Abstract
We propose a routine protocol based on size fractionation of pelagic samples and the use of the fluorochrome calcofluor white (which binds to β-1,3 and β-1,4 polysaccharides) for diagnosing, identifying, and counting chitinaceous fungal parasites (i.e., the sporangia of chytrids) of phytoplankton. The protocol was applied to freshwater samples collected during different seasons (spring and summer/autumn) in two lakes whose trophic statuses varied. Because few samples were collected (i.e., two dates per site), the findings are considered preliminary and mainly a “proof of concept” rather than a valid comparison of sites versus seasons. The results from the proposed protocol indicate higher diversity of infected host and parasite communities than in previous studies. Chytrid epidemics were omnipresent, infecting diverse phytoplankton host communities, primarily diatoms, chlorophytes, and colonial and filamentous cyanobacteria. The diversity and numerical abundance of sporangia and of hosts, and the prevalence of infection (range, <1 to 24% of total host cells) as well, increased from the oligotrophic Lake Pavin to the eutrophic Lake Aydat, while the temporal changes in parasites were apparently more influenced by the host community composition. We conclude that the proposed protocol offers a valid method for the quantitative ecology of chytrid epidemics in aquatic ecosystems and food web dynamics.
Fungal infections are recurrent in aquatic ecosystems (15, 42, 46). Organisms belonging to the order of Chytridiales (i.e., chytrids) are known mainly as phytoplankton parasites (10, 22, 44). Recently, we have unveiled a large reservoir of unexpected fungal diversity in freshwater lakes, primarily of chytrids (31-33). Parasitic chytrids and other zoosporic true fungi sensu Barr (3) produce zoospores and are often host specific, highly infective, and extremely virulent (13, 16). They are considered relevant not only for the evolution of their hosts but also for the population dynamics and successions of phytoplankton communities (14, 48). Studies of chytrid fungal parasitism carried out in the English Lake District indicate that infection of diatoms, desmids, and other green algae is fairly common (10). Significant chytrid parasitism has also been recorded in other lakes, affecting primarily the diatom Asterionella formosa (12, 29, 37, 48). However, fungal parasitism on plankton has rarely been studied and has mostly been restricted to descriptive taxonomy. Full descriptions of parasitic chytrids have been given since the middle of the last century (8-9) but even today, their impact on the dynamics of host populations and the related biogeochemical cycling and energetics remain largely unknown (16), mainly because of methodological difficulties (17).
Various approaches have been used to study fungal parasites, but routine techniques for reliably identifying and counting these organisms are lacking in the context of aquatic microbial ecology (31-33). Some of them have been misidentified as protistan bacterivorous nanoflagellates, e.g., flagellates in the group of Bicosoeca, which are attached to phytoplankton but do not harm the algae (31-33). A few studies have demonstrated epidemic outbreaks of chytrids on phytoplankton, but mainly with particular emphasis on host-parasite interactions and coevolution (21, 30, 48). Thus far, observations of parasitic fungi were obtained by using phase-contrast light microscopy with live or Lugol's iodine preserved samples (30, 39, 48). Such conventional microscopy allows observation of fungal sporangia or similar forms (especially in laboratory cultures) but is a poor approach for characterizing chytrid parasites in natural samples, at the complex community level. For example, a simple light microscopy observation of fungal rhizoidal systems, i.e., a pertinent criterion for identifying chytrids (10, 13, 44), is very difficult, a situation which usually leads to confusion of chytrids with protistan flagellates such as choanoflagellates (31-33). Most chytrids occupy the most basal branch of the kingdom Fungi, a finding consistent with choanoflagellate-like ancestors (25, 26).
Methodological limitations for the study of the ecological dynamics of chytrid populations can be overcome with epifluorescence microscopy coupled to a specific fluorochrome targeting molecular tracers (i.e., some types of polysaccharides) of chitinaceous fungal structures, primarily sporangia, the presence of an opercule, and the extent of the rhizoidal system. We propose here a simple procedure based on the fluorochrome calcofluor white (CFW) and epifluorescence microscopy for diagnosing, identifying, and counting parasitic chytrids within phytoplanktonic communities.
MATERIALS AND METHODS
Sites and sampling.
Samples were collected in two freshwater lakes, which differed in trophic status and were located in the French Massif Central. Lake Pavin (45°29′41″N, 002°53′12″E) is an oligotrophic deep volcanic mountain lake (Zmax = 92 m), characterized by small surface (44 ha) and small drainage basin (50 ha) areas. Lake Aydat (45°39′48″N, 002°59′04″E) is a small eutrophic lake (Zmax = 15 m, surface area = 60 ha), with a larger catchment area (3 × 104 ha). For both lakes, samples were taken near the center of the lake, at the point of maximum depth. Lake Pavin was first sampled on 26 October 2006 for testing different methodological protocols, and both lakes were subsequently sampled twice in 2007 for the application of the optimal protocol retained (i.e., the size-fractionated community approach using 1% CFW final concentration; see below) to the natural community dynamics. The latter samples were collected during the spring and autumn (22 March and 2 October 2007) for the oligotrophic Lake Pavin, and during spring and late summer (22 May and 28 August 2007) for the eutrophic Lake Aydat. All samples were collected in triplicates, i.e., from three independent sampling operations per sampling date.
During all sampling occasions, ca. 21 liters of the whole euphotic layers of the two lakes (estimated from Secchi depths) was sampled manually using a flexible plastic tube (diameter, 4 cm) provided by a rope connecting the ballasted bottom of the tube with a surface manipulator. With this system, the euphotic water column samples are collected by simple capillarity. This technique of sampling is rapid, easy, and inexpensive (43). Analytic samples were thus considered as integrated samples representative of the euphotic layers of the lakes, i.e., 0 to 20 m for Lake Pavin and 0 to 5 m for Lake Aydat. Collected samples were immediately prefiltered through a 150-μm-pore-size nylon filter (to eliminate the predatory metazoan zooplankton) when poured into clean transparent recipients previously washed with the lake water and then transferred immediately to the laboratory for processing.
