Abstract
Hyperoxia is cytotoxic and depresses many cellular metabolic functions including protein synthesis. Translational control is exerted primarily during initiation by two mechanisms: 1) through inhibition of translation initiation complex formation via sequestration of the cap-binding protein, eukaryotic initiation factor (eIF) 4E, with inhibitory 4E-binding proteins (4E-BP); and 2) by prevention of formation and eIF2B activity by phosphorylated eIF2α. In this report, exposure of human lung fibroblasts to 95% O2 decreased the incorporation of thymidine into DNA at 6 h and the incorporation of leucine into protein beginning at 12 h. The reductions in DNA and protein synthesis were accompanied by increased phosphorylation of eIF4E protein and reduced phosphorylation of 4E-BP1. At 24 h, hyperoxia shifted 4E-BP1 phosphorylation to lesser-phosphorylated isoforms, increased eIF4E expression, and increased the association of eIF4E with 4E-BP1. Although hyperoxia did not change eIF2α expression, it increased its phosphorylation at Ser51, but not until 48 h. In addition, the activation of eIF2α was not accompanied by the formation of stress granules. These findings suggest that hyperoxia diminishes protein synthesis by increasing eIF4E phosphorylation and enhancing the affinity of 4E-BP1 for eIF4E.
Keywords: eukaryotic translation initiation factors, protein synthesis, lung, stress granules
Exposure of the developing lung to high concentrations of inspired oxygen is known to inhibit lung growth, both acutely and chronically (7, 26). Cell culture and whole animal studies indicate that hyperoxia inhibits not only DNA synthesis but also protein synthesis (2, 7, 15, 23). In adult rats breathing 95% O2, the fractional rate of protein synthesis and the rate of protein synthesis per ribosome are depressed by 30% within 6 h (7). Similarly, whole lung slices from rats exposed to hyperoxia for 24 and 48 h show 25% reductions in protein synthetic capacity of both total protein and newly synthesized proteins (15). At the cellular level, Jornot and colleagues (12) revealed that exposure to 95% O2 inhibited the incorporation of leucine into protein, delayed the ribosomal transit time, and marginally increased the polysome content of cultured endothelial cells. Yet despite the near universality of the inhibitory effect of hyperoxia on protein synthesis, few reports have focused on the mechanisms whereby hyperoxia hinders translation.
In mammalian cells, translation is principally regulated by a family of eukaryotic initiation factors (eIF) that control the assembly of the ribosome on the mRNA. The efficiency of ribosome/mRNA binding is governed by the availability of the 5′ m7-GpppX (where X is any nucleotide) mRNA cap binding protein, eIF4E, which, together with eIF4G and eIF4A, comprises the heterotrimeric eIF4F initiation complex (reviewed in Refs. 29 and 34). The eIF4F complex, along with eIF3, facilitates the assembly of the 43S ribosomal subunit on the 5′ end of the mRNA. The low abundance of eIF4E relative to the other 4F components renders it rate limiting for eIF4F formation. The level of free eIF4E is regulated by a group of repressor proteins, known as 4E-binding proteins (4E-BP), that serve to exclude eIF4E from the initiation complex by competing for the eIF4G binding site. In turn, the affinity of 4E-BP for eIF4E is diminished through phosphorylation of numerous residues, of which Ser65 appears to be of central importance (10, 24). eIF4E activity is also regulated by its own phosphorylation. The majority of stimuli that enhance translation increases the phosphorylation of eIF4E (reviewed in Ref. 34). Although phosphorylated eIF4E predominates in the eIF4F complex and the inability to phosphorylate eIF4E stunts Drosophila larval development, in vitro analysis indicates that phosphorylation at Ser209 actually reduces cap affinity 10-fold (19, 36). At present, therefore, the precise role of eIF4E phosphorylation in translational regulation remains to be determined.
Initiation is also regulated by the degree of phosphorylation of eIF2α. eIF2α is a subunit of eIF2, the factor responsible for bringing the initial to the 40S subunit (reviewed in Ref. 11). Phosphorylation of eIF2α converts eIF2 from a substrate to a competitive inhibitor of the guanine-nucleotide exchange factor, eIF2B, and thereby prevents the regeneration of GTP necessary to form . This regulatory pathway appears to be particularly relevant to hyperoxia as it is commonly activated by stress conditions including hypoxia, heat shock, and starvation. These stressors trigger the unfolded protein response within the endoplasmic reticulum and induce the formation of discrete cytoplasmic foci of untranslated mRNA, eIFs, and protein, known as stress granules (13). Stress granules have been theorized to represent stalled translation initiation complexes, thereby representing an additional regulatory pathway within initiation (13, 17).
If the initiation phase proceeds unimpeded, translation may still be regulated at the level of peptide chain elongation. The principal regulatory pathway during elongation occurs via the phosphorylation of eEF2 at Ser59. Activation of this site inhibits peptide chain lengthening by blocking peptidyl-tRNA translocation (reviewed in Ref. 4).
Given the paucity of information surrounding hyperoxia-mediated repression of protein synthesis, coupled with our previous report that H2O2 alters the phosphorylation of eIF4E and 4E-BP1 in a lung cancer cell line, we sought to investigate the translation initiation factors that regulate protein synthesis during prolonged hyperoxia in normal lung fibroblasts (32). Our results demonstrate that the hyperoxia-mediated decrements in protein synthesis result from an increase in eIF4E phosphorylation and a reduction in 4E-BP1 phosphorylation.
MATERIALS AND METHODS
Reagents and supplies
Medium was purchased from Cellgro/Mediatech (Herndon, VA). Antibodies were purchased as follows: 4E-BP1, phospho(Ser65)-4E-BP1, phospho(Ser51)-eIF2α, eEF2, and phospho(Ser59)-eEF2 were from Cell Signaling Technologies (Beverly, MA); eIF4E was from BD/Transduction Laboratories (San Diego, CA); eIF2α was from BioSource International (Camarillo, CA); phospho(Ser209)-eIF4E was from Cell Signaling Technologies or Santa Cruz Biotechnology (Santa Cruz, CA); eIF4G was from Santa Cruz; T-cell intracytoplasmic antigen, cytotoxic granule-associated RNA binding protein (TIA-1) was from Abcam (Cambridge, MA); and goat anti-mouse Cy2-conjugated IgG was from Jackson ImmunoResearch Laboratories (West Grove, PA). Tritiated leucine and thymidine, mouse and rabbit horseradish peroxidase IgG, and m7-GTP-Sepharose beads were obtained from Amersham Pharmacia Biotech (Piscataway, NJ). Bis-Tris and Tris-acetate gels were purchased from Invitrogen (Carlsbad, CA), and chemiluminescence detection was received from Perkin-Elmer (Boston, MA). Thapsigargin was obtained from LC Laboratories (Woburn, MA). Protein concentrations were determined using the bicinchoninic acid (BCA) assay kit from Pierce Biotechnology (Rockford, IL). The remaining reagents and chemicals were obtained from Sigma Chemical (St. Louis, MO).
