Abstract
Molecular data show that the filamentous bacterium Eikelboom type 0092, frequently seen in Australian activated sludge plants, is a member of the phylum Chloroflexi. Fluorescence in situ hybridization (FISH) probes designed against cloned 16S rRNA sequences from a full-scale enhanced biological phosphate removal-activated sludge plant community, where this was a dominant filament morphotype, suggest that it can exist as two variants, differing in their trichome diameter. When applied to samples from several treatment plants in eastern Australia, each FISH probe targeted only the type 0092 filament morphotype against which it was designed. The patterns of FISH signals generated with both were consistent with the ribosomes not being evenly distributed but arranged as intracellular aggregates. The FISH survey data showed that these two variants appeared together in most but not all of the plants examined. None stained positively for intracellular presence of either poly-β-hydroxyalkanoates or polyphosphate.
Most activated sludge plants suffer from the operational disorders of bulking and foaming, both of which are caused by excessive growth of certain filamentous bacteria. Several different filament morphotypes have been described from systems treating domestic and industrial wastes (17, 18) but, in the absence of pure cultures, many of these have never been characterized sufficiently to resolve their taxonomy or provide them with valid names. Hence, they are often still referred to as numerical types persisting from the study of Eikelboom (18). Success has been achieved with some cultured and uncultured filaments in elucidating their phylogeny from 16S rRNA sequence analyses (6, 9, 10, 31, 50) and providing them with valid names (37, 49). Furthermore, with such sequence information, rRNA targeted oligonucleotide probes have been designed for their in situ identification and, together with microautoradiography (MAR) and other techniques (29, 40), their ecophysiology may be elucidated (25, 27, 28).
Type 0092, originally described by Eikelboom (18), appears prominently in many filament surveys carried out on plants around the world, where microscopy was used to identify them (35, 48). These morphotypes have been associated especially with long sludge age (>15-day) operational conditions (22) and thus frequently appear in enhanced biological phosphate removal (EBPR) systems (see, for example, reference 11), where the biomass is recycled repeatedly through anaerobic: aerobic zones. Consequently, this filament morphotype was classified as an “all-zone” grower by Wanner and Grau (52), able in their view to grow under aerobic, anoxic, and anaerobic conditions. However, its physiology from pure culture studies was described as being strictly aerobic (13, 21). These isolates were never deposited in recognized culture collections, and so confirmation of their identity is difficult. Similarly, the precise identification of the type 0092 filaments claimed to have been cultured by Ramothokang et al. (43) is unclear.
Type 0092 has very distinctive morphological features and so can be readily “identified” microscopically by its positive Neisser staining reaction and its short blunt-ended trichomes extending from the flocs or suspended in the bulk liquid (22). On the basis of 16S rRNA sequenced micromanipulated cultures, Bradford et al. (12) suggested it was a member of the Bacteroidetes, and yet filaments with the morphological features of type 0092 never fluoresced in situ with 16S rRNA targeted fluorescence in situ hybridization (FISH) probes designed against this sequence (37; E. M. Seviour, unpublished data). Consequently, it now seems unlikely that the organisms that grew from the micromanipulated filament with the type 0092 morphotype are type 0092 but rather are contaminating faster-growing filaments.
Regular microscopic examination of an EBPR full scale plant in Bendigo, Victoria, Australia, revealed that the biomass was always heavily dominated by a Neisser-positive filament fitting the microscopic description of type 0092 (see Fig. 1a). Therefore, attempts were made with FISH and 16S rRNA clone library construction to determine whether this filament morphotype could identified. We describe it here as a member of the phylum Chloroflexi and detail FISH probes designed for its in situ identification.
FIG. 1.
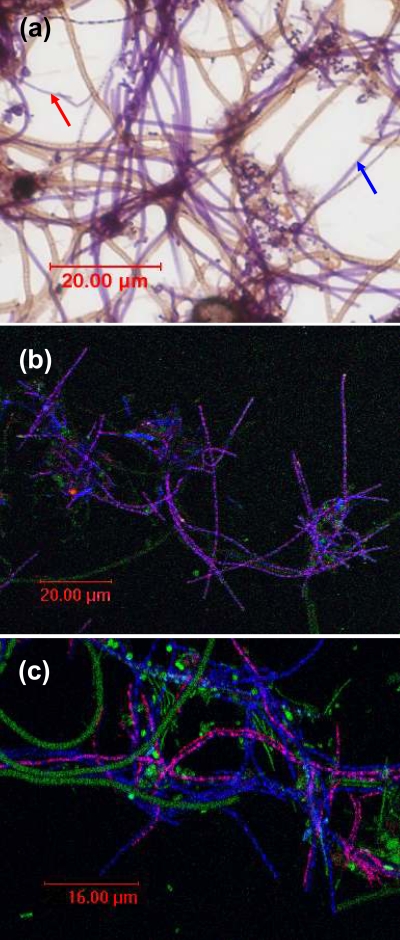
(a) Neisser-stained biomass from the Bendigo wastewater treatment plant showing Neisser-positive type 0092 filaments. Blue arrow = thicker type 0092; red arrow = thinner type 0092 filaments. (b) Confocal laser-scanning microscopy image of FISH-probed biomass from the Bendigo EBPR plant. Magenta cells are those responding to both GNSB941/CFX1223 mix (CY5) and CFX197 (CY3) FISH probes for Chloroflexi type 0092. Note the localized fluorescence signal. Blue cells are those responding to the GNSB941/CFX1223 mix probes alone. Note the absence of EUBmix FISH target sites; green cells are those responding only to the EUBmix (FLUOS) probes; light blue cells are those responding to EUBmix plus GNSB941/CFX1223 mix probes together. (c) Confocal laser-scanning microscopy image of FISH probed biomass from the Bendigo EBPR plant. Magenta cells are those responding to both GNSB941/CFX1223 mix (CY5) and CFX223 (CY3) FISH probes for Chloroflexi type 0092. Note again the localized fluorescence signal. Blue cells are those responding to the GNSB941/CFX1223 mix probes alone. Blue and magenta cells lack EUBmix FISH target sites; green cells are those responding only to the EUBmix (FLUOS) probes; light blue cells are those responding to EUBmix plus GNSB941/CFX1223 mix probes together.
