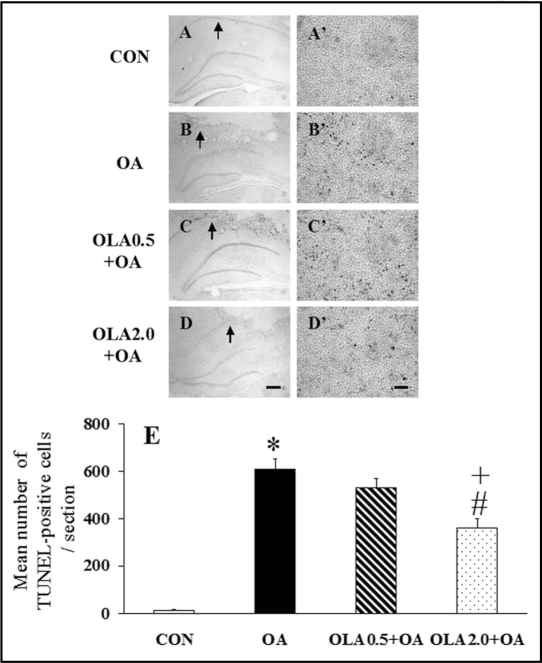
Figure 5.
(A–D, A′–D′) Representative photomicrographs of TUNEL staining in the injected hippocampus of rats in the CON (A and A′), OA (B and B′), OLA0.5 + OA (C and C′) and OLA2.0 + OA (D and D′) groups. The high magnification of right photomicrographs (A′–D′) are enlargement of selected sections of (A–D), respectively. Arrows on the low magnification panels indicate the location of the high magnification images. In the hippocampi of OA-injected groups (B, B′; C, C′; and D, D′), the TUNEL-positive cells are visible in different frequency, whereas almost no TUNEL-positive cells are evident in the hippocampus of the control group (A and A′). The scale bar represents 300 µm in (A–D) and 30 µm in (A′–D′). (E) Quantitative analysis of the effect of olanzapine on the OA-induced increase of TUNEL-positive cells in the injected hippocampus. The number of TUNEL-positive cells in the hippocampus was counted at 400× magnification. Results are expressed as means ± S.E.M. (n = 6–7 in each group). *p < 0.05 vs CON, #p < 0.01 vs OA and +p <0 .05 vs OLA0.5 + OA.
OA-induced spatial memory impairment in the present paradigm is likely due to the secondary effect of OA-induced hippocampal cell death.94 The ability of olanzapine to improve OA-induced spatial memory impairment in rats may be subsequent to its attenuating effects on OA-induced hippocampal cell death.99 Olanzapine in rats induces an increase of acetylcholine release in the medial prefrontal cortex and hippocampus, a possible contributing factor to cognitive improvement in schizophrenia.108,109 Therefore, the effects of olanzapine on acetylcholine may be an additional contributor to its ability to improve OA-induced memory impairment.
Effect of Quetiapine on Global Cerebral Ischemia-Induced Cognitive and Non-Cognitive Behavioral Impairments and Hippocampal Neurodegeneration
Cerebral ischemia is one of the major leading causes of morbidity and mortality worldwide. Cognitive deficits, neuropsychiatric disorders and brain damages occur in global cerebral ischemia (GCI) subjects.110–113 Post-stroke depression, following cerebrovascular lesions, along with post-stroke anxiety, inhibit physical and cognitive recovery.112–117 The ischemia-induced brain damage is believed to be associated with cognitive and memory dysfunction.114,115,118–120 In animal studies, GCI induced by transient occlusion of common carotid arteries causes spatial memory impairment and hippocampal neurodegeneration, and induces changes in depressive and anxiety-like behaviors.121–123
Our study shows the administration of quetiapine attenuates GCI-induced spatial memory impairment in a water maze test and neurodegeneration in the hilus of hippocampus in mice, suggesting quetiapine's neuroprotective effects may contribute to its beneficial effect on memory impairment.122 In this study, quetiapine is pre-administrated two weeks before GCI, so it may act to attenuate cell death rather than “improve memory” after disease onset. Quetiapine may attenuate GCI-induced neurotoxicity by upregulating neuroprotective proteins or regulating NMDA receptors, thus leading to the downregulation of oxidative stress.122
Quetiapine effectively attenuates GCI-induced changes in depressive and anxiety-like behaviors in mice.123 Dysfunction of neurotransmitter systems is the major cause of the depressive-like behavior in ischemic mice, and these depressive-like behaviors are relevant to the low levels of norepinephrine and dopamine.124 Serotonin deficiency is also postulated to be relevant to the pathophysiology of depression after stroke.125,126 In addition, GCI-induced injury of dopaminergic and serotoninergic systems in mice may cause anxiety-like behavioral changes;123 therefore, the neuroprotective effects of quetiapine on dopaminergic and serotoninergic system damage may be involved in its action to regulate the depressive- and anxiety-like behaviors. In fact, quetiapine can alleviate the GCI-induced neurodegeneration and neuron loss as well as attenuate the GCI-induced decrease of striatal TH immunostaining.122,123
Neuroprotective Mechanisms of Atypical Antipsychotics
Atypical antipsychotics upregulate the level of BDNF, an important neurotrophin mainly expressed and distributed in brain neurons. Neurotrophins are growth factors that act directly on neurons to support their growth, differentiation and survival.127 Chronic administration (28 days) of clozapine (10 mg/kg) and olanzapine (2.7 mg/kg) upregulates BDNF mRNA expression in the hippocampus of rats.89 Quetiapine attenuates the immobilization stress-induced decrease of BDNF expression in rat hippocampus51 and chronic administration of olanzapine accelerates the restoration of BDNF in hippocampal neurons from decrease induced by repeated restraint stress.50 Atypical antipsychotics also modulate the levels of other growth factors, such as fibroblast growth factor 2 (FGF-2) and nerve growth factor (NGF), that may play important roles in changing synaptic plasticity, normalizing cognitive deficits, and preventing cell degeneration.128,129
