Abstract
The blacklegged tick, Ixodes scapularis, is of significant public health importance as a vector of Borrelia burgdorferi, the agent of Lyme borreliosis. The timing of seasonal activity of each immature I. scapularis life stage relative to the next is critical for the maintenance of B. burgdorferi because larvae must feed after an infected nymph to efficiently acquire the infection from reservoir hosts. Recent studies have shown that some strains of B. burgdorferi do not persist in the primary reservoir host for more than a few weeks, thereby shortening the window of opportunity between nymphal and larval feeding that sustains their enzootic maintenance. We tested the hypothesis that climate is predictive of geographic variation in the seasonal activity of I. scapularis, which in turn differentially influences the distribution of B. burgdorferi genotypes within the geographic range of I. scapularis. We analyzed the relationships between climate, seasonal activity of I. scapularis, and B. burgdorferi genotype frequency in 30 geographically diverse sites in the northeastern and midwestern United States. We found that the magnitude of the difference between summer and winter daily temperature maximums was positively correlated with the degree of seasonal synchrony of the two immature stages of I. scapularis. Genotyping revealed an enrichment of 16S-23S rRNA intergenic spacer restriction fragment length polymorphism sequence type 1 strains relative to others at sites with lower seasonal synchrony. We conclude that climate-associated variability in the timing of I. scapularis host seeking contributes to geographic heterogeneities in the frequencies of B. burgdorferi genotypes, with potential consequences for Lyme borreliosis morbidity.
An increasingly important area of research in infectious disease epidemiology is the influence of pathogen strain diversity on patterns of disease risk and clinical outcome. Strain-specific pathogenicity or transmissibility can be important clinical and epidemiological parameters; for example, only a subset of Neisseria meningitidis strains are responsible for invasive infections leading to meningitis (1). Geography and environmental features influence the genetic structure of certain pathogens by regulating their distribution, dispersal, or population size (8, 31, 49). Accordingly, a heterogeneous environment will result in spatial structuring of genotype frequencies, with possible epidemiological implications.
Lyme borreliosis is a tick-borne zoonosis caused by Borrelia burgdorferi, a spirochetal bacterium that exhibits genetic diversity throughout its range in eastern North America (12, 60), where it is maintained in a horizontal transmission cycle between its vector, the blacklegged tick Ixodes scapularis, and vertebrate reservoir hosts. I. scapularis has a two-year life cycle in which it takes three blood meals, one per life stage, with the two subadult stages responsible for the enzootic maintenance of B. burgdorferi (2, 3, 51). Larval ticks hatch uninfected from eggs (41) and acquire the spirochetes from infected reservoir hosts. Infected larvae maintain the spirochetes transstadially, allowing them to transmit B. burgdorferi to uninfected reservoirs during their nymphal blood meal the following summer. The seasonal timing of activity, or phenology, of each tick life stage relative to the next is a critical factor in the maintenance of B. burgdorferi because larvae typically must feed after an infected nymph in order to acquire the bacteria (32).
Previous studies in Europe of tick-borne encephalitis virus have shown that seasonal synchrony of immature ticks is necessary for the maintenance of the virus in natural enzootic cycles because nonsystemic infections are transmitted from nymphs to larvae feeding in close proximity on the same individual reservoir rodent (48). Furthermore, seasonal synchrony of immature tick activity, a prerequisite of cofeeding, was found to be correlated with climate (47). Although it is possible for an I. scapularis larva to become infected with B. burgdorferi by simultaneously feeding in close proximity to an infected nymph, a role for cofeeding transmission in the enzootic maintenance of B. burgdorferi in North America has not been established (43). Rather, until recently, the existing evidence indicated that B. burgdorferi causes life-long systemic infections in reservoirs that allow for its maintenance in the absence of seasonal synchrony of I. scapularis immatures (18). However, recent studies suggest that this may not always be the case (34) and that there are differences in the duration of infectiousness that are strain specific (16, 28).
We hypothesized that large-scale, climate-driven geographic variability in the host seeking phenology of immature I. scapularis ticks is associated with heterogeneity in the frequencies of strains acquired by larval ticks. Using regression models and accounting for spatial autocorrelation, we examined the relationships between climate, the temporal synchrony of larval and nymphal seasonal host seeking activity, and B. burgdorferi genotype frequency in ticks collected from 30 geographically diverse sites systematically selected for their locations throughout the northeastern and midwestern United States.
Here we present empirical evidence that climate patterns, specifically, regional variation in summer and winter temperature cycle extremes, are associated with variation in the seasonal synchrony of I. scapularis larval and nymphal host seeking activity. Furthermore, both climate and the differences in the seasonal synchrony of the two immature tick stages are related to geographic variation in B. burgdorferi genotype frequency. Our results point to the impact of climate upon the natural dynamics of enzootic transmission and population genetic structure of an important vector-borne human pathogen, with possible implications for the distribution of human disease risk and epidemiology.
MATERIALS AND METHODS
Tick collections.
Host seeking ticks were collected between mid-May and late September in the years 2004 to 2006. Potential collection sites were selected according to a stratified random selection procedure described previously (17). Over the 3-year period, 304 sampling sites were selected throughout the range of I. scapularis in the United States. Host seeking I. scapularis ticks were found in 103 of these sites and were concentrated around two major foci of activity in the Northeast and Upper Midwest (see Fig. S1 in the supplemental material). A subset of these sites were sampled in multiple years, resulting in 115 total site-year collections. Included in the present analysis were all collections where larvae were found on a minimum of three visits over the course of a sampling season. The lowest 10th percentile of site-year collections in terms of larval counts was dropped from the analysis, yielding a minimum sample size of 19 larvae per site per year. These selection criteria resulted in 34 site-year collections that were adequate for examining seasonal host seeking activity. The 34 collections represented 30 unique sites; 2 of the 30 sites were visited in two different years of the study, and one site was visited in all three years.
Each site was visited repeatedly at approximately even intervals throughout the summer months, with a median of five visits during the season. At each visit, host seeking ticks were collected from vegetation by using a 1-m2 drag cloth over five 200-meter transects, for a total of 1,000 m2 sampled per visit. The cloth was inspected for ticks every 20 m, and nymphal ticks were preserved in transect-specific vials of 70% ethanol. Larvae were either placed in the vial or collected using adhesive tape and stored in plastic bags. All nymphs were identified to species using the key by Durden and Keirans (20). Due to the large number of larvae collected and the difficulty involved in identifying larvae of the genus Ixodes to species, all larval specimens were identified to genus. Within the 3-year sampling period, only 6 of 3,923 Ixodes spp. nymphs in the 34 collections included in this study were not I. scapularis. Therefore, we estimated that less than 0.2% of Ixodes spp. larvae would be a species other than I. scapularis and included all Ixodes spp. larvae in this analysis.