MATERIALS AND METHODS
Examination of biomass samples.
Biomass samples were collected from 17 full-scale plants from eastern states in Australia and fixed in 4% (wt/vol) paraformaldehyde for FISH (2). Neisser staining was carried out as described by Jenkins et al. (22) on unfixed samples to determine whether they contained filamentous bacteria with the diagnostic morphological and staining properties of type 0092 filaments.
Preparation of 16S rRNA clone library.
The DNA was extracted from a fresh sample of biomass from the Bendigo (Victoria, Australia) full-scale modified University of Cape Town EBPR plant which contained type 0092 as a dominant filament. Three different DNA extraction methods were used in attempts to minimize any potential biases associated with each, and the extracts were combined. These three methods included those of McVeigh et al. (38) and McIlroy et al. (36) and a FASTDNA spin kit (Qbiogene, Melbourne, Australia). The former two were selected because they had performed best in comparative trials at recovering DNA from marker activated sludge populations known to resist many DNA extraction protocols (S. McIlroy, K. Porter, S. Schroeder, R. J. Seviour, and D. Tillett, unpublished data).
Five PCRs were performed on the DNA from each extraction method, and all of the resulting PCR products were pooled to minimize any PCR-associated biases, using a GeneAmp PCR System 9700 thermal cycler (Applied Biosystems). The 16S rRNA gene sequences were amplified with the primers U27f (5′-GAGTTTGATCMTGGCTCAG-3′) and U1492r (5′-GGYTACCTTGTTACGACTT-3′) under the following PCR conditions: 1 cycle of 10 min at 95°C; 30 cycles of 30 s at 94°C, 30 s at 50°C, and 2 min at 72°C; and 1 cycle of 10 min at 72°C. Each PCR mixture (50 μl) contained 1 μl of template DNA, 0.2 μM each primer, 0.2 mM each deoxynucleoside triphosphate, 5 μl of 10× PCR buffer, 2.5 mM MgCl2, and 1.25 U of AmpliTaq Gold (Applied Biosystems). All of the amplified PCR products were then combined and run on 1.5% agarose gels. Bands of ∼1,450 bp were excised with a clean razor and purified with the Wizard SV gel and PCR clean-up system (Promega, Melbourne, Australia) according to the manufacturer's instructions.
Clone library construction.
Purified PCR products were cloned into the pGEM-T Easy vector system (Promega). The presence of correctly sized inserts was checked by agarose gel electrophoresis of the clone colony PCR products using the PCR conditions described above. Plasmids from each clone were extracted with the Wizard Plus SV Minipreps DNA purification system (Promega). Initially, partial sequencing (approximately the first 500 bp) of the inserts was carried out by AGRF, Brisbane, Australia, and clones of interest were selected for complete sequencing based on the presence or absence of particular signature sequences of interest, as detailed below in Results. Possible chimeric sequences were assessed by using Bellerophon v3 (16), Mallard (5), and Pintail (4) software, and all putative chimeras were eliminated from subsequent analyses. The remainder were added to ARB (32) and aligned. A maximum-likelihood phylogenetic tree was constructed from these and selected related sequences.
FISH analyses.
FISH was performed on biomass samples according to the protocol of Amann (2). The probes used are listed in Table 1, and the hybridization conditions applied were those detailed in the original publications for each. FISH probes developed during the present study were designed with the ARB software package (32). They were validated against biomass samples by incrementally increasing the formamide concentrations until only filaments with the desired morphotype fluoresced and before their fluorescent signal strength began to decrease. All probes were purchased from Proligo (Melbourne, Victoria, Australia) and were fluorescently tagged with CY3 and CY5 fluorochromes as detailed in Results.
TABLE 1.
