Abstract
The stable, low-molecular-weight (LMW) RNA fractions of several rhizobial isolates of Phaseolus vulgaris grown in the soil of Lanzarote, an island of the Canary Islands, were identical to a less-common pattern found within Sinorhizobium meliloti (assigned to group II) obtained from nodules of alfalfa and alfalfa-related legumes grown in northern Spain. The P. vulgaris isolates and the group II LMW RNA S. meliloti isolates also were distinguishable in that both had two conserved inserts of 20 and 46 bp in the 16S-23S internal transcribed spacer region that were not present in other strains of S. meliloti. The isolates from P. vulgaris nodulated bean but not Medicago sativa, while those recovered from Medicago, Melilotus, and Trigonella spp. nodulated both host legumes. The bean isolates also were distinguished from those of Medicago, Melilotus, and Trigonella spp. by nodC sequence analysis. The nodC sequences of the bean isolates were most similar to those reported for S. meliloti bv. mediterranense and Sinorhizobium fredii bv. mediterranense (GenBank accession numbers DQ333891 and AF217267, respectively). None of the evidence placed the bean isolates from Lanzarote in the genus Rhizobium, which perhaps is inconsistent with seed-borne transmission of Rhizobium etli from the Americas to the Canaries as an explanation for the presence of bean-nodulating rhizobia in soils of Lanzarote.
A remarkable attribute of Phaseolus vulgaris (common bean) is its ability to nodulate with rhizobia from at least 20 different legume genera (summarized in reference 1). Of particular relevance is the report by Ishizawa (16), who described P. vulgaris nodulation ranging from doubtful to good by 14 strains recovered from Medicago sativa, Medicago denticulata, and Melilotus alba, while nodulation of the latter three legumes by four bean strains was negative.
At the time of the host range experiments, such as those described by Ishizawa (16), rhizobial nomenclature depended on the legume host of origin; the taxonomy of the strains was based on cross-inoculation groups. Consequently, no information was available about the genetic relationships among the rhizobial strains that originated from the different host legume genera and formed nodules on P. vulgaris. Eventually, rhizobial nomenclature based on the cross-inoculation groups was abandoned because of the many unexplainable and incongruous nodulation data (44). The cross-inoculation groups consisted of different rhizobial species within the single genus Rhizobium. Eventually, rhizobial taxonomy was expanded to several different genera based on estimates of their phylogeny (38). Phylogenies of bean-nodulating rhizobia were estimated from variations in the 16S rRNA gene sequence (39), even though subsequently it became clear that this method is significantly limited by histories of genetic exchange and recombination (6, 40). Most reported phylogenies of rhizobia nodulating P. vulgaris have placed them in the genus Rhizobium (3, 39), but several surveys with isolates from North Africa and Spain have demonstrated that rhizobia in the genus Sinorhizobium also nodulate this legume species (12, 23, 24, 25, 41), supporting the nodulation data originally published by Ishizawa (16). The number of isolates described as originating from nodules of P. vulgaris in the genus Sinorhizobium is small, and for the most part, from the published evidence, it has been suggested that they are affiliated with Sinorhizobium fredii. However, nodules of P. vulgaris growing in a single Tunisian soil where beans are cultivated yielded four isolates that, according to the data, appeared to support an affiliation with Sinorhizobium meliloti rather than S. fredii (25). Whether these four cultures were of the same rhizobial genotype constituting a single example of S. meliloti isolated from P. vulgaris is unknown.
P. vulgaris was introduced into Europe as a crop plant as early as the 16th century (31) but never became a very important part of agriculture in Lanzarote, one of the Canary Islands that lie in the Atlantic Ocean to the west of the North African coast. Since there is no record of any nodulation studies with P. vulgaris cultivated on Lanzarote Island, the first objective of this study was to examine bean plants that had grown in Lanzarote soil for nodulation. Considering that the diversity of rhizobia able to nodulate bean plants is extremely wide, the second objective was to characterize the isolates originating from the nodules of plants grown in Lanzarote soil.
(Part of this work was presented as a poster at the First International Meeting on Microbial Phosphate Solubilization, Salamanca, Spain, July 2002.)
MATERIALS AND METHODS
Isolation and nodulation tests.
Isolates were made from effective root nodules of P. vulgaris (Table 1), according to the method of Vincent (42), by using yeast mannitol agar as the bacterial growth medium. Seeds of P. vulgaris var. “pinta” were surface sterilized with sodium hypochlorite for 20 min, washed 10 times with sterile distilled water, and sown in pots with a soil from Guatiza Vega (Lanzarote, Canary Islands) that had been collected in a location where this legume has not recently been cultivated. Plants were grown in a greenhouse in the Canaries without supplemental lighting for 30 days before being harvested; nodules were selected from five randomly chosen plants. Isolation and characterization of rhizobia from Medicago, Melilotus, and Trigonella spp. originating from León, Spain, have been described by del Villar et al. (5).
TABLE 1.