Host seeking seasonality.
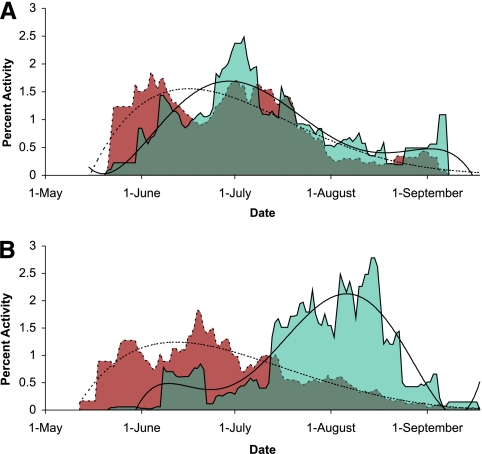
For each site, the percent activity of larvae and nymphs was plotted for each collection date, yielding overlapping activity curves for the two stages. Percent activity was measured as the total number of I. scapularis ticks of a given stage collected at a visit over the total number collected at the site throughout the season. Seasonal synchrony was measured as the overlapping area under both larval and nymphal percent activity curves. Seasonal synchrony was calculated for each site, as well as each sampling year for repeated sites. To more closely inspect patterns of host seeking seasonality, we pooled observations into two groups based on the median value of seasonal synchrony and plotted the 2-week moving average of larval and nymphal seasonal percent activity for each group (see Fig. 2).
FIG. 2.
Seasonal activity of larvae and nymphs. Seasonal activity curves of immature tick host seeking based on 2-week moving averages of larval (solid lines/blue shading) and nymphal (dashed lines/red shading) host seeking. Observations of seasonal activity were pooled into two groups divided at the median of the seasonal synchrony index. Fifth-order polynomial trend lines were fitted for illustration purposes and are shown in black.
Environmental covariates.
Previous research on ticks belonging to the Ixodes ricinus species complex, of which I. scapularis is a member, has shown that temperature can affect the seasonal activity of immature life stages (6, 35, 38, 40, 42, 45-48). In addition, vapor pressure deficit has been shown to affect the distribution, survivorship, and host seeking behavior of I. scapularis (11, 35, 57). Because the timing of host seeking in ticks is thought to be a genetic adaptation to long-term climate patterns (9, 22), we used detrended averages of ground-collected climate data recorded between 1950 and 2006. Spatially continuous meteorological surfaces of all variables, at an 8-km spatial resolution, were derived by interpolation of daily data from meteorological stations by the Ecological Forecasting Laboratory at NASA Ames Research Center (http://ecocast.arc.nasa.gov/) using the Surface Observation and Gridding System (29) and the DAYMET algorithms (55). We included in our analyses the monthly average of the daily maximum and minimum temperatures and the monthly average vapor pressure deficit at each of the sites. In addition to the monthly means, we calculated the detrended annual and biannual Fourier component magnitude and phase of the maximum and minimum temperature and vapor pressure deficit variables by using ERDAS Imagine version 9.1 (Leica Geosystems Atlanta, GA). The temporal Fourier processing removes noise from the seasonal data, achieves data reduction, and produces a set of orthogonal outputs while retaining a description of seasonality. It is commonly used to study the distribution of vectors (44, 50, 53). Processed raster surfaces were then overlaid with sampling points, and point values were extracted by using ArcGIS 9 (ESRI, Inc., Redlands, CA). Photoperiod has been shown to be the main trigger of nymphal emergence from diapause for I. scapularis ticks (7). Therefore, we included latitude as a covariate in our analyses.
DNA extraction and PCR.
Total DNA was extracted from individual nymphal I. scapularis ticks using ammonium hydroxide (NH4OH). Ticks were incubated for 2 h in 5 μl of 1.4 M NH4OH at room temperature and then crushed with a pipette tip. Ninety-five microliters of water was added, followed by a second incubation at 95°C for 30 min. The material was centrifuged to separate tick debris from the DNA solution, and the supernatant was transferred to clean vials containing 1 μl of 0.1 M EDTA and stored at −20°C.
All extracts were tested for the presence of B. burgdorferi DNA by using real-time PCR targeting the 16S rRNA (rrs) gene, and positive samples were subjected to a second real-time PCR to identify 16S-23S rRNA intergenic spacer restriction fragment length polymorphism sequence type 1 (RST 1) strains as described previously (56). Our choice of genotyping method, therefore, discriminated between two broad groups of strains, RST 1 strains and RST 2 and 3 strains. The reasons for this choice were twofold. (i) RST 1 strains, a genetically conserved and monophyletic assemblage that includes the temporally persistent infection-causing strain B206, have been linked with a greater range and severity of clinical symptoms than RST 2 and RST 3 strains, which together form a genetically more diverse group (30, 54, 61, 62); therefore, this genotyping modality has epidemiological and ecological relevance to our study question. (ii) By using a single real-time PCR, we were able to assay a larger number of B. burgdorferi samples than would have been possible using a sequencing-based method. One limitation of our genotyping approach is that any ticks that were coinfected with both RST 1 and RST 2 and 3 strains would have been scored as positive for RST 1, thereby in effect underestimating the prevalence of RST 2 and 3 strains. Using finer-scale sequence-based genotyping at multiple loci, including the 16S-23S rRNA intergenic spacer region, we have found a rate of mixed infections of approximately 20% in all sites, with no geographic patterning (A. G. Gatewood, unpublished data). Therefore, this potential source of bias appears to be systematic and distributed evenly across our study area.
Statistical analyses.
Linear regression was used to model the synchrony in larval and nymphal seasonal activity using climate variables as predictors. Multiple regression models, including all combinations of two or more variables, were not significant, likely due to small sample size and to the covariance inherent in climate variables. The best-fit univariate model according to Akaike's information criterion (AIC) was selected as a basis for logistic regression analyses to examine the relationships between seasonal synchrony, climate, and RST 1 strain presence/absence in infected ticks.