FISH probes used in this study and their hybridization conditions
| Probe | Target | Sequence (5′-3′) | Formamide (%) | Source or reference |
|---|---|---|---|---|
| EUB338 Ib | All Bacteria | GCTGCCTCCCGTAGGAGT | 0-50 | 3 |
| EUB338 IIb | Planctomycetales | GCAGCCACCCGTAGGTGT | 0-50 | 14 |
| EUB338 IIIb | Verrucomicrobiales | GCTGCCACCCGTAGGTGT | 0-50 | 14 |
| HHY | Haliscomenobacter hydrossis | GCCTACCTCAACCTGATT | 20 | 51 |
| BAC303 | Bacteroidetes | CCAATGTGGGGGACCTT | 0 | 33 |
| PLA886 | Planctomycetales | GCCTTGCGACCATACTCCC | 35 | 39 |
| BET42A | Betaproteobacteria | GCCTTCCCACTTCGTTT | 35 | 34 |
| CFB719 | Cytophaga-Flavobacterium-Bacteroides | AGCTGCCTTCGCAATCGG | 30 | 53 |
| CF319a | Flavobacteria; some Bacteroidetes; some sphingobacteria | TGGTCCGTGTCTCAGTAC | 35 | 33 |
| CHL1851 | Type 1851 filamentous bacterium | AATTCCACAACCTCTCCA | 35 | 6 |
| CFX109 | Chloroflexi subgroup 3 | CACGTGTTCCTCAGCCGT | 30 | 8 |
| CFX784 | Chloroflexi subgroup 1 | ACCGGGGTCTCTAATCCC | 35 | 8 |
| CFX1223 | All Chloroflexi | CCATTGTAGCGTGTGTGTMG | 35 | 8 |
| GNSB941 | All Chloroflexi | AAACCACACGCTCCGCT | 35 | 20 |
| CFX197 | Chloroflexi OTU A (clones A26, B1, and B45) (variant A) | TCCCGGAGCGCCTGAACT | 40 | This study |
| CFX197comp | Competitor probe against sequences with accession no. ZA3635c and ZA3612c | TCCCGAAGCGCCTGAACTa | This study | |
| CFX223 | Chloroflexi OTU B (clone A58) (variant B) | GGTGCTGGCTCCTCCCAG | 35 | This study |
| CFX223 H202 | Helper probe | AGCGCCTGAGCTTCAGTCATC | This study | |
| CFX223 H241 | Helper probe | CGTTACCTTACCAACTAGCTGATGG | This study |
The mismatching base of the CFX197comp probe to use with the CFX197 probe is underlined.
EUB338 I, II, and III used in equimolar amounts as EUBmix.
Staining.
To detect polyphosphate (polyP) and poly-β-hydroxyalkanoates (PHA) in cells, DAPI (4′,6′-diamidino-2-phenylindole) (23) and Nile blue A (42), respectively, were used as detailed in Ahn et al. (1).
Nucleotide sequence accession numbers.
The nucleotide sequence data reported in the present study were deposited in the DDBJ/EMBL/GenBank nucleotide sequence databases with the accession numbers AB445103 to AB445106.
RESULTS
Identification of type 0092.
Most of the biomass samples taken from Australian EBPR and non-EBPR plants located in three eastern states (Table 2) contained Neisser-positive short filaments extending from the floc into the bulk liquid or, occasionally, freely suspended in the bulk liquid. These fitted the morphological description of type 0092 (Fig. 1a). FISH analyses of biomass from the Bendigo plant showed that filaments with this distinctive morphology failed to respond to the EUB338 I, II, and III probes designed to target all Bacteria (14), regardless of whether these were applied individually or in combination. Of the other FISH probes tested (Table 1), this filament morphotype fluoresced only with the GNSB941 and CFX1223 probes designed to target members of the Chloroflexi (Fig. 1b). Another slightly thinner Neisser-positive filament (trichome ∼0.67 μm in diameter compared to ∼0.80 μm) also fluoresced with these two probes (Fig. 1c) and, in each case, the fluorescent signal generated was unevenly distributed and granular in appearance. Neither of these two Neisser-positive filament variants responded to the CHL1851 or CFX109 and CFX784 probes targeting Eikelboom type 1851 and the subgroups 3 and 1a of the Chloroflexi, respectively (Table 1). Also present in this sample were Neisser-negative filaments more similar in appearance to type 0092 than to the much thinner Haliscomenobacter hydrossis.
TABLE 2.
Results of FISH-based survey of Australian EBPR and non-EBPR full-scale plants for type 0092 using the CFX197 and CFX223 probes
| Biomass samplea | Filament countb
|
||
|---|---|---|---|
| Neisser staining | CFX197 | CFX223 | |
| Bendigo (VIC) | 6 | 5 | 3 |
| Logan (QLD) | 4 | 4 | 3 |
| Thorneside (QLD) | 2 | 2 | 1 |
| Coolum (QLD) | 3 | 3 | 2 |
| Nambour (QLD) | 3 | 3 | 2 |
| Merrimac (QLD) | 5 | 3 | 2 |
| Kyneton (VIC) | 1 | 1 | 0 |
| Castlemaine (VIC) | 1 | 1 | 0 |
| Watella (QLD) | 5 | 5 | 3 |
| Mornington (VIC)c | 3 | 3 | 0 |
| Daylesford (VIC)c | 2 | 2 | 0 |
| Carrum (VIC) | 0 | 0 | 0 |
| Maroochydore (QLD) | 2 | 2 | 1 |
| Dalby (QLD) | 5 | 4 | 3 |
| Morpeth (NSW) | 2 | 3 | 1 |
| Orange (NSW) | 3 | 3 | 0 |
VIC, Victoria, Australia; QLD, Queensland, Australia; NSW, New South Wales, Australia.
Grading was as described in Jenkins et al. (22): 1, few; 2, some; 3, common; 4, very common; 5, abundant; and 6, excessive. The uptake of Neisser stain by floc EPS material meant the visualization of filaments within flocs was difficult. Therefore, estimations based only on staining may underestimate type 0092 abundance.
Non-EBPR plant.
Design of FISH probes against Chloroflexi filaments.