Characteristics of the strains analyzed in this study
| Strain(s) | Geographical origin | Host of isolation | RAPD pattern | LMW RNA group | Plasmid profile | Nodulation ofc:
|
|
|---|---|---|---|---|---|---|---|
| Alfalfa | Common bean | ||||||
| GVPV01, GVPV02, GVPV04, GVPV05, GVPV06, GVPV16 | Guatiza Vegaa | P. vulgaris | a | II | A | − | + |
| GVPV08, GVPV09, | Guatiza Vega | P. vulgaris | b | II | A | − | + |
| GVPV11 | Guatiza Vega | P. vulgaris | b | II | B | − | + |
| GVPV12, GVPV13 | Guatiza Vega | P. vulgaris | c | II | B | − | + |
| RMA20, RMA30, RMA31, RMA32, RMA33 | Riego de la Vegab | Melilotus alba | NDd | II | C | + | + |
| RMOF01 | Riego de la Vega | Melilotus officinalis | ND | II | C | + | + |
| RTM02, RTM06, RTM08, RTM11, RTM15, RTM17 | Riego de la Vega | T. monspelliaca | ND | II | C | + | + |
| RTF15 | Riego de la Vega | T. foenum-graecum | ND | II | C | + | + |
| RMSA04, RMSA36, RMSA42 | Riego de la Vega | M. sativa | ND | II | C | + | + |
| S. meliloti ATCC 9930T | Virginia | M. sativa | d | I | D | + | + |
Guatiza Vega, Lanzarote, Canary Islands, Spain.
Riego de la Vega, Spain.
+, positive; −, negative.
ND, not determined.
The isolates were examined for their effectiveness for nitrogen fixation with P. vulgaris var. “pinta” and M. sativa var. “Aragon” cultivated in a growth chamber using modified Leonard jars (21) filled with vermiculite moistened with N-free Rigaud and Puppo nutrient solutions (29). Noninoculated nitrogen-free and nitrogen-supplemented plants were used as controls. Five replicates for each treatment were used, and plants were harvested 6 weeks after planting to determine the shoot dry weight and the number of nodules. Rhizobium etli CFN42T and S. meliloti ATCC 9930T were included for reference.
RAPD analysis.
Randomly amplified polymorphic DNA (RAPD) PCR was done on the isolates and controls using the primer M13 (5′-GAGGGTGGCGGTTCT-3′) according to the method described by Rivas et al. (30). The PCR products were separated according to molecular size by horizontal agarose gel electrophoresis (30) using Standard VI (Boehringer-Roche, Indianapolis, IN) as a size marker.
LMW RNA analysis.
Low-molecular-weight (LMW) RNA extractions were done as described by Höfle (13). The LMW RNA mixtures in each sample were separated according to molecular size by staircase electrophoresis (4) using 400- by 360- by 0.4-mm gels in a vertical slab unit (Poker Face SE 1500 Sequencer; Hoeffer Scientific Instruments, San Francisco, CA) as described by Velázquez et al. (41). Molecular size markers used were obtained from Boehringer Mannheim (Mannheim, Germany) and Sigma (St. Louis, MO) and included 5S rRNA from Escherichia coli MRE 600, tRNA specific for tyrosine from E. coli, and tRNA specific for valine from E. coli. After electrophoresis was complete, the gels were silver stained according to the method described by Haas et al. (11).
DNA-DNA hybridization analysis.
The DNA-DNA hybridization analysis was done according to the method described by Ezaki et al. (7) by following the recommendations of Willems et al. (43). The analysis was done with representative strains from each of the RAPD groups and the type strain of S. meliloti, LMG 6133T.
Sequence analyses of 16S rRNA genes, nodC, and the 16S-23S ITS regions.
PCR amplification and sequence analysis of 16S rRNA genes, nodC, and the 16S-23S internal transcribed spacer (ITS) regions were carried out as previously described (19, 20). Selected sequences in GenBank, obtained by searches using the BLASTN program (2), were aligned with the DNA sequences obtained for the isolates by using Clustal W (35). Distances calculated according to Kimura's two-parameter model (17) were used to infer phylogenetic trees by using the neighbor-joining method (34) with MEGA 2.1 software (18). Confidence values for nodes in the trees were generated by bootstrap analysis using 500 permutations of the data sets.
Plasmid profile analysis.
Rhizobial cells were incubated in tryptone-yeast extract medium at 25°C at 180 rpm on a rotary shaker until cultures reached a concentration of 1 × 106 cells/ml. Cells were collected from 1.5 ml of broth in a centrifuge set at 9,000 × g for 5 min. Separation of plasmids by electrophoresis was done according to the method described by Plazinski et al. (28), with the exception that the conditions used were 2 V cm−1 for 90 min followed by 3 V cm−1 for 60 min and finally 6 V cm−1 for 240 min. The pRmeGR4b (205 kb) and pRmeGR4a (175 kb) plasmids of S. meliloti GR4 (36) were used as molecular markers.
RESULTS AND DISCUSSION
Isolation of rhizobia from bean nodules.