Cell culture and conditions
Nontransformed, human lung fibroblasts (passages 4–10), obtained from a several-month-old infant, were utilized for all experiments (35). Cells were grown in a 1:1 mixture of MEM for cell suspension and Leibowitz L-15 medium supplemented with 10% FBS, 100 IU/ml penicillin, 100 mg/ml streptomycin, and 2 mM glutamine. Cells were allowed to attach overnight, and the following morning, medium was refreshed and plates were divided into room air control (CON) and hyperoxic (OX) groups. Plates in the CON group were returned to the incubator to grow in room air plus 5% CO2, while plates in the OX group were loaded into a humidified, sealed chamber (Billups-Rothenberg, Del Mar, CA) and flushed twice daily for 15 min with a mixture of 95% O2-5% CO2.
Proliferation
Cell number was determined by counting cells on a hemacytometer. Percent viability was assessed via exclusion of 0.4% trypan blue (50:50 vol/vol). Synthesis of DNA was determined by thymidine incorporation in cells incubated with 2 μCi/ml of [3-methyl-3H]thymidine. Cells were labeled with thymidine for the final hour of each 6-h interval during the first 24 h and also for the entirety of each 24-h period. After incubation, monolayers were rinsed with ice-cold PBS before precipitation with 10% trichloroacetic acid for 15 min. Precipitated proteins were then dissolved in 0.5 N NaOH, and the corresponding radioactivity was measured by liquid scintillation counting.
Flow cytometry was used to assess DNA content. The combination of floating and attached cells was rinsed twice with ice-cold PBS and fixed in cold 70% ethanol. After fixation, cells were stained for 30 min with propidium iodine (10 μg/ml) plus RNase A (250 μg/ml). Fixed cells were analyzed within 4 h of staining on a FACscan flow cytometer (Becton-Dickinson, San Jose, CA).
Protein synthesis/content
Protein synthesis was assessed by the incorporation of [4,5-3H]leucine (4 μCi/ml) into cells labeled during the final hour of each 6-h interval during the first 24 h and for the entire 24 h of each 24-h period. Samples were then handled as for thymidine, and counts were normalized to micrograms of protein. Protein concentrations were determined using the colorimetric BCA assay.
Immunoblotting
Cell monolayers were rinsed with PBS, trypsinized, and counted on a hemacytometer. Duplicate wells were then lysed in buffer A [125 mM Tris · HCl, pH 6.8, 4% SDS, 10% glycerol, 2% 2-mercaptoethanol, 1 mM 4-(2-aminoethyl)benzenesul-fonylfluoride-HCl (AEBSF), 0.8 μM aprotinin, 50 μM bestatin, 15 μM E-64, 20 μM leupeptin, and 10 μM pepstatin A (final concentrations)] at a ratio of 2,000 cells/μl. Protein extracts from equal cell numbers were resolved on either 12% or 4–12% Bis-Tris or 3–8% Tris-acetate gels. Resolved proteins were transferred to polyvinylidene difluoride (PVDF) membranes and incubated overnight at 4°C with primary antibodies (all at 1:1,000, except β-actin at 1:5,000) in blocking buffer. After being rinsed, membranes were incubated with the corresponding horseradish peroxidase-conjugated secondary antibody (1:5,000), and the signal was detected by chemiluminescence. Values were normalized to β-actin expression and listed as change from baseline (time 0) values.
m7-GTP affinity chromatography
To purify eIF4E, monolayers were rinsed with ice-cold PBS and scraped into buffer B (0.1% Nonidet P-40, 10 mM HEPES-KOH, pH 7.9, 10 mM KCl, 0.1 mM EDTA, 0.5 mM DTT, 2 mM MgCl2, 0.5 mM PMSF, 0.5 M sucrose, 1 mM AEBSF, 0.8 μM aprotinin, 50 μM bestatin, 15 μM E-64, 20 μM leupeptin, and 10 μM pepstatin A) in a ratio of 2,000 cells/μl of buffer. After incubation for 10 min on ice, lysates were sonicated on high power (four 5-s cycles) and pelleted at 10,000 g for 10 min. Lysates from equal cell numbers were then added to 25 μl of m7-GTP-Sepharose beads in 250 μl of buffer C (20 mM Tris·HCl, pH 7.4, 0.2 mM EDTA, 100 mM KCl, 1 mM PMSF, and 7 mM 2-mercaptoethanol) and incubated for 2 h at 4°C with agitation. The beads were pelleted and washed three times with buffer C. Thereafter, bound proteins were removed from the beads by being boiled in 20 μl of buffer D (50 mM Tris·HCl, pH 6.8, 100 mM DTT, 2% SDS, 0.1% bromphenol blue, and 10% glycerol). Eluted proteins were then resolved by PAGE using 3–8% Tris-acetate or 12% Bis-Tris gels. The presence of eIF4G, eIF4E, and 4E-BP1 was subsequently identified by immunoblotting.
Immunofluorescent staining
To assess the effect of hyperoxia on stress granule formation, fibroblasts were grown on plastic slides and exposed to either 95% O2 or room air, and the presence of the stress granules was determined by immunofluorescent microscopy as previously described (14). Briefly, cells were rinsed with PBS and fixed in 2% paraformaldehyde for 10 min. The paraformaldehyde was then removed, and cells were immersed in 100% methanol at −20°C for 10 min. After several rinses in PBS, cells were blocked for 1 h in 5% normal horse serum before overnight incubation with primary antibodies. Monoclonal mouse antibodies were diluted in 2% BSA/PBS at a concentration of 1:150 for both eIF4E and TIA-1. The next morning, cells were washed three times in PBS and incubated with Cy2-conjugated anti-mouse secondary antibody (1:200). Cells were then thoroughly rinsed with PBS, mounted with 30% glycerol, and immediately examined using a Nikon Diaphot-TMD microscope (Tokyo, Japan) equipped with epifluorescence optics and appropriate filters. Digital images were obtained using a QColor3 camera and QCapture software (QImaging, Burnaby, BC, Canada). Incubation of cells with 1 μM thapsigargin for 50 min was used as a positive control for stress granule formation (13). Control studies performed without primary antibodies or secondary antibodies revealed no significant staining with either primary antibody but some nonspecific nuclear staining with Cy2 alone.
Statistics
All studies were performed a minimum of three times. Data were analyzed using either Student’s t-tests or repeated-measures analysis of variance with Fisher’s least significant differences testing to determine individual differences when appropriate. Data are listed as means ±SE, and the level of significance is set at P < 0.05.
