Abstract
Four bifidobacteria, each representing a cluster of strains with specific inulin-type-fructan degradation capacities, were grown in coculture fermentations with Bacteroides thetaiotaomicron LMG 11262, a strain able to metabolize both oligofructose and inulin. In a medium for colon bacteria with inulin as the sole added energy source, the ability of the bifidobacteria to compete for this substrate reflected phenotypical variation. Bifidobacterium breve Yakult, a strain that was not able to degrade oligofructose or inulin, was outcompeted by B. thetaiotaomicron LMG 11262. Bifidobacterium adolescentis LMG 10734, a strain that could degrade oligofructose (displaying a preferential breakdown mechanism) but that did not grow on inulin, managed to become competitive when oligofructose and short fractions of inulin started to accumulate in the fermentation medium. Bifidobacterium angulatum LMG 11039T, a strain that was previously shown to degrade all oligofructose fractions simultaneously and to be able to partially break down inulin, was competitive from the beginning of the fermentation, consuming short fractions of inulin from the moment they appeared. Bifidobacterium longum LMG 11047, representing a cluster of bifidobacteria that shared both high fructose consumption and oligofructose degradation rates and were able to perform partial breakdown of inulin, was the dominating strain in a coculture with B. thetaiotaomicron LMG 11262. These observations indicate that distinct subgroups within the large-intestinal Bifidobacterium population will be stimulated by different groups of prebiotic inulin-type fructans, a variation that could be reflected in differences concerning their health-promoting effects.
The vast complexity of the human colon microbiota, the key element of the large-intestinal ecosystem, has inspired researchers to describe it as a postnatally acquired microbial organ located inside a host organ (1, 46). The microbial colon community is estimated to be composed of up to 100 trillion microorganisms, a number exceeding 10 times the total number of somatic and germ cells of a human adult (18, 38). The human microbiome is thought to contain more than 100 times the total number of human genes (1, 18). It not only broadens the digestive abilities of the host (18, 22, 40) but also influences body processes far beyond digestion (7, 33). In spite of its fundamental impact on human health and disease, the human gastrointestinal ecosystem remains largely unexplored (7, 8).
Despite the fact that the present knowledge of the composition of the human large-intestinal microbiota is partial, fragmented, and undetailed, the consistency of some observations allows them to be generalized as facts (8, 28, 47). Notwithstanding the huge diversity at the strain level, up to 87% of the human colon inhabitants belong to only two bacterial phyla, the Bacteroidetes and the Firmicutes (1, 8, 14). Within the group of large-intestinal Bacteroidetes, large variations between individuals have been reported (8). However, Bacteroides spp. generally seem to account for up to 20% of the human colon microbiota (26, 32). Moreover, the presence of Bacteroides thetaiotomicron appears to be universal (8, 21). This species, which has been isolated only from human and rodent intestines or feces up to now, has gained importance as a perfect example of a flexible, niche-adapted, human symbiont with a wide carbohydrate consumption range (3, 4, 40).
Although B. thetaiotaomicron is considered a human symbiont contributing to the stability of the colon ecosystem, the Bacteroides genus also harbors some notorious pathogens that are linked with severe extraintestinal infections and that have been mentioned as causal agents of acute diarrhea (30, 35). Moreover, besides their enormous saccharolytic potential, Bacteroides spp. are also capable of proteolytic fermentation (22). These considerations make them unsuited as target organisms for stimulation by prebiotics such as inulin-type fructans (23, 31).