Atypical antipsychotics upregulate the level of Bcl-2 and modulate the Bcl-XL/Bax ratio in brain. Bcl-2, a neuroprotective protein, inhibits apoptosis by sequestering proforms of death-driving caspases and preventing the release of mitochondrial apoptotic factors into the cytoplasm.49,80,130 The mRNA and protein expression of Bcl-2 in rat frontal cortex and hippocampus are increased after chronic atypical antipsychotic treatment.49 Olanzapine prevents METH-induced Bcl-2 decrease and accelerates the restoration of Bcl-2 in hippocampal neurons from the repeated restraint stress-induced decrease.20,50 Atypical antipsychotics attenuate neurotoxicity of β-amyloid by modulating Bax and Bcl-XL/S expression and localization in PC12 cells.28 In animal studies, quetiapine attenuates the phencyclidine-induced decrease in the Bcl-XL/Bax ratio in the posterior cingulate cortex in rats.23
Atypical antipsychotics have an antioxidant capacity. Clozapine, olanzapine, quetiapine and risperidone increase the gene expression of superoxide dismutase (SOD1) in PC12 cells, and prevent cell death after serum withdrawal.25,106 As such, atypical antipsychotics may have a common antioxidant action responsible for their cytoprotective effects in reducing PC12 cell death induced by serum withdrawal or by addition of hydrogen peroxide, β-amyloid peptide, or MPP+.25–28 Olanzapine and quetiapine prevent PC12 cells from Aβ-induced apoptosis and the overproduction of intracellular reactive oxygen species, attenuate Aβ-induced activity changes of the antioxidant enzymes (SOD1, catalase and glutathione peroxidase), and block Aβ-induced decrease in mitochondrial membrane potential in PC12 cells.68 Furthermore, atypical antipsychotics may demonstrate other aspects of neuroprotective effect. For example, the treatment effect of olanzapine may be associated with its effects on brain gray matter volume and psychopathology in schizophrenia.131
Atypical Antipsychotics Upregulate Brain Neurogenesis
Neurogenesis (neuronal regeneration) is a process of generating functionally integrated neurons from progenitor cells.132 Atypical antipsychotics can increase cell proliferation and neurogenesis in adult rat brain.133,134 In addition, quetiapine reverses the suppression of hippocampal neurogenesis caused by repeated restraint stress.24 Although the function of neurogenesis in the hippocampus of transgenic mice under physiological or pathological conditions is unknown, new neurons from the adult human hippocampus have shown some function.135 The formation of some types of memory relies on the continuous production of new hippocampal neurons throughout adulthood.136 Therefore, the beneficial behavioral effects of atypical antipsychotics may be linked to their upregulation of neurogenesis. However, the effect of atypical antipsychotics on the hippocampal neurogenesis is still controversial. Other labs using different dosages and schedules show atypical antipsychotics have no effect on hippocampal neurogenesis (as reviewed by Newton and Duman137).
Summary
Atypical antipsychotics attenuate both cognitive and non-cognitive behavioral impairments in different animal models of neurotoxicity. Their beneficial behavioral effects are not only related to their dopamine and serotonin receptor blockade effects, but also to their effects on neuroprotection, neurotrophins and neurogenesis. The neuroprotective potential of atypical antipsychotics may contribute to their therapeutic effects in treating cognitive and non-cognitive impairments in schizophrenia and other neurodegenerative diseases.
Acknowledgements
This work was supported by the Canadian Psychiatry Research Foundation, the Canadian Institutes of Health Research, NeuroScience Canada and the Saskatchewan Health Research Foundation. The authors thank Gabriel Stegeman for her excellent technical assistance, and are grateful to Yvonne Wilkinson for her helpful comments during the preparation of this manuscript.
Abbreviations
- Bcl-2
B cell lymphoma protein-2
- BDNF
brain-derived neurotrophic factor
- CPu
caudate putamen
- GCI
global cerebral ischemia
- METH
methamphetamine
- MPP+
N-methyl-4-phenylpyridinium ion
- NMDA
N-methyl-D-aspartate
- OA
okadaic acid
- PCP
phencyclidine
- 5-HT
serotonin
- SOD1
superoxide dismutase
- TUNEL
terminal deoxynucleutidyl transferase-mediated biotinylated UTP nick end labeling
- TH
tyrosine hydroxylase
Footnotes
Previously published online as a Cell Adhesion & Migration E-publication: http://www.landesbioscience.com/journals/celladhesion/article/7401
References
- 1.Velligan DI, Newcomer J, Pultz J, Csernansky J, Hoff AL, Mahurin R, et al. Does cognitive function improve with quetiapine in comparison to haloperidol? Schizophr Res. 2002;53:239–248. doi: 10.1016/s0920-9964(01)00268-7. [DOI] [PubMed] [Google Scholar]
- 2.Purdon SE, Malla A, Labelle A, Lit W. Neuropsychological change in patients with schizophrenia after treatment with quetiapine or haloperidol. J Psychiatry Neurosci. 2001;26:137–149. [PMC free article] [PubMed] [Google Scholar]
- 3.Beasley CM, Jr, Tollefson GD, Tran PV. Efficacy of olanzapine: An overview of pivotal clinical trials. J Clin Psychiatry. 1997;58:7–12. [PubMed] [Google Scholar]
- 4.Cuesta MJ, Peralta V, Zarzuela A. Effects of olanzapine and other antipsychotics on cognitive function in chronic schizophrenia: A longitudinal study. Schizophr Res. 2001;48:17–28. doi: 10.1016/s0920-9964(00)00112-2. [DOI] [PubMed] [Google Scholar]
- 5.Jann MW. Implications for atypical antipsychotics in the treatment of schizophrenia: Neurocognition effects and a neuroprotective hypothesis. Pharmacotherapy. 2004;24:1759–1783. doi: 10.1592/phco.24.17.1759.52346. [DOI] [PubMed] [Google Scholar]