RESULTS
Effect of hyperoxia on proliferation
The capacity of hyperoxia to inhibit DNA synthesis is well documented. In the present study, exposure to 95% O2 inhibited cell proliferation by 24 h such that the O2-treated cells displayed no change in cell number thereafter. Decrements in thymidine incorporation became evident at 6 h (CON: 51 ± 2; OX: 31 ±1 dpm/1,000 cells; P < 0.001) and were progressive over time. As previously reported in smooth muscle and epithelial cell lines, the decline in cell proliferation and DNA synthesis was accompanied by a marked prolongation of S phase of the cell cycle and little change in the DNA histograms beyond 24 h (28, 33). The lack of pre-G0 peaks in the DNA histograms and the similarity of trypan blue staining between the groups imply that cell death was unlikely to be responsible for the diminished proliferation. Nonetheless, there was a trend toward slightly lower survival at 72 h in the OX group (CON: 98.5 ± 0.5; OX: 94.0 ± 0.4% viable), which could represent either necrotic or apoptotic events.
Effect of hyperoxia on protein metabolism
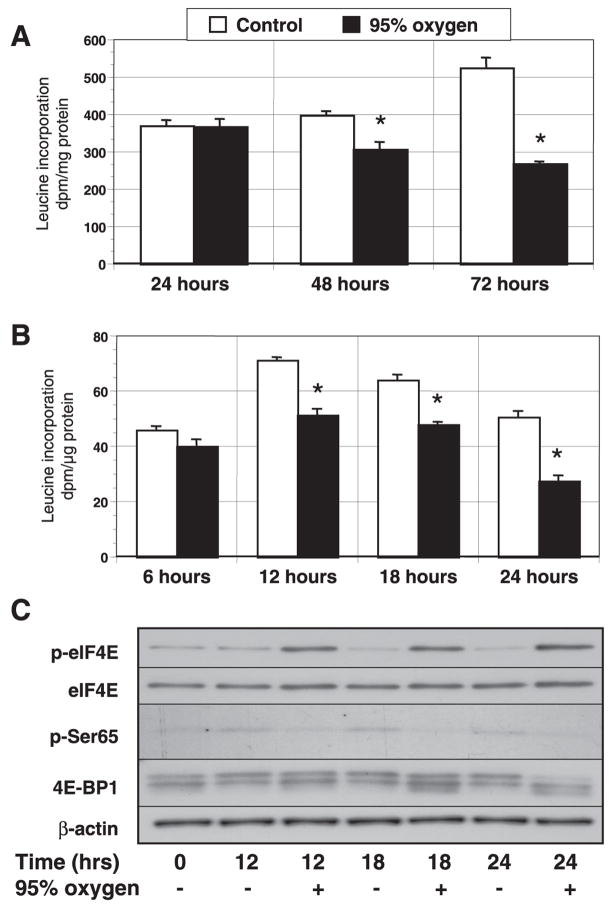
Using a 24-h labeling period, we found that O2 exposure decreased leucine incorporation at 48 and 72 h but not at 24 h (Fig. 1A). Because lengthy labeling periods “dilute” changes in protein synthesis occurring late in the interval and incorporate changes in protein turnover, we shortened both the exposure and labeling periods to examine the final hour of each 6-h interval during the first day. This methodology revealed that hyperoxia did not significantly impact protein synthesis until 12–24 h. During the 24th hour, hyperoxia inhibited protein synthesis by 47% (CON: 51±2 vs. OX: 27 ± 2 dpm/μg protein, P < 0.01; Fig. 1B).
Fig. 1.
Effect of hyperoxia on protein synthesis in lung fibroblasts. Proliferating cells were incubated in room air (control) + 5% CO2 or a mixture of 95% O2 + 5% CO2 for 72 h. Time 0 represents values before the initiation of the study protocol. Cells were labeled with [4,5-methyl-3H]leucine for the entire 24 h (A) or for the last hour of each 6-h interval during the initial 24-h period of exposure (B). After trichloroacetic acid precipitation, cells were dissolved in NaOH, and the radioactivity of the retained label was determined by scintillation (dpm/μg protein). Shown is 1 of 3 similar experiments with n = 4 per time and condition. *Differences between control and 95% oxygen-treated cells, indicating P < 0.05. Columns represent means and bars represent SE. C: representative immunoblot of cell lysates corresponding to control and hyperoxic groups at 12–24 h. Protein extracts from equal cell numbers were separated by SDS-PAGE and transferred to polyvinylidene difluoride (PVDF; n = 3–4) before being probed with eukaryotic initiation factor (eIF)4E, phospho(Ser209) eIF4E, phospho(Ser65) 4E-binding protein (BP)1 (p-Ser65), 4E-BP1, and β-actin antibodies.
Effect of hyperoxia on eIF, eEF, and 4E-BP1 expression and phosphorylation
Translation is principally regulated at the level of initiation through the stabilization of the ribosome with the mRNA, scanning of the ribosomal complex to the start codon, and the introduction of the primary . Key factors in this regulatory cascade include the cap-binding protein eIF4E, the scaffolding protein eIF4G, and the eIF2B regulatory protein eIF2α. To determine whether changes in protein synthesis during hyperoxic exposure were secondary to alterations in initiation factors, we examined the expression, phosphorylation, and complex formation of the principal regulators of initiation.
To screen for factors affected early in the course of hyperoxia, we assessed the expression and phosphorylation of regulators of initiation every 6 h during the first 24 h of hyperoxic exposure. Before 12 h, hyperoxia appeared to have no effect on the expression or phosphorylation of eIF4E, eIF2α, eEF2, 4E-BP1, or eIF4G. At the time corresponding to the decline in leucine incorporation, however, hyperoxia was associated with increased eIF4E phosphorylation, decreased expression of phosphorylated 4E-BP1 at Ser65, contraction of the phosphorylation bands of 4E-BP1, and no change in the phosphorylation of eEF2 or eIF2α (Fig. 1C). These trends were maintained at 18 h.