The residuals of all models were tested for the presence of spatial autocorrelation, which would violate the underlying assumption of regression analysis that residual errors are uncorrelated. Spatial autocorrelation was assessed at different distance classes by calculating Moran's I (13) over equally sized spatial lags, with the smallest lag distance chosen to be the maximum distance between any point and its nearest neighbor. When evidence of spatial autocorrelation was found, it was adjusted for by including a spatial autocovariate in the model. The spatial autocovariate was an inverse distance-weighted average of the response variable that was calculated over a set of neighbors for each point, with the neighborhood defined by the spatial extent of autocorrelation. Finally, for all models, standard errors were adjusted to account for repeated sampling of the sites in more than one year in three by using the cluster option in Stata (STATA SE 10; Stata Corporation), which corrects for within-group autocorrelation. All distance calculations were performed on geographic data projected with a Lambert azimuthal equal area projection in ArcGIS (ESRI, Inc., Redlands, CA). All statistical calculations were done in Stata and in R using the spdep and ncf packages. R procedures for generating autocovariate terms were adapted from Dormann et al. (19).
RESULTS
We estimated the seasonal overlap of host seeking I. scapularis larvae and nymphs by using collections of 31,917 Ixodes spp. larvae and 3,929 I. scapularis nymphs at 30 geographically diverse sites in the northeastern and midwestern United States (see Table S1 in the supplemental material). In general, host seeking Ixodes spp. ticks were concentrated in the Northeast and Upper Midwest and were not found in and around the state of Ohio despite extensive and systematic sampling throughout the northern and eastern quadrant of the United States (see Fig. S1 in the supplemental material). This distribution has been observed both in the sampling associated with the present study (17) and in other studies (11, 15). Seasonal synchrony, quantified as an index calculated as the area of overlap in larval and nymphal seasonal host seeking activity curves, was higher in midwestern sites than in northeastern sites, although there was variability within each region (Fig. 1A). Dividing the observations into halves at the median value of the seasonal synchrony index allowed for a more-detailed illustration of host seeking seasonality patterns (Fig. 2). Nymphal host seeking activity reached a peak early in the season and then tapered off slowly over the summer months over all sites. Larval host seeking activity reached a peak soon after the nymphal peak in highly synchronous sites (Fig. 2A), while in less-synchronous sites, larvae peaked toward the end of the season, after the nymphal peak.
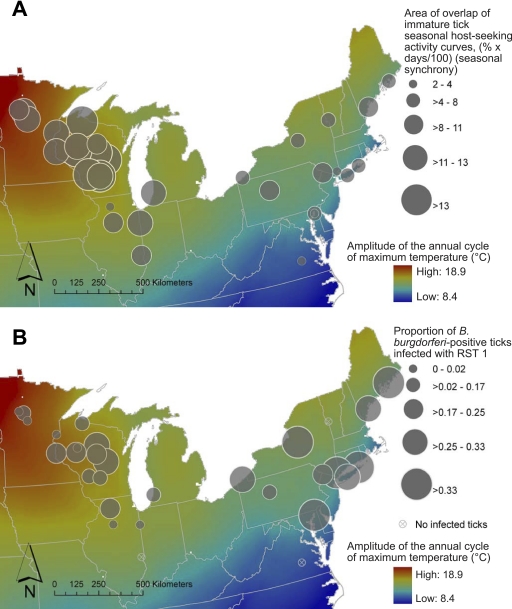
FIG. 1.
Maps of seasonal synchrony and RST 1 strain prevalence. (A) Seasonal synchrony, measured as the area of overlap of immature tick seasonal host seeking activity curves, mapped by site. Large circles are sites characterized by a high degree of seasonal synchrony of immature tick stage activity, and small circles represent sites with little seasonal overlap. Data are divided into categories by quantiles. Background shading corresponds to the amplitude of the annual cycle of maximum temperature in degrees Celsius. Warm colors represent regions with extreme annual temperature cycles, while areas shaded with cooler colors are characterized by milder seasonal climates. (B) RST 1 strain infection prevalence in B. burgdorferi-positive ticks, mapped by site. Larger circles represent sites with high rates of RST 1 strain infection, while smaller circles indicate sites with low RST 1 strain prevalence in infected ticks. Data are divided into categories by quantiles. Map background shading is as described for panel A.
Of the 3,923 nymphs, 817 (20.8%) were infected with B. burgdorferi, and of those, 212 (25.9%) had RST 1 strain infections (see Table S1 in the supplemental material). Infected nymphs were found in 27 of the 30 sites, and RST 1 strains were present in 25 sites. There was a general trend of increased RST 1 strain prevalence in northeastern sites (Fig. 1B), where there was less seasonal synchrony (Fig. 1A).
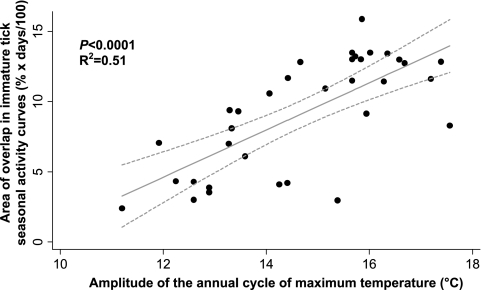
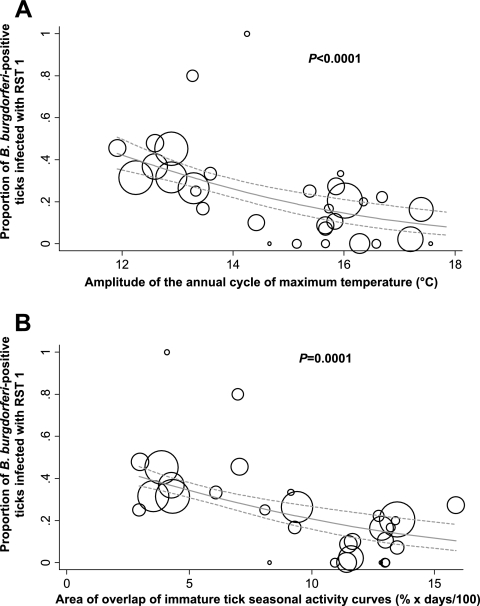
Using regression modeling, we identified the amplitude of the annual cycle of maximum daily temperature as the best predictor of seasonal synchrony (Table 1) based on AIC. Seasonal synchrony increased with increasing amplitude of the annual cycle of maximum temperature [F(1, 29) = 37.32, P < 0.0001, R2 = 0.51] (Fig. 3). This temporal Fourier-derived variable is a descriptor of the magnitude of the difference between winter and summer temperatures. Despite all of the climate variables exhibiting high covariance (data not shown), this variable outperformed the next-most-predictive variable by more than 6 points of AIC. We then used both seasonal synchrony and the amplitude of the annual cycle of maximum temperature in separate logistic regressions to predict the presence/absence of RST 1 strains. We observed a significant negative relationship between both variables and the probability of RST 1 strain presence (P < 0.001 and P = 0.001, respectively) (Fig. 4).