Protocols tested for concentrating and staining fungal parasites.
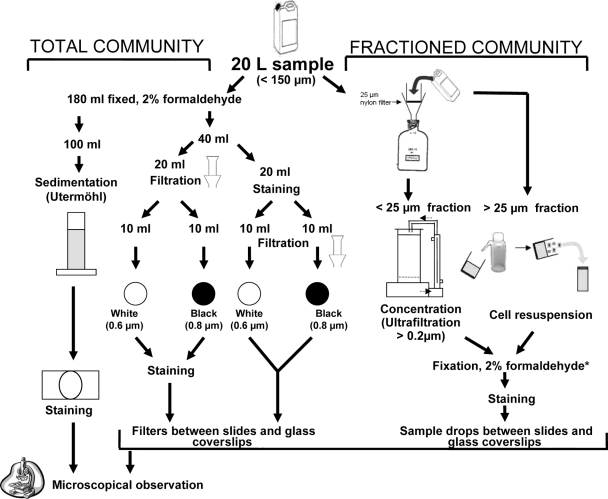
Back in the laboratory, different procedures were tested on the experimental samples collected on 26 October 2006 in Lake Pavin for concentrating phytoplankton cells before the staining tests of chytrids. Concentrating phytoplankton is essential for an accurate assessment of morphological characteristics (general shape of sporangia, the rhizoidal system, the attachment of peduncle, and the presence of operculum), which include typical identification keys of fungal parasites. The sample processing is summarized in Fig. 1.
FIG. 1.
Sample partitioning and different concentration procedures tested for CFW staining and epifluorescence microscopy observation of phytoplankton parasitic chytrids. *, the fixation step for the fractionated community approach is facultative, i.e., can be avoided when observations are made without delay.
Concentration of host cells.
Two different approaches were tested on samples collected on 26 October 2006 in Lake Pavin: the total community approach and the size-fractionated community approach (Fig. 1). For the former approach, 180-ml portions of experimental samples were fixed with formaldehyde (2% final concentration), and aliquots were concentrated in three different ways: (i) by simple gravity according to the Utermöhl method (47) before the chyrids were stained, (ii) by vacuum pressure on two different filters before staining directly onto filters, and (iii) by vacuum pressure on the same two types of filters but after being stained in solution. For the Utermöhl method, 100 ml of fixed samples was settled for at least 24 h. For each of the two filter-vacuum pressure methods, 10 ml of fixed samples was filtered onto polycarbonate white filters (pore size, 0.6 μm; catalog no. DTTP02500 [Millipore]) and Nuclepore polycarbonate black filters (pore size, 0.8 μm; catalog no. 110659 [Whatman]) by using gentle vacuum (20 kPa).
For the size-fractionated community approach, 20 liters of the experimental samples was passed through a 25-μm-pore-size nylon filter (Fig. 1). Large phytoplankton cells in the >25-μm size fraction were collected by washing the filter with 40 ml of 0.2-μm-pore-size-filtered lake water and fixed with formaldehyde (2% final concentration) before staining and analysis. Nanoplanktonic cells in the <25-μm size fraction (i.e., the 20-liter filtrate) were concentrated ∼20-fold by ultrafiltration to a volume of ∼1 liter using a high-performance concentration/diafiltration system (model DC 10LA; Amicon, Epernon, France) equipped with a reusable hollow fiber cartridge (0.2-μm cutoff, surface area of 0.45 m2; Amicon) and an entry pressure of 0.9 bar. About 180 ml of the ultrafiltrate retentate was then fixed with formaldehyde (2% final concentration) before staining and analysis. The size-fractionated community approach gave optimal results and was used for the samples collected in 2007 in both Lake Pavin and Lake Aydat.
Staining of parasites.
Sporangia and rhizoids of parasitic chytrids in concentrated subsamples were stained with the fluorochrome CFW (C40H44N12O10S2 Fluorescent Brightener 28; Sigma catalog no. F3543). CFW is used as whitening agent by the paper industry and selectively binds to cellulose and chitin. The dye fluoresces when exposed to UV light and offers a very sensitive method for direct microscopic examination of skin scrapings, hairs, nails, and other clinical specimen for fungal elements (19, 20). Here, we have adapted the technique to environmental aquatic samples. Before staining, a stock solution of CFW was prepared as modified from an original protocol (20; http://www.mycology.adelaide.edu.au/) by adding 35 mg of CFW to 7 ml of sterile distilled water and a few drops of 10 N NaOH to increase the pH to between 10 and 11, because CFW does not dissolve well in neutral solutions. The final volume of stock solution was then adjusted to 10 ml with sterile distilled water, divided into small aliquots (150 μl), and stored in the dark at −20°C until use.
For the experimental samples collected on 26 October 2006 in Lake Pavin, four final concentrations of CFW (20, 10, 3, and 1% [vol/vol]) relative to the stock solution were tested. For the Utermöhl method, settled phytoplankton cells were stained by replacing an appropriate volume of the supernatant water with the stock solution of CFW directly into the Utermöhl chamber, so as to yield the target dye final concentrations tested. For the vacuum pressure method, phytoplankton cells concentrated onto DTTP and black filters were stained by flooding the filters with the dye (CFW stock solution was diluted at the four final concentrations tested) for 10 min, followed by thorough washing with <0.2-μm-pore-size-filtered lake water. In the variant of this method, CFW stock solution was added to subsamples of suspended phytoplanktonic cells (to reach the appropriate concentrations) for 10 min, before concentrating stained cells onto DTTP and black filters, following by washing with <0.2-μm-pore-size-filtered lake water. All filters were then mounted between microscope slides and glass coverslips using a nonfluorescent immersion oil (Cargille type A). For the fractionated samples (i.e., >25 μm and <25 μm), aliquots (150 μl) of concentrated and fixed materials were stained in solution for 10 min (as previously described for suspended cells), and drops (10 μl) of the stained samples were mounted between glass slides and coverslips for observations and counting (Fig. 1).