Most in vivo studies regarding the effect of the addition of inulin or oligofructose to the diet on the composition of the human colon microbiota reveal that Bacteroides spp. are neither stimulated nor repressed through administration of these prebiotics (34). However, at least some Bacteroides spp. are able to degrade inulin-type fructans, including B. thetaiotaomicron (13, 44). Since this species accounts for up to 6% of the colon microbiota (8), it is at least surprising that its numbers are hardly influenced by an increased availability of these prebiotics as substrates for large-intestinal fermentation. A possible explanation for these contradicting observations is to be found in the mechanism of inulin degradation, which in the case of Bacteroides is presumed to be periplasmic or even extracellular (37, 44). Leakage of free fructose toward the extracellular environment appears to be inherent in such breakdown mechanisms (10, 25, 44). Hence, extracellular fructan degraders inevitably provide opportunistic competitors, which are not able to degrade inulin-type fructans themselves, with a valuable source of energy (2, 10, 19). In contrast, a cell-associated or intracellular degradation mechanism is thought to be widespread among Bifidobacterium spp., which are still considered the main target organisms for prebiotic stimulation by inulin-type fructans (15, 16, 39, 44). This mechanism is often reflected in a clearly preferential breakdown of different-chain-length fractions of oligofructose, which approaches degradation of the long fractions only when short ones are depleted (10, 42, 44). The main disadvantage of such a cell-associated or intracellular degradation strategy seems to be the bifidobacterial incapacity to grow on long-chain-length fractions of inulin (36). Reports of the latter are indeed scarce: kinetic pure culture studies report an upper chain length limit for inulin degradation by Bifidobacterium spp., a disadvantage that will presumably not affect extracellular fructan degraders, such as Bacteroides spp. (9). Although the prebiotic effect of inulin-type fructans on the colon Bifidobacterium population is well documented, in vivo stimulation studies usually tend to consider the bifidobacterial community as a whole, ignoring interspecies differences (23). However, since the early days of in vitro prebiotic studies, a large variation in fructan degradation capacities of different Bifidobacterium strains has been reported (17, 36). It is likely that this variety is translated to the in vivo environment, implying that not all bifidobacteria are equally subject to prebiotic stimulation (5, 45). In a recent study, the kinetics of growth, carbohydrate consumption, and metabolite production of 18 Bifidobacterium spp., 17 of which were human intestinal isolates, have been statistically analyzed (9). The existence of four phenotypically distinct clusters among the tested strains, probably reflecting niche-specific adaptation, has been revealed. This rather limited variation was hypothesized to influence the susceptibilities of various bifidobacteria toward prebiotic stimulation by inulin-type fructans and their fitness to compete for these substrates in a complex environment, such as the colon ecosystem (44).
The present study aimed at mapping the fructan degradation capacity of B. thetaiotaomicron LMG 11262 growing on oligofructose or inulin. In vitro competitiveness trials with bifidobacterial strains belonging to the different phenotypical clusters mentioned above were designed to investigate the abilities of these strains to compete for inulin in a coculture with an inulin-degrading B. thetaiotaomicron strain.
MATERIALS AND METHODS
Microorganisms and media.
B. thetaiotaomicron LMG 11262 was used throughout this study. For the coculture experiments, four bifidobacterial strains were selected, each representing a cluster of bifidobacteria exhibiting a distinct fructan degradation profile, referred to as clusters A to D (Table 1). All strains were purchased from the Belgian Coordinated Collections of Microorganisms/Laboratory for Microbiology Ghent (Ghent, Belgium), except for Bifidobacterium breve Yakult, which was kindly provided by Yakult Honsha Co. Ltd. (Tokyo, Japan). For inoculum preparation and storage purposes, bifidobacteria were grown anaerobically in reinforced clostridial medium (RCM) (Oxoid Ltd., Basingstoke, United Kingdom), while B. thetaiotaomicron LMG 11262 was cultured in Wilkins-Chalgren anaerobe broth (WCB) (Oxoid) under the same conditions. Strains were stored at −80°C in the appropriate medium, supplemented with 25% (vol/vol) glycerol as a cryoprotectant.
TABLE 1.
