Abstract
The composition of the methanogenic archaeal community in the foregut contents of Tammar wallabies (Macropus eugenii) was studied using 16S rRNA and methyl coenzyme reductase subunit A (mcrA) gene clone libraries. Methanogens belonging to the Methanobacteriales and a well-supported cluster of uncultivated archaeon sequences previously observed in the ovine and bovine rumens were found. Methanogen densities ranged from 7.0 × 105 and 3.9 × 106 cells per gram of wet weight.
Kangaroos and wallabies belong to the marsupial family Macropodidae and are native to Australia. Because of their geographical isolation, macropod marsupials have evolved separately from other herbivorous animals, such as ruminants, but like ruminants, macropods have a complex gut microbiome that includes fungi, archaea, bacteria, and protozoa to coordinate plant biomass breakdown (11). The macropod foregut is functionally analogous to the rumen, yet for reasons unknown, macropod species produce relatively low levels of methane compared to ruminants (5, 13, 30).
New species of bacteria (21) and protozoa (2-4) have been indentified in the macropod foregut, and the presence of fungi has also been reported (5). Preliminary studies have shown that methanogens are present in the kangaroo foregut (1) but can be absent or at levels below detection limits (22). This study represents the first attempt to describe the diversity of methanogens residing in the macropod foregut by using 16S rRNA and methyl coenzyme reductase A (mcrA) clone libraries in combination with quantitative real-time PCR.
Sample preparation and library construction.
Foregut contents were collected from a captive colony of female Tammar wallabies (Macropus eugenii). Eight wallabies, aged between 1.5 and 4 years, were euthanized at two different sampling dates, November 2006 (five wallabies) and May 2007 (three wallabies), and stomach contents collected. DNA from foregut contents was pooled by sampling date and was extracted using the cetyltrimethylammonium bromide method of Wright et al. (32). Archaeal 16S rRNA genes were PCR amplified using the gene primer set and protocol of Wright and Pimm (33), whereas the methanogen-specific methyl coenzyme reductase A (mcrA) gene was PCR amplified using the primer set and protocol of Luton et al. (18). PCR products were cloned using a TOPO TA cloning kit according to the manufacturer's instructions (Invitrogen Corporation, San Diego, CA).
In total, 191 16S rRNA clone inserts were sequenced using the primers Met448F (33), Met1027F (33), and Met780R (5′-TTCGTCCCTCACCGTC-3′). The 95 mcrA genes obtained were sequenced using the mcrA primer set of Luton et al. (18). All products were sequenced using a BigDye Terminator cycle sequencing kit (version 3.1) with an ABI 3730 genetic analyzer (Applied Biosystems). Pooled samples were used only for clone library analysis, but real-time PCR analysis was performed on DNA extractions from individual animals.
Phylogeny and real-time PCR.
All DNA sequence reads were edited manually and assembled into contiguous sequences by using SEQMAN (DNASTAR) and checked for chimeras using the program Bellerophon (10). Sequences were imported into the ARB software package (16), release 07.07.11, and aligned, and similarity matrices were constructed using the Kimura-2 parameter correction method (14). Neighbor-joining dendrograms (25) for both mcrA and 16S rRNA gene sequences were constructed using PHYLIP (7) with 1,000 bootstrap resamplings. Methanogen cell densities were estimated from foregut contents of individual wallabies with the real-time PCR primers and calculation methods of Denman et al. (6).
Methanogen isolation.
A single methanogen isolate, WBY1, was purified from May 2007 wallaby foregut contents by using the modified RF30 medium and anaerobic dilution techniques of Skillman et al. (26). The 16S rRNA and mcrA genes from the methanogen isolate were PCR amplified and resulting PCR products sequenced.
Clone library analysis.