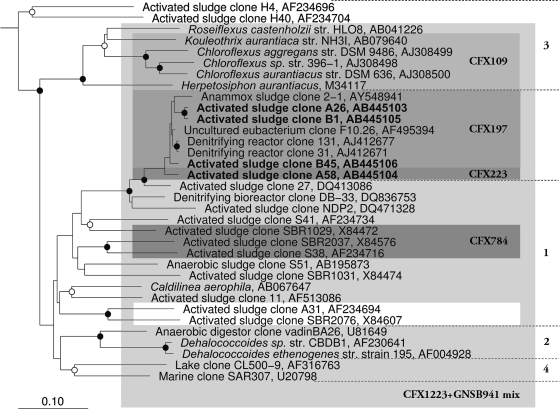
A library of 75 partial 16S rRNA gene sequences generated from the Bendigo EBPR plant biomass contained 11 Chloroflexi clones lacking the EUBmix signature probe target sites (Table 3). Representatives of the four OTUs they formed (based on shared 99% similarities) were then fully sequenced. Clone A58 is representative of an OTU of six clones, clones B1 and B54 each represent an OTU containing two clones, and A26 represents an OTU of a single clone. They formed two clusters, A (clones A26, B1, and B45) and cluster B (clone A58) in the phylogenetic tree (Fig. 2). All of these sequences also lacked the CHL1851, CFX109, and CFX784 target site sequences but contained those for the GNSB941 and CFX1223 probes (Table 1), which increased confidence in them being derived from the two filament morphotype variants suggested from the FISH data described above.
TABLE 3.
FISH probes target site mismatches used to screen Chloroflexi 16S rRNA clone sequences to identify tentatively those derived from type 0092, as assessed by FISH analysis of Bendigo activated sludge biomass
| Probe(s) | 16S rRNA target sequencea | Reference |
|---|---|---|
| EUB338 I | ACTCCTACGGGAGGCAGC | 3 |
| Clones A26, A58, B1, and B45 | ATACCTACGGGTAGCAGC | |
| EUB338 II | ACACCTACGGGTGGCTGC | 14 |
| Clones A26, A58, B1, and B45 | ATACCTACGGGTAGCAGC | |
| EUB338 III | ACACCTACGGGTGGCAGC | 14 |
| Clones A26, A58, B1, B45 | ATACCTACGGGTAGCAGC | |
| CFX109 | ACGGCTGAGGAACACGTG | 8 |
| Clone A58 | ACGGGTGAGTAACATGTT | |
| Clones A26 and B45 | ACGGGTGAGTAACGCGTT | |
| Clone B1 | ACGGGTGAGTAATGCGTT | |
| CFX784 | GGGATTAGAGACCCCGGT | 8 |
| Clones A26, A58, B1, and B45 | GGGATTAGAAACCCCGGT |
Mismatches are underlined.
FIG. 2.
Maximum-likelihood phylogenetic tree of the 16S rRNA gene sequences obtained in the present study and representatives from the Chloroflexi phylum. All sequences were at least 1,200 bp long except for AF495394, which was added in later using the quick-add function in ARB. Shading illustrates the coverage of each individual FISH probe. The scale bar corresponds to 0.1 substitutions per nucleotide position. Bootstrap values are calculated as a percentage of 1,000 analysis and are only indicated for values of ≥75%. Symbols: ○, bootstrap value of ≥75%; •, bootstrap value of ≥95%.
Two FISH probes were then designed against their sequences, CFX197 and CFX223, with the former designed to target the Chloroflexi clones in cluster A (Fig. 1b), and the latter the single clone A58 in cluster B (Fig. 1c). Their sequences are given in Table 1. A competitor probe CFX197comp (Table 1) was used in combination with the CXF197 probe to reduce the likelihood of false positives with sequences containing a single known mismatch with its target site (sequence accession numbers ZA3635c and ZA3612c from uncultured bacterioplankton). Its effectiveness could not be assessed here. After validation against biomass from the Bendigo EBPR plant, formamide concentrations of 35 and 40% were selected for the CFX223 and CFX197 probes, respectively. Two helper probes CFX H202 and CFX H241, whose sequences are given in Table 1 were also designed for use with the CFX223 probe designed from the A58 clone sequence, which by necessity targets the relatively inaccessible region IV of the 16S rRNA of Escherichia coli (19). However, no obvious increase in fluorescence signal strength was noticed when these helper probes were used either individually or in combination with the CFX223 probe on biomass from the Bendigo plant.
When both targeted probes were applied to samples from Bendigo, filaments with the typical type 0092 morphotype (variant A) fluoresced with the CFX197 probe (Fig. 1b), while the CFX223 probe lit up the thinner (variant B) Neisser-positive Chloroflexi filaments (Fig. 1c). Whether these two variants represent different taxa of this filament morphotype is not clear from the phylogenetic data, and such a decision should be delayed until more sequence data become available. Again, FISH fluorescent signals were distinctively uneven and granular. No other cells fluoresced with either probe that did not also fluoresce with the non-EUB probe.
polyP and PHA staining reactions.
None of the type 0092 filaments stained positively for either polyP or PHA in any of the biomass samples examined.
FISH-based plant surveys.