Effect of prolonged hyperoxia on eIF, eEF, and 4E-BP1 expression, phosphorylation, and binding
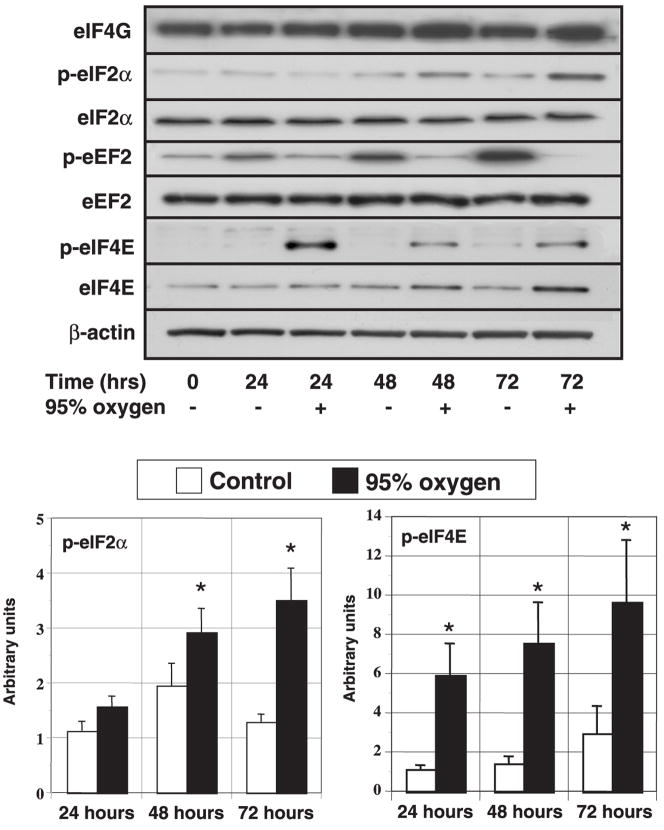
Beyond 24 h, only the expression of eIF4E protein increased with hyperoxia, although the changes were modest (72 h, CON: 1.53 ± 0.42 vs. OX: 2.71 ± 0.75 arbitrary units, P < 0.05). The early enhancement of eIF4E phosphorylation continued with prolonged O2 exposure, being three- to fivefold greater in the OX cells throughout the 72-h study period. Although unaffected acutely, prolonged exposure enhanced the phosphorylation of eIF2α at Ser51 after 24 h, culminating in a 2.5-fold greater level at 72 h (Fig. 2).
Fig. 2.
Effect of prolonged hyperoxic exposure on the expression and phosphorylation of translation initiation and elongation factors. Proliferating human lung fibroblasts were incubated in either room air (control) or 95% O2 for 72 h. Cell number was determined on duplicate wells, and the cells were lysed directly into lysis buffer. Protein extracts from equal cell numbers were subjected to immunoblotting on PVDF membranes (n = 4–8). Top: representative immunoblots. Bottom: histograms show the effect of hyperoxia on the phosphorylation of eIF2α and eIF4E corrected for the expression of β-actin at each time point. Values are expressed in arbitrary units. Columns represent means and bars denote SE. *P < 0.05 between control and 95% oxygen-treated cells.
Because translational regulation also occurs during the elongation phase through the blockade peptidyl-tRNA, we investigated the phosphorylation of eEF2 at Ser59 (reviewed in Ref. 4). As mentioned previously, eEF2 expression was unaffected by hyperoxia during the initial 24 h. Although there was a modest degree of interexperiment variation in the levels of eEF2 phosphorylation beyond 24 h, the collective results revealed an increase in eEF2 phosphorylation in the CON cells relative to time 0 cells and a trend toward greater phosphorylation in CON cells than OX cells (P = 0.087). Because cell density has been shown to directly influence eEF2 phosphorylation, we compared CON and OX cells at similar densities (that is, time 0 cells vs. OX at 24, 48, and 72 h) (27). Using this strategy, we were still unable to show either a positive or negative effect of hyperoxia on eEF2 phosphorylation.
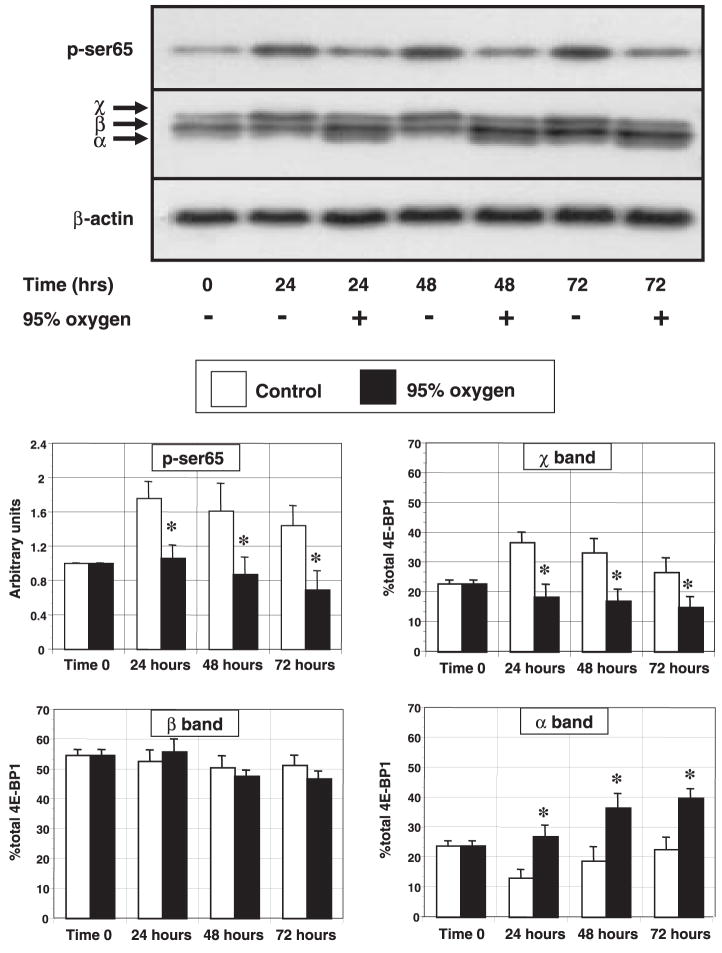
During prolonged hyperoxia, the expression of 4E-BP1 protein was unaltered by time or hyperoxia. The phosphorylation of 4E-BP1 on multiple sites results in the appearance of three to four distinct bands during electrophoresis, with the more slowly migrating species representing more highly phosphorylated isoforms. In control cells, the distribution of the phosphorylation bands shifted to favor the more highly phosphorylated isoforms at 12 h and beyond, perhaps secondarily to the effect of replenishment with fresh medium (Fig. 1C). By contrast, hyperoxia induced a shift in phosphorylation from the highly phosphorylated χ-isoform to the lesser phosphorylated α-isoform, an event subtly appearing within 12 h and becoming quantifiable by 24 h (Fig. 3). To further substantiate the apparent decrease in the highly phosphorylated isoforms during hyperoxia, we immunoblotted lysates with an antibody specific to the Ser65 phosphorylation site of 4E-BP1. Phosphorylation at this site has been consistently associated with the release of eIF4E from 4E-BP1 (29, 34). Using this strategy, we found that hyperoxia decreased the expression of the Ser65 phosphorylated isoform beginning at 12 h. By 24–72 h, OX cells displayed a 37–50% reduction in the expression of the Ser65 phosphorylated isoform compared with control cells and a 30% reduction compared with time 0 (Fig. 4).
Fig. 3.