The bean plants that had grown in soil from Lanzarote for 30 days were well nodulated, indicating the presence of bean-nodulating rhizobia in the soil of Lanzarote. Nodules for isolation of the rhizobia were randomly selected from five plants and yielded cultures that were numbered with the prefix GVPV (Table 1). The observation of bean-nodulating rhizobia in the soil of Lanzarote is similar to reports of bean-nodulating rhizobia in different soils of Andalucía (12, 32), because in each case, there had been no recent history of bean cultivation. The results of studies from Spain are different from those from Tunisia, where normal-sized nodules were formed only when beans were grown in soils that had a recent history of bean cultivation (22, 23, 24). Even though there is no recent history of bean cultivation or of rhizobial inoculation in the Spanish locations, both Herrera-Cervera et al. (12) and Rodríguez-Navarro et al. (32) postulated that bean rhizobia may well have been introduced into Spanish soils from the Americas during the last five centuries, because Pérez-Ramírez et al. (27) reported that bean seeds naturally carry rhizobia on their testa. In support of their hypothesis were the high numbers of isolates (54% and 71%, respectively) they categorized as being members of R. etli.
RAPD, DNA-DNA hybridization, and 16S rRNA gene sequence analyses.
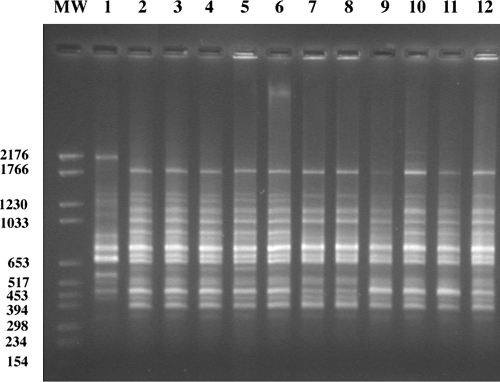
RAPD analysis, as used in other rhizobial studies to determine diversity (15, 26, 37), was applied to the bean isolates from Lanzarote. From the fingerprint patterns obtained with this approach, evidence was obtained that all isolates were almost identical and could be placed into only three highly similar groups (Fig. 1 and Table 1).
FIG. 1.
Horizontal agarose gel electrophoresis of RAPD using DNA of bean isolates from Guatiza Vega (Lanzarote) as the template with results for S. meliloti LMG 6133, GVPV01, GVPV02, GVPV04, GVPV05, GVPV06, GVPV08, GVPV09, GVPV11, GVPV12, GVPV13, and GVPV16 shown in lanes 1 through 12, respectively.
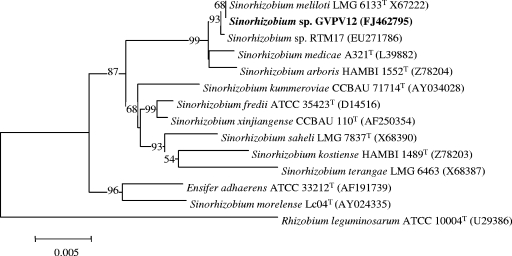
The 16S rRNA gene sequences obtained with a representative isolate from each of the three almost-indistinguishable RAPD groups were identical to each other. Also, the 16S rRNA gene sequences of the bean isolates were 99.8% similar to the gene sequence of the S. meliloti type strain USDA 1002T (ATCC 9930, LMG 6133). Since genus affiliation commonly is decided from the 16S rRNA gene sequence (9), and because histories of recombination (40) exclude inferences of species boundaries, perhaps a suggestion that the bean isolates obtained from Lanzarote should be grouped in the genus Sinorhizobium would be justified (Fig. 2). Further supporting evidence was obtained by a DNA-DNA hybridization analysis, because a mean homology value of 75% was obtained between the bean isolate GVPV12 and the type strain of S. meliloti, LMG 6133T. Similarly, isolates from nodules of P. vulgaris that had grown in Southern Spain as well as Tunisia have been placed in the genus Sinorhizobium (12, 22, 23, 24, 32). None of the representatives of the isolates of bean from Lanzarote were placed in the genus Rhizobium, a finding which is significantly different from the reported prevalence of Rhizobium in the nodules of P. vulgaris in the other studies. Consequently, seed-borne transmission of bean rhizobia in the genus Rhizobium from the Americas to the Canaries and subsequent extensive interspecific symbiotic gene exchange, as inferred by Herrera-Cervera et al. (12) to explain the diversity of bean-nodulating rhizobia in European soils, appears to have less support in the case of the isolates from Lanzarote.
FIG. 2.
Placement of the bean isolates on a neighbor-joining tree constructed from variations in the 16S rRNA gene sequences. Permutations of the data set (1,000) were used in a bootstrap analysis to derive a majority-rule consensus tree, and the levels of support for the presence of nodes are indicated. The alignment length of the sequences was 1,479 nucleotides.
LMW RNA analysis.
Gel electrophoresis of the stable, LMW RNA fraction of single bacterial strains is a high-resolution method for rapid genotypic identification and classification of bacteria (13). This approach was applied to obtain additional evidence for the placement of the bean isolates from Lanzarote in the genus Sinorhizobium and to provide further support for their close affiliation with S. meliloti. A representative LMW RNA group II strain of S. meliloti (5) originating from Trigonella monspelliaca (RTM17) was included in the analysis in addition to the type strains for S. fredii, Sinorhizobium medicae, and S. meliloti (Fig. 3). The 5S rRNA zones of the bean-nodulating isolate GVPV12 from Lanzarote, S. fredii USDA 205T, S. medicae USDA 1037T, and S. meliloti LMG 6133T were identical and supported the placement of the bean isolates in the genus Sinorhizobium. The class 1 and class 2 tRNA patterns of RTM17 and the bean isolate were identical, while the two tRNA patterns produced in the lanes with the type strains for S. fredii, S. medicae, and S. meliloti were dissimilar. Therefore, the bean isolates from Lanzarote were placed in LMW RNA group II of S. meliloti as described recently by del Villar et al. (5).