When biomass samples from full-scale EBPR and non-EBPR plants in eastern Australia were screened by FISH after Neisser staining for the presence of the type 0092 morphotype, most contained high levels of this filament. In all samples, type 0092 filaments fluoresced strongly with the CFX197 probe (Fig. 1b). Application of the CFX223 probe to the same samples revealed the presence of a thinner Neisser-positive type 0092 filament (Fig. 1c) in most of them (Table 2). They were less frequently seen in the non-EBPR biomasses and always at much lower abundances (Daylesford and Carrum).
DISCUSSION
This study has resolved the phylogeny of the Neisser-positive filament morphotype Eikelboom type 0092 commonly seen in Australian activated sludge plants is a member of the Chloroflexi. It also describes 16S rRNA targeted oligonucleotide sequences for unequivocal in situ identification by FISH of its two morphological variants, differing in their filament diameter. Application of these FISH probes to biomass samples from several Australian EBPR plants several thousand kilometers apart and with different operating configurations suggests that these two type 0092 variants each consist of a single phylotype, since they always responded to either the CFX197 or the CFX223 probes. However, all FISH-probed type 0092 cells had an unusual appearance, where the fluorescent signal from each was localized, a finding consistent with their ribosomes being in aggregates and not uniformly distributed within their cells (Fig. 1b and c). A similar arrangement has not been reported previously, but some Planctomycetes cells also show uneven FISH signal distribution, thought to arise from ribosome association with peripheral internal membranes (14). The Neisser negative filaments did not respond to any of the Chloroflexi targeted probes but instead fluoresced with the CFB719 probe designed against the Bacteroidetes. Whether these are the same filaments described by Lemmer et al. (30) or Kragelund et al. (26) was not examined here.
The successful eventual phylogenetic placement of type 0092 exploited preliminary FISH data, where screening by probing showed both were members of the Chloroflexi. The Chloroflexi 16S rRNA clones possessing or lacking these and other known probe signature target sequences could then be identified (Table 3). Thus, neither of the two filament morphotype variants identified here responded to any of the three EUBmix FISH probes designed to target members of the domain Bacteria (14), a feature shared by several other members of this phylum (8, 27). Such an outcome must impact on FISH quantification of Chloroflexi in natural communities if, as commonly used, it is based on calculating their biovolume percentages of EUBmix fluorescing cells (15). Redesigning the EUBmix probes to accommodate such populations is not straightforward, since the sequence diversity of the EUBmix probe target sites among them is substantial (T. Nittami, unpublished data; P. H. Nielsen, unpublished data). Several additional probes would be required to embrace this diversity.
The selected Chloroflexi sequences generated in the present study did not cluster closely with sequences in the preexisting divisions 1 and 3 of the Chloroflexi (8), thought to contain most of the activated sludge members of this phylum, but instead form a distinct adjacent grouping (Fig. 2). Thus, these type 0092 are not closely related to the other described Chloroflexi activated sludge filamentous bacteria Eikelboom type 1851 and “Kouleothrix aurantiaca” (6, 27) or the “Nostocoida limicola” morphotype of Schade et al. (44), whose 16S rRNA sequence is only ca. 80% similar. The FISH probes described here for type 0092 should now be applied more widely to samples from plants in other parts of the world. This will resolve the important question of whether members of this single filament morphotype are phylogenetically diverse, as the reports of Lemmer et al. (30) and Schade et al. (45) might suggest. A similar situation has been reported with several filament morphotypes including, for example, “Nostocoida limicola” II (31, 37, 44), type 021N (31, 49), and Haliscomenobacter hydrossis (26).
Several reports have suggested that the Chloroflexi and type 0092 are frequent members of EBPR communities (7, 8, 24), although whether they play any role in phosphate removal in these communities is uncertain. None in samples taken at the end of the aerobic stage stained positively for polyP in our study. Both variants occurred together in most of the EBPR plant samples examined, suggesting that they share an ecophysiology well suited to the alternative anaerobic:aerobic feast: famine conditions deliberately established in these processes (41, 46), although they were also seen in non-EBPR biomass samples (Table 2). Which features might be competitively advantageous to such populations would include an ability for anaerobic substrate assimilation for the synthesis of storage material such as poly β-hydroxyalkanoates. This may enable them to grow in the aerobic zone of EBPR plants in the absence of other exogenous metabolizable substrates (47) and consequently thrive in a highly competitive environment. However, Nile blue A staining failed to reveal the presence of PHA in these FISH-probed filaments in any biomass sample examined. Whether they possess a capacity for anaerobic substrate assimilation may be elucidated with FISH in combination with MAR (FISH/MAR). Where FISH/MAR was applied to other unidentified Chloroflexi in non-EBPR-activated sludge communities treating industrial wastes (27), the data suggested that they assimilated substrates actively only under aerobic conditions. This probably does not apply to all activated sludge populations, and certainly anaerobic Chloroflexi have been isolated from a range of environments (54). No mention was made (27) as to whether any of their activated sludge Chloroflexi synthesized PHA or polyP in situ. Clearly, more work is needed with FISH/MAR before their frequent appearance in EBPR plants can be explained, and their functional roles better understood. The FISH probes described here should make a valuable contribution to this important task.
Acknowledgments
This study was supported by an ARC Discovery grant, and S.S. was funded from the Victorian State Government Smartwater fund and La Trobe University. T.N. was supported by an overseas study program of Yokohama National University, and S.M. was the recipient of an Australian Government APA Ph.D. scholarship.
Footnotes
Published ahead of print on 13 February 2009.