Effect of prolonged hyperoxic exposure on 4E-BP1 phosphorylation. Growing cells exposed to either room air (control) or 95% O2 were harvested every 24 h and analyzed for the expression of 4E-BP1 and the Ser65 phosphorylated isoform of 4E-BP1. 4E-BP1 migrates as 3–4 distinct bands during electrophoresis secondary to differences in the degree of phosphorylation. Greek letters represent the fastest migrating, least phosphorylated isoform (α) through the slowest migrating, most phosphorylated isoform (χ). Top: representative immunoblots for Ser65 4E-BP1 and total 4E-BP1. Bottom: histograms represent the effects of hyperoxia on Ser65 phosphorylation (corrected to β-actin expression, n = 6) and the distribution (% of total band density) of the χ-isoforms of 4E-BP1 (n = 4). Columns represent means and bars denote SE. *P < 0.05 between control and 95% oxygen-treated cells.
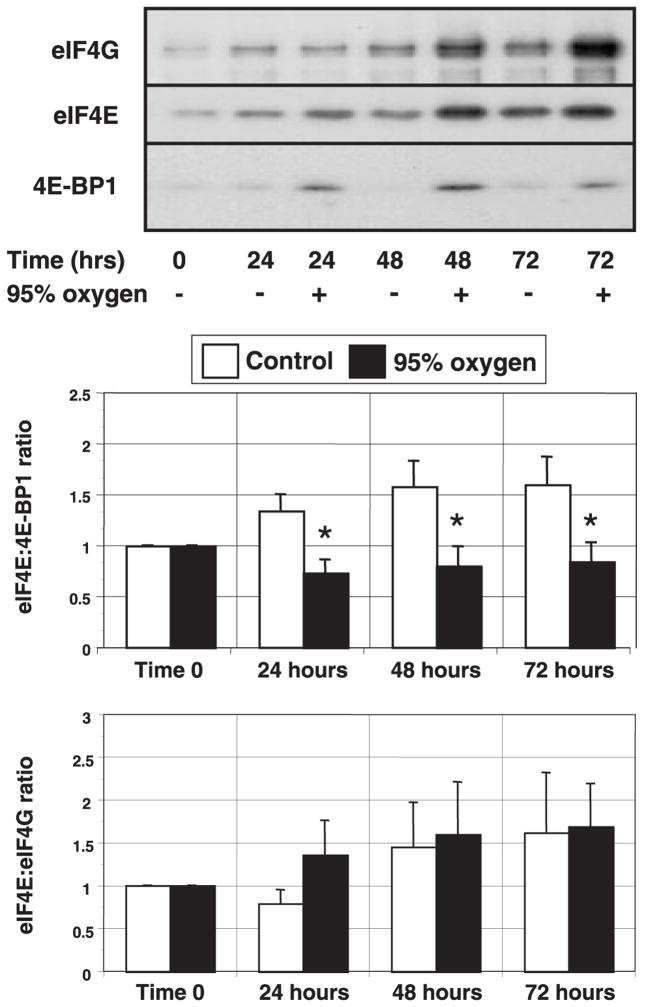
Fig. 4.
Effect of prolonged hyperoxic exposure on eIF4E binding. Proliferating lung fibroblasts incubated in room air (control) or 95% O2 for 72 h were harvested in nonreducing lysis buffer as described in MATERIALS AND METHODS. Lysates from equal cell numbers were then affinity purified using m7-GTP Sepharose. Purified extracts were subjected to electrophoresis, and the resulting immunoblots were probed for eIF4E, eIF4G, and 4E-BP1. Top: characteristic immunoblots of each protein. Bottom: histograms show the relative ratios of eIF4E with 4E-BP1 and eIF4E with eIF4G, calculated as densitometric ratio. Columns represent means and bars denote SE (n = 4). *P < 0.05 between control and 95% oxygen-treated cells.
Effect of hyperoxia on eIF4E:4E-BP1 binding
Control experiments revealed that our m7-GTP affinity chromatography protocol captured at least 90% of eIF4E in the samples and that the bound fraction was free of contamination by β-actin and eIF2α. As determined by immunoblotting (Fig. 2), we noted that OX samples contained 60–80% more eIF4E than samples from the CON group during prolonged hyperoxia. Nevertheless, the densitometric ratios of eIF4E:4E-BP1 were lower at all times in the cells exposed to hyperoxia. One would then expect the ratios of eIF4E:eIF4G to be higher in the OX group. As shown in Fig. 4, the overall eIF4E:eIF4G ratios were not different between the groups, although there was a trend toward a reduction in the amount of eIF4G associated with eIF4E at 24 h in the OX group. It would appear then that the increased expression of eIF4E adequately compensated for the enhanced 4E-BP1 affinity, thereby maintaining consistent ratios of associated eIF4E:eIF4G beyond 24 h.
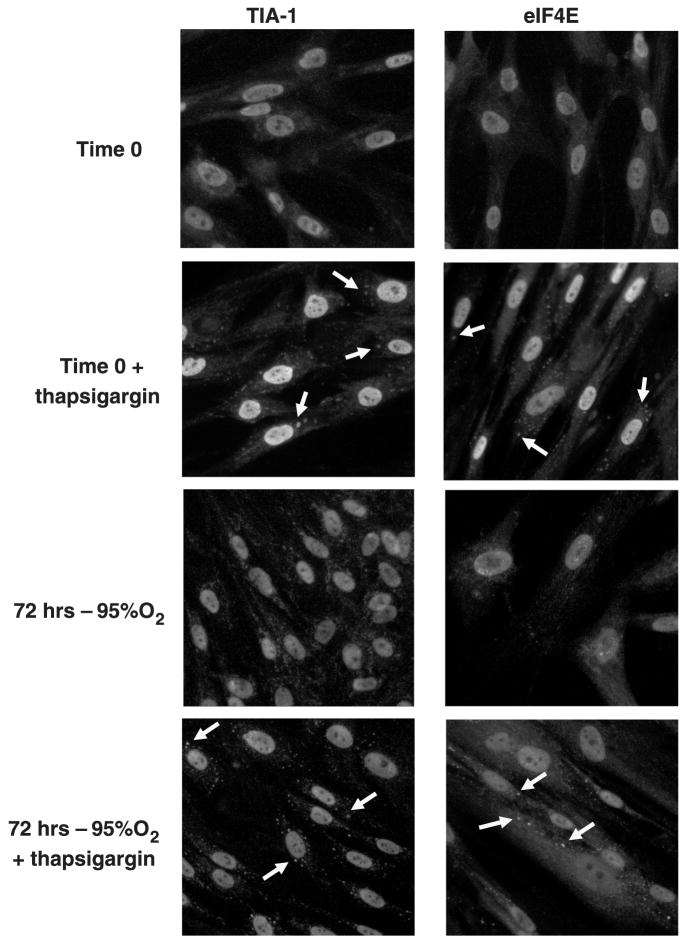
Effect of hyperoxia on stress granule formation
Stress granules represent an additional mechanism regulating translation, whereby formed initiation complexes, along with untranslated mRNA, are shuttled into discrete, cytoplasmic aggregates in response to environmental stress (14). Both RNA-binding proteins [TIA-1, TIAR, poly(A) binding protein-1] and eIFs have been shown to localize to the granules (13). In the current study, TIA-1 and eIF4E immunofluorescence appeared throughout the cytoplasm of the fibroblasts at time 0 (Fig. 5). Upon stimulation with 1 μM thapsigargin, both TIA-1 and eIF4E accumulated into punctate loci within the cytoplasm, a process consistent with stress granule formation (14). In fibroblasts exposed to hyperoxia from 12–72 h, the cytoplasmic distribution of TIA-1 appeared more granular than at baseline, yet stress granules were clearly absent. Likewise, the distribution of eIF4E appeared unaltered by hyperoxia. Nonetheless, upon stimulation with thapsigargin, OX cells readily formed stress granules, indicating that the lack of granule formation during hyperoxia was not secondary to a fundamental inability of the cells to form these aggregates.