FIG. 3.
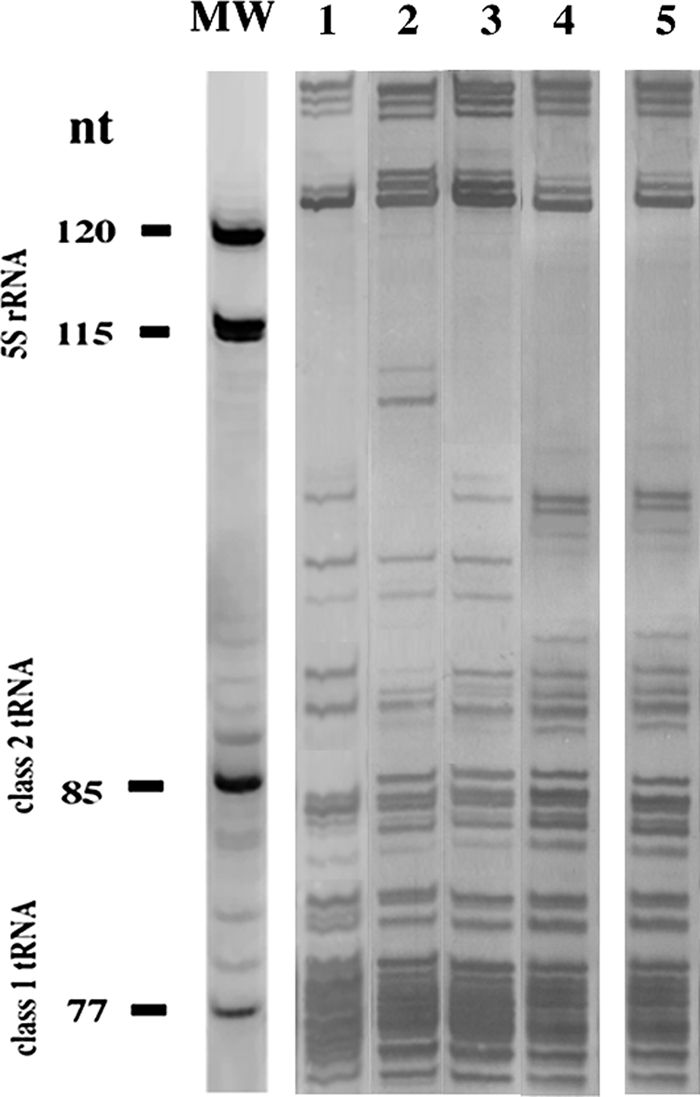
LMW RNA patterns of S. fredii USDA 205T (lane 1), S. medicae USDA 1037T (lane 2), S. meliloti LMG 6133T (lane 3), RTM17 (lane 4), and GVPV12 (lane 5).
Analysis of ITS sequences.
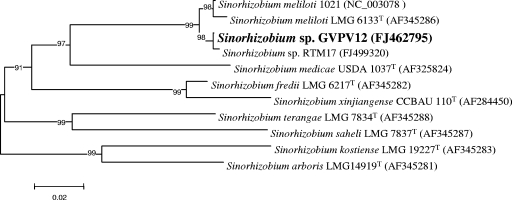
Further evidence for the placement of the bean isolates was gathered by sequence analysis of the ITS (Fig. 4) in representatives of both LMW RNA I and II groups of S. meliloti, since the 16S-23S ITS region has sections that are hypervariable and useful for distinguishing intraspecific groups (19, 33, 37). Two inserts, of 20 and 46 bp, located between positions 565 to 585 and 644 to 690 relative to the sequence of the type strain of S. meliloti, LMG 6133T (GenBank accession number AF345286), were present in the ITS of all LMW RNA group II rhizobia, irrespective of their host of origin. The ITS in the LMW RNA group II isolates RTM17 and GVPV12 were 94.8% and 95% similar to the region in S. meliloti LMG 6133T (LMW RNA type I), respectively. Within each LMW RNA group, the ITS regions were more than 99.5% similar. Therefore, an analysis of ITS regions provided further evidence for the placement of the bean isolates in the genus Sinorhizobium, perhaps supporting an inferred description of them as S. meliloti, but recombination as an explanation for the presence of similar ITS sequences should not be disregarded.
FIG. 4.
Placement of LMW RNA group II isolates on a neighbor-joining tree constructed from sequence variation of 16S-23S ITS fragments. Permutations of the data set (1,000) were used in a bootstrap analysis to derive a majority-rule consensus tree, and the levels of support for the presence of nodes are indicated. The alignment length was 726 nucleotides.
Host range for nodulation, plasmid content, and nodC sequence analysis.