The optimal CFW final concentration (i.e., 1%) staining and the size-fractionated community approach were then applied to the samples collected in 2007 in both Lake Pavin and Lake Aydat, for diagnosing, identifying, and counting phytoplankton fungal parasites using direct epifluorescence microscope observations.
Direct observation and counting.
For all samples, stained chytrids were observed in a dark room under an inverted epifluorescence microscope (Leica DMIRB model) equipped with a ×1,250 (i.e., 100/1.25) objective lens, an HBO-100W mercury lamp, and a set of different optic filters, including filters (340 to 380 nm) for UV light excitation. For picture capture and processing, the microscope was equipped with a Leica color video camera (model DC 300F) and a Leica Q500 personal computer.
Experimental samples were mounted between slides and glass coverslips either as concentrates onto filters (i.e., the total community approach) or directly as concentrated liquid drops (5 to 15 μl, i.e., the size-fractionated approach). Slides were then inverted for light excitation and observation under the inverted Leica epifluorescence microscope. For each replicate analyzed, at least 500 phytoplanktonic cells (calculated standard error of <10%) were inspected for fungal infection (i.e., the presence of fixed sporangia), and the morphological characteristics of parasites were noted for identification using the software for image analysis Leica Qwin. The natural abundance and composition of phytoplankton hosts were determined from raw fixed parallel samples by using the Utermöhl method (47).
Species identification and fungal infectivity parameters.
During microscopic observations, phytoplanktonic cells were identified, often to the species level, using morphological taxonomic keys known from references, e.g., Bourelly (6), Huber-Pestalozzi (23), and Prescott (38). For fungal parasites, identification was similarly based on phenotypic keys known from classical manuals, primarily those in Sparrow (44), Canter (10), and Canter and Lund (13). To estimate the infectivity parameters of ecological interest, several algorithms were used according to formulas proposed by Bush et al. (7). These parameters include the prevalence of infection (Pr), i.e., the proportion of individuals in a given phytoplankton population with one or more sporangia or rhizoids, expressed as Pr (%) = [(Ni/N) × 100], where Ni is the number of infected host cells, and N is the total number of host cells. The second parameter is the mean intensity of infection (I), calculated as I = Np/Ni where Np is the number of parasites, and Ni is the number of the infected individuals within a host population.
RESULTS AND DISCUSSION
Handling, staining, and observation of chytrids.
In the present study CFW clearly appears to be a good candidate for diagnosing, identifying, and counting phytoplankton parasitic chytrids in pelagic samples (see, for example, Fig. 2 and 3). This complements the idea that this dye offers a very sensitive method for direct microscopic examination of skin scrapings, hairs, nails, and other clinical specimens for fungal elements known from clinical mycology (19, 20), cytopathology (34, 35), ophthalmology (49), or parasitology (36). CFW binds to β1-3 and β1-4 polysaccharides such as those found in cellulose or in chitin which commonly occur in the fungal cell wall (42, 46). CFW also stains tissue elements such as keratin, collagen, and elastin, providing useful markers for their examination (35). The absorption spectrum for aqueous CFW solution peaked at 347 nm (19, 20) and, when excited with UV radiation, fluoresces with an intense blue color (e.g., Fig. 2 and 3). In our effort to search for an accurate routine procedure for simultaneous study of population dynamics of parasitic chytrids and their hosts in the plankton, it was clear that the quality of observations and counting depended on the concentration of the stain and on the approach used for concentrating phytoplankton hosts.
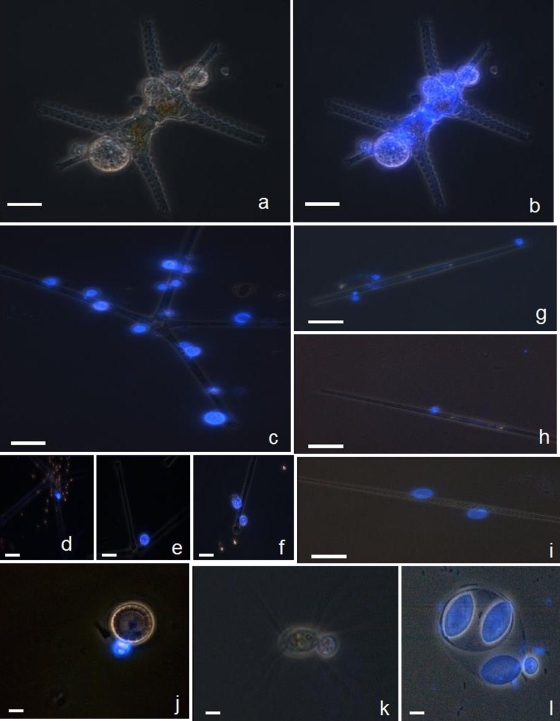
FIG. 2.
Examples of microscopic micrographs of phytoplankton eukaryotes with CFW-stained chytrid parasites, obtained via the fractionated community approach. Typical morphological taxonomic keys are visible under white light for host cell (i.e., the chlorophyte Staurastrum sp.) (a) and under UV light for the specific parasitic chytrids (b). Under UV light, chytrid epidemics were diagnosed for a diversified host populations, including both large size (e.g., the chlorophyte Staurastrum sp. [a and b] and the diatoms A. formosa [c to f] and Synedra sp. [g to i]) and small size (e.g., the diatom Cyclotella sp. [j] and the chlorophytes C. ciliata [k] and O. lacustris [l]) hosts. Multiple infectious chytrids are visible in most micrographs and different development stages as well, e.g., young sporangia with visible rhizoidal system (d and g), mature sporangia containing zoospores (e and h), a mature sporangium discharging its zoospore contents (f), and empty sporangia with chitinaceous wall visible (i). Scale bar, 10 μm.