From the first sampling date, both 16S rRNA (96 sequences) and mcrA (35 sequences) gene libraries revealed the same three species, Methanobrevibacter gottschalkii (20), Methanosphaera stadtmanae (19), and an uncultivated archaeon, ON-CAN.17, from bovine rumen contents (35). The 16S rRNA gene sequences for the unknown archaeon were dominant at the November sampling (91.7% of the 96 sequences), compared to those for the Methanobrevibacter sp. (6.2%). However, with only 35 sequences from the November mcrA library, the unknown archaeon was as prevalent (48.6%) as the Methanobrevibacter sp. (45.7%). Analysis of the 16S rRNA (95 sequences) and mcrA (60 sequences) genelibraries from the May sampling revealed the same three species. However, Methanobrevibacter gottschalkii was now dominant (91.6%), with the unknown archaeon representing only 6.3% of the sequences. The proportions of these three species in the May mcrA gene library were similar to those in the May 16S rRNA gene library, with the Methanobrevibacter sp. and the unknown archaeon accounting for 66.7% and 20.0% of sequences, respectively. Methanosphaera sequences were the minority in each of the four clone libraries. These data imply a likely difference in community proportion between the two sampling dates, as Methanobrevibacter gottschalkii was predominant at the May (autumn) sampling while the novel archaeon phylotype was predominant at the November (spring) sampling.
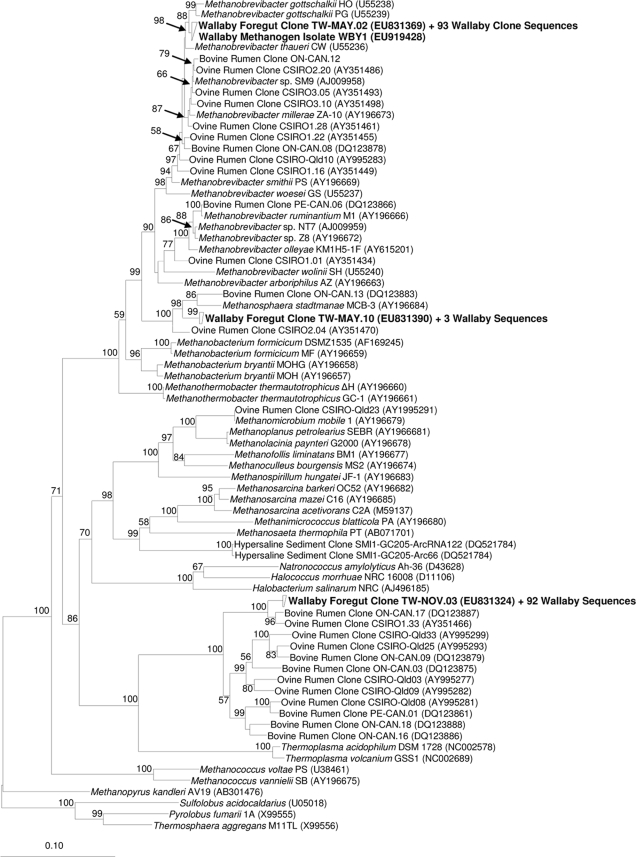
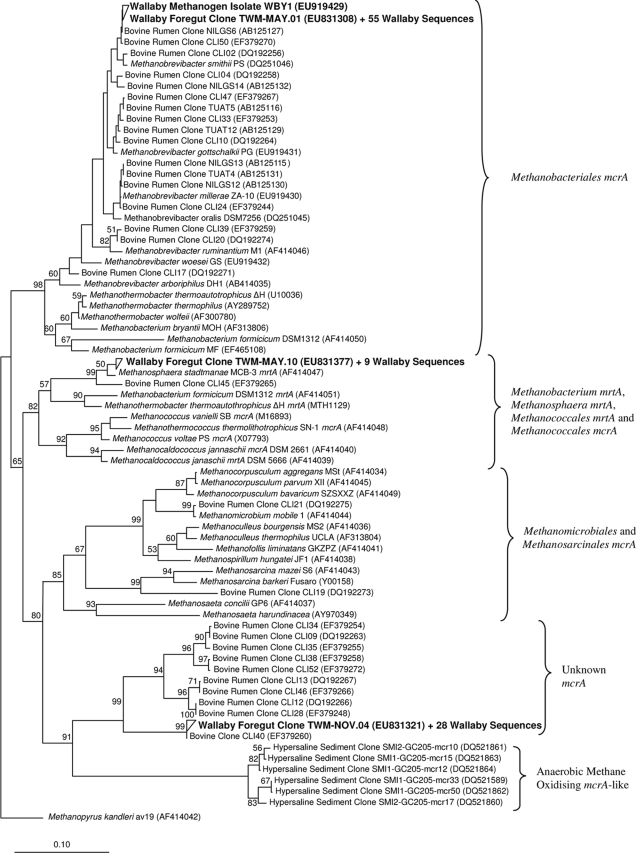
Overall, TW-MAY.02 represented 94 of the 191 sequences (99.4 to 100% identity) (Fig. 1) and had 98.9% identity to Methanobrevibacter gottschalkii, a methanogen isolated from the feces of a pig (20). Also, 56 of 95 mcrA sequences were 100% identical to each other, represented by TWM-MAY.01 (Fig. 2), and were 97.6% similar to Methanobrevibacter smithii PST, a near neighbor of Methanobrevibacter gottschalkii PGT. The reason for the dominance of a single Methanobrevibacter phylotype in the wallaby foregut is unknown, but environmental conditions and ecological pressures in the foregut likely favor this species over all others. Similarly, 420 methanogen clone sequences from chicken gut contents were found to be almost identical to each other (24). In contrast, Methanobrevibacter species from the rumen appear to form a continuum of species rather than discreet groups (12). The 16S rRNA gene of an isolate, WBY1, had 100% sequence identity to the Methanobrevibacter sp. clone library sequences (Fig. 1). Also, the mcrA sequence of WBY1 was 100% identical to those from the mcrA clone library (Fig. 2).