LMW RNA group II isolates originating from bean or alfalfa-related legume hosts were used in reciprocal cross-inoculation studies to determine their host range. Nodulation levels of bean by GVPV04 and GVPV12, representing the bean isolates from Lanzarote with different plasmid profiles, and the type strain for R. etli (CFN42T), were not significantly different (Table 2). Also, there were no significant differences in plant dry weights or in total plant nitrogen levels among the treatments. Significantly fewer nodules on bean resulted from inoculation with RMA31 and RTM17, which are LMW RNA group II isolates from Melilotus alba and T. monspelliaca, respectively. However, there were no significant differences in plant dry weights or nitrogen contents among any of these treatments. Nodulation levels for M. sativa by RMA31, RTM17, and the type strain for S. meliloti (ATCC 9930T) were similar. Plant dry weights and total nitrogen levels produced by RMA31 and RTM17 were significantly higher than those for ATCC9930T. The bean isolates from Lanzarote failed to nodulate M. sativa (Table 2), indicating that they have a more-restricted range for nodulation than LMW RNA group II rhizobia that originated from alfalfa-related legumes grown in soil from Riego de la Vega, Northern Spain.
TABLE 2.
Results of inoculation experimentsa
| Treatment |
P. vulgaris
|
M. sativa
|
||||
|---|---|---|---|---|---|---|
| NN | SDW (g) | PN (mg) | NN | SDW (mg) | PN (mg) | |
| GVPV12 | 97 (c) | 1.3 (a) | 325 (a) | 0 | NA | NA |
| GVPV04 | 89 (c) | 1.0 (a) | 250 (a) | 0 | NA | NA |
| RTM17 | 37 (b) | 1.2 (a) | 300 (a) | 7 (a) | 57.2 (b) | 2.0 (b) |
| RMA31 | 12 (a) | 1.3 (a) | 325 (a) | 8 (a) | 65.3 (b) | 2.3 (b) |
| S. meliloti ATCC 9930T | 37 (b) | 1.4 (a) | 350 (a) | 9 (a) | 38.0 (a) | 1.0 (a) |
| R. etli CFN42T | 97 (c) | 1.1 (a) | 275 (a) | ND | ND | ND |
Letters in parentheses indicate groups of values that are not significantly different from each other (P = 0.05, Fisher's protected least significant differences). NN, number of nodules per plant; SDW, shoot dry weight per plant; PN, total nitrogen per plant; ND, not determined; NA, not applicable.
Cellular plasmid contents of LMW RNA group II isolates of S. meliloti were compared because of the difference in the nodulation responses with M. sativa. RTM17, chosen to represent LMW group II isolates from Medicago, Melilotus, and Trigonella, harbored a single plasmid (Fig. 5, lane 3). GVPV04 and GVPV12, chosen to represent LMW RNA group II isolates from bean, harbored four plasmids each (Fig. 5, lanes 1 and 2). Based upon relative mobilities, two different plasmid contents were observed among the bean isolates, ranging from approximately 200 to 1,500 kb or 90 to 1,500 kb, respectively. Three plasmids, ranging from approximately 60 to 1,700 kb, were observed with the reference strain of S. meliloti, GR4. Therefore, isolates GVPV04 and GVPV12 originating from bean appeared to harbor three additional plasmids compared to RTM17 despite the implied similarity of their chromosomes.
FIG. 5.
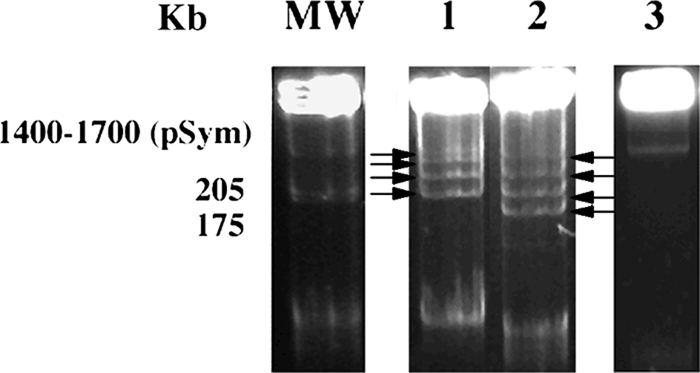
Plasmids of GVPV04 (lane 1), GVPV12 (lane 2), and RTM17 (lane 3). MW, S. meliloti GR4. Plasmids in lanes 1 and 2 are indicated with arrows.