Fig. 5.
Effect of hyperoxia on stress granule formation. Growing lung fibroblasts were exposed to room air (control) or 95% O2 for 72 h and fixed with 2% paraformaldehyde/100% methanol as described in MATERIALS AND METHODS. Slides were then incubated overnight with monoclonal antibodies to T-cell intracytoplasmic antigen; cytotoxic granule-associated RNA binding protein (TIA-1) and eIF4E followed by Cy2-labeled secondary antibody. Slides were examined with a Nikon Diaphot-TMD fluorescent microscope, and the digital images were captured using a charge-coupled device camera and imaging software. Time 0 photomicrographs illustrate basal immunostaining of TIA-1 and eIF4E. Treatment with 1 μM thapsigargin for 50 min was used to induce stress granule formation (arrows) at time 0 and every 24 h for 72 h.
DISCUSSION
Exposure of cells to hyperoxia is cytotoxic, secondary to the production of reactive oxygen species, which interact with and modify proteins, lipids, carbohydrates, and DNA. These reactive species include those derived directly from molecular oxygen-superoxide anion, singlet oxygen, H2O2, and hydroxyl radical as well as species formed from secondary reactions with other reactive species or substrates such as lipid peroxides, peroxynitrite, hypochlorous acid, and others (reviewed in Ref. 20). Whereas a great deal has been learned regarding the activation of cell cycle checkpoints and DNA repair pathways by hyperoxia, little attention has been paid to the process whereby hyperoxia impairs translation. The present study found that hyperoxia-induced inhibition of protein synthesis coincided with changes in the expression and phosphorylation of factors controlling translation initiation and not with changes in elongation factors, as has been suggested from studies on ribosomal transit times (12).
Translational control is principally exerted by regulating the integrity of the cap-dependent translation initiation complex. Of the components of the eIF4F complex, eIF4E is considered rate limiting owing to its low abundance relative to eIF4G and eIF4A (reviewed in Ref. 34). The availability of “free” eIF4E is generally regulated by inhibitory 4E-BP, which competes with eIF4G for eIF4E binding. During exposure to hyperoxia, we found an acute reduction in 4E-BP1 phosphorylation, illustrated by the shift from the highly phosphorylated χ-isoforms to the non- or lesser-phosphorylated χ-isoforms and by the reduction in Ser65 phosphorylation. These changes continued for 3 days and correlated with an increase in the proportion of eIF4E associated with 4E-BP1 during that time. Although the diminished 4E-BP1 phosphorylation may signify reductions in specific kinase or phosphatase activities, it may also reflect the observed alterations in cell cycle distribution. Heesom et al. (10) recently reported that bound eIF4E dissociates from 4E-BP1 during mitosis and remains free throughout G1-phase, an event that correlates with the phosphorylation of 4E-BP1 at Ser65. Because only 25% of our hyperoxia-treated cells remained in G1-phase, alterations in the amount of 4E-BP1 associated with eIF4E could simply be a manifestation of the prolonged cell cycle transit time. This would appear unlikely given that inhibition of DNA synthesis occurs by 6 h, a time when alterations in protein synthesis and 4E-BP1 phosphorylation have yet to occur.
Aside from 4E-BP1 binding, the activity of eIF4E is also regulated by eIF4E expression and phosphorylation. Altered expression of eIF4E is a common finding in malignancies of the lung and colon but has not been routinely identified in noncancerous conditions (3, 31). Thus we were surprised to find that hyperoxia increased eIF4E protein expression in both nonpurified cell lysates and m7-GTP-purified lysates. Whereas downregulation of eIF4E protein has been reported in response to TNF-α, cortisone, and ischemia, only mammary gland development appears to enhance eIF4E expression under physiological conditions (5, 8, 21). Nonetheless, given that hyperoxia enhances activator protein (AP)-1 transcription, it is conceivable that binding of AP-1 to the AP-1 motifs in the eIF4E gene could upregulate eIF4E expression (20, 22).
In conjunction with the enhanced expression of eIF4E, we found that hyperoxia induced a fourfold increase in eIF4E phosphorylation at Ser209. Previous studies have shown that H2O2 and oxidized low-density lipoprotein potently phosphorylate eIF4E (6, 27, 32). Phosphorylation of eIF4E is mediated by activation of MAPK interacting kinases 1 and 2 (MNK-1 and -2). These eIF4E kinases lie downstream of hyperoxia-inducible signaling pathways containing ERK1/2 and p38 MAPK (18). Early reports indicated that eIF4E phosphorylation coincided with the formation of stable eIF4F complexes and with greater affinity of eIF4E for the 5′ cap. This view integrated nicely with crystalline structure data illustrating that the phosphorylation of eIF4E at Ser209 would produce a salt bridge with Lys159 to form a functional “clamp” on the mRNA (reviewed in Ref. 34). More recently, however, reports have found both enhanced eIF4E phosphorylation in the absence of increased translation and a fourfold reduction in cap affinity by phosphorylation of eIF4E on Ser209 (30, 36). It is conceivable, therefore, that hyperoxia-mediated increases in eIF4E phosphorylation actually inhibit, rather than promote, translation initiation, as has been demonstrated recently using a low-molecular-weight MNK-1 inhibitor and constitutively active mutants of MNK-1 and -2 (18).
Although the enhanced sequestration of eIF4E by 4E-BP1 and the increased phosphorylation of eIF4E are consistent with the reduction in eIF4F complex formation at 24 h, they do not provide a basis for the reduction in protein synthesis thereafter, given the normalization of eIF4E:eIF4G binding. Similar changes in protein synthesis could occur from the increased phosphorylation of eIF2α, the competitive inhibitor of eIF2B. Both H2O2 and nitric oxide have been shown to increase eIF2α phosphorylation and to block protein synthesis (1, 16). Several kinases phosphorylate eIF2α at Ser51 in response to stress and induce the formation of stress granules, cytoplasmic domains of RNA, and proteins (13, 17). In addition to mRNA, stress granules contain 40S ribosomal subunits, eIF4E, eIF4G, eIF3, poly(A) binding protein, the RNA binding proteins TIA-1 and TIAR, and eIF2α itself (13, 17). As eIF4E:eIF4G binding and the formation of the 48S preinitiation complex precedes binding, enhanced phosphorylation of eIF2α would be expected to stall initiation by inhibiting the activity of eIF2B (17). Under these circumstances, eIF4E:eIF4G binding could be maintained or even enhanced in the face of reduced initiation.