- 6.Brugue E, Vieta E. Atypical antipsychotics in bipolar depression: Neurobiological basis and clinical implications. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:275–282. doi: 10.1016/j.pnpbp.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 7.Pae CU, Lim HK, Peindl K, Ajwani N, Serretti A, Patkar AA, et al. The atypical antipsychotics olanzapine and risperidone in the treatment of posttraumatic stress disorder: A meta-analysis of randomized, double-blind, placebo-controlled clinical trials. Int Clin Psychopharmacol. 2008;23:1–8. doi: 10.1097/YIC.0b013e32825ea324. [DOI] [PubMed] [Google Scholar]
- 8.Madhusoodanan S, Shah P, Brenner R, Gupta S. Pharmacological treatment of the psychosis of alzheimer's disease: What is the best approach? CNS Drugs. 2007;21:101–115. doi: 10.2165/00023210-200721020-00002. [DOI] [PubMed] [Google Scholar]
- 9.Keefe RS, Sweeney JA, Gu H, Hamer RM, Perkins DO, McEvoy JP, et al. Effects of olanzapine, quetiapine and risperidone on neurocognitive function in early psychosis: A randomized, double-blind 52-week comparison. Am J Psychiatry. 2007;164:1061–1071. doi: 10.1176/ajp.2007.164.7.1061. [DOI] [PubMed] [Google Scholar]
- 10.Juncos JL, Roberts VJ, Evatt ML, Jewart RD, Wood CD, Potter LS, et al. Quetiapine improves psychotic symptoms and cognition in parkinson's disease. Mov Disord. 2004;19:29–35. doi: 10.1002/mds.10620. [DOI] [PubMed] [Google Scholar]
- 11.Wong AH, Van Tol HH. Schizophrenia: From phenomenology to neurobiology. Neurosci Biobehav Rev. 2003;27:269–306. doi: 10.1016/s0149-7634(03)00035-6. [DOI] [PubMed] [Google Scholar]
- 12.Kapur S, Zipursky RB, Remington G. Clinical and theoretical implications of 5-HT2 and D2 receptor occupancy of clozapine, risperidone and olanzapine in schizophrenia. Am J Psychiatry. 1999;156:286–293. doi: 10.1176/ajp.156.2.286. [DOI] [PubMed] [Google Scholar]
- 13.Seeman P, Tallerico T. Rapid release of antipsychotic drugs from dopamine D2 receptors: An explanation for low receptor occupancy and early clinical relapse upon withdrawal of clozapine or quetiapine. Am J Psychiatry. 1999;156:876–884. doi: 10.1176/ajp.156.6.876. [DOI] [PubMed] [Google Scholar]
- 14.Woods BT, Yurgelun-Todd D, Benes FM, Frankenburg FR, Pope HG, Jr, McSparren J. Progressive ventricular enlargement in schizophrenia: Comparison to bipolar affective disorder and correlation with clinical course. Biol Psychiatry. 1990;27:341–352. doi: 10.1016/0006-3223(90)90008-p. [DOI] [PubMed] [Google Scholar]
- 15.Waddington JL, O'Callaghan E, Buckley P, Larkin C, Redmond O, Stack J, et al. The age dependencies of MRI abnormalities in schizophrenia suggest early ventricular enlargement but later prominence of cortical atrophy. Schizophr Res. 1991;5:188–189. doi: 10.1016/0920-9964(91)90064-x. [DOI] [PubMed] [Google Scholar]
- 16.DeLisi LE, Sakuma M, Tew W, Kushner M, Hoff AL, Grimson R. Schizophrenia as a chronic active brain process: A study of progressive brain structural change subsequent to the onset of schizophrenia. Psychiatry Res. 1997;74:129–140. doi: 10.1016/s0925-4927(97)00012-7. [DOI] [PubMed] [Google Scholar]
- 17.Arnold SE, Trojanowski JQ. Recent advances in defining the neuropathology of schizophrenia. Acta Neuropathol. 1996;92:217–231. doi: 10.1007/s004010050512. [DOI] [PubMed] [Google Scholar]
- 18.Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- 19.Harvey PD. Comprehensive clinical psychology. In: Bellack AS, Hersen M, editors. Schizophrenia and related disorders in late life. Amsterdam: Elsevier; 1998. pp. 74–86. [Google Scholar]
- 20.He J, Xu H, Yang Y, Zhang X, Li XM. Neuroprotective effects of olanzapine on methamphetamine-induced neurotoxicity are associated with an inhibition of hyperthermia and prevention of bcl-2 decrease in rats. Brain Res. 2004;1018:186–192. doi: 10.1016/j.brainres.2004.05.060. [DOI] [PubMed] [Google Scholar]
- 21.He J, Yang Y, Yu Y, Li X, Li XM. The effects of chronic administration of quetiapine on the methamphetamine-induced recognition memory impairment and dopaminergic terminal deficit in rats. Behav Brain Res. 2006;172:39–45. doi: 10.1016/j.bbr.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 22.He J, Xu H, Yang Y, Zhang X, Li XM. Chronic administration of quetiapine alleviates the anxiety-like behavioural changes induced by a neurotoxic regimen of dl-amphetamine in rats. Behav Brain Res. 2005;160:178–187. doi: 10.1016/j.bbr.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 23.He J, Xu H, Yang Y, Rajakumar D, Li X, Li XM. The effects of chronic administration of quetiapine on the phencyclidine-induced reference memory impairment and decrease of bcl-XL/Bax ratio in the posterior cingulate cortex in rats. Behav Brain Res. 2006;168:236–242. doi: 10.1016/j.bbr.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 24.Luo C, Xu H, Li XM. Quetiapine reverses the suppression of hippocampal neurogenesis caused by repeated restraint stress. Brain Res. 2005;1063:32–39. doi: 10.1016/j.brainres.2005.09.043. [DOI] [PubMed] [Google Scholar]
- 25.Bai O, Wei Z, Lu W, Bowen R, Keegan D, Li XM. Protective effects of atypical antipsychotic drugs on PC12 cells after serum withdrawal. J Neurosci Res. 2002;69:278–283. doi: 10.1002/jnr.10290. [DOI] [PubMed] [Google Scholar]
- 26.Qing H, Xu H, Wei Z, Gibson K, Li XM. The ability of atypical antipsychotic drugs vs. haloperidol to protect PC12 cells against MPP+-induced apoptosis. Eur J Neurosci. 2003;17:1563–1570. doi: 10.1046/j.1460-9568.2003.02590.x. [DOI] [PubMed] [Google Scholar]