Bifidobacterium spp. included in the present studya
| Cluster | Strain | Inulin-type fructan degradation profile |
|---|---|---|
| A | B. breve Yakult | Neither oligofructose nor inulin degradation |
| B | B. adolescentis LMG 10734 | Oligofructose degradation with preferential breakdown of short fractions; oligofructose degradation slower than fructose consumption; no inulin degradation |
| C | B. angulatum LMG 11039T | Nonpreferential oligofructose degradation; oligofructose degradation faster than fructose consumption; degradation of short fractions of inulin |
| D | B. longum LMG 11047 | Nonpreferential oligofructose degradation; equally fast fructose consumption and oligofructose degradation; degradation of short fractions of inulin |
Each species represents a phenotypical cluster of bifidobacteria exhibiting distinct inulin-type fructan degradation profiles (9).
The fermentation experiments were performed in a medium for colon bacteria (MCB), developed by Van der Meulen et al. (44), to support growth of various human colon bacteria when supplemented with an adequate energy source. The pH of the medium was adjusted to 6.3 before autoclaving was carried out at 210 kPa and 121°C for 20 min. After sterilization, fructose (VWR International GmbH, Darmstadt, Germany), oligofructose (OraftiP95; BENEO-Orafti NV, Tienen, Belgium), or inulin (OraftiHP; BENEO-Orafti) was added aseptically as the sole energy source at a concentration of 50 mM fructose equivalents (FE). Fructose was autoclaved under the same conditions as the MCB; oligofructose and inulin were sterilized through membrane filtration using Minisart filters (pore size, 0.2 μm; Sartorius AG, Göttingen, Germany). OraftiP95 and OraftiHP are commercial powders derived from chicory roots. OraftiP95 is obtained through enzymatic hydrolysis of chicory inulin. It consists mainly of oligofructose (≥93.2% [wt/wt]) but also contains some minor amounts of glucose, fructose, and sucrose (<6.8% [wt/wt]). The degree of polymerization (DP) of the oligofructose fractions varies between 2 and 8, with an average of 4. OraftiHP contains inulin (≥99.5%, wt/wt), with a DP ranging from 12 to 65, and minor amounts of glucose, fructose, and sucrose (<0.5% [wt/wt]). The average DP of the inulin chains exceeds 23 due to removal of the smaller molecules during processing.
Solid RCM and WCB were prepared by adding 1.5% (wt/vol) of agar (Oxoid) to the respective broths. Gram-negative anaerobe supplement (Oxoid) was added to WCB agar to prevent bifidobacterial growth and allow selective enumeration of Bacteroides bacteria. No bacteroidal growth was observed on RCM agar.
Fermentation experiments.
Mono- and coculture fermentations were carried out in 2-liter Biostat B-DCU fermentors (Sartorius) containing 1.5 liter of MCB supplemented with the energy source under study. Inocula were prepared as follows: strains were transferred from −80°C to the appropriate medium and incubated anaerobically at 37°C for 12 h in a modular atmosphere-controlled system (MG anaerobic work station; Don Whitley Scientific Ltd., West Yorkshire, United Kingdom) that was continuously sparged with a mixture of 80% N2, 10% CO2, and 10% H2 (Air Liquide, Paris, France). Subsequently, the strains were propagated twice in MCB with fructose as the sole energy source and finally added to the fermentor. During inoculum build-up, the transferred volume was always 5% (vol/vol). Anaerobic conditions were assured during fermentation experiments by continuously sparging the medium with a mixture of 90% N2 and 10% CO2 (Air Liquide). The fermentation temperature was kept constant at 37°C. A constant pH of 6.3 was imposed and controlled automatically, using 1.5 M solutions of NaOH and H3PO4. To keep the medium homogeneous, a gentle stirring of 100 rpm was applied. Temperature, pH, and agitation speed were controlled online (MFCS/win 2.1; Sartorius). Fermentations were followed up during a 48-h period; samples for further analysis were taken at regular time intervals. All fermentations were performed in duplicate; the results and figures presented hereinafter are representative for both fermentations.
Analysis of bacterial growth, carbohydrate consumption, and metabolite production. (i) Bacterial growth.