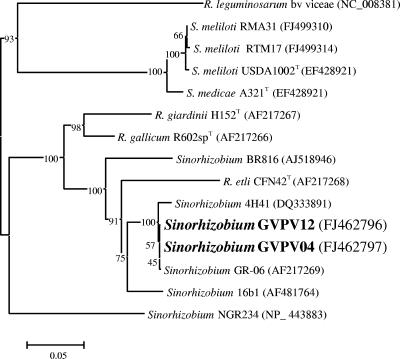
In addition to differentiation based on nodulation of M. sativa and plasmid content, distinct nodC gene sequences were observed among the LMW RNA group II isolates (Fig. 6). The nodC gene sequences of isolates RTM17 and RMA31 (data for RMSA36, RMOF01, and RTF15 not shown) from T. monspelliaca, M. sativa, Melilotus alba, Melilotus officinalis, and Trigonella foenum-graecum, respectively, were almost identical to each other and to the sequence of the type strain for S. meliloti, USDA 1002T (GenBank accession number EF428922). The nodC gene sequence of bean isolates GVPV04 and GVPV12 from Lanzarote and GR-06 from Andalucía (12) (GenBank accession number AF217269) were identical to one another but different from RTM17, RMSA36, RMA31, RMOF01, and RTF15, which were isolated from different alfalfa-related legumes in northern Spain (Fig. 6). Placement of bean isolate GR-06 in S. fredii has been proposed (20), and the biovar mediterranense has been suggested to distinguish it from the soybean-nodulating strains of S. fredii (25). However, the nodC sequences of the bean isolate 16b1 from Tunisia (GenBank accession number AF481764), also with a proposed placement in S. fredii (23), and GVPV04 and GVPV12 were dissimilar (Fig. 6). Evidence for placement of both isolate GR-06 and isolate 16b1 in S. fredii was provided by restriction fragment length polymorphism analysis of PCR-amplified 16S rRNA genes and by a 536-bp sequence of 16S rRNA gene in the case of strain GR-06 (20, 23). The nodC gene sequence of GVPV04 and GVPV12 also was similar to that of the bean isolate LILM4H41 (GenBank accession number DQ333891) placed in S. meliloti with evidence provided from 16S rRNA gene sequence analysis (25). Based on nodC gene sequence analysis, Mnasri et al. (25) proposed to distinguish the bean-nodulating isolate LILM4H41 from Medicago-nodulating S. meliloti by assigning it the biovar mediterranense, as was done for S. fredii bean-nodulating isolates from Andalucía and Tunisia. Additional evidence for the placement of GR-06, 16b1, and LILMH4H41 in the species S. fredii and S. meliloti was not provided. This limitation is significant, because Gevers et al. (8) have indicated that classification of prokaryotic species by rRNA gene sequence alone is unsatisfactory since sequence similarity is subject both to simple stochastic variation and to the influence of recombination or horizontal gene transfer. Certainly, evidence has been provided that sections within the 16S rRNA genes of rhizobia have undergone recombination, influencing the placement of species on a phylogenetic tree (40). Consequently, it may have been premature to propose biovars of S. fredii and S. meliloti, because perhaps insufficient evidence was provided to suggest that GR-06, 16b1, and LILMH4H41 are rhizobia belonging to these two prokaryotic species. Also, consideration should perhaps be given to whether it is sensible to assign biovar status to different rhizobia that nodulate P. vulgaris, since this host legume is so promiscuous that it nodulates with rhizobia of legumes that range from the temperate shrub Caragana arborescens, native to Siberia and Manchuria (10), to the tropical shrub Leucaena leucocephala (14).
FIG. 6.
Comparative sequence analysis of nodC gene sequences from strains isolated in this study and related representative strains from GenBank. The significance of each branch is indicated by a bootstrap value calculated for 500 subsets. The alignment length was 834 nucleotides.
Acknowledgments
This work was supported by MICYT (central Spanish Government) and JCyL (regional Spanish Government).
Footnotes
Published ahead of print on 13 February 2009.
REFERENCES
- 1.Alexander, M., and M. T. Lieberman. 1981. Cross-inoculation patterns of legumes and isolates of Rhizobium. Report to the U.S. Agency for International Development. Contract number AID/csd 2834. U.S. Agency for International Development, Washington, DC.
- 2.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 3.Amarger, N., V. Macheret, and G. Laguerre. 1997. Rhizobium gallicum sp. nov. and Rhizobium giardinii sp. nov., from Phaseolus vulgaris n odules. Int. J. Syst. Bacteriol. 47:996-1006. [DOI] [PubMed] [Google Scholar]
- 4.Cruz-Sánchez, J. M., E. Velázquez, P. F. Mateos, E. Velázquez, and E. Martínez-Molina. 1997. Enhancement of resolution of low molecular weight RNA profiles by staircase electrophoresis. Electrophoresis 18:1909-1911. [DOI] [PubMed] [Google Scholar]