Although the spectrum of alterations in eIF expression and phosphorylation found in the present study fit well into the stress granule model, we were unable to detect stress granules during hyperoxia using TIA-1 and eIF4E as markers (13, 17). Despite this, the fundamental capacity of the cells to form stress granules was maintained, as indicated by the continued response to thapsigargin. These findings suggest that hyperoxia, unlike thapsigargin and ischemia/reperfusion, increases eIF2α phosphorylation through a nonpancreatic endoplasmic reticulum eIF2α kinase-dependent pathway (9, 13). Given that nonphosphorylatable eIF2α mutants ablate stress-induced inhibition of protein synthesis, it would seem improbable that eIF2α phosphorylation does not decrease eIF2B activity (25). Still, measurement of the activities of the specific eIF2α kinases, in addition to that of eIF2B, will be required to determine whether hyperoxia inhibits protein synthesis by an eIF2α-dependent mechanism analogous to the unfolded protein response (13).
In conclusion, the data presented in this study suggest that the regulation of protein synthesis during hyperoxia is complex, involving decreases in 4E-BP1 phosphorylation and the sequestration of eIF4E, increases in the phosphorylation of eIF4E, and possibly the increased phosphorylation of eIF2α. Unresolved issues surrounding eIF2B activity and elongation factors lend caution to the overall interpretation that inhibition of protein synthesis occurs exclusively by these mechanisms. Future studies utilizing isolated eIF and reticulocyte lysates, in conjunction with polysome profiling and eIF4E binding assays, are required to firmly delineate the role of initiation in the inhibition of translation by hyperoxia.
Acknowledgments
This work was supported by National Heart, Lung, and Blood Institute Grant K08-HL-071905 (to J. S. Shenberger).
References
- 1.Alirezaei M, Marin P, Nairn AC, Glowinski J, Premont J. Inhibition of protein synthesis in cortical neurons during exposure to hydrogen peroxide. J Neurochem. 2001;76:1080–1088. doi: 10.1046/j.1471-4159.2001.00105.x. [DOI] [PubMed] [Google Scholar]
- 2.Balin AK, Goodman BP, Rasmussen H, Cristofalo VJ. The effect of oxygen tension on the growth and metabolism of WI-38 cells. J Cell Physiol. 1976;89:235–249. doi: 10.1002/jcp.1040890207. [DOI] [PubMed] [Google Scholar]
- 3.Berkel HJ, Turbat-Herrera EA, Shi R, de Benedetti A. Expression of the translation initiation factor eIF4E in the polyp-cancer sequence in the colon. Cancer Epidemiol Biomarkers Prev. 2001;10:663–666. [PubMed] [Google Scholar]
- 4.Browne GJ, Proud CG. Regulation of peptide-chain elongation in mammalian cells. Eur J Biochem. 2002;269:5360–5368. doi: 10.1046/j.1432-1033.2002.03290.x. [DOI] [PubMed] [Google Scholar]
- 5.Cheema IR, Jones DL. TNF-α and cortisone impair peptide chain initiation by altering the availability of initiation factor EIF-4E. Front Biosci. 2003;8:S1051–S1055. doi: 10.2741/1160. [DOI] [PubMed] [Google Scholar]
- 6.Duncan RF, Peterson H, Hagedorn CH, Sevanian A. Oxidative stress increases eukaryotic initiation factor 4E phosphorylation in vascular cells. Biochem J. 2003;369:213–225. doi: 10.1042/BJ20020435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gacad G, Massaro D. Hyperoxia: influence on lung mechanics and protein synthesis. J Clin Invest. 1973;52:559–565. doi: 10.1172/JCI107216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia L, Burda J, Hrehorovska M, Burda R, Martin ME, Salinas M. Ischaemic preconditioning in the rat brain: effect on the activity of several initiation factors, Akt and extracellular signal-regulated protein kinase phosphorylation, and GRP78 and GADD34 expression. J Neurochem. 2004;88:136–147. doi: 10.1111/j.1471-4159.2004.02188.x. [DOI] [PubMed] [Google Scholar]
- 9.Hayashi T, Saito A, Okuno S, Ferrand-Drake M, Dodd RL, Nishi T, Maier CM, Kinouchi H, Chan PH. Oxidative damage to the endoplasmic reticulum is implicated in ischemic neuronal cell death. J Cereb Blood Flow Metab. 2003;23:1117–1128. doi: 10.1097/01.WCB.0000089600.87125.AD. [DOI] [PubMed] [Google Scholar]
- 10.Heesom KJ, Gampel A, Mellor H, Denton RM. Cell cycle-dependent phosphorylation of the translational repressor eIF-4E binding protein-1 (4E-BP1) Curr Biol. 2001;11:1374–1379. doi: 10.1016/s0960-9822(01)00422-5. [DOI] [PubMed] [Google Scholar]
- 11.Hinnebusch A. Mechanism and regulation of initiator methionyl-tRNA binding to ribosomes. In: Sonenberg NHJ, Hershey JWB, Mathews MB, editors. Translational Control of Gene Expression. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 2000. pp. 185–243. [Google Scholar]
- 12.Jornot L, Mirault ME, Junod AF. Protein synthesis in hyperoxic endothelial cells: evidence for translational defect. J Appl Physiol. 1987;63:457–464. doi: 10.1152/jappl.1987.63.2.457. [DOI] [PubMed] [Google Scholar]
- 13.Kedersha N, Anderson P. Stress granules: sites of mRNA triage that regulate mRNA stability and translatability. Biochem Soc Trans. 2002;30:963–969. doi: 10.1042/bst0300963. [DOI] [PubMed] [Google Scholar]
- 14.Kedersha N, Chen S, Gilks N, Li W, Miller IJ, Stahl J, Anderson P. Evidence that ternary complex [eIF2-GTP-tRNA(i)(Met)]-deficient preinitiation complexes are core constituents of mammalian stress granules. Mol Biol Cell. 2002;13:195–210. doi: 10.1091/mbc.01-05-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelly FJ. Effect of hyperoxic exposure on protein synthesis in the rat. Biochem J. 1988;249:609–612. doi: 10.1042/bj2490609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim YM, Son K, Hong SJ, Green A, Chen JJ, Tzeng E, Hierholzer C, Billiar TR. Inhibition of protein synthesis by nitric oxide correlates with cytostatic activity: nitric oxide induces phosphorylation of initiation factor eIF-2α. Mol Med. 1998;4:179–190. [PMC free article] [PubMed] [Google Scholar]