Growth was followed throughout all fermentations by plating on the appropriate selective agar medium. Plates were incubated for 24 h under anaerobic conditions as indicated above.
(ii) Carbohydrate, organic acid, and ethanol determinations.
Residual concentrations of glucose, fructose, oligofructose, and inulin (the latter two expressed in mM FE), as well as concentrations of acetate, formate, and ethanol, were determined through high-performance liquid chromatography as described previously (9). All samples were analyzed in triplicate.
For the fermentations with OraftiHP as the sole added energy source, concentrations of free fructose were determined by high-performance liquid chromatography analysis, following the same procedure. However, to prevent fructan hydrolysis, proteins were removed through the addition of 350 μl of Carrez A [36 g liter−1 of K4Fe(CN)6·3H2O] and 350 μl of Carrez B (72 g liter−1 of ZnSO4·7H2O) reagents to 700 μl of sample.
Succinate and lactate were separated through high-performance anion-exchange chromatography (HPAEC) and subsequently quantified by conductivity measurement with ion suppression using an ICS3000 chromatograph (Dionex Corp., Sunnyvale, CA). An AS-19 column (Dionex) was used. The mobile phase, at a flow rate of 1.0 ml min−1, consisted of ultrapure water (0.015 μS cm−1; eluent A) and 0.1 M KOH (eluent B). The following gradient was applied: 0 min, 96% A and 4% B; 20 min, 96% A and 4% B; and 60 min, 0% A and 100% B. The injection volume was 10 μl, and the column temperature was kept constant at 30°C. Sample preparation involved centrifugation (21,036 × g, 20 min), followed by protein removal using an isovolume of acetonitrile, centrifugation (21,036 × g, 20 min), appropriate dilution, and filtration (pore size, 0.2 μm; Minisart RC4 filters; Sartorius). All samples were analyzed in triplicate.
(iii) Breakdown of oligofructose and inulin.
Detailed analysis of the breakdown of the different oligofructose fractions of OraftiP95 was performed by gas chromatography (GC) using a 5300-HT high-resolution gas chromatograph (Carlo Erba, Rodina, Italy). The GC was equipped with an SGE Aluminum Clad-5 capillary column (Achrom NV, Zulte, Belgium), a cooled on-column autoinjector (AS-550), and a flame ionization detector (detector temperature of 447°C). The oven temperature varied linearly from 105 to 440°C at 10°C min−1. Samples were derivatized following a procedure involving oximation and silylation (20). The oxime-trimethylsilyl sugar derivatives were extracted using iso-octane; the resulting iso-octane phase was injected (1 μl) into the GC. The same procedure was carried out for reference samples containing reference oligofructose (RaftiloseP95X; BENEO-Orafti), glucose, fructose, and sucrose as external standards.
Qualitative analysis of inulin breakdown in fermentations with OraftiHP as the sole added energy source was performed using HPAEC with pulsed amperometric detection (PAD) with a DX500 chromatograph (Dionex). A CarboPac PA100 column (Dionex) was used with a gradient of three eluent solutions as a mobile phase: 0.1 M NaOH (eluent A), 0.1 M NaOH and 0.4 M NaCH3COOH (eluent B), and 1 M NaOH (eluent C). The following gradient was applied: 0 min, 96% A and 4% B; 5 min, 96% A and 4% B; 15 min, 60% A and 40% B; 35 min, 30% A and 70% B; 50 min, 10% A and 90% B; 60 min, 100% B; 85 min, 100% B; 90 min, 100% C; 95 min, 100% C; 96 min, 96% A and 4% B; and 101 min, 96% A and 4% B. The injection volume was 50 μl. Sample preparation (700 μl) involved centrifugation (21,036 × g, 20 min), followed by protein removal through addition of 350 μl of Carrez A and 350 μl of Carrez B, centrifugation (21,036 × g, 20 min), appropriate dilution, and filtration (Minisart RC4). Samples were analyzed in duplicate.