REFERENCES
- 1.Ahn, J., S. Schroeder, M. Beer, S. McIlroy, R. C. Bayly, J. W. May, G. Vasiliadis, and R. J. Seviour. 2007. Ecology of the microbial community removing phosphate from wastewater under continuously aerobic conditions in a sequencing batch reactor. Appl. Environ. Microbiol. 73:2257-2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amann, R. I. 1995. In situ identification of microorganisms by whole cell hybridization with rRNA-targeted nucleic acid probes, p. 3.3.6/1-3.3.6/15. In A. D. L. Akkermans, J. D. van Elsas, and F. J. de Bruijn (ed.), Molecular microbial ecology manual. Kluwer Academic Publishers, Boston, MA.
- 3.Amann, R. I., B. J. Binder, R. J. Olson, S. W. Chisolm, R. Devereux, and D. A. Stahl. 1990. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl. Environ. Microbiol. 56:1919-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashelford, K. E., N. A. Chuzhanova, J. C. Fry, A. J. Jones, and A. J. Weightman. 2005. At least 1 in 20 16S rRNA sequence records currently held in public repositories is estimated to contain substantial anomalies. Appl. Environ. Microbiol. 71:7724-7736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashelford, K. E., N. A. Chuzhanova, J. C. Fry, A. J. Jones, and A. J. Weightman. 2006. New screening software shows that most recent large 16S rRNA gene clone libraries contain chimeras. Appl. Environ. Microbiol. 72:5734-5741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beer, M., E. M. Seviour, Y. Kong, M. Cunningham, L. L. Blackall, and R. J. Seviour. 2002. Phylogeny of the filamentous bacterium Eikelboom type 1851, and design and application of a 16S rRNA targeted oligonucleotide probe for its fluorescence in situ identification in activated sludge. FEMS Microbiol. Lett. 207:179-183. [DOI] [PubMed] [Google Scholar]
- 7.Beer, M., H. Stratton, P. Griffiths, and R. Seviour. 2006. Which are the polyphosphate accumulating organisms in full-scale activated sludge enhanced biological phosphate removal systems in Australia? J. Appl. Microbiol. 100:223-243. [DOI] [PubMed] [Google Scholar]
- 8.Björnsson, L., P. Hugenholtz, G. W. Tyson, and L. L. Blackall. 2002. Filamentous Chloroflexi (green non-sulfur bacteria) are abundant in wastewater treatment processes with biological nutrient removal. Microbiology 148:2309-2318. [DOI] [PubMed] [Google Scholar]
- 9.Blackall, L., E. Seviour, D. Bradford, S. Rossetti, V. Tandoi, and R. Seviour. 2000. “Candidatus Nostocoida limicola,” a filamentous bacterium from activated sludge. Int. J. Syst. Evol. Microbiol. 50:703-709. [DOI] [PubMed] [Google Scholar]
- 10.Blackall, L. L. 1994. Molecular identification of activated sludge foaming bacteria. Water Sci. Technol. 29:35-42. [Google Scholar]
- 11.Blackbeard, J., D. Gabb, G. Ekama, and G. Marais. 1988. Identification of filamentous organisms in nutrient removal activated sludge plants in South Africa. Water SA 14:1-18. [Google Scholar]
- 12.Bradford, D., P. Hugenholtz, E. M. Seviour, M. A. Cunningham, H. Stratton, R. J. Seviour, and L. L. Blackall. 1996. 16S rRNA analysis of isolates obtained from gram-negative, filamentous bacteria micromanipulated from activated sludge. Syst. Appl. Microbiol. 19:334-343. [Google Scholar]
- 13.Bu'ali, A. M., and N. J. Horan. 1988. Variable morphology in certain filamentous bacteria and implications for this for theories of activated sludge bulking. Environ. Technol. Lett. 10:941-950. [Google Scholar]
- 14.Daims, H., A. Brühl, R. Amann, K. Schleifer, and M. Wagner. 1999. The domain-specific probe EUB338 is insufficient for the detection of all Bacteria: development and evaluation of a more comprehensive probe set. Syst. Appl. Microbiol. 22:434-444. [DOI] [PubMed] [Google Scholar]
- 15.Daims, H., K. Stoecker, and M. Wagner. 2005. Fluorescence in situ hybridization for the detection of prokaryotes, p. 213-239. In A. M. Osborn and C. J. Smith (ed.), Molecular microbial ecology. Taylor & Francis, New York, NY.
- 16.DeSantis, T. Z., P. Hugenholtz, N. Larsen, M. Rojas, E. L. Brodie, K. Keller, T. Huber, D. Dalevi, P. Hu, and G. L. Andersen. 2006. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 72:5069-5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eikelboom, D., and B. Geurkink. 2002. Filamentous microorganisms observed in industrial activated sludge plants. Water Sci. Technol. 46:535-542. [PubMed] [Google Scholar]
- 18.Eikelboom, D. H. 1975. Filamentous organisms observed in activated sludge. Water Res. 9:365-388. [Google Scholar]
- 19.Fuchs, B. M., G. Wallner, W. Beisker, I. Schwippl, W. Ludwig, and R. Amman. 1998. Flow cytometric analysis of the in situ accessibility of Escherichia coli 16S rRNA for fluorescently labeled oligonucleotide probes. Appl. Environ. Microbiol. 64:4973-4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gich, F., J. Garcia-Gil, and J. Overmann. 2001. Previously unknown and phylogenetically diverse members of the green nonsulfur bacteria are indigenous to freshwater lakes. Arch. Microbiol. 177:1-10. [DOI] [PubMed] [Google Scholar]
- 21.Horan, N. J., A. M. Bu'ali, and C. R. Eccles. 1988. Isolation, identification and characterisation of filamentous and floc-forming bacteria from activated sludge flocs. Environ. Technol. Lett. 9:449-457. [Google Scholar]
- 22.Jenkins, D., M. G. Richard, and G. T. Daigger. 2004. Manual on the causes and control of activated sludge bulking, foaming, and other solids separation problems, 3rd ed. CRC Press LLC, London, England.