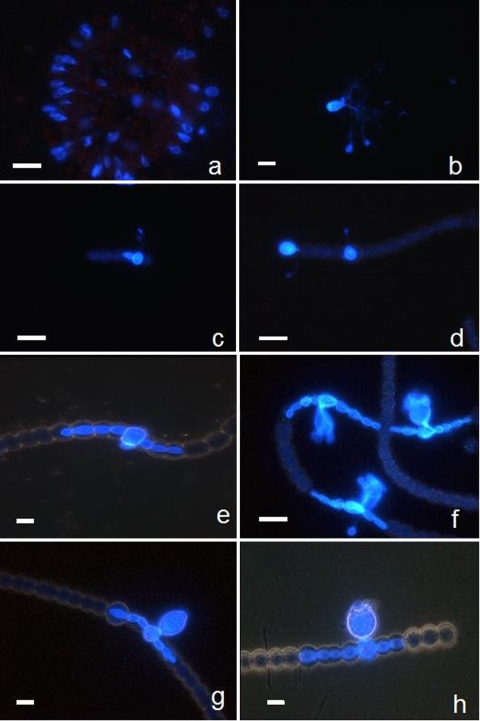
FIG. 3.
Examples of microscopic micrographs of phytoplankton prokaryotes (cyanobacteria) with CFW-stained chytrid parasites, obtained using the fractionated community approach. Typical morphological taxonomic characteristics are visible under white light for host cells (micrographs not shown) and under UV light for parasitic chytrids on their hosts identified as colonial Gomphosphaeria sp. (a) and Microcystis sp. (b) and as the filamentous Anabaena flosaquae (c to h). The branched rhizoidal system of the parasite is visible on Microcystis sp. (b). On A. flosaquae, young endobiotic thalli and encysted zoospores attached by long slender penetration tubes to the host are visible (c and d). In addition, tubular vegetative structure (e), mature sporangia of irregular pyriformic shape (f), and mature sporangia with protruding papilla for discharging zoospores (g and h) are also visible. Scale bar, 10 μm.
For the total community approach using the classical Utermöhl method (47), visualization of fungal parasites was very difficult, and most of the time it was practically impossible for all of the stain concentrations tested. The main reason was that staining directly in the Utermöhl chamber resulted in very poor-quality specimens of parasites observed in any given sample. Other disadvantages of the procedure include the relatively long sedimentation time and the difficulty of increasing the volume analyzed. For these reasons, we decided to exclude the procedure based on the Utermöhl method from the comparisons. The alternative total-community approaches based on vacuum pressure concentrations on polycarbonate filters, i.e., white (0.6-μm-pore-size) and black (0.8-μm-pore-size) filters, yielded similar-quality images of fungal parasites, either when CFW staining was done before (i.e., in solution) or after (i.e., on filters) concentrating phytoplankton host cells onto filters. However, substantial differences were noted depending both on the type of the filter and on the concentration of the stain. In general, for the two types of filters, high levels of background fluorescence were obtained when CFW was used at final concentrations of 3, 10, or 20%, precluding any accurate assessment of numerical and phenotypic characteristics of both host cells and their fungal parasites (data not shown). For this reason, experimental samples stained at these CFW concentrations were not analyzed further. Staining with 1% CFW final concentration substantially improved the viewing of chytrids on filters, with an increasing contrast from the white DTTP Millipore filters to the black Whatman filters (data not shown). However, none of the membrane-retaining approaches yielded satisfactory images of morphological and cellular features of the host cells, e.g., the presence of chloroplast or viability of the host cell. In addition, almost all of the infected phytoplankton individuals observed with the total community approach appeared to be large diatoms. Accordingly, procedures from the total community approach were excluded from comparisons. We will thereafter focus on the size-fractionation approach using a 1% (vol/vol) CFW final concentration (from the stock solution) which substantially enhanced the observational results. We consider this protocol to be optimal for the diagnosis and quantitative assessment of phytoplanktonic chytrid infections in natural samples.
Indeed, the latter procedure yielded the best images for identification and quantitative assessment of both phytoplankton host cells present in the two size classes (>25 μm and < 25 μm) under white light illumination, and their fungal parasites after switching light to UV excitation. Illustrations from samples collected in 2006 in Lake Pavin and in 2007 in Lakes Pavin and Aydat are provided on Fig. 2 and 3. Under white light, diverse phytoplankton host cells were identified based on phenotypic features and on their viability through the integrity of cell wall and the presence of chloroplasts. Under UV excitation, the wall of chytrid sporangia is well visible because of the presence of chitin (19, 20), allowing the assessment of phenotypic keys for identification. These keys include the shape of thallus (e.g., Fig. 3c,d), the rhizoidal system (e.g., Fig. 3e to h) and, at times, different development stages of parasites (e.g., Fig. 2d to f and 3e to f) (10, 13, 44). Furthermore, the size-fractionation approach allowed the diagnosis of fungal phytoplankton infections not only for large hosts such as diatoms and colonial and filamentous cyanobacteria in the >25-μm size fractions but also for small nanophytoplankton cells such as Cyclotella sp., Chodatella ciliata, or Oocystis lacustris (e.g., Fig. 2j to l) in the <25-μm size fractions.
Comparative data from the optimal protocol proposed are presented in the following sections only for the field survey, i.e., samples collected in 2007 in both lakes, mainly as a “proof of concept” rather than a valid comparison of sites versus seasons.
Preliminary data on the natural dynamics of hosts versus parasites using the proposed protocol. (i) Host community composition.