- 5.Del Villar, M., R. Rivas, A. Peix, P. F. Mateos, E. Martínez-Molina, P. van Berkum, A. Willems, and E. Velázquez. 2008. Stable low molecular weight RNA profiling showed variations within Sinorhizobium meliloti and Sinorhizobium medicae nodulating different legumes from the alfalfa cross-inoculation group. FEMS Microbiol. Lett. 282:273-281. [DOI] [PubMed] [Google Scholar]
- 6.Eardly, B. D., S. M. Nour, P. van Berkum, and R. K. Selander. 2005. Rhizobial 16S rRNA and dnaK genes: mosaicism and the uncertain phylogenetic placement of Rhizobium galegae. Appl. Environ. Microbiol. 71:1328-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ezaki, T., Y., Hashimoto, and E. Yabuchi. 1989. Fluorometric deoxyribonucleic acid-deoxyribonucleic acid hybridization in microdilution wells as an alternative to membrane filter hybridization in which radioisotopes are used to determine genetic relatedness among bacterial strains. Int. J. Syst. Bacteriol. 39:224-229. [Google Scholar]
- 8.Gevers, D., F. M. Cohan, J. G. Lawrence, B. G. Spratt, T. Coenye, E. J. Feil, E. Stackebrandt, Y. Van de Peer, P. Vandamme, F. L. Thompson, and J. Swings. 2005. Re-evaluating prokaryotic species. Nat. Rev. Microbiol. 3:733-739. [DOI] [PubMed] [Google Scholar]
- 9.Graham, P. H., M. J. Sadowsky, H. H. Keyser, Y. M. Barnet, R. S. Bradley, J. E. Cooper, D. J. de Ley, B. D. W. Jarvis, E. B. Roslycky, B. W. Strijdom, and J. P. W. Young. 1991. Proposed minimum standards for the description of new genera and species of root- and stem-nodulating bacteria. Int. J. Syst. Bacteriol. 41:582-587. [Google Scholar]
- 10.Gregory, K. F., and O. N. Allen. 1953. Physiological variations and host plant specificities of rhizobia isolated from Caragana arborescens L. Can. J. Bot. 31:730-738. [Google Scholar]
- 11.Haas, H., B. Budowle, and G. Weiler. 1994. Horizontal polyacrylamide gel electrophoresis for the separation of DNA fragments. Electrophoresis 15:153-158. [DOI] [PubMed] [Google Scholar]
- 12.Herrera-Cervera, J. A., J. Caballero-Mellado, G. Laguerre, H. V. Tichy, N. Requena, N. Amarger, E. Martínez-Romero, J. Olivares, and J. Sanjuán. 1999. At least five rhizobial species nodulate Phaseolus vulgaris in a Spanish soil. FEMS Microbiol. Ecol. 30:87-97. [Google Scholar]
- 13.Höfle, M. G. 1988. Identification of bacteria by low molecular weight RNA profiles: a new chemotaxonomic approach. J. Microbiol. Methods 8:235-248. [Google Scholar]
- 14.Hungría, M., A. A. Franco, and J. I. Sprent. 1993. New sources of high-temperature tolerant rhizobia for Phaseolus vulgaris L. Plant Soil 149:103-109. [Google Scholar]
- 15.Iglesias, O., R. Rivas, P. García-Fraile, A. Abril, P. F. Mateos, E. Martínez-Molina, and E. Velázquez. 2007. Genetic characterization of fast-growing rhizobia able to nodulate Prosopis alba in North Spain. FEMS Microbiol. Lett. 277:210-216. [DOI] [PubMed] [Google Scholar]
- 16.Ishizawa, S. 1954. Studies on root-nodule bacteria of leguminous plants. II. The relationship between nodule bacteria and leguminous plants, part I. From the view of nodule production. a. Cross-inoculation test. J. Sci. Soil Manure (Tokyo) 24:297-302. [Google Scholar]
- 17.Kimura, M. 1980. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 16:111-120. [DOI] [PubMed] [Google Scholar]
- 18.Kumar, S., K. Tamura, I. B. Jakobsen, and M. Nei. 2001. MEGA2: molecular evolutionary genetic analysis software. Bioinformatics 17:1244-1245. [DOI] [PubMed] [Google Scholar]
- 19.Kwon, S. W., J. Y. Park, J. S. Kim, J. W. Kang, Y. H. Cho, C. K. Lim, M. A. Parker, and G. B. Lee. 2005. Phylogenetic analysis of the genera Bradyrhizobium, Mesorhizobium, Rhizobium and Sinorhizobium on the basis of 16S rRNA gene and internally transcribed spacer region sequences. Int. J. Syst. Evol. Microbiol. 55:263-270. [DOI] [PubMed] [Google Scholar]
- 20.Laguerre, G., S. M. Nour, V. Macheret, J. Sanjuan, P. Drouin, and N. Amarger. 2001. Classification of rhizobia based on nodC and nifH gene analysis reveals a close phylogenetic relationship among Phaseolus vulgaris symbionts. Microbiology 147:981-993. [DOI] [PubMed] [Google Scholar]
- 21.Leonard, L. T. 1943. A simple assembly for use in the testing for cultures of rhizobia. J. Bacteriol. 45:523-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mhamdi, R., M. Jebara, M. E. Aouani, R. Ghrir, and M. Mars. 1999. Genotypic diversity and symbiotic effectiveness of rhizobia isolated from root nodules of Phaseolus vulgaris L. grown in Tunisian soils. Biol. Fertil. Soils 28:313-320. [Google Scholar]
- 23.Mhamdi, R., G. Laguerre, M. Elarbi-Aouani, M. Mars, and N. Amarger. 2002. Different species and symbiotic genotypes of field rhizobia can nodulate Phaseolus vulgaris in Tunisian soils. FEMS Microbiol. Ecol. 41:77-84. [DOI] [PubMed] [Google Scholar]