- 23.Kawaharasaki, M., A. Manome, T. Kanagawa, and K. Nakamura. 2002. Flow cytometric sorting and RFLP analysis of phosphate accumulating bacteria in an enhanced biological phosphorus removal system. Water Sci. Technol. 46:139-144. [PubMed] [Google Scholar]
- 24.Kong, Y., Y. Xia, J. Nielsen, and P. Nielsen. 2007. Structure and function of the microbial community in a full-scale enhanced biological phosphorus removal plant. Microbiology 153:4061-4073. [DOI] [PubMed] [Google Scholar]
- 25.Kragelund, C., Y. Kong, J. van der Waarde, K. Thelen, D. Eikelboom, V. Tandoi, T. Thomsen, and P. Nielsen. 2006. Ecophysiology of different filamentous Alphaproteobacteria in industrial wastewater treatment plants. Microbiology 152:3003-3012. [DOI] [PubMed] [Google Scholar]
- 26.Kragelund, C., C. Levantesi, A. Borger, K. Thelen, D. Eikelboom, V. Tandoi, Y. Kong, J. Krooneman, P. Larsen, T. Thomsen, and P. Nielsen. 2008. Identity, abundance and ecophysiology of filamentous bacteria belonging to the Bacteroidetes present in activated sludge plants. Microbiology 154:886-894. [DOI] [PubMed] [Google Scholar]
- 27.Kragelund, C., C. Levantesi, A. Borger, K. Thelen, D. Eikelboom, V. Tandoi, Y. Kong, J. van der Waarde, J. Krooneman, S. Rossetti, T. Thomsen, and P. Nielsen. 2007. Identity, abundance and ecophysiology of filamentous Chloroflexi species present in activated sludge treatment plants. FEMS Microbiol. Ecol. 59:671-682. [DOI] [PubMed] [Google Scholar]
- 28.Kragelund, C., J. Nielsen, T. Thomsen, and P. Nielsen. 2005. Ecophysiology of the filamentous alphaproteobacterium Meganema perideroedes in activated sludge. FEMS Microbiol. Ecol. 54:111-122. [DOI] [PubMed] [Google Scholar]
- 29.Lee, N., P. Nielsen, K. Andreasen, S. Juretschko, J. Nielsen, K. Schleifer, and M. Wagner. 1999. Combination of fluorescent in situ hybridization and microautoradiography: a new tool for structure-function analyses in microbial ecology. Appl. Environ. Microbiol. 65:1289-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lemmer, H., G. Lind, E. Müller, and M. Schade. 2005. Non-famous scum bacteria: biological characterization and troubleshooting. Acta Hydrochim. Hydrobiol. 33:197-202. [Google Scholar]
- 31.Levantesi, C., C. Beimfohr, B. Geurkink, S. Rossetti, K. Thelen, J. Krooneman, J. Snaidr, J. van der Waarde, and V. Tandoi. 2004. Filamentous Alphaproteobacteria associated with bulking in industrial wastewater treatment plants. Syst. Appl. Microbiol. 27:716-727. [DOI] [PubMed] [Google Scholar]
- 32.Ludwig, W., O. Strunk, R. Westram, L. Richter, H. Meier, Yadhukumar, A. Buchner, T. Lai, S. Steppi, G. Jobb, W. Förster, I. Brettske, S. Gerber, A. W. Ginhart, O. Gross, S. Grumann, S. Hermann, R. Jost, A. König, T. Liss, R. Lüssmann, M. May, B. Nonhoff, B. Reichel, R. Strehlow, A. Stamatakis, N. Stuckmann, A. Vilbig, M. Lenke, T. Ludwig, A. Bode, and K.-H. Schleifer. 2004. ARB: a software environment for sequence data. Nucleic Acids Res. 32:1363-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manz, W., R. Amann, W. Ludwig, M. Vancanneyt, and K. Schleifer. 1996. Application of a suite of 16S rRNA-specific oligonucleotide probes designed to investigate bacteria of the phylum Cytophaga-Flavobacter-Bacteroides in the natural environment. Microbiology 142:1097-1106. [DOI] [PubMed] [Google Scholar]
- 34.Manz, W., R. Amann, W. Ludwig, M. Wagner, and K.-H. Scheifer. 1992. Phylogenetic oligodeoxynucleotide probes for the major subclasses of proteobacteria: problems and solutions. Syst. Appl. Microbiol. 15:593-600. [Google Scholar]
- 35.Martins, A., K. Pagilla, J. Heijnen, and M. van Loosdrecht. 2004. Filamentous bulking sludge: a critical review. Water Res. 38:793-817. [DOI] [PubMed] [Google Scholar]
- 36.McIlroy, S., K. Porter, R. J. Seviour, and D. Tillett. 2008. Simple and safe method for simultaneous isolation of microbial RNA and DNA from problematic populations. Appl. Environ. Microbiol. 74:6806-6807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKenzie, C., E. Seviour, P. Schumann, A. Maszenan, J. Liu, R. Webb, P. Monis, C. Saint, U. Steiner, and R. Seviour. 2006. Isolates of “Candidatus Nostocoida limicola” Blackall et al. 2000 should be described as three novel species of the genus Tetrasphaera, as Tetrasphaera jenkinsii sp. nov., Tetrasphaera vanveenii sp. nov., and Tetrasphaera veronensis sp. nov. Int. J. Syst. Evol. Microbiol. 56:2279-2290. [DOI] [PubMed] [Google Scholar]