CR.
Carbon recovery (CR) (expressed in percentages) was calculated by dividing the total amount of carbon recovered in the sugar metabolites by the total amount of carbon present in the added energy source. For B. thetaiotaomicron LMG 11262, the production of one mole of CO2 for every mole of acetate formed was taken into account, as was as the uptake of one mole of CO2 for every mole of succinate formed (6, 10, 29). In coculture fermentation experiments, the competitiveness of each strain was evaluated by calculating the percentage of carbon that could be recovered as an end product of its metabolism.
RESULTS
Monocultures of Bifidobacterium breve Yakult, Bifidobacterium adolescentis LMG 10734, Bifidobacterium angulatum LMG 11039T, and Bifidobacterium longum LMG 11047 in MCB supplemented with fructose, oligofructose, or inulin.
Results of Bifidobacterium monoculture fermentations have been discussed in detail in a previous article (9).
Monoculture of Bacteroides thetaiotaomicron LMG 11262 in MCB supplemented with fructose, oligofructose, or inulin.
B. thetaiotaomicron LMG 11262 was able to grow on fructose, oligofructose, and inulin (Table 2). Besides gases, acetate and succinate were the only metabolites produced. Degradation of inulin was slower than that of fructose or oligofructose. A shift in the succinate/acetate production ratio was observed, from 1.49 for growth in MCB with fructose as the sole added energy source, to 1.91 for growth on oligofructose and 2.64 in MCB supplemented with inulin.
TABLE 2.
Growth, carbohydrate consumption, and metabolite production of Bacteroides thetaiotaomicron LMG 11262a
| Carbohydrate | Mean consumption ± SD (mM FE) of substrate (after 48 h) | Mean production ± SD (mM) of metabolites (after 48 h) |
CRb (%) | Substrate depletion time (h) | Maximal cell population (CFU ml−1)c | |
|---|---|---|---|---|---|---|
| Succinate | Acetate | |||||
| Fructose | 49.9 ± 0.4 | 54.3 ± 0.3 | 36.5 ± 0.3 | 92.5 | 6-9 | 8.5 × 109 |
| Oligofructose | 56.3 ± 0.1 | 65.1 ± 1.2 | 34.1 ± 1.5 | 90.6 | 6-9 | 1.1 × 1010 |
| Inulin | 47.4 ± 0.2 | 61.7 ± 1.5 | 23.4 ± 1.5 | 91.6 | 18-24 | 1.6 × 1010 |
Bacteria were grown in a medium for colon bacteria supplemented with 50 mM FE of fructose, oligofructose (OraftiP95), or inulin (OraftiHP).
Including theoretical carbon metabolism (37).
Assessed at 9 h for fructose or oligofructose supplementation or at 24 h for inulin.
Detailed quantitative analysis of oligofructose breakdown by the strain revealed simultaneous degradation of fractions of all different chain lengths, accompanied by an accumulation of free fructose in the fermentation medium (Fig. 1A). A similar simultaneous degradation profile was observed after qualitative analysis of inulin degradation (Fig. 1B). During inulin breakdown, fructose (maximal concentration of 8.8 ± 0.7 mM after 15 h of fermentation), oligofructose, and short fractions of inulin accumulated in the fermentation medium. For each fermentation, the CR was above 90% (Table 2).
FIG. 1.
Fructan degradation by Bacteroides thetaiotaomicron LMG 11262 in a medium for colon bacteria supplemented with 50 mM FE of oligofructose (OraftiP95) or inulin (OraftiHP). (A) Oligofructose degradation. F, fructose; G, glucose. (B) Qualitative inulin degradation. An HPAEC-PAD chromatogram is shown.
Coculture of Bacteroides thetaiotaomicron LMG 11262 and Bifidobacterium breve Yakult in MCB supplemented with inulin.