- 27.Wei Z, Bai O, Richardson JS, Mousseau DD, Li XM. Olanzapine protects PC12 cells from oxidative stress induced by hydrogen peroxide. J Neurosci Res. 2003;73:364–368. doi: 10.1002/jnr.10668. [DOI] [PubMed] [Google Scholar]
- 28.Wei Z, Mousseau DD, Richardson JS, Dyck LE, Li XM. Atypical antipsychotics attenuate neurotoxicity of beta-amyloid(25–35) by modulating bax and bcl-X(l/s) expression and localization. J Neurosci Res. 2003;74:942–947. doi: 10.1002/jnr.10832. [DOI] [PubMed] [Google Scholar]
- 29.Cancela LM, Basso AM, Martijena ID, Capriles NR, Molina VA. A dopaminergic mechanism is involved in the ‘anxiogenic-like’ response induced by chronic amphetamine treatment: A behavioral and neurochemical study. Brain Res. 2001;909:179–186. doi: 10.1016/s0006-8993(01)02680-4. [DOI] [PubMed] [Google Scholar]
- 30.Lapin IP. Anxiogenic effect of phenylethylamine and amphetamine in the elevated plus-maze in mice and its attenuation by ethanol. Pharmacol Biochem Behav. 1993;44:241–243. doi: 10.1016/0091-3057(93)90305-d. [DOI] [PubMed] [Google Scholar]
- 31.Lin HQ, Burden PM, Christie MJ, Johnston GA. The anxiogenic-like and anxiolytic-like effects of MDMA on mice in the elevated plus-maze: A comparison with amphetamine. Pharmacol Biochem Behav. 1999;62:403–408. doi: 10.1016/s0091-3057(98)00191-9. [DOI] [PubMed] [Google Scholar]
- 32.Simon P, Panissaud C, Costentin J. Anxiogenic-like effects induced by stimulation of dopamine receptors. Pharmacol Biochem Behav. 1993;45:685–690. doi: 10.1016/0091-3057(93)90525-x. [DOI] [PubMed] [Google Scholar]
- 33.Dawson GR, Crawford SP, Collinson N, Iversen SD, Tricklebank MD. Evidence that the anxiolytic-like effects of chlordiazepoxide on the elevated plus maze are confounded by increases in locomotor activity. Psychopharmacology (Berl) 1995;118:316–323. doi: 10.1007/BF02245961. [DOI] [PubMed] [Google Scholar]
- 34.Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl) 1987;92:180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- 35.Abekawa T, Ohmori T, Koyama T. Tolerance to the neurotoxic effect of methamphetamine in rats behaviorally sensitized to methamphetamine or amphetamine. Brain Res. 1997;767:34–44. doi: 10.1016/s0006-8993(97)00542-8. [DOI] [PubMed] [Google Scholar]
- 36.Eisch AJ, Marshall JF. Methamphetamine neurotoxicity: Dissociation of striatal dopamine terminal damage from parietal cortical cell body injury. Synapse. 1998;30:433–445. doi: 10.1002/(SICI)1098-2396(199812)30:4<433::AID-SYN10>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 37.Ellison G, Eison MS, Huberman HS, Daniel F. Long-term changes in dopaminergic innervation of caudate nucleus after continuous amphetamine administration. Science. 1978;201:276–278. doi: 10.1126/science.26975. [DOI] [PubMed] [Google Scholar]
- 38.Angrist BM, Gershon S. The phenomenology of experimentally induced amphetamine psychosis—preliminary observations. Biol Psychiatry. 1970;2:95–107. [PubMed] [Google Scholar]
- 39.Angrist B, Sathananthan G, Wilk S, Gershon S. Amphetamine psychosis: Behavioral and biochemical aspects. J Psychiatr Res. 1974;11:13–23. doi: 10.1016/0022-3956(74)90064-8. [DOI] [PubMed] [Google Scholar]
- 40.Fulton B, Goa KL. Olanzapine. A review of its pharmacological properties and therapeutic efficacy in the management of schizophrenia and related psychoses. Drugs. 1997;53:281–298. doi: 10.2165/00003495-199753020-00007. [DOI] [PubMed] [Google Scholar]
- 41.Grant S, Fitton A. Risperidone. A review of its pharmacology and therapeutic potential in the treatment of schizophrenia. Drugs. 1994;48:253–273. doi: 10.2165/00003495-199448020-00009. [DOI] [PubMed] [Google Scholar]
- 42.Mechanic JA, Wasielewski JA, Carl KL, Holloway FA. Attenuation of the amphetamine discriminative cue in rats with the atypical antipsychotic olanzapine. Pharmacol Biochem Behav. 2002;72:767–777. doi: 10.1016/s0091-3057(02)00766-9. [DOI] [PubMed] [Google Scholar]
- 43.Rush CR, Stoops WW, Hays LR, Glaser PE, Hays LS. Risperidone attenuates the discriminative-stimulus effects of d-amphetamine in humans. J Pharmacol Exp Ther. 2003;306:195–204. doi: 10.1124/jpet.102.048439. [DOI] [PubMed] [Google Scholar]
- 44.Kapur S, Seeman P. Antipsychotic agents differ in how fast they come off the dopamine D2 receptors. implications for atypical antipsychotic action. J Psychiatry Neurosci. 2000;25:161–166. [PMC free article] [PubMed] [Google Scholar]
- 45.Richelson E, Souder T. Binding of antipsychotic drugs to human brain receptors focus on newer generation compounds. Life Sci. 2000;68:29–39. doi: 10.1016/s0024-3205(00)00911-5. [DOI] [PubMed] [Google Scholar]
- 46.Briley M, Chopin P, Moret C. Effect of serotonergic lesion on “anxious” behaviour measured in the elevated plus-maze test in the rat. Psychopharmacology (Berl) 1990;101:187–189. doi: 10.1007/BF02244124. [DOI] [PubMed] [Google Scholar]
- 47.Tye NC, Everitt BJ, Iversen SD. 5-hydroxytryptamine and punishment. Nature. 1977;268:741–743. doi: 10.1038/268741a0. [DOI] [PubMed] [Google Scholar]
- 48.Tye NC, Iversen SD, Green AR. The effects of benzodiazepines and serotonergic manipulations on punished responding. Neuropharmacology. 1979;18:689–695. doi: 10.1016/0028-3908(79)90036-4. [DOI] [PubMed] [Google Scholar]
- 49.Bai O, Zhang H, Li XM. Antipsychotic drugs clozapine and olanzapine upregulate bcl-2 mRNA and protein in rat frontal cortex and hippocampus. Brain Res. 2004;1010:81–86. doi: 10.1016/j.brainres.2004.02.064. [DOI] [PubMed] [Google Scholar]