FIG. 1.
Phylogenetic tree of archaeal 16S rRNA gene sequences, with representative wallaby foregut clone sequences and the wallaby methanogen isolate WBY1. Wallaby foregut sequences are in bold type. Only bootstrapping values greater than 50% are shown. The scale bar represents 10% sequence divergence.
FIG. 2.
Phylogenetic tree of mcrA and mcrA-like gene sequences, with representative wallaby foregut clone sequences and the wallaby methanogen isolate WBY1 mcrA gene sequence. Wallaby foregut sequences are in bold type. Only bootstrapping values greater than 50% are shown. The scale bar represents 10% sequence divergence.
In addition, TW-NOV.03 represented 93 of the 191 16S rRNA gene sequences (99.5 to 100% identity) (Fig. 1) and had 97.2% identity to ON-CAN.17 (35), an uncultured archaeon from bovine rumen contents. The validly described species closest to TW-NOV.03 was Thermoplasma acidophilum, with 73.8% identity. The 29 mcrA sequences that formed a grouping represented by TWM-NOV.04 (96.9 to 100% identity) (Fig. 2) had 99.8% identity to the bovine rumen clone CLI40 (6) and only 74.5% similarity to the nearest known methanogen, Methanosaeta concilii GP6T. These mcrA sequences formed a monophyletic clade distantly related to mcrA-like sequences from a mixed culture containing archaea that oxidized methane (9). Given that the uncultivated archaeon sequences have genuine mcrA genes, which are known only to exist in methanogenic archaea, it is likely that these archaea are indeed methanogens, although further evidence to support this notion is required.
Four 16S rRNA genes, represented by TW-MAY.10 (99.4 to 100% identity) (Fig. 1), had 95.9% similarity to Methanosphaera stadtmanae MCB-3T, which was isolated from human fecal material (19). Other Methanosphaera spp. have previously been detected in bovine (29, 31) and ovine (34) rumen contents. Ten mrtA clone library sequences, represented by TWM-MAY.10 (95.8 to 100% identity) (Fig. 2), had 95.8% similarity to Methanosphaera stadtmanae MCB-3T. A search of the Methanosphaera stadtmanae MCB-3T genome sequence (8) revealed the absence of mcrA genes but the presence of the mrtA isoform (data not shown). Although the mcrA gene has been shown to be a possible alternative to the 16S rRNA gene phylogeny (15, 17, 18, 27), anomalies due to conserved mcrA primer sites (6, 18) in the mrtA genes of Methanosphaera, Methanobacterium, Methanococcales, and Methanothermobacter spp. exist (Fig. 2). Because the mrtA gene is expressed at greater levels, with increased hydrogen concentrations, than the mcrA gene (23), under increased hydrogen concentrations, like that found in wallaby and kangaroo foreguts (5), Methanosphaera stadtmanae methanogens could be more prevalent than methanogens only possessing the mcrA gene.
Real-time PCR analysis.