- 38.McVeigh, H., J. Munro, and T. Embley. 1996. Molecular evidence for the presence of novel actinomycete lineages in a temperate forest soil. J. Ind. Microbiol. Biotechnol. 17:197-204. [Google Scholar]
- 39.Neef, A., R. Amann, H. Schlesner, and K.-H. Schleifer. 1998. Monitoring a widespread bacterial group: in situ detection of planctomycetes with 16S rRNA-targeted probes. Microbiology 144:3257-3266. [DOI] [PubMed] [Google Scholar]
- 40.Nielsen, J. L., and P. H. Nielsen. 2005. Advances in microscopy: microautoradiography of single cells. Methods Enzymol. 397:237-256. [DOI] [PubMed] [Google Scholar]
- 41.Oehmen, A., P. C. Lemos, G. Carvalho, Z. Yuan, J. Keller, L. L. Blackall, and M. A. M. Reis. 2007. Advances in enhanced biological phosphorus removal: from micro to macro scale. Water Res. 41:2271-2300. [DOI] [PubMed] [Google Scholar]
- 42.Ostle, A., and J. Holt. 1982. Nile blue A as a fluorescent stain for poly-β-hydroxybutyrate. Appl. Environ. Microbiol. 44:238-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramothokang, T., G. Drysdale, and F. Bux. 2003. Isolation and cultivation of filamentous bacteria implicated in activated sludge bulking. Water SA 29:405-410. [Google Scholar]
- 44.Schade, M., C. Beimfohr, and H. Lemmer. 2002. Phylogenetic and physiological characterization of a “Nostocoida limicola”-like organism isolated from activated sludge. Water Sci. Technol. 46:91-97. [PubMed] [Google Scholar]
- 45.Schade, M., and H. Lemmer. 2006. In situ enzyme activities of filamentous scum bacteria in municipal activated sludge wastewater treatment plants. Acta Hydrochim. Hydrobiol. 34:480-490. [Google Scholar]
- 46.Seviour, R. J., and S. McIlroy. 2008. The microbiology of phosphorus removal in activated sludge processes: the current state of play. J. Microb. 46:115-124. [DOI] [PubMed] [Google Scholar]
- 47.Seviour, R. J., T. Mino, and M. Onuki. 2003. The microbiology of biological phosphorus removal in activated sludge systems. FEMS Microbiol. Rev. 27:99-127. [DOI] [PubMed] [Google Scholar]
- 48.Tandoi, V., D. Jenkins, and J. Wanner. 2006. Activated sludge separation problems. IWA Publishing, London, England.
- 49.Thomsen, T., L. Blackall, M. de Muro, J. Nielsen, and P. Nielsen. 2006. Meganema perideroedes gen. nov., sp. nov., a filamentous alphaproteobacterium from activated sludge. Int. J. Syst. Evol. Microbiol. 56:1865-1868. [DOI] [PubMed] [Google Scholar]
- 50.Thomsen, T., B. Kjellerup, J. Nielsen, P. Hugenholtz, and P. Nielsen. 2002. In situ studies of the phylogeny and physiology of filamentous bacteria with attached growth. Environ. Microbiol. 4:383-391. [DOI] [PubMed] [Google Scholar]
- 51.Wagner, M., R. Amann, P. Kämpfer, B. Assmus, A. Hartmann, P. Hutzler, N. Springer, and K.-H. Schleifer. 1994. Identification and in situ detection of gram-negative filamentous bacteria in activated sludge. Syst. Appl. Microbiol. 17:405-417. [Google Scholar]
- 52.Wanner, J., and P. Grau. 1989. Identification of filamentous microorganisms from activated sludge: a compromise between wishes, needs and possibilities. Water Res. 23:883-891. [Google Scholar]
- 53.Weller, R., F. Glöckner, and R. Amann. 2000. 16S rRNA-targeted oligonucleotide probes for the in situ detection of members of the phylum Cytophaga-Flavobacterium-Bacteroides. Syst. Appl. Microbiol. 23:107-114. [DOI] [PubMed] [Google Scholar]
- 54.Yamada, T., H. Imachi, A. Ohashi, H. Harada, S. Hanada, Y. Kamagata, and Y. Sekiguchi. 2007. Bellilinea caldifistulae gen. nov., sp. nov. and Longilinea arvoryzae gen. nov., sp. nov., strictly anaerobic, filamentous bacteria of the phylum Chloroflexi isolated from methanogenic propionate-degrading consortia. Int. J. Syst. Evol. Microbiol. 57:2299-2306. [DOI] [PubMed] [Google Scholar]