Our size-fractionation approach resulted in an apparent increase in the diversity of infected phytoplankton populations, compared to previous studies. With this approach, a total of nine phytoplankton species belonging to Cyanobacteria, Chrysophyceae, and Chlorophyceae were found with fungal parasites (Fig. 2 and 3). The major hosts were the diatoms A. formosa, Synedra spp., Fragilaria crotonensis, and Cyclotella sp., and the Chlorophyceae Staurastrum paradoxum, Staurodesmus incus, and C. ciliata (see Table 2). For a long time, diatoms, primarily Asterionella spp., have been described from light microscopy as the preferred hosts for chytrid infections in several lakes: English Lake District (12) and Shearwater Lake (39), United Kingdom; Lake Leman, Switzerland (37); Lake Maarsseveen, The Netherlands (48); and two lakes in Colorado (29). Reports on the chytrid epidemic on green algae are more episodic, including host species O. lacustris in Lake Walensee, Switzerland (22), and the desmids Staurastrum spp. in Lake Windermere (11) and Shearwater Lake (40), United Kingdom. Our finding of chytrid infections among prokaryotes is new. This occurred only in summer in the eutrophic Lake Aydat and affected three species of cyanobacteria: Anabaena flosaquae, Gomphosphaeria sp., and Microcystis sp. (Fig. 3). The latter two species were detected once in one of the replicate samples from Lake Aydat. This corroborates our recent suggestion that chytrid infections and the related biogeochemical processes in aquatic systems, primarily through parasitisms and saprophytism, may represent ecologically important driving forces in the food web dynamics (17, 31-33).
TABLE 2.
Prevalence and intensity of chytrid infection for different phytoplanktonic populationsa
| Species | Lake Pavin
|
Lake Aydat
|
||||
|---|---|---|---|---|---|---|
| Date | Pr (%) | I (%) | Date | Pr (%) | I (%) | |
| Asterionella formosa | 22/03/2007 | 6.32 | 1.09 | 22/05/2007 | 24.19 | 1.21 |
| Synedra sp. | 22/03/2007 | 3.23 | 1 | 22/05/2007 | 10 | 1 |
| Melosira italica | 22/03/2007 | 1 | 1 | 22/05/2007 | 1.43 | 1 |
| Fragilaria crotononensis | 22/05/2007 | 3.03 | 1.07 | |||
| 28/08/2007 | 4.95 | 1 | ||||
| Cyclotella sp. | 02/10/2007 | <1 | 1 | |||
| Staurastrum sp. | 02/10/2007 | <1 | 1 | 28/08/2007 | 10.82 | 1.25 |
| Oocystis lacustris | 02/10/2007 | 4.63 | 1.28 | |||
| Chodatella ciliata | 02/10/2007 | 15.8 | 1 | |||
| Anabaena flosaquae | 28/08/2007 | <1 | 1 | |||
Samples were collected on two occasions during different seasons in the euphotic layer of Lake Pavin and Lake Aydat, and the percent prevalence (Pr) and intensity (I) values were determined. Dates are expressed in the format day/month/year.
(ii) Parasite community composition.
Based on morphological features, the majority of fungal parasites were tentatively identified as monocentric (i.e., with one center of growth and development) and eucarpic (using part of the thallus for the fruit-body and with a specialized rhizoidal system). This is characteristic of the order Chytridiales, with two families, four genera, and about ten different species (cf. Table 1 and the identification keys) recorded in our natural samples. The family Phlyctidiaceae contains two genera: Rhizosiphon, which comprised typical parasites of cyanobacteria and normally harbors tubular rhizoids that radiate from the bodies of sporangia (Fig. 3c to h), and Rhizophidium, which is the largest and most complex genus of chytrids parasitizing diatoms and chlorophytes, especially desmids (10, 44). The second family (i.e., Chytridiaceae) was represented by two genera (Chytridium and Zygorhizidium) (Table 1) with species known mainly as parasites of diatoms and green algae, which have the ability to maintain the wall of their sporangia after zoospore discharge, as does Rhizophidium (e.g., Fig. 2i) (10, 44).
TABLE 1.
Morphological features and occurrence of phytoplankton chytrid parasites in the euphotic layer of Lakes Pavin and Aydat sampled each on two occasions during different seasonsa
| Classification | Sporangium | Thallus | Rhizoid | Lake Pavin
|
Lake Aydat
|
||
|---|---|---|---|---|---|---|---|
| Mar. | Oct. | May | Aug. | ||||
| Order Chytridiales | Operculate or inoperculate | Holocarpic or eucarpic | Branched or unbranched | ||||
| Spherical or pyriform | Monocentric | ||||||
| Sessile or epibiotic | |||||||
| Family Phlyctidiaceae | Inoperculate | Eucarpic | Branched or unbranched | ||||
| Sessile or epibiotic | |||||||
| Genus Rhizophydium | Spherical | ||||||
| R. planktonicum | Sessile or epibiotic | Unbranched | X | X | X | ||
| R. couchii | Sessile | Branched | X | X | |||
| R. cyclotellae | Sessile-subspherical | Branched | X | X | X | ||
| R. melosirae | Epibiotic | Branched | X | X | |||
| R. fulgens | Sessile | X | |||||
| R. fragilariae | Spherical to subspherical | Unbranched | X | X | |||
| Genus Rhizosiphon | Sessile | Unbranched | |||||
| R. crassum | Pyriform | Unbranched and tubular | X | ||||
| Family Chytridiaceae | Operculate | Eucarpic | |||||
| Sessile or epibiotic | |||||||
| Genus Chytridium | Spherical or pyriform | Extremely variable | |||||
| Epibiotic | |||||||
| C. versatile | Obpyriform | Branched | X | X | |||
| C. oocystidis | Unbranched | X | |||||
| Genus Zygorhizidium | Sessile | ||||||
| Z. melosirae | Ovate | Unbranched | X | X | |||
Tentative identifications are based on phenotypic keys (see the text).