- 50.Luo C, Xu H, Li XM. Post-stress changes in BDNF and bcl-2 immunoreactivities in hippocampal neurons: Effect of chronic administration of olanzapine. Brain Res. 2004;1025:194–202. doi: 10.1016/j.brainres.2004.06.089. [DOI] [PubMed] [Google Scholar]
- 51.Xu H, Qing H, Lu W, Keegan D, Richardson JS, Chlan-Fourney J, et al. Quetiapine attenuates the immobilization stress-induced decrease of brain-derived neurotrophic factor expression in rat hippocampus. Neurosci Lett. 2002;321:65–68. doi: 10.1016/s0304-3940(02)00034-4. [DOI] [PubMed] [Google Scholar]
- 52.Albers DS, Sonsalla PK. Methamphetamine-induced hyperthermia and dopaminergic neurotoxicity in mice: Pharmacological profile of protective and nonprotective agents. J Pharmacol Exp Ther. 1995;275:1104–1114. [PubMed] [Google Scholar]
- 53.Bowyer JF, Davies DL, Schmued L, Broening HW, Newport GD, Slikker W, Jr, et al. Further studies of the role of hyperthermia in methamphetamine neurotoxicity. J Pharmacol Exp Ther. 1994;268:1571–1580. [PubMed] [Google Scholar]
- 54.Farfel GM, Seiden LS. Role of hypothermia in the mechanism of protection against serotonergic toxicity I. experiments using 3,4-methylenedioxymethamphetamine, dizocilpine, CGS 19755 and NBQX. J Pharmacol Exp Ther. 1995;272:860–867. [PubMed] [Google Scholar]
- 55.Miller DB, O'Callaghan JP. Environment-, drug- and stress-induced alterations in body temperature affect the neurotoxicity of substituted amphetamines in the C57BL/6J mouse. J Pharmacol Exp Ther. 1994;270:752–760. [PubMed] [Google Scholar]
- 56.Schmidt CJ, Black CK, Abbate GM, Taylor VL. Methylenedioxymethamphetamine-induced hyperthermia and neurotoxicity are independently mediated by 5-HT2 receptors. Brain Res. 1990;529:85–90. doi: 10.1016/0006-8993(90)90813-q. [DOI] [PubMed] [Google Scholar]
- 57.Lan KC, Lin YF, Yu FC, Lin CS, Chu P. Clinical manifestations and prognostic features of acute methamphetamine intoxication. J Formos Med Assoc. 1998;97:528–533. [PubMed] [Google Scholar]
- 58.O'Dell SJ, Weihmuller FB, Marshall JF. Multiple methamphetamine injections induce marked increases in extracellular striatal dopamine which correlate with subsequent neurotoxicity. Brain Res. 1991;564:256–260. doi: 10.1016/0006-8993(91)91461-9. [DOI] [PubMed] [Google Scholar]
- 59.Wallace TL, Gudelsky GA, Vorhees CV. Methamphetamine-induced neurotoxicity alters locomotor activity, stereotypic behavior and stimulated dopamine release in the rat. J Neurosci. 1999;19:9141–9148. doi: 10.1523/JNEUROSCI.19-20-09141.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Davidson C, Gow AJ, Lee TH, Ellinwood EH. Methamphetamine neurotoxicity: Necrotic and apoptotic mechanisms and relevance to human abuse and treatment. Brain Res Brain Res Rev. 2001;36:1–22. doi: 10.1016/s0165-0173(01)00054-6. [DOI] [PubMed] [Google Scholar]
- 61.Simon SL, Domier C, Carnell J, Brethen P, Rawson R, Ling W. Cognitive impairment in individuals currently using methamphetamine. Am J Addict. 2000;9:222–231. doi: 10.1080/10550490050148053. [DOI] [PubMed] [Google Scholar]
- 62.Bisagno V, Ferguson D, Luine VN. Short toxic methamphetamine schedule impairs object recognition task in male rats. Brain Res. 2002;940:95–101. doi: 10.1016/s0006-8993(02)02599-4. [DOI] [PubMed] [Google Scholar]
- 63.Schroder N, O'Dell SJ, Marshall JF. Neurotoxic methamphetamine regimen severely impairs recognition memory in rats. Synapse. 2003;49:89–96. doi: 10.1002/syn.10210. [DOI] [PubMed] [Google Scholar]
- 64.Jayanthi S, Deng X, Bordelon M, McCoy MT, Cadet JL. Methamphetamine causes differential regulation of pro-death and anti-death bcl-2 genes in the mouse neocortex. FASEB J. 2001;15:1745–1752. doi: 10.1096/fj.01-0025com. [DOI] [PubMed] [Google Scholar]
- 65.Cadet JL, Ordonez SV, Ordonez JV. Methamphetamine induces apoptosis in immortalized neural cells: Protection by the proto-oncogene, bcl-2. Synapse. 1997;25:176–184. doi: 10.1002/(SICI)1098-2396(199702)25:2<176::AID-SYN8>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 66.Kita T, Wagner GC, Nakashima T. Current research on methamphetamine-induced neurotoxicity: Animal models of monoamine disruption. J Pharmacol Sci. 2003;92:178–195. doi: 10.1254/jphs.92.178. [DOI] [PubMed] [Google Scholar]
- 67.Howard S, Bottino C, Brooke S, Cheng E, Giffard RG, Sapolsky R. Neuroprotective effects of bcl-2 overexpression in hippocampal cultures: Interactions with pathways of oxidative damage. J Neurochem. 2002;83:914–923. doi: 10.1046/j.1471-4159.2002.01198.x. [DOI] [PubMed] [Google Scholar]
- 68.Wang H, Xu H, Dyck LE, Li XM. Olanzapine and quetiapine protect PC12 cells from beta-amyloid peptide(25-35)-induced oxidative stress and the ensuing apoptosis. J Neurosci Res. 2005;81:572–580. doi: 10.1002/jnr.20570. [DOI] [PubMed] [Google Scholar]
- 69.Bowyer JF, Tank AW, Newport GD, Slikker W, Jr, Ali SF, Holson RR. The influence of environmental temperature on the transient effects of methamphetamine on dopamine levels and dopamine release in rat striatum. J Pharmacol Exp Ther. 1992;260:817–824. [PubMed] [Google Scholar]
- 70.Fukumura M, Cappon GD, Pu C, Broening HW, Vorhees CV. A single dose model of methamphetamine-induced neurotoxicity in rats: Effects on neostriatal monoamines and glial fibrillary acidic protein. Brain Res. 1998;806:1–7. doi: 10.1016/s0006-8993(98)00656-8. [DOI] [PubMed] [Google Scholar]
- 71.Javitt DC. Negative schizophrenic symptomatology and the PCP (phencyclidine) model of schizophrenia. Hillside J Clin Psychiatry. 1987;9:12–35. [PubMed] [Google Scholar]