Because no estimates of archaeon cell density from macropod foregut contents exist, densities from bovine rumen contents were also estimated using the methods of Denman et al. (6). The estimate of 9.8 × 108 cells per gram of rumen contents (Table 1) compares favorably to the estimate made by Denman et al. (6) (1.3 × 109 cells per gram of rumen contents obtained using the mcrA gene) as well as estimates for sheep (26) and reindeer (28). Densities at the November sampling were similar to those at the May sampling (Table 1), but this difference was not significant, because of low sample numbers and estimate variation between wallabies (Table 1). Overall, estimates of archaeal cell densities varied between 7.0 × 105 and 3.9 × 106 cells per gram of wet weight (Table 1) and were between 400- and 1,400-fold less than estimates from rumen contents from the present study (Table 1). The lower methanogen densities may be why methane emissions in Tammar wallabies were only 1 to 2% of digestible energy (30), compared to the 10.5% of digestible energy in the sheep rumen (13).
TABLE 1.
Quantitative real-time PCR estimates of methanogen cell densities of individual wallabies for first and second sampling times and bovine rumen contents
| Digesta sample group | Mo and yr of sample collection | Methanogen cell densitya (g−1 wet wt) |
|---|---|---|
| By origin | ||
| Tammar wallaby 1 | November 2006 | 1.0 × 106 |
| Tammar wallaby 2 | November 2006 | 2.4 × 106 |
| Tammar wallaby 3 | November 2006 | 3.9 × 106 |
| Tammar wallaby 4 | November 2006 | 7.1 × 105 |
| Tammar wallaby 5 | November 2006 | 2.3 × 106 |
| Tammar wallaby 6 | May 2007 | 2.5 × 106 |
| Tammar wallaby 7 | May 2007 | 7.0 × 105 |
| Tammar wallaby 8 | May 2007 | 1.7 × 106 |
| By time point | ||
| November 2006 | November 2006 | 2.8 × 106 ± 5.7 × 105 |
| May 2007 | May 2007 | 3.2 × 106 ± 5.2 × 105 |
| Bovine rumen content control | Unknown | 9.8 × 108 |
Errors are standard errors of the means.
In conclusion, methanogens and the unknown archaeon sequences were similar to those previously identified in gut environments and based on 16S rRNA gene sequences. Methanobrevibacter spp. were predominant at the May sampling date, while sequences from the unknown archaeon were most numerous at the November sampling.
Nucleotide sequence accession numbers.
Of the 191 16S rRNA gene sequences, 79 different sequences were deposited in the GenBank database (under accession numbers EU831322 to EU831401). Of the 95 mcrA gene sequences, 14 different sequences were deposited in the GenBank database (under accession numbers EU831308 to EU831321). The deposited sequences were designated TW for 16S rRNA and TWM mcrA genes and either NOV or MAY for the two sampling dates. Sequence data from the new methanogen isolate, WBY1, for the 16S rRNA gene and the mcrA gene were deposited in the GenBank database under accession numbers EU919428 and EU919429, respectively. mcrA gene sequences from Methanobrevibacter millerae ZA-10 (accession number EU919430), Methanobrevibacter gottschalkii PG (accession number EU919431), and Methanobrevibacter woesei GS (accession number EU919432) were deposited in the GenBank database.
Footnotes
Published ahead of print on 13 February 2009.
REFERENCES
- 1.Baker, S. K., T. Schoep, N. J. Edwards, and A.-D. G. Wright. 2002. Methanogens in kangaroos. Reprod. Nutr. Dev. 42:S77. [Google Scholar]
- 2.Cameron, S. L., and P. J. O'Donoghue. 2002. Trichostome ciliates from Australian marsupials. I. Bandia gen. nov. (Litostomatea: Amylovoracidae). Eur. J. Protistol. 38:405-429. [Google Scholar]
- 3.Cameron, S. L., and P. J. O'Donoghue. 2003. Trichostome ciliates from Australian marsupials. II. Polycosta gen. nov. (Litostomatea: Polycostidae fam. nov.). Eur. J. Protistol. 39:83-100. [Google Scholar]
- 4.Cameron, S. L., and P. J. O'Donoghue. 2003. Trichostome ciliates from Australian marsupials. III. Megavestibulum gen. nov. (Litostomatea: Macropodiniidae). Eur. J. Protistol. 39:123-138. [Google Scholar]
- 5.Dellow, D. W., I. D. Hume, R. T. J. Clarke, and T. Bauchop. 1988. Microbial activity in the forestomach of free-living macropodid marsupials: comparisons with laboratory studies. Aust. J. Zool. 36:383-395. [Google Scholar]
- 6.Denman, S. E., N. W. Tomkins, and C. S. McSweeney. 2007. Quantitation and diversity analysis of ruminal methanogenic populations in response to the antimethanogenic compound bromochloromethane. FEMS Microbiol. Ecol. 62:313-322. [DOI] [PubMed] [Google Scholar]
- 7.Felsenstein, J. 1993. PHYLIP (Phylogeny Inference Package) version 3.5c. Department of Genetics, University of Washington, Seattle.