- 24.Mhousine, B., J. Prell, A. Filali-Matouf, U. B. Priefer, and J. Aurag. 2007. Diversity, phylogeny and distribution of bean rhizobia in salt-affected soils of North-West Morocco. Symbiosis 43:86-93. [Google Scholar]
- 25.Mnasri, B., M. Mrabet, G. Laguerre, M. E. Aouani, and R. Mhamdi. 2007. Salt-tolerant rhizobia isolated from a Tunisian oasis that are highly effective for symbiotic N2-fixation with Phaseolus vulgaris constitute a novel biovar (bv. mediterranense) of Sinorhizobium meliloti. Arch. Microbiol. 187:79-85. [DOI] [PubMed] [Google Scholar]
- 26.Moschetti, G., A. Peluso, A. Protopapa, M. Anastasio, O. Pepe, and R. Defez. 2005. Use of nodulation pattern, stress tolerance, nodC gene amplification, RAPD-PCR and RFLP-16S rDNA to discriminate genotypes of Rhizobium leguminosarum biovar viciae. Syst. Appl. Microbiol. 28:619-631. [DOI] [PubMed] [Google Scholar]
- 27.Pérez-Ramírez, N. O., M. A. Rogel, E. Wang, J. Z. Castellanos, and E. Martínez-Romero. 1998. Seeds of Phaseolus vulgaris bean carry Rhizobium etli. FEMS Microbiol. Ecol. 26:289-296. [Google Scholar]
- 28.Plazinski, J., Y. H. Cen, and B. G. Rolfe. 1985. General method for the identification of plasmid species in fast-growing soil microorganisms. Appl. Environ. Microbiol. 49:1001-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rigaud, J., and A. Puppo. 1975. Indole 3 acetic acid catabolism by soybean bacteroids. J. Gen. Microbiol. 88:223-228. [Google Scholar]
- 30.Rivas, R., A. Peix, P. F. Mateos, M. E. Trujillo, E. Martínez-Molina, and E. Velázquez. 2006. Biodiversity of populations of phosphate solubilizing rhizobia that nodulate chickpea in different Spanish soils. Plant Soil 287:23-33. [Google Scholar]
- 31.Rodino, A. P., and J.-J. Drevon. 2004. Migration of a grain legume, Phaseolus vulgaris, in Europe, p. 61-72. In D. Werner (ed.), Biological resources and migration. Springer-Verlag, New York, NY.
- 32.Rodríguez-Navarro, D. N., A. M. Buendía, M. Camacho, M. M. Lucas, and C. Santamaría. 2000. Characterization of Rhizobium spp. bean isolates from southwest Spain. Soil Biol. Biochem. 32:1601-1613. [Google Scholar]
- 33.Safronova, V. I., G. Piluzza, A. A. Belimov, and S. Bullitta. 2004. Phenotypic and genotypic analysis of rhizobia isolated from pasture legumes native of Sardinia and Asinara Island. Antonie van Leeuwenhoek 85:115-127. [DOI] [PubMed] [Google Scholar]
- 34.Saitou, N., and M. Nei. 1987. A neighbour-joining method: a new method for reconstructing phylogenetics trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 35.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The CLUSTAL_X Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Toro, N., and J. Olivares. 1986. Characterization of a large plasmid of Rhizobium meliloti involved in enhancing nodulation. Mol. Gen. Genet. 202:331-335. [Google Scholar]
- 37.Valverde, A., J. M. Igual, A. Peix, E. Cervantes, and E. Velázquez. 2006. Rhizobium lusitanum sp. nov. a bacterium that nodulates Phaseolus vulgaris. Int. J. Syst. Evol. Microbiol. 56:2631-2637. [DOI] [PubMed] [Google Scholar]
- 38.van Berkum, P., and B. D. Eardly. 1998. Molecular evolutionary systematics of the Rhizobiaceae, p. 1-24. In H. Spaink, A. Kondorosi, and P. Hooykaas (ed.), The Rhizobiaceae. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 39.van Berkum, P., D. Beyene, and B. D. Eardly. 1996. Phylogenetic relationships among Rhizobium species nodulating the common bean (Phaseolus vulgaris L.). Int. J. Syst. Bacteriol. 46:240-244. [DOI] [PubMed] [Google Scholar]
- 40.van Berkum, P., Z. Terefework, L. Paulin, S. Suomalainen, K. Lindström, and B. D. Eardly. 2003. Discordant phylogenies within the rrn loci of rhizobia. J. Bacteriol. 185:2988-2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Velázquez, E., E. Martínez-Romero, D. N. Rodríguez-Navarro, M. E. Trujillo, A. Daza, P. F. Mateos, E. Martinez-Molina, and P. van Berkum. 2001. Characterization of rhizobial isolates of Phaseolus vulgaris by staircase electrophoresis of low-molecular-weight RNA. Appl. Environ. Microbiol. 67:1008-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vincent, J. M. 1970. The cultivation, isolation and maintenance of rhizobia, p. 1-13. In J. M. Vincent (ed.), A manual for the practical study of root-nodule bacteria. Blackwell Scientific Publications, Oxford, United Kingdom.
- 43.Willems, A., F. Doignon-Bourcier, J. Goris, R. Coopman, P. De Lajudie, and M. Gillis. 2001. DNA-DNA hybridization study of Bradyrhizobium strains. Int. J. Syst. Evol. Microbiol. 51:1315-1322. [DOI] [PubMed] [Google Scholar]
- 44.Wilson, J. K. 1944. Over five hundred reasons for abandoning the cross-inoculation groups of the legumes. Soil Sci. 58:61-69. [Google Scholar]