- 72.Campbell UC, Lalwani K, Hernandez L, Kinney GG, Conn PJ, Bristow LJ. The mGluR5 antagonist 2-methyl-6-(phenylethynyl)-pyridine (MPEP) potentiates PCP-induced cognitive deficits in rats. Psychopharmacology (Berl) 2004;175:310–318. doi: 10.1007/s00213-004-1827-5. [DOI] [PubMed] [Google Scholar]
- 73.Handelmann GE, Contreras PC, O'Donohue TL. Selective memory impairment by phencyclidine in rats. Eur J Pharmacol. 1987;140:69–73. doi: 10.1016/0014-2999(87)90635-2. [DOI] [PubMed] [Google Scholar]
- 74.Kesner RP, Dakis M, Bolland BL. Phencyclidine disrupts long- but not short-term memory within a spatial learning task. Psychopharmacology (Berl) 1993;111:85–90. doi: 10.1007/BF02257411. [DOI] [PubMed] [Google Scholar]
- 75.Corso TD, Sesma MA, Tenkova TI, Der TC, Wozniak DF, Farber NB, et al. Multifocal brain damage induced by phencyclidine is augmented by pilocarpine. Brain Res. 1997;752:1–14. doi: 10.1016/s0006-8993(96)01347-9. [DOI] [PubMed] [Google Scholar]
- 76.Olney JW, Labruyere J, Price MT. Pathological changes induced in cerebrocortical neurons by phencyclidine and related drugs. Science. 1989;244:1360–1362. doi: 10.1126/science.2660263. [DOI] [PubMed] [Google Scholar]
- 77.Sharp FR, Butman M, Koistinaho J, Aardalen K, Nakki R, Massa SM, et al. Phencyclidine induction of the hsp 70 stress gene in injured pyramidal neurons is mediated via multiple receptors and voltage gated calcium channels. Neuroscience. 1994;62:1079–1092. doi: 10.1016/0306-4522(94)90345-x. [DOI] [PubMed] [Google Scholar]
- 78.Mitchell IJ, Cooper AJ, Griffiths MR, Barber DJ. Phencyclidine and corticosteroids induce apoptosis of a subpopulation of striatal neurons: A neural substrate for psychosis? Neuroscience. 1998;84:489–501. doi: 10.1016/s0306-4522(97)00534-4. [DOI] [PubMed] [Google Scholar]
- 79.Wang C, McInnis J, Ross-Sanchez M, Shinnick-Gallagher P, Wiley JL, Johnson KM. Long-term behavioral and neurodegenerative effects of perinatal phencyclidine administration: Implications for schizophrenia. Neuroscience. 2001;107:535–550. doi: 10.1016/s0306-4522(01)00384-0. [DOI] [PubMed] [Google Scholar]
- 80.Adams JM, Cory S. The bcl-2 protein family: Arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 81.Wang C, Kaufmann JA, Sanchez-Ross MG, Johnson KM. Mechanisms of N-methyl-D-aspartate-induced apoptosis in phencyclidine-treated cultured forebrain neurons. J Pharmacol Exp Ther. 2000;294:287–295. [PubMed] [Google Scholar]
- 82.Pandya DN, Yeterian EH. Proposed neural circuitry for spatial memory in the primate brain. Neuropsychologia. 1984;22:109–122. doi: 10.1016/0028-3932(84)90055-1. [DOI] [PubMed] [Google Scholar]
- 83.Sutherland RJ, Whishaw IQ, Kolb B. Contributions of cingulate cortex to two forms of spatial learning and memory. J Neurosci. 1988;8:1863–1872. doi: 10.1523/JNEUROSCI.08-06-01863.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Griffiths MR, Cooper AJ, Barber DJ, Mitchell IJ. Pharmacological mechanisms mediating phencyclidine-induced apoptosis of striatopallidal neurons: The roles of glutamate, dopamine, acetylcholine and corticosteroids. Brain Res. 2000;855:1–10. doi: 10.1016/s0006-8993(99)01917-4. [DOI] [PubMed] [Google Scholar]
- 85.Hertel P, Mathe JM, Nomikos GG, Iurlo M, Mathe AA, Svensson TH. Effects of D-amphetamine and phencyclidine on behavior and extracellular concentrations of neurotensin and dopamine in the ventral striatum and the medial prefrontal cortex of the rat. Behav Brain Res. 1995;72:103–114. doi: 10.1016/0166-4328(96)00138-6. [DOI] [PubMed] [Google Scholar]
- 86.Farber NB, Price MT, Labruyere J, Nemnich J, St Peter H, Wozniak DF, et al. Antipsychotic drugs block phencyclidine receptor-mediated neurotoxicity. Biol Psychiatry. 1993;34:119–121. doi: 10.1016/0006-3223(93)90265-f. [DOI] [PubMed] [Google Scholar]
- 87.Olney JW, Farber NB. Efficacy of clozapine compared with other antipsychotics in preventing NMDA-antagonist neurotoxicity. J Clin Psychiatry. 1994;55:43–46. [PubMed] [Google Scholar]
- 88.Seeman P. Atypical antipsychotics: Mechanism of action. Can J Psychiatry. 2002;47:27–38. [PubMed] [Google Scholar]
- 89.Bai O, Chlan-Fourney J, Bowen R, Keegan D, Li XM. Expression of brain-derived neurotrophic factor mRNA in rat hippocampus after treatment with antipsychotic drugs. J Neurosci Res. 2003;71:127–131. doi: 10.1002/jnr.10440. [DOI] [PubMed] [Google Scholar]
- 90.Ishihara H, Martin BL, Brautigan DL, Karaki H, Ozaki H, Kato Y, et al. Calyculin A and okadaic acid: Inhibitors of protein phosphatase activity. Biochem Biophys Res Commun. 1989;159:871–877. doi: 10.1016/0006-291x(89)92189-x. [DOI] [PubMed] [Google Scholar]
- 91.Cohen P, Holmes CF, Tsukitani Y. Okadaic acid: A new probe for the study of cellular regulation. Trends Biochem Sci. 1990;15:98–102. doi: 10.1016/0968-0004(90)90192-e. [DOI] [PubMed] [Google Scholar]
- 92.Cagnoli CM, Kharlamov E, Atabay C, Uz T, Manev H. Apoptosis induced in neuronal cultures by either the phosphatase inhibitor okadaic acid or the kinase inhibitor staurosporine is attenuated by isoquinolinesulfonamides H-7, H-8 and H-9. J Mol Neurosci. 1996;7:65–76. doi: 10.1007/BF02736849. [DOI] [PubMed] [Google Scholar]
- 93.Van Dam AM, Bol JG, Binnekade R, van Muiswinkel FL. Acute or chronic administration of okadaic acid to rats induces brain damage rather than alzheimer-like neuropathology. Neuroscience. 1998;85:1333–1335. doi: 10.1016/s0306-4522(97)00696-9. [DOI] [PubMed] [Google Scholar]