- 17.Kimball SR, Horetsky RL, Ron D, Jefferson LS, Harding HP. Mammalian stress granules represent sites of accumulation of stalled translation initiation complexes. Am J Physiol Cell Physiol. 2003;284:C273–C284. doi: 10.1152/ajpcell.00314.2002. [DOI] [PubMed] [Google Scholar]
- 18.Knauf U, Tschopp C, Gram H. Negative regulation of protein translation by mitogen-activated protein kinase-interacting kinases 1 and 2. Mol Cell Biol. 2001;21:5500–5511. doi: 10.1128/MCB.21.16.5500-5511.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lachance PE, Miron M, Raught B, Sonenberg N, Lasko P. Phosphorylation of eukaryotic translation initiation factor 4E is critical for growth. Mol Cell Biol. 2002;22:1656–1663. doi: 10.1128/MCB.22.6.1656-1663.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee PJ, Choi AM. Pathways of cell signaling in hyperoxia. Free Radic Biol Med. 2003;35:341–350. doi: 10.1016/s0891-5849(03)00279-x. [DOI] [PubMed] [Google Scholar]
- 21.Long E, Capuco AV, Zhao X. Cloning of bovine eukaryotic translation initiation factor 4E (eIF-4E) and its expression in the bovine mammary gland at different physiological stages. DNA Seq. 2001;12:319–329. doi: 10.3109/10425170109084455. [DOI] [PubMed] [Google Scholar]
- 22.Makhlouf AA, Namboodiri AM, McDermott PJ. Transcriptional regulation of the rat eIF4E gene in cardiac muscle cells: the role of specific elements in the promoter region. Gene. 2001;267:1–12. doi: 10.1016/s0378-1119(01)00399-7. [DOI] [PubMed] [Google Scholar]
- 23.McGrath SA. Induction of p21WAF/CIP1 during hyperoxia. Am J Respir Cell Mol Biol. 1998;18:179–187. doi: 10.1165/ajrcmb.18.2.2964m. [DOI] [PubMed] [Google Scholar]
- 24.Mothe-Satney I, Yang D, Fadden P, Haystead TA, Lawrence JC., Jr Multiple mechanisms control phosphorylation of PHAS-I in five (S/T)P sites that govern translational repression. Mol Cell Biol. 2000;20:3558–3567. doi: 10.1128/mcb.20.10.3558-3567.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murtha-Riel P, Davies MV, Scherer BJ, Choi SY, Hershey JW, Kaufman RJ. Expression of a phosphorylation-resistant eukaryotic initiation factor 2 α-subunit mitigates heat shock inhibition of protein synthesis. J Biol Chem. 1993;268:12946–12951. [PubMed] [Google Scholar]
- 26.Northway WH, Jr, Petriceks R, Shahinian L. Quantitative aspects of oxygen toxicity in the newborn: inhibition of lung DNA synthesis in the mouse. Pediatrics. 1972;50:67–72. [PubMed] [Google Scholar]
- 27.Patel J, McLeod LE, Vries RG, Flynn A, Wang X, Proud CG. Cellular stresses profoundly inhibit protein synthesis and modulate the states of phosphorylation of multiple translation factors. Eur J Biochem. 2002;269:3076–3085. doi: 10.1046/j.1432-1033.2002.02992.x. [DOI] [PubMed] [Google Scholar]
- 28.Rancourt RC, Keng PC, Helt CE, O’Reilly MA. The role of p21(CIP1/WAF1) in growth of epithelial cells exposed to hyperoxia. Am J Physiol Lung Cell Mol Physiol. 2001;280:L617–L626. doi: 10.1152/ajplung.2001.280.4.L617. [DOI] [PubMed] [Google Scholar]
- 29.Raught B, Gingras AC. eIF4E activity is regulated at multiple levels. Int J Biochem Cell Biol. 1999;31:43–57. doi: 10.1016/s1357-2725(98)00131-9. [DOI] [PubMed] [Google Scholar]
- 30.Scheper GC, van Kollenburg B, Hu J, Luo Y, Goss DJ, Proud CG. Phosphorylation of eukaryotic initiation factor 4E markedly reduces its affinity for capped mRNA. J Biol Chem. 2002;277:3303–3309. doi: 10.1074/jbc.M103607200. [DOI] [PubMed] [Google Scholar]
- 31.Seki N, Takasu T, Mandai K, Nakata M, Saeki H, Heike Y, Takata I, Segawa Y, Hanafusa T, Eguchi K. Expression of eukaryotic initiation factor 4E in atypical adenomatous hyperplasia and adenocarcinoma of the human peripheral lung. Clin Cancer Res. 2002;8:3046–3053. [PubMed] [Google Scholar]
- 32.Shenberger JS, Adams MH, Zimmer SG. Oxidant-induced hypertrophy of A549 cells is accompanied by alterations in eukaryotic translation initiation factor 4E and 4E-binding protein-1. Am J Respir Cell Mol Biol. 2002;27:250–256. doi: 10.1165/ajrcmb.27.2.4785. [DOI] [PubMed] [Google Scholar]
- 33.Shenberger JS, Dixon PS. Oxygen induces S-phase growth arrest and increases p53 and p21(WAF1/CIP1) expression in human bronchial smooth muscle cells. Am J Respir Cell Mol Biol. 1999;21:395–402. doi: 10.1165/ajrcmb.21.3.3604. [DOI] [PubMed] [Google Scholar]
- 34.Sonenberg N, Dever TE. Eukaryotic translation initiation factors and regulators. Curr Opin Struct Biol. 2003;13:56–63. doi: 10.1016/s0959-440x(03)00009-5. [DOI] [PubMed] [Google Scholar]
- 35.Stancombe BB, Walsh WF, Derdak S, Dixon P, Hensley D. Induction of human neonatal pulmonary fibroblast cytokines by hyperoxia and Ureaplasma urealyticum. Clin Infect Dis. 1993;17(Suppl 1):S154–S157. doi: 10.1093/clinids/17.supplement_1.s154. [DOI] [PubMed] [Google Scholar]
- 36.Zuberek J, Wyslouch-Cieszynska A, Niedzwiecka A, Dadlez M, Stepinski J, Augustyniak W, Gingras AC, Zhang Z, Burley SK, Sonenberg N, Stolarski R, Darzynkiewicz E. Phosphorylation of eIF4E attenuates its interaction with mRNA 5′ cap analogs by electrostatic repulsion: intein-mediated protein ligation strategy to obtain phosphorylated protein. RNA. 2003;9:52–61. doi: 10.1261/rna.2133403. [DOI] [PMC free article] [PubMed] [Google Scholar]