TABLE 1.
Univariate regression models to predict seasonal synchronya
| Model | Parameter | Parameter estimate | Pr > |t| | Pr > F | AIC value |
|---|---|---|---|---|---|
| Latitude | Intercept | −32.21 | 0.002 | <0.001 | 179 |
| Slope | 0.96 | <0.001 | |||
| Monthly mean Tmax | Intercept | 22.36 | <0.001 | <0.001 | 179.4 |
| Slope | −0.92 | <0.001 | |||
| Tmaxannual cycle amplitude | Intercept | −15.78 | <0.001 | <0.001 | 168.23 |
| Slope | 1.7 | <0.001 | |||
| Tmax annual cycle phase | Intercept | 248.47 | <0.001 | <0.001 | 178.67 |
| Slope | −1.11 | <0.001 | |||
| Tmax biannual cycle amplitude | Intercept | 3.14 | 0.043 | <0.001 | 176.37 |
| Slope | 6.31 | <0.001 | |||
| Tmax biannual cycle phase | Intercept | 11.68 | 0.574 | 0.9 | 193.77 |
| Slope | −0.01 | 0.903 | |||
| Monthly mean Tmin | Intercept | 11.79 | <0.001 | <0.001 | 178.44 |
| Slope | −0.87 | <0.001 | |||
| Tmin annual cycle magnitude | Intercept | −12.06 | 0.011 | <0.001 | 174.43 |
| Slope | 1.59 | <0.001 | |||
| Tmin annual cycle phase | Intercept | 207.07 | <0.001 | <0.001 | 183.66 |
| Slope | −0.9 | 0.001 | |||
| Tmin biannual cycle amplitude | Intercept | 5.72 | <0.001 | <0.001 | 182.13 |
| Slope | 4.77 | 0.001 | |||
| Tmin biannual cycle phase | Intercept | 22.76 | <0.001 | <0.001 | 186.82 |
| Slope | −0.06 | <0.001 | |||
| Monthly mean VPD | Intercept | 22.19 | <0.001 | 0.005 | 187.78 |
| Slope | −0.02 | 0.005 | |||
| VPD annual cycle magnitude | Intercept | −9.52 | 0.08 | 0.002 | 187.49 |
| Slope | 0.04 | 0.002 | |||
| VPD annual cycle phase | Intercept | 219.12 | 0.001 | 0.002 | 185.17 |
| Slope | −0.98 | 0.002 | |||
| VPD biannual cycle amplitude | Intercept | 3.08 | 0.304 | 0.04 | 188.3 |
| Slope | 0.13 | 0.04 | |||
| VPD biannual cycle phase | Intercept | 14.22 | 0.005 | 0.29 | 192.2 |
| Slope | −0.06 | 0.29 |
Results for the model with the lowest AIC are shown in boldface. Pr > F, P value associated with the F score for the model; Pr > |t|, 2-tailed P value for testing the null hypothesis that the parameter estimate is 0; Tmax, maximum daily temperature; Tmin, minimum daily temperature; VPD, vapor pressure deficit.
FIG. 3.
Relationship between seasonal synchrony and climate. Linear regression plot of the relationship between the area of overlap of immature tick seasonal host seeking activity curves and the amplitude of the annual cycle of maximum temperature. Solid gray line represents regression prediction. Dashed gray lines show the 95% confidence interval for the prediction line.
FIG. 4.
Relationships between RST 1 strain prevalence, climate, and seasonal synchrony. Logistic regression plot showing relationship between proportions of ticks infected with RST 1 strains and the amplitude of the annual cycle of maximum temperature (A) or seasonal synchrony measured as the area of overlap of immature tick seasonal host seeking activity curves (B). Circles are proportional to sample size. Solid gray lines are predicted probabilities of RST 1 strain infection. Dashed gray lines show 95% confidence intervals for predictions.
DISCUSSION
Our results show that seasonal synchrony of the immature stages of I. scapularis is significantly related to average maximum and minimum temperatures and vapor pressure deficit, as well as variables that capture seasonal climate patterns (Table 1). These climate-associated differences in seasonal synchrony are also correlated with the relative frequencies of RST 1 genotypes of B. burgdorferi circulating in natural enzootic cycles, resulting in an uneven geographic distribution of strains.
A previous study of the host seeking phenology of all three life stages of I. scapularis in a single site in Westchester County, NY, showed that the majority of the seasonal activity of larvae occurs in the late summer to early fall and is preceded by a much smaller peak of activity that is coincident with nymphal activity in early June (23). Because larvae do not hatch from eggs until July, it has been hypothesized that the small, earlier June peak in larval activity comprises larvae that were unsuccessful at finding a host in the preceding autumn and resumed activity in spring after overwintering (14). We observed a similar pattern among sites characterized by asynchronous peaks in immature ticks host seeking in the present study (Fig. 2B). Interestingly, the pattern is reversed in sites that are characterized by greater synchrony between stages, where we also observed a bimodal distribution of larval activity, but with the early peak much more pronounced than the late-season peak (Fig. 2A).
Abiotic factors, such as ground temperature, can affect the seasonality of subadult life stages of I. scapularis (39) and I. ricinus, the vector of B. burgdorferi and tick-borne encephalitis virus in Europe (41, 45-48). The temporal Fourier-processed climate variables evaluated in this study are summary descriptors of seasonal climate patterns and have been used previously in mapping vector seasonal activity (47). In sites characterized by a high amplitude of the annual cycles of maximum monthly temperature, there is a large difference between winter and summer temperatures. These higher extremes result in a more abrupt autumnal cooling, which has been implicated previously as a factor leading to unfed larvae entering diapause (5, 47). It is also possible that more-extreme seasonal temperatures delay ovipositioning and larval hatching, further shortening the window of opportunity for larvae to acquire a blood meal later in the same season. Our observation of a dominant peak in larval activity occurring in the spring in the more-continental sites may, in part, reflect a cohort of ticks that overwintered as unfed larvae as an adaptation to local climate conditions.