Based on the limited number of samples analyzed, few differences in the occurrence of parasites were noted between the two lakes sampled, where the more common species were in the genera Rhizophidium and Chytridium (Table 1). The Rhizosiphon species (i.e., R. crassum) was observed only in the eutrophic Lake Aydat as a parasite of the cyanobacteria A. flosaquae (Fig. 3c to h), while Rhizophidium fulgens and Chytridium oocystidis were observed only in Lake Pavin (Table 1) as a parasite of the small chlorophyta O. lacustris and C. ciliata (Fig. 2k and l). Only one species in Lake Pavin (R. planktonicum) and two in Lake Aydat (Rhizophidium cyclotella and C. versatile) were present during the two sampling times (Table 1). In our samples, one parasite species often was found on one host species. An exception was R. planktonicum, which infected different species of diatoms (A. formosa and Synedra spp). In contrast, the diatom F. crotonensis was found infected by two different parasite species in Lake Aydat, i.e., Rhizophidium fragilaria and C. versatile. These findings corroborate the complexity of parasitic ecology (2, 5, 15, 17) and lifestyles in chytrids, which can be facultative parasitic (1), hyperparasitic (28, 41), promiscuous (18), symbiotic (45), and/or multispecific within a genus (4) but species specific in the majority of cases (24, 27).
(iii) Quantitative data.
In Lake Aydat, the total abundance of phytoplankton increased from 4.5 to 18.5 × 106 cells liter−1 between May and August and were higher than those recorded in Lake Pavin in March (2.5 × 106 cells liter−1) and October (3 × 106 cells liter−1) (Fig. 4a). In samples collected during spring, phytoplankton communities were dominated by diatoms in both lakes, accounting for ca. 75 and 65% of the total abundance in Lakes Pavin and Aydat, respectively (Fig. 4b). The main diatom species were A. formosa (32% of total phytoplankton abundance) and Synedra spp. (24%) in Lake Pavin and Melosira italica (57%) in Lake Aydat. In the summer and autumn, diatoms were replaced by cyanobacteria, the species A. flosaquae in Lake Aydat and Cylindrospermum maius (i.e., with no infection noted) in Lake Pavin, with an increasing relative abundance from ca. 65% (of the total abundance) in Lake Pavin to 95% in Lake Aydat. In addition, chlorophytes were also quantitatively important in Lake Pavin in autumn (27% of the total abundance), with O. lacustris (23% of total abundance) as the major species.
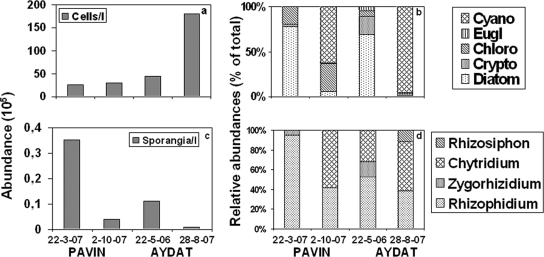
FIG. 4.
Variations in the numerical (a and c) and relative (b and d) abundances of phytoplankton and their chytrid parasite (i.e., sporangia) communities. Samples were collected in triplicate (i.e., from three independent sampling operations for each sampling date) on two occasions during two different seasons in the eutrophic Lake Aydat and in the oligotrophic Lake Pavin. The variability between replicates was low, with a coefficient of variation that was always <10%.
The total abundance of fungal sporangia increased from the oligotrophic Lake Pavin to the eutrophic Lake Aydat, similar to the total abundance of phytoplankton. This may represent a universal pattern, although few quantitative seasonal studies on fungal parasites are sufficiently complete to permit generalization (21). Indeed, the abundance of sporangia was higher in spring than in summer-autumn. The counts were 32.9 (March) and 3.9 (October) × 103 sporangia liter−1 for Lake Pavin and 10.7 (May) and 1.1 (August) × 103 sporangia liter−1 for Lake Aydat. The fluctuation in the number of sporangia thus seems to increase with the trophic status of the lake but may instead be related to the seasonal changes in the host community composition. Indeed, the numerical abundance of sporangia appeared to increase with the increasing relative importance of diatoms within the phytoplankton communities due to the infection from Rhizophidium spp., Zygorhizidium melosirae, and C. versatile (Fig. 4b to d). Diatoms are well known as preferred hosts for chytrid epidemics in the plankton (24, 27), likely because of the large cell size and capacity of diatoms to form blooms, thereby increasing the probability for fungal propagule attachment and development (39). This may help explain why, in contrast to their abundances, the host community was less diverse in Lake Pavin, where fewer species dominated host communities than in Lake Aydat (Fig. 4b and d).
The data on the prevalence and intensity of chytrid infection show that the prevalence increased with increasing trophic status and ranged from <1 to 16% in Lake Pavin and from 1 to 24% in Lake Aydat. The more vulnerable populations were the chlorophyte C. ciliata recorded in October in Pavin and the diatom A. formosa recorded in May in Aydat. Other highly exposed host populations (i.e., prevalence > 5%) included the A. formosa diatom in Lake Pavin and the Synedra spp. diatoms and Staurastrum spp. chlorophytes in Lake Aydat (Table 2), confirming the importance of diatoms and other bloom-forming phytoplankton as preferred hosts for chytrid epidemics (10, 24, 27, 39, 44). The calculation of the intensity of infection revealed the occurrence of multiple infections (i.e., >1 parasites per host cell) for A. formosa in both lakes, O. lacustris in Lake Pavin, and F. crotonensis and Staurastrum spp. in Lake Aydat (Table 2 and Fig. 2).
Conclusions.
The present study describes a routine size-fractionation, CFW staining approach for diagnosing, identifying, and counting phytoplankton chytrid parasites in pelagic samples. The approach is based on the concentration of large initial volumes and size partitioning of samples, a step that we judged necessary in order to yield good analytic images of infectious sporangia for an accurate diagnosing and identification of parasites. In addition, our approach yields freeze-conserved particulate DNA samples for quantifying the propagule stages (i.e., zoospores) of chytrids via FISH targeting of a specific rRNA oligonucleotide probe that we have recently designed (M. Jobard et al., unpublished data). Our protocol can therefore be combined with modern molecular biology protocols such as fluorescence in situ hybridization-targeting or cloning/sequencing. Applied to field samples, the approach provides quantitative preliminary data on infectious sporangia within phytoplankton communities in two contrasted lake environments, which were consistent with ecological considerations known from pelagic habitats and host versus parasite populations. When the samples are analyzed immediately, the approach does not require toxic fixatives and the related disadvantages such as losses during sample storage.