In a coculture of B. thetaiotaomicron LMG 11262 and B. breve Yakult, all added substrate was consumed after 30 h of fermentation (Fig. 2A). Apart from gases, acetate and succinate were the main metabolites produced. Also, traces of formate (4.4 ± 0.5 mM), ethanol (2.0 ± 0.5 mM), and lactate (1.2 ± 1.4 mM) were found.
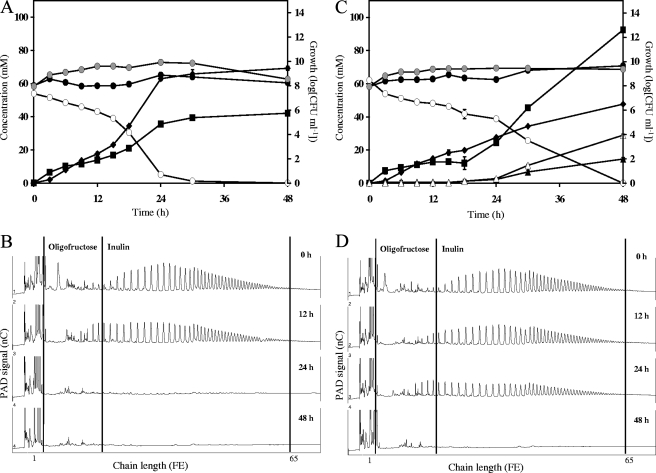
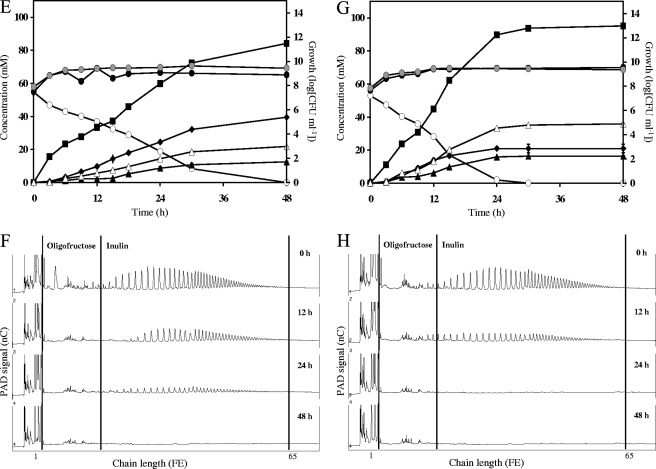
FIG. 2.
Growth, carbohydrate consumption, and metabolite production by a coculture of Bacteroides thetaiotaomicron LMG 11262 with Bifidobacterium breve Yakult (A and B), with Bifidobacterium adolescentis LMG 10734 (C and D), with Bifidobacterium angulatum LMG 11039T (E and F), or with Bifidobacterium longum LMG 11047 (G and H) in a medium for colon bacteria supplemented with 50 mM FE of inulin (OraftiHP). (A, C, E, and G) ○, inulin (FE); ♦, succinate; ▪, acetate; ▵, formate; ▴, ethanol; , growth of Bacteroides; •, growth of Bifidobacterium. (B, D, F, and H) Qualitative inulin degradation. An HPAEC-PAD chromatogram is shown.
Qualitative analysis of inulin degradation revealed a profile similar to the one found for B. thetaiotaomicron LMG 11262 monoculture fermentations (Fig. 2B). Free fructose concentrations peaked after 18 h of fermentation (11.0 ± 0.1 mM).
Since Bifidobacterium species produce only minor amounts of succinate (few μM [50 mM FE substrate]−1) under fermentation conditions similar to those applied in the present study (10, 41), all succinate detected in the coculture fermentation could be regarded as originating from the Bacteroides metabolism. Assuming that B. thetaiotaomicron LMG 11262 behaved similarly in mono- and coculture fermentations with inulin as the sole added energy source (succinate-to-acetate ratio of 2.64) and considering the strain could not (co)metabolize lactate (data not shown