- 8.Fricke, W. F., H. Seedorf, A. Henne, M. Krüer, H. Liesegang, R. Hedderich, G. Gottschalk, and R. K. Thauer. 2006. The genome sequence of Methanosphaera stadtmanae reveals why this human intestinal archaeon is restricted to methanol and H2 for methane formation and ATP synthesis. J. Bacteriol. 188:642-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hallam, S. J., P. R. Girguis, C. M. Preston, P. M. Richardson, and E. F. DeLong. 2003. Identification of methyl coenzyme M reductase A (mcrA) genes associated with methane-oxidizing archaea. Appl. Environ. Microbiol. 69:5483-5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huber, T., G. Faulkner, and P. Hugenholtz. 2004. Bellerophon: a program to detect chimeric sequences in multiple sequence alignments. Bioinformatics 20:2317-2319. [DOI] [PubMed] [Google Scholar]
- 11.Hume, I. D. 1984. Microbial fermentation in herbivorous marsupials. BioScience 34:435-440. [Google Scholar]
- 12.Janssen, P. H., and M. Kirs. 2008. Structure of the archaeal community of the rumen. Appl. Environ. Microbiol. 74:3619-3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kempton, T. J., R. M. Murray, and R. A. Leng. 1976. Methane production and digestibility measurements in the grey kangaroo and sheep. Aust. J. Biol. Sci. 29:209-214. [DOI] [PubMed] [Google Scholar]
- 14.Kimura, M. 1980. A simple method of estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 16:111-120. [DOI] [PubMed] [Google Scholar]
- 15.Lehmacher, A., and H. P. Klenk. 1994. Characterization and phylogeny of mcrII, a gene cluster encoding an isozyme of methyl coenzyme M reductase from hyperthermophilic Methanothermus fervidus. Mol. Gen. Genet. 243:198-206. [DOI] [PubMed] [Google Scholar]
- 16.Ludwig, W., O. Strunk, R. Westram, L. Richter, H. Meier, Yadhukumar, A. Buchner, T. Lai, S. Steppi, G. Jobb, W. Forster, I. Brettske, S. Gerber, A. W. Ginhart, O. Gross, S. Grumann, S. Hermann, R. Jost, A. Konig, T. Liss, R. Lussmann, M. May, B. Nonhoff, B. Reichel, R. Strehlow, A. Stamatakis, N. Stuckmann, A. Vilbig, M. Lenke, T. Ludwig, A. Bode, and K. H. Schleifer. 2004. ARB: a software environment for sequence data. Nucleic Acids Res. 32:1363-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lueders, T., K. J. Chin, R. Conrad, and M. Friedrich. 2001. Molecular analyses of methyl-coenzyme M reductase alpha-subunit (mcrA) genes in rice field soil and enrichment cultures reveal the methanogenic phenotype of a novel archaeal lineage. Environ. Microbiol. 3:194-204. [DOI] [PubMed] [Google Scholar]
- 18.Luton, P. E., J. M. Wayne, R. J. Sharp, and P. W. Riley. 2002. The mcrA gene as an alternative to 16S rRNA in the phylogenetic analysis of methanogen populations in landfill. Microbiology 148:3521-3530. [DOI] [PubMed] [Google Scholar]
- 19.Miller, T. L., and M. J. Wolin. 1985. Methanosphaera stadtmaniae gen-nov, sp-nov: a species that forms methane by reducing methanol with hydrogen. Arch. Microbiol. 141:116-122. [DOI] [PubMed] [Google Scholar]
- 20.Miller, T. L., and C. Lin. 2002. Description of Methanobrevibacter gottschalkii sp. nov., Methanobrevibacter thaueri sp. nov., Methanobrevibacter woesei sp. nov. and Methanobrevibacter wolinii sp. nov. Int. J. Syst. Evol. Microbiol. 52:819-822. [DOI] [PubMed] [Google Scholar]
- 21.Ouwerkerk, D., A. V. Klieve, R. J. Forster, J. M. Templeton, and A. J. Maguire. 2005. Characterization of culturable anaerobic bacteria from the forestomach of an eastern grey kangaroo, Macropus giganteus. Lett. Appl. Microbiol. 41:327-333. [DOI] [PubMed] [Google Scholar]