Our finding that RST 1 strains are more prevalent in sites where immature tick activity is less synchronous is consistent with the hypothesis that a temporal gap between nymphal and larval feeding confers a higher relative fitness to these strains than other strains. This is of particular interest in light of two recent studies that compared the transmission dynamics of two strains of B. burgdorferi, an invasive RST 1 strain (BL206) isolated from human blood (58) and a slowly disseminating RST 3 strain (B348) isolated from an erythema migrans lesion (59). A population simulation study (38) found that BL206 was weakly favored when immature tick feeding was synchronous but was more strongly favored when the temporal gap between peak nymphal and larval feeding was long. Our study provides empirical evidence that supports this theoretical finding. Another recent study experimentally compared the fitness of BL206 and B348 by measuring the duration of infection with each strain in mice by their ability to infect larval ticks (28). The authors found that the duration of BL206 infection in the white-footed mouse, Peromyscus leucopus, lasted for at least 79 days, while B348 infection declined dramatically within 40 days. This confirmed a result of an earlier study, which found that B348 infection dropped sharply by 21 days and approached zero by day 42 (16). It should be noted that P. leucopus is an important reservoir host for B. burgdorferi but that several other vertebrates are involved in its enzootic maintenance. Further studies are needed to determine if there are strain-specific differences in persistence in other vertebrate hosts, including birds.
The truncated infectious period of B348 observed in previous studies does not preclude its establishment under conditions favoring asynchronous immature tick activity. In fact, B348 was originally isolated from a patient in Westchester County, NY, the same county where asynchronous immature tick activity was described previously (23) and where we observed low seasonal overlap of immature tick activity in this study. In the absence of phenotypic data from other isolates or an understanding of the genetic basis of persistence, we cannot assume that all RST 1 strains share the high relative persistence of infectiousness observed in BL206. In genotyping studies, RST 1 strains generally form a well-supported monophyletic clade (12, 54, 59, 61), while RST 2 and 3 strains are for the most part diverged from RST 1 strains and exhibit more genetic diversity than do RST 1 strains, which are relatively more conserved (12, 54). We postulate that the invasive and persistent BL206 is likely to be somewhat representative of RST 1 strains, on average, while RST 3 strains, and perhaps also RST 2 strains, are more likely to exhibit a wider range of phenotypes. This pattern is also suggested by studies of the outer-surface protein C (ospC) locus, which is linked with the 16S-23S rRNA intergenic spacer region. Both ospC major groups, A and B, which correspond to RST 1, have been shown to be associated with highly invasive disease, while only a few of the more diverse and more numerous ospC genotype groups that correspond to RST 2 and 3 have been shown to be more capable of invasive infections (21, 52, 61).
Previous studies paint a complex and incomplete picture of strain-specific variation in dissemination and persistence for B. burgdorferi; nevertheless, they form the foundation for the hypothesis that less-invasive and -persistent strains would have a relative fitness disadvantage in regions with low seasonal overlap of subadult tick activity, thereby allowing the more-persistent strains to reach a higher prevalence than they would under synchronous conditions. Our finding that RST 1 strains are more prevalent in areas with low seasonal synchrony of immature tick host seeking activity is consistent with the hypothesis that seasonality-related drivers of selection for persistent strains in part underlie the distribution of RST 1 strains that we observed in this study. One likely source of noise in our model is the possibility that observations of RST 1 absence included in this analysis represent RST 2 or 3 strains that are highly persistent either in mice or other reservoir species. Despite this potential source of error, we observed a highly significant trend of a relative enrichment of RST 1 strains in asynchronous sites.
If RST 1 strains have a fitness advantage in an asynchronous environment, it could be argued that they should also have a fitness advantage over other strains in an environment with synchronous feeding on the basis that longer-persisting strains will be more likely to be transmitted under any conditions. Based on the findings of this and previous studies, we hypothesize that any fitness advantage conferred by long persistence is more beneficial in sites with asynchronous immature tick activity but that balancing selection is maintaining a degree of strain diversity in both environments. Two mutually compatible mechanisms for balancing selection in B. burgdorferi have been proposed by Brisson and Dykhuizen (10). First, B. burgdorferi appears to be under negative-frequency-dependent selection acting on the ospC gene (10, 60). Because a strong secondary immune response protects individual reservoir hosts from reinfection with the same ospC type but not from different ospC types (4), rare ospC types could have a selective advantage, resulting in the maintenance of a variety of ospC genotypes in a B. burgdorferi population (10, 60). Although the 16S-23S intergenic spacer is not subject to such selective pressures, its strong linkage with ospC (12, 28, 30, 59) suggests that the maintenance of a diverse set of ospC genotypes will generally result in RST polymorphism in the population. It should be noted that, although ospC and 16S-23S spacer region sequences correlate with the varied pathogenic and immunodynamic properties of B. burgdorferi, the precise mechanisms driving these patterns are not known.
Second, although nearly all B. burgdorferi strains are capable of infecting the known range of reservoir hosts in the environment (27), there is evidence that B. burgdorferi exhibits some strain-mediated host specialization (10). If strains have greater fitness in host species to which they are specialized, then the diverse host community available to I. scapularis in our study area is expected to drive the maintenance of diversity in the B. burgdorferi population, a process termed multiple-niche polymorphism (33). It follows, then, that differences in B. burgdorferi reservoir host communities between the two regions of our study area may be in part responsible for the differences in RST frequencies that we observed. However, this is unlikely to be a major source of variation since these two regions are similar in the host communities they support (24, 36).
Undoubtedly, diverse evolutionary forces determine the frequencies of B. burgdorferi strain types that we observe in host seeking ticks. With this in mind, we propose that: (i) environmental features, such as climate, can modify tick phenology to select for certain strains of B. burgdorferi over others; (ii) these selective pressures are moderated by the opposing forces of balancing selection that drive the maintenance of diversity; and (iii) the relative strengths of these and other evolutionary forces in a given environment determine the resulting frequencies of different B. burgdorferi strains. Future studies aimed at elucidating the relationships between climate, vector phenology, and pathogen genotype distribution within the context of the diversity of factors regulating vector-borne disease systems are warranted.