Acknowledgments
S.R. and M.J. were supported by Ph.D. fellowships from the French Ministère de la Recherche et de la Technologie and from the Grand Duché du Luxembourg (Ministry of Culture, High School, and Research), respectively. This study was supported by a grant from the French ANR Programme Blanc DREP (Diversité et Rôles des Eumycètes dans le Pélagos) (T.S.-N.).
We thank C. Portelli and D. Sargos for their logistic, technical, and field assistance.
Footnotes
Published ahead of print on 20 February 2009.
REFERENCES
- 1.Alster, A., and T. Zohary. 2007. Interactions between the bloom-forming dinoflagellate Peridinium gatunense and the chytrid fungus Phlyctochytrium sp. Hydrobiologia 578:131-139. [Google Scholar]
- 2.Amon, J. P., and R. D. Arthur. 1981. Nutritional studies of a marine Phlyctochytrium sp. Mycologia 73:1049-1055. [Google Scholar]
- 3.Barr, D. J. S. 2001. Chytridiomycota, p. 93-112. In D. J. McLaughlin, E. G. McLaughlin, and P. A. Lemke (ed.), The mycota, vol. VII, part A. Springer-Verlag, New York, NY. [Google Scholar]
- 4.Barr, D. J. S., and C. J. Hickman. 1967. Chytrids and algae I: host-substrate range, and morphological variation of species of Rhizophydium. Can. J. Bot. 45:423-430. [Google Scholar]
- 5.Booth, T. 1971. Distribution of certain soil inhabiting chytrid and chytridiaceous species related to some physical and chemical factors. Can. J. Bot. 49:1743-1755. [Google Scholar]
- 6.Bourelly, P. 1970. Les algues d'eau douce. N. Boubée & Cie, Paris, France.
- 7.Bush, A. O., K. D. Lafferty, J. M. Lotz, and A.W. Shostak. 1997. Parasitology meets ecology on its own terms: Margolis et al. revisited. J. Parasitol. 83:575-583. [PubMed] [Google Scholar]
- 8.Canter, H. M. 1946. Studies on British chytrids. I. Dangeardia mammillata Schrödor. Trans. Br. Mycol. Soc. 29:128-134. [Google Scholar]
- 9.Canter, H. M. 1947. Studies on British chytrids. II. Some new monocentric chytrids. Trans. Br. Mycol. Soc. 31:94-105. [Google Scholar]
- 10.Canter, H. M. 1950. Fungal parasites of the phytoplankton. I. Studies on British chytrids X. Ann. Bot. 14:263-289. [Google Scholar]
- 11.Canter, H. M. 1968. Studies on British chytrids XXVII. Rhizophydium fugax sp. nov. a parasite of planktonic cryptomonads with additional notes and records of planktonic fungi. Trans. Br. Mycol. Soc. 51:699. [Google Scholar]
- 12.Canter, H. M., and J. W. G. Lund. 1948. Studies on plankton parasites. I. Fluctuations in the numbers of Asterionella formosa Hass in relation to fungal epidemics. New Phytol. 47:238-261. [Google Scholar]
- 13.Canter, H. M., and J. W. G. Lund. 1951. Studies on plankton parasites. III. Examples of the interaction between parasitism and other factors determining the growth of diatoms. Ann. Bot. 15:359-371. [Google Scholar]
- 14.De Bruin, A. 2006. The potential for coevolution in aquatic host-parasite system. Ph.D. thesis. Netherlands Institute of Ecology of the Royal Academy of Arts and Sciences, Amsterdam, The Netherlands.
- 15.Dix, N. J., and J. Webster. 1995. Fungal ecology. Chapman & Hall, London, United Kingdom.
- 16.Gleason, F. H., and D. Macarthur. 2008. The chytrid epidemic revisited. Inoculum 59:1-3. [Google Scholar]
- 17.Gleason, F. H., M. Kagami, E. Lefèvre, and T. Sime-Ngando. 2008. The ecology of chytrids in aquatic ecosystems: roles in food web dynamics. Fungal Biol. Rev. 22:17-25. [Google Scholar]
- 18.Gromov, B. V., A. V. Plujusch, and K. A. Mamkaeva. 1999. Morphology and possible host range of Rhizophydium algavorum sp. nov. (Chytridiales): an obligate parasite of algae. Protistology 1:61-65. [Google Scholar]
- 19.Hageage, G. J., and B. J. Harrington. 1984. Use of calcofluor white in clinical mycology. Lab. Med. 15:109-112. [Google Scholar]
- 20.Hageage, G. J., and B. J. Harrington. 2003. Calcofluor white: a review of its uses and application in clinical mycology and parasitology. Lab. Med. 34:361-367. [Google Scholar]
- 21.Holfeld, H. 1998. Fungal infections of the phytoplankton: seasonality, minimum host density, and specificity in a mesotrophic lake. New Phytol. 138:507-517. [Google Scholar]
- 22.Huber-Pestalozzi, G. 1944. Chytridium oocystidis (spec. nova?) ein Parasit auf Oocystis lacustris Chodat. Aquat. Sci. 10:117-120. [Google Scholar]
- 23.Huber-Pestalozzi, G. 1983. Das Phytoplankton des Süsswassers. Schweizerbart, Stuttgart, Germany.