- 22.Ouwerkerk, D., A. J. Maguire, and A. V. Klieve. 2005. Reductive acetogenesis in the foregut of macropod marsupials in Australia, p. 98-101. In C. R. Soliva, J. Takahashi, and M. Kreuzer (ed.), Publication series, vol. 27. Institute of Animal Science, ETH, Zurich, Switzerland. [Google Scholar]
- 23.Pennings, J. L., P. Vermeij, L. M. de Poorter, J. T. Keltjens, and G. D. Vogels. 2000. Adaptation of methane formation and enzyme contents during growth of Methanobacterium thermoautotrophicum (strain ΔH) in a fed-batch fermentor. Antonie van Leeuwenhoek 77:281-291. [DOI] [PubMed] [Google Scholar]
- 24.Saengkerdsub, S., R. C. Anderson, H. H. Wilkinson, W.-K. Kim, D. J. Nisbet, and R. C. Ricke. 2007. Identification and quantification of methanogenic archaea in adult chicken ceca. Appl. Environ. Microbiol. 73:353-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for constructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 26.Skillman, L. C., P. N. Evans, G. E. Naylor, B. Morvan, G. N. Jarvis, and K. N. Joblin. 2004. 16S ribosomal DNA-directed PCR primers for ruminal methanogens and identification of methanogens colonising young lambs. Anaerobe 10:277-285. [DOI] [PubMed] [Google Scholar]
- 27.Springer, E., M. S. Sachs, C. R. Woese, and D. R. Boone. 1995. Partial gene sequences for the A subunit of methyl-coenzyme M reductase (mcrI) as a phylogenetic tool for the family Methanosarcinaceae. Int. J. Syst. Bacteriol. 45:554-559. [DOI] [PubMed] [Google Scholar]
- 28.Sundset, M. A., J. E. Edwards, Y. F. Cheng, R. S. Senosiain, M. N. Fraile, K. N. Northwood, K. E. Præsteng, T. Glad, S. D. Mathiesen, and A.-D. G. Wright. 2009. Molecular diversity of the rumen microbiome of Norwegian reindeer on natural summer pasture. Microb. Ecol. 57:335-348. [DOI] [PubMed] [Google Scholar]
- 29.Tajima, K., T. Nagamine, H. Matsui, M. Nakamura, and R. I. Aminov. 2001. Phylogenetic analysis of archaeal 16S rRNA libraries from the rumen suggests the existence of a novel group of archaea not associated with known methanogens. FEMS Microbiol. Lett. 200:67-72. [DOI] [PubMed] [Google Scholar]
- 30.von Engelhardt, W., S. Wolter, H. Lawrenz, and J. A. Hemsley. 1978. Production of methane in two non-ruminant herbivores. Comp. Biochem. Physiol. A 60:309-311. [Google Scholar]
- 31.Whitford, M. F., R. M. Teather, and R. J. Forster. 2001. Phylogenetic analysis of methanogens from the bovine rumen. BMC Microbiol. 1:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wright, A.-D. G., B. A. Dehority, and D. H. Lynn. 1997. Phylogeny of the rumen ciliates Entodinium, Epidinium and Polyplastron (Litostomatea: Entodiniomorphida) inferred from small subunit ribosomal RNA sequences. J. Eukaryot. Microbiol. 44:61-67. [DOI] [PubMed] [Google Scholar]
- 33.Wright, A.-D. G., and C. Pimm. 2003. Improved strategy for presumptive identification of methanogens using 16S riboprinting. J. Microbiol. Methods 55:337-349. [DOI] [PubMed] [Google Scholar]
- 34.Wright, A.-D. G., A. J. Williams, B. Winder, C. T. Christophersen, S. L. Rodgers, and K. D. Smith. 2004. Molecular diversity of rumen methanogens from sheep in Western Australia. Appl. Environ. Microbiol. 70:1263-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wright, A.-D. G., C. H. Auckland, and D. H. Lynn. 2007. Molecular diversity of methanogens in feedlot cattle from Ontario and Prince Edward Island, Canada. Appl. Environ. Microbiol. 73:4206-4210. [DOI] [PMC free article] [PubMed] [Google Scholar]