Several recent studies have shown that global climate change is influencing the seasonality of different plant and animal species, including ticks and other arthropods (25, 26, 37). The present study provides empirical evidence that supports previous reports that tick phenology is associated with climate and, importantly, that both climate and tick seasonality are associated with variation in B. burgdorferi genotype frequency. The prevalence of RST 1 strains relative to other strains in our study sites that are characterized by a milder climate reveals an increased risk for infection with RST 1 strains for people living in these areas compared with other areas. Previous studies have demonstrated that, as a group, RST 1 strains are more likely to be associated with disseminated infections and a wider range of clinical manifestations in humans (30, 62). Therefore, the climate-associated heterogeneities in the relative frequencies of RST 1 strains of B. burgdorferi observed in this study have potential epidemiological consequences.
Supplementary Material
Acknowledgments
We gratefully acknowledge many field assistants for tick collections and identifications; Roland Geerken for assistance with temporal Fourier analysis; Hany Mattaous, Paul Vu, and Bridgit Travinsky for technical assistance; Stephen Bent, Lindsay Rollend, Heidi Brown, and James Childs for helpful discussions; and Graham Hickling, Ned Walker, Joseph Piesman, and John Brownstein for their contributions to the early stages of this work and for ongoing support.
This research was supported by the U.S. Centers for Disease Control and Prevention (CDC) under cooperative agreement no. 5U01CI000171-04, the U.S. Department of Agriculture Agricultural Research Service under cooperative agreement no. 58-0790-5-068, and the G. Harold and Leila Y. Mathers Foundation. A.G.G. acknowledges support from the CDC Fellowship Training Program in Vector-Borne Diseases.
Footnotes
Published ahead of print on 27 February 2009.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Achtman, M. 1995. Global epidemiology of meningococcal disease, p. 159-175. In K. Cartwright (ed.), Meningococcal disease. John Wiley & Sons, Ltd., Chichester, England.
- 2.Anderson, J. F., and L. A. Magnarelli. 2008. Biology of ticks. Infect. Dis. Clin. N. Am. 22:195-215. [DOI] [PubMed] [Google Scholar]
- 3.Barbour, A. G., and D. Fish. 1993. The biological and social phenomenon of Lyme disease. Science 260:1610-1616. [DOI] [PubMed] [Google Scholar]
- 4.Barthold, S. W. 1999. Specificity of infection-induced immunity among Borrelia burgdorferi sensu lato species. Infect. Immun. 67:36-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belozerov, V. N. 1982. Diapause and biological rhythms in ticks, p. 469-500. In F. D. Obenchain and R. Galun (ed.), Physiology of ticks. Pergamon Press, Oxford, United Kingdom.
- 6.Belozerov, V. N. 1967. Larval diapause in the tick Ixodes ricinus L. and its dependence on external conditions. Entomol. Rev. 46:447-451. [Google Scholar]
- 7.Belozerov, V. N., and R. L. Naumov. 2002. Nymphal diapause and its photoperiodic control in the tick Ixodes scapularis (Acari: Ixodidae). Folia Parasitol. 49:314-318. [DOI] [PubMed] [Google Scholar]
- 8.Biek, R., J. C. Henderson, L. A. Waller, C. E. Rupprecht, and L. A. Real. 2007. A high-resolution genetic signature of demographic and spatial expansion in epizootic rabies virus. Proc. Natl. Acad. Sci. USA 104:7993-7998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bradshaw, W. E., and C. M. Holzapfel. 2008. Genetic response to rapid climate change: it's seasonal timing that matters. Mol. Ecol. 17:157-166. [DOI] [PubMed] [Google Scholar]
- 10.Brisson, D., and D. E. Dykhuizen. 2004. ospC diversity in Borrelia burgdorferi: different hosts are different niches. Genetics 168:713-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brownstein, J. S., T. R. Holford, and D. Fish. 2003. A climate-based model predicts the spatial distribution of the Lyme disease vector Ixodes scapularis in the United States. Environ. Health Perspect. 111:1152-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bunikis, J., U. Garpmo, J. Tsao, J. Berglund, D. Fish, and A. G. Barbour. 2004. Sequence typing reveals extensive strain diversity of the Lyme borreliosis agents Borrelia burgdorferi in North America and Borrelia afzelii in Europe. Microbiology 150:1741-1755. [DOI] [PubMed] [Google Scholar]
- 13.Cliff, A. D., and J. K. Ord. 1973. Spatial autocorrelation. Pion Press, London, United Kingdom.
- 14.Daniels, T. J., R. C. Falco, K. L. Curran, and D. Fish. 1996. Timing of Ixodes scapularis (Acari: Ixodidae) oviposition and larval activity in southern New York. J. Med. Entomol. 33:140-147. [DOI] [PubMed] [Google Scholar]
- 15.Dennis, D. T., T. S. Nekomoto, J. C. Victor, W. S. Paul, and J. Piesman. 1998. Reported distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the United States. J. Med. Entomol. 35:629-638. [DOI] [PubMed] [Google Scholar]
- 16.Derdakova, M., V. Dudioak, B. Brei, J. S. Brownstein, I. Schwartz, and D. Fish. 2004. Interaction and transmission of two Borrelia burgdorferi sensu stricto strains in a tick-rodent maintenance system. Appl. Environ. Microbiol. 70:6783-6788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diuk-Wasser, M. A., A. G. Gatewood, M. R. Cortinas, S. Yaremych-Hamer, J. Tsao, U. Kitron, G. Hickling, J. S. Brownstein, E. Walker, J. Piesman, and D. Fish. 2006. Spatiotemporal patterns of host-seeking Ixodes scapularis nymphs (Acari: Ixodidae) in the United States. J. Med. Entomol. 43:166-176. [DOI] [PubMed] [Google Scholar]
- 18.Donahue, J. G., J. Piesman, and A. Spielman. 1987. Reservoir competence of white-footed mice for Lyme disease spirochetes. Am. J. Trop. Med. Hyg. 36:92-96. [DOI] [PubMed] [Google Scholar]
- 19.Dormann, C. F., J. M. McPherson, M. B. Araujo, R. Bivand, J. Bolliger, G. Carl, R. G. Davies, A. Hirzel, W. Jetz, W. D. Kissling, I. Kuhn, R. Ohlemuller, P. R. Peres-Neto, B. Reineking, B. Schroder, F. M. Schurr, and R. Wilson. 2007. Methods to account for spatial autocorrelation in the analysis of species distributional data: a review. Ecography 30:609-628. [Google Scholar]
- 20.Durden, L. A., and J. E. Keirans. 1996. Nymphs of the genus Ixodes (Acari: Ixodidae) of the United States: taxonomy, identification key, distribution, hosts and medical/veterinary importance. Entomological Society of America, Lanham, MD.