- 24.Ibelings, B. W., A. de Bruin, M. Kagami, M. Rijkeboer, M. Brehm, and E. van Donk. 2004. Host parasite interactions between freshwater phytoplankton and chytrid fungi (Chytridiomycota). J. Phycol. 40:437-453. [Google Scholar]
- 25.James, T. Y., F. Kauff, C. L. Schoch, P. B. Matheny, V. Hofstetter, C. J. Cox, G. Celio, C. Gueidan, E. Fraker, J. Miadlikowska, and H. T. Lumbsch. 2006. Reconstructing the early evolution of Fungi using a six-gene phylogeny. Nature 443:818-822. [DOI] [PubMed] [Google Scholar]
- 26.James, T. Y., D. Porter, C. A. Leander, R. Vilgalys, and J. E. Longcore. 2000. Molecular phylogenetics of the Chytridiomycota supports the utility of ultrastructural data in chytrid systematics. Can. J. Bot. 78:336-350. [Google Scholar]
- 27.Kagami, M., A. de Bruin, B. W. Ibelings, and E. van Donk. 2007. Parasitic chytrids: their effects on phytoplankton communities and food-web dynamics. Hydrobiologia 578:113-129. [Google Scholar]
- 28.Karling, J. S. 1942. Parasitism among the chytrids. Am. J. Bot. 29:24-35. [Google Scholar]
- 29.Koob, D. B. 1966. Parasitism of Asterionella formosa Hass by a chytrid in two lakes of Rawah wild area of Colorado. J. Phycol. 2:41-45. [DOI] [PubMed] [Google Scholar]
- 30.Kudoh, S., and M. Takahashi. 1990. Fungal control of population-changes of the planktonic diatom Asterionella formosa in a shallow eutrophic lake. J. Phycol. 26:239-244. [Google Scholar]
- 31.Lefèvre, E. 2007. Taxonomic and functional diversity of heterotrophic flagellates in lakes: molecular approaches. Ph.D. thesis. Université Blaise Pascal, Clermont-Ferrand, France.
- 32.Lefèvre, E., B. Roussel, C. Amblard, and T. Sime-Ngando. 2008. The molecular diversity of freshwater picoeukaryotes reveals high occurrence of putative parasitoids in the plankton. PLoS ONE 3:e2324. doi: 10.1371/journal.pone.0002324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lefèvre, E., C. Bardot, C. Noel, J. F. Carrias, E. Viscogliosi, C. Amblard, and T. Sime-Ngando. 2007. Unveiling fungal zooflagellates as members of freshwater picoeukaryotes: evidence from a molecular diversity study in a deep meromictic lake. Environ. Microbiol. 9:61-71. [DOI] [PubMed] [Google Scholar]
- 34.Luna, B. S., B. K. Stewart, D. L. Bergeron, C. R. Crausen, J. J. Plorde, and T. R. Fritsche. 1995. Use of the fluorochrome calcofluor white in the screening of stool specimens for spores of microsporidia. Am. J. Clin. Pathol. 103:656-659. [DOI] [PubMed] [Google Scholar]
- 35.Monheit, J. G., G. Brown, M. M. Kott, W. A. Schmidt, and D. G. Moore. 1986. Calcofluor white detection of fungi in cytopathology. Am. J. Clin. Pathol. 85:222-225. [DOI] [PubMed] [Google Scholar]
- 36.Müller, U., and P. Sengbusch. 1983. Visualization of aquatic fungi (Chytridiales) parasitizing on algae by means of induced fluorescence. Arch. Hydrobiol. 97:471-485. [Google Scholar]
- 37.Pongratz, E. 1966. De quelques champignons parasites d'organismes planctoniques du Léman. Aquat. Sci. 28:104-132. [Google Scholar]
- 38.Prescott, G. W. 1961. Algae of the western great lakes area. W. M. C. Company Publishers, Dubuque, IA.
- 39.Sen, B. 1987. Fungal parasitism of planktonic algae in Shearwater. I. Occurrence of Zygorhizidium affluens Canter on Asterionella formosa Hass in relation to the seasonal periodicity of the alga. Arch. Hydrobiol. 76:101-127. [Google Scholar]
- 40.Sen, B. 1988. Fungal parasitism of planktonic algae in Shearwater. V. Fungal parasites of the green algae. Arch. Hydrobiol. Suppl. 79:185-205. [Google Scholar]
- 41.Seymour, R. L. 1971. Studies on mycoparasitic chytrids. I. The genus Septosperma. Mycologia 63:83-93. [Google Scholar]
- 42.Sigee, D. C. 2005. Freshwater microbiology. John Wiley & Sons, Ltd., Chichester, England.
- 43.Sime-Ngando, T., and H. J. Hartmann. 1991. Short-term variations of the abundance and biomass of planktonic ciliates in a eutrophic lake. Eur. J. Protistol. 27:249-263. [DOI] [PubMed] [Google Scholar]
- 44.Sparrow, F. K. 1960. Aquatic phycomycetes, 2nd ed. University of Michigan Press, Ann Arbor.
- 45.Trinci, A. P. J., D. R. Davies, K. Gull, M. I. Lawrence, B. B. Nielsen, A. Rickers, and M. K. Theodorou. 1994. Anaerobic fungi in herbivorous animals. Mycol. Res. 98:129-152. [Google Scholar]
- 46.Tsui, C. K. M., and K. D. Hyde. 2003. Freshwater mycology. Fungal Diversity Press, Hong Kong, China.
- 47.Utermöhl, H. 1958. Zur Vervollkommung der quantitative Phytoplankton Methodik. Mitt. Int. Verein. Limnol. 9:1-38. [Google Scholar]
- 48.Van Donk, E., and J. Ringelberg. 1983. The effect of fungal parasitism on the succession of diatoms in Lake Maarsseveen I (The Netherlands). Freshw. Biol. 13:241-251. [Google Scholar]
- 49.Wilhelmus, K. R., M. S. Osato, R. L. Font, et al. 1986. Rapid diagnosis of Acanthamoeba keratitis using calcofluor white. Arch. Ophthalmol. 104:1309-1312. [DOI] [PubMed] [Google Scholar]