- 94.He J, Yamada K, Zou LB, Nabeshima T. Spatial memory deficit and neurodegeneration induced by the direct injection of okadaic acid into the hippocampus in rats. J Neural Transm. 2001;108:1435–1443. doi: 10.1007/s007020100018. [DOI] [PubMed] [Google Scholar]
- 95.Arendt T, Holzer M, Fruth R, Bruckner MK, Gartner U. Phosphorylation of tau, abetaformation, and apoptosis after in vivo inhibition of PP-1 and PP-2A. Neurobiol Aging. 1998;19:3–13. doi: 10.1016/s0197-4580(98)00003-7. [DOI] [PubMed] [Google Scholar]
- 96.Arendt T, Holzer M, Fruth R, Bruckner MK, Gartner U. Paired helical filament-like phosphorylation of tau, deposition of beta/A4-amyloid and memory impairment in rat induced by chronic inhibition of phosphatase 1 and 2A. Neuroscience. 1995;69:691–698. doi: 10.1016/0306-4522(95)00347-l. [DOI] [PubMed] [Google Scholar]
- 97.Zhao W, Bennett P, Sedman GL, NG KT. The impairment of long-term memory formation by the phosphatase inhibitor okadaic acid. Brain Res Bull. 1995;36:557–561. doi: 10.1016/0361-9230(94)00244-u. [DOI] [PubMed] [Google Scholar]
- 98.Tian Q, Lin ZQ, Wang XC, Chen J, Wang Q, Gong CX, et al. Injection of okadaic acid into the meynert nucleus basalis of rat brain induces decreased acetylcholine level and spatial memory deficit. Neuroscience. 2004;126:277–284. doi: 10.1016/j.neuroscience.2004.03.037. [DOI] [PubMed] [Google Scholar]
- 99.He J, Yang Y, Xu H, Zhang X, Li XM. Olanzapine attenuates the okadaic acid-induced spatial memory impairment and hippocampal cell death in rats. Neuropsychopharmacology. 2005;30:1511–1520. doi: 10.1038/sj.npp.1300757. [DOI] [PubMed] [Google Scholar]
- 100.Sweatt JD. Hippocampal function in cognition. Psychopharmacology (Berl) 2004;174:99–110. doi: 10.1007/s00213-004-1795-9. [DOI] [PubMed] [Google Scholar]
- 101.Alvarado MC, Bachevalier J. Selective neurotoxic damage to the hippocampal formation impairs performance of the transverse patterning and location memory tasks in rhesus macaques. Hippocampus. 2005;15:118–131. doi: 10.1002/hipo.20037. [DOI] [PubMed] [Google Scholar]
- 102.Benito A, Lerga A, Silva M, Leon J, Fernandez-Luna JL. Apoptosis of human myeloid leukemia cells induced by an inhibitor of protein phosphatases (okadaic acid) is prevented by bcl-2 and bcl-X(L) Leukemia. 1997;11:940–944. doi: 10.1038/sj.leu.2400699. [DOI] [PubMed] [Google Scholar]
- 103.Cabado AG, Leira F, Vieytes MR, Vieites JM, Botana LM. Cytoskeletal disruption is the key factor that triggers apoptosis in okadaic acid-treated neuroblastoma cells. Arch Toxicol. 2004;78:74–85. doi: 10.1007/s00204-003-0505-4. [DOI] [PubMed] [Google Scholar]
- 104.Nuydens R, Dispersyn G, Van Den Keiboom G, de Jong M, Connors R, Ramaekers F, et al. Bcl-2 protects against apoptosis-related microtubule alterations in neuronal cells. Apoptosis. 2000;5:43–51. doi: 10.1023/a:1009685609275. [DOI] [PubMed] [Google Scholar]
- 105.Manna SK, Zhang HJ, Yan T, Oberley LW, Aggarwal BB. Overexpression of manganese superoxide dismutase suppresses tumor necrosis factor-induced apoptosis and activation of nuclear transcription factor-kappaB and activated protein-1. J Biol Chem. 1998;273:13245–13254. doi: 10.1074/jbc.273.21.13245. [DOI] [PubMed] [Google Scholar]
- 106.Li XM, Chlan-Fourney J, Juorio AV, Bennett VL, Shrikhande S, Keegan DL, et al. Differential effects of olanzapine on the gene expression of superoxide dismutase and the low affinity nerve growth factor receptor. J Neurosci Res. 1999;56:72–75. doi: 10.1002/(SICI)1097-4547(19990401)56:1<72::AID-JNR9>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 107.Matias WG, Traore A, Bonini M, Sanni A, Creppy EE. Oxygen reactive radicals production in cell culture by okadaic acid and their implication in protein synthesis inhibition. Hum Exp Toxicol. 1999;18:634–639. doi: 10.1191/096032799678839473. [DOI] [PubMed] [Google Scholar]
- 108.Ichikawa J, Dai J, O'Laughlin IA, Fowler WL, Meltzer HY. Atypical, but not typical, antipsychotic drugs increase cortical acetylcholine release without an effect in the nucleus accumbens or striatum. Neuropsychopharmacology. 2002;26:325–339. doi: 10.1016/S0893-133X(01)00312-8. [DOI] [PubMed] [Google Scholar]
- 109.Shirazi-Southall S, Rodriguez DE, Nomikos GG. Effects of typical and atypical antipsychotics and receptor selective compounds on acetylcholine efflux in the hippocampus of the rat. Neuropsychopharmacology. 2002;26:583–594. doi: 10.1016/S0893-133X(01)00400-6. [DOI] [PubMed] [Google Scholar]
- 110.Kurz AF. Uncommon neurodegenerative causes of dementia. Int Psychogeriatr. 2005;17:35–49. doi: 10.1017/s1041610205001936. [DOI] [PubMed] [Google Scholar]
- 111.Nunn JA, LePeillet E, Netto CA, Hodges H, Gray JA, Meldrum BS. Global ischaemia: Hippocampal pathology and spatial deficits in the water maze. Behav Brain Res. 1994;62:41–54. doi: 10.1016/0166-4328(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 112.Chemerinski E, Levine SR. Neuropsychiatric disorders following vascular brain injury. Mt Sinai J Med. 2006;73:1006–1014. [PubMed] [Google Scholar]
- 113.Williams LS, Ghose SS, Swindle RW. Depression and other mental health diagnoses increase mortality risk after ischemic stroke. Am J Psychiatry. 2004;161:1090–1095. doi: 10.1176/appi.ajp.161.6.1090. [DOI] [PubMed] [Google Scholar]
- 114.Bokura H, Robinson RG. Long-term cognitive impairment associated with caudate stroke. Stroke. 1997;28:970–975. doi: 10.1161/01.str.28.5.970. [DOI] [PubMed] [Google Scholar]