- 21.Dykhuizen, D. E., D. Brisson, S. Sandigursky, G. P. Wormser, J. Nowakowski, R. B. Nadelman, and I. Schwartz. 2008. The propensity of different Borrelia burgdorferi sensu stricto genotypes to cause disseminated infections in humans. Am. J. Trop. Med. Hyg. 78:806-810. [PMC free article] [PubMed] [Google Scholar]
- 22.Estrada-Pena, A., J. M. Martinez, C. Sanchez Acedo, J. Quilez, and E. Del Cacho. 2004. Phenology of the tick, Ixodes ricinus, in its southern distribution range (central Spain). Med. Vet. Entomol. 18:387-397. [DOI] [PubMed] [Google Scholar]
- 23.Fish, D. 1993. Population ecology of Ixodes dammini. In H. S. Ginsberg. (ed.), Ecology and environmental management of Lyme disease. Rutgers University Press, New Brunswick, NJ.
- 24.Fish, D., and T. J. Daniels. 1990. The role of medium-sized mammals as reservoirs of Borrelia burgdorferi in southern New York. J. Wildl. Dis. 26:339-345. [DOI] [PubMed] [Google Scholar]
- 25.Forister, M. L., and A. M. Shapiro. 2003. Climatic trends and advancing spring flight of butterflies in lowland California. Global Change Biol. 9:1130-1135. [Google Scholar]
- 26.Gray, J. S. 2008. Ixodes ricinus seasonal activity: Implications of global warming indicated by revisiting tick and weather data. Int. J. Med. Microbiol. 298:19-24. [Google Scholar]
- 27.Hanincova, K., K. Kurtenbach, M. Diuk-Wasser, B. Brei, and D. Fish. 2006. Epidemic spread of Lyme borreliosis, northeastern United States. Emerg. Infect. Dis. 12:604-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanincova, K., N. H. Ogden, M. Diuk-Wasser, C. J. Pappas, R. Iyer, D. Fish, I. Schwartz, and K. Kurtenbach. 2008. Fitness variation of Borrelia burgdorferi sensu stricto strains in mice. Appl. Environ. Microbiol. 74:153-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jolly, W. M., J. M. Graham, A. Michaelis, R. Nemani, and S. W. Running. 2005. A flexible, integrated system for generating meteorological surfaces derived from point sources across multiple geographic scales. Environ. Model. Software 20:873-882. [Google Scholar]
- 30.Jones, K. L., L. J. Glickstein, N. Damle, V. K. Sikand, G. McHugh, and A. C. Steere. 2006. Borrelia burgdorferi genetic markers and disseminated disease in patients with early Lyme disease. J. Clin. Microbiol. 44:4407-4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kassen, R., and P. B. Rainey. 2004. The ecology and genetics of microbial diversity. Ann. Rev. Microbiol. 58:207-231. [DOI] [PubMed] [Google Scholar]
- 32.Kurtenbach, K., K. Hanincova, J. I. Tsao, G. Margos, D. Fish, and N. H. Ogden. 2006. Fundamental processes in the evolutionary ecology of Lyme borreliosis. Nat. Rev. Microbiol. 4:660-669. [DOI] [PubMed] [Google Scholar]
- 33.Levene, H. 1953. Genetic equilibrium when more than one ecological niche is available. Am. Nat. 87:331-333. [Google Scholar]
- 34.Lindsay, L. R., I. K. Barker, G. A. Surgeoner, S. A. McEwen, and G. D. Campbell. 1997. Duration of Borrelia burgdorferi infectivity in white-footed mice for the tick vector Ixodes scapularis under laboratory and field conditions in Ontario. J. Wildl. Dis. 33:766-775. [DOI] [PubMed] [Google Scholar]
- 35.Lindsay, L. R., S. W. Mathison, I. K. Barker, S. A. Mcewen, T. J. Gillespie, and G. A. Surgeoner. 1999. Microclimate and habitat in relation to Ixodes scapularis (Acari: Ixodidae) populations on Long Point, Ontario, Canada. J. Med. Entomol. 36:255-262. [DOI] [PubMed] [Google Scholar]
- 36.Mannelli, A., U. Kitron, C. J. Jones, and T. L. Slajchert. 1993. Ixodes dammini (Acari: Ixodidae) infestation on medium-sized mammals and blue jays in northwestern Illinois. J. Med. Entomol. 30:950-952. [DOI] [PubMed] [Google Scholar]
- 37.Miller-Rushing, A. J., R. B. Primack, D. Primack, and S. Mukunda. 2006. Photographs and herbarium specimens as tools to document phenological changes in response to global warming. Am. J. Bot. 93:1667-1674. [DOI] [PubMed] [Google Scholar]
- 38.Ogden, N. H., M. Bigras-Poulin, C. J. O'Callaghan, I. K. Barker, K. Kurtenbach, L. R. Lindsay, and D. F. Charron. 2007. Vector seasonality, host infection dynamics and fitness of pathogens transmitted by the tick Ixodes scapularis. Parasitology 134:209-227. [DOI] [PubMed] [Google Scholar]
- 39.Ogden, N. H., M. Bigras-Poulin, C. J. O'Callaghan, I. K. Barker, L. R. Lindsay, A. Maarouf, K. E. Smoyer-Tomic, D. Waltner-Toews, and D. Charron. 2005. A dynamic population model to investigate effects of climate on geographic range and seasonality of the tick Ixodes scapularis. Int. J. Parasitol. 35:375-389. [DOI] [PubMed] [Google Scholar]
- 40.Ogden, N. H., L. R. Lindsay, G. Beauchamp, D. Charron, A. Maarouf, C. J. O'Callaghan, D. Waltner-Toews, and I. K. Barker. 2004. Investigation of relationships between temperature and developmental rates of tick Ixodes scapularis (Acari: Ixodidae) in the laboratory and field. J. Med. Entomol. 41:622-633. [DOI] [PubMed] [Google Scholar]
- 41.Patrican, L. A. 1997. Absence of Lyme disease spirochetes in larval progeny of naturally infected Ixodes scapularis (Acari: Ixodidae) fed on dogs. J. Med. Entomol. 34:52-55. [DOI] [PubMed] [Google Scholar]
- 42.Perret, J. L., O. Rais, and L. Gern. 2004. Influence of climate on the proportion of Ixodes ricinus nymphs and adults questing in a tick population. J. Med. Entomol. 41:361-365. [DOI] [PubMed] [Google Scholar]