- 115.Bradvik B, Sonesson B, Holtas S. Spatial impairment following right hemisphere transient ischaemic attacks in patients without carotid artery stenosis. Acta Neurol Scand. 1989;80:411–418. doi: 10.1111/j.1600-0404.1989.tb03902.x. [DOI] [PubMed] [Google Scholar]
- 116.Desmond DW, Moroney JT, Sano M, Stern Y. Incidence of dementia after ischemic stroke: Results of a longitudinal study. Stroke. 2002;33:2254–2260. doi: 10.1161/01.str.0000028235.91778.95. [DOI] [PubMed] [Google Scholar]
- 117.Desmond DW, Moroney JT, Sano M, Stern Y. Mortality in patients with dementia after ischemic stroke. Neurology. 2002;59:537–543. doi: 10.1212/wnl.59.4.537. [DOI] [PubMed] [Google Scholar]
- 118.Martin LJ. Neuronal cell death in nervous system development, disease and injury (review) Int J Mol Med. 2001;7:455–478. [PubMed] [Google Scholar]
- 119.Caccamo D, Campisi A, Curro M, Li Volti G, Vanella A, Ientile R. Excitotoxic and post-ischemic neurodegeneration: Involvement of transglutaminases. Amino Acids. 2004;27:373–379. doi: 10.1007/s00726-004-0117-1. [DOI] [PubMed] [Google Scholar]
- 120.Hartman RE, Lee JM, Zipfel GJ, Wozniak DF. Characterizing learning deficits and hippocampal neuron loss following transient global cerebral ischemia in rats. Brain Res. 2005;1043:48–56. doi: 10.1016/j.brainres.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 121.Jaspers RM, Block F, Heim C, Sontag KH. Spatial learning is affected by transient occlusion of common carotid arteries (2VO): Comparison of behavioural and histopathological changes after ‘2VO’ and ‘four-vessel-occlusion’ in rats. Neurosci Lett. 1990;117:149–153. doi: 10.1016/0304-3940(90)90135-v. [DOI] [PubMed] [Google Scholar]
- 122.Yan B, Bi X, He J, Zhang Y, Thakur S, Xu H, et al. Quetiapine attenuates spatial memory impairment and hippocampal neurodegeneration induced by bilateral common carotid artery occlusion in mice. Life Sci. 2007;81:353–361. doi: 10.1016/j.lfs.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 123.Yan B, He J, Xu H, Zhang Y, Bi X, Thakur S, et al. Quetiapine attenuates the depressive and anxiolytic-like behavioural changes induced by global cerebral ischemia in mice. Behav Brain Res. 2007;182:36–41. doi: 10.1016/j.bbr.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 124.Yan B, Wang DY, Xing DM, Ding Y, Wang RF, Lei F, et al. The antidepressant effect of ethanol extract of radix puerariae in mice exposed to cerebral ischemia reperfusion. Pharmacol Biochem Behav. 2004;78:319–325. doi: 10.1016/j.pbb.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 125.Bryer JB, Starkstein SE, Votypka V, Parikh RM, Price TR, Robinson RG. Reduction of CSF monoamine metabolites in poststroke depression: A preliminary report. J Neuropsychiatry Clin Neurosci. 1992;4:440–442. doi: 10.1176/jnp.4.4.440. [DOI] [PubMed] [Google Scholar]
- 126.Mayberg HS, Parikh RM, Morris PL, Robinson RG. Spontaneous remission of post-stroke depression and temporal changes in cortical S2-serotonin receptors. J Neuropsychiatry Clin Neurosci. 1991;3:80–83. doi: 10.1176/jnp.3.1.80. [DOI] [PubMed] [Google Scholar]
- 127.Yuen EC, Mobley WC. Therapeutic potential of neurotrophic factors for neurological disorders. Ann Neurol. 1996;40:346–354. doi: 10.1002/ana.410400304. [DOI] [PubMed] [Google Scholar]
- 128.Parikh V, Evans DR, Khan MM, Mahadik SP. Nerve growth factor levels in never medicated first episode psychotic patients and medicated chronic schizophrenic patients. Schizophr Res. 2003;60:117–123. doi: 10.1016/s0920-9964(02)00434-6. [DOI] [PubMed] [Google Scholar]
- 129.Fumagalli F, Molteni R, Bedogni F, Gennarelli M, Perez J, Racagni G, et al. Quetiapine regulates FGF-2 and BDNF expression in the hippocampus of animals treated with MK-801. NeuroReport. 2004;15:2109–2112. doi: 10.1097/00001756-200409150-00022. [DOI] [PubMed] [Google Scholar]
- 130.Bruckheimer EM, Cho SH, Sarkiss M, Herrmann J, McDonnell TJ. The bcl-2 gene family and apoptosis. Adv Biochem Eng Biotechnol. 1998;62:75–105. doi: 10.1007/BFb0102306. [DOI] [PubMed] [Google Scholar]
- 131.Lieberman JA, Tollefson GD, Charles C, Zipursky R, Sharma T, Kahn RS, et al. Antipsychotic drug effects on brain morphology in first-episode psychosis. Arch Gen Psychiatry. 2005;62:361–370. doi: 10.1001/archpsyc.62.4.361. [DOI] [PubMed] [Google Scholar]
- 132.Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- 133.Kodama M, Fujioka T, Duman RS. Chronic olanzapine or fluoxetine administration increases cell proliferation in hippocampus and prefrontal cortex of adult rat. Biol Psychiatry. 2004;56:570–580. doi: 10.1016/j.biopsych.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 134.Wakade CG, Mahadik SP, Waller JL, Chiu FC. Atypical neuroleptics stimulate neurogenesis in adult rat brain. J Neurosci Res. 2002;69:72–79. doi: 10.1002/jnr.10281. [DOI] [PubMed] [Google Scholar]
- 135.Roy NS, Wang S, Jiang L, Kang J, Benraiss A, Harrison-Restelli C, et al. In vitro neurogenesis by progenitor cells isolated from the adult human hippocampus. Nat Med. 2000;6:271–277. doi: 10.1038/73119. [DOI] [PubMed] [Google Scholar]
- 136.Bruel-Jungerman E, Rampon C, Laroche S. Adult hippocampal neurogenesis, synaptic plasticity and memory: Facts and hypotheses. Rev Neurosci. 2007;18:93–114. doi: 10.1515/revneuro.2007.18.2.93. [DOI] [PubMed] [Google Scholar]
- 137.Newton SS, Duman RS. Neurogenic actions of atypical antipsychotic drugs and therapeutic implications. CNS Drugs. 2007;21:715–725. doi: 10.2165/00023210-200721090-00002. [DOI] [PubMed] [Google Scholar]