- 43.Piesman, J., and C. M. Happ. 2001. The efficacy of co-feeding as a means of maintaining Borrelia burgdorferi: a North American model system. J. Vector Ecol. 26:216-220. [PubMed] [Google Scholar]
- 44.Purse, B. V., P. S. Mellor, D. J. Rogers, A. R. Samuel, P. P. Mertens, and M. Baylis. 2005. Climate change and the recent emergence of bluetongue in Europe. Nat. Rev. Microbiol. 3:171-181. [DOI] [PubMed] [Google Scholar]
- 45.Randolph, S. E. 2004. Tick ecology: processes and patterns behind the epidemiological risk posed by ixodid ticks as vectors. Parasitology 129:S37-S65. [DOI] [PubMed] [Google Scholar]
- 46.Randolph, S. E., R. M. Green, A. N. Hoodless, and M. F. Peacey. 2002. An empirical quantitative framework for the seasonal population dynamics of the tick Ixodes ricinus. Int. J. Parasitol. 32:979-989. [DOI] [PubMed] [Google Scholar]
- 47.Randolph, S. E., R. M. Green, M. F. Peacey, and D. J. Rogers. 2000. Seasonal synchrony: the key to tick-borne encephalitis foci identified by satellite data. Parasitology 121:15-23. [DOI] [PubMed] [Google Scholar]
- 48.Randolph, S. E., D. Miklisova, J. Lysy, D. J. Rogers, and M. Labuda. 1999. Incidence from coincidence: patterns of tick infestations on rodents facilitate transmission of tick-borne encephalitis virus. Parasitology 118:177-186. [DOI] [PubMed] [Google Scholar]
- 49.Real, L. A., J. C. Henderson, R. Biek, J. Snaman, T. L. Jack, J. E. Childs, E. Stahl, L. Waller, R. Tinline, and S. Nadin-Davis. 2005. Unifying the spatial population dynamics and molecular evolution of epidemic rabies virus. Proc. Natl. Acad. Sci. USA 102:12107-12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rogers, D. J., and S. E. Randolph. 2003. Studying the global distribution of infectious diseases using GIS and RS. Nat. Rev. Microbiol. 1:231-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwan, T. G., and J. Piesman. 2002. Vector interactions and molecular adaptations of Lyme disease and relapsing fever spirochetes associated with transmission by ticks. Emerg. Infect. Dis. 8:115-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seinost, G., D. E. Dykhuizen, R. J. Dattwyler, W. T. Golde, J. J. Dunn, I. N. Wang, G. P. Wormser, M. E. Schriefer, and B. J. Luft. 1999. Four clones of Borrelia burgdorferi sensu stricto cause invasive infection in humans. Infect. Immun. 67:3518-3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tatem, A. J., M. Baylis, P. S. Mellor, B. V. Purse, R. Capela, I. Pena, and D. J. Rogers. 2003. Prediction of bluetongue vector distribution in Europe and north Africa using satellite imagery. Vet. Microbiol. 97:13-29. [DOI] [PubMed] [Google Scholar]
- 54.Terekhova, D., R. Iyer, G. P. Wormser, and I. Schwartz. 2006. Comparative genome hybridization reveals substantial variation among clinical isolates of Borrelia burgdorferi sensu stricto with different pathogenic properties. J. Bacteriol. 188:6124-6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thornton, P. E., S. W. Running, and M. A. White. 1997. Generating surfaces of daily meteorological variables over large regions of complex terrain. J. Hydrol. 190:214-251. [Google Scholar]
- 56.Tsao, J. I., J. T. Wootton, J. Bunikis, M. G. Luna, D. Fish, and A. G. Barbour. 2004. An ecological approach to preventing human infection: vaccinating wild mouse reservoirs intervenes in the Lyme disease cycle. Proc. Natl. Acad. Sci. USA 101:18159-18164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vail, S. C., and G. Smith. 2002. Vertical movement and posture of blacklegged tick (Acari: Ixodidae) nymphs as a function of temperature and relative humidity in laboratory experiments. J. Med. Entomol. 39:842-846. [DOI] [PubMed] [Google Scholar]
- 58.Wang, G., C. Ojaimi, R. Iyer, V. Saksenberg, S. A. McClain, G. P. Wormser, and I. Schwartz. 2001. Impact of genotypic variation of Borrelia burgdorferi sensu stricto on kinetics of dissemination and severity of disease in C3H/HeJ mice. Infect. Immun. 69:4303-4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang, G., C. Ojaimi, H. Wu, V. Saksenberg, R. Iyer, D. Liveris, S. A. McClain, G. P. Wormser, and I. Schwartz. 2002. Disease severity in a murine model of Lyme borreliosis is associated with the genotype of the infecting Borrelia burgdorferi sensu stricto strain. J. Infect. Dis. 186:782-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang, I. N., D. E. Dykhuizen, W. Qiu, J. J. Dunn, E. M. Bosler, and B. J. Luft. 1999. Genetic diversity of ospC in a local population of Borrelia burgdorferi sensu stricto. Genetics 151:15-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wormser, G. P., D. Brisson, D. Liveris, K. Hanincova, S. Sandigursky, J. Nowakowski, R. B. Nadelman, S. Ludin, and I. Schwartz. 2008. Borrelia burgdorferi genotype predicts the capacity for hematogenous dissemination during early Lyme disease. J. Infect. Dis. 198:1358-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wormser, G. P., D. Liveris, J. Nowakowski, R. B. Nadelman, L. F. Cavaliere, D. McKenna, D. Holmgren, and I. Schwartz. 1999. Association of specific subtypes of Borrelia burgdorferi with hematogenous dissemination in early Lyme disease. J. Infect. Dis. 180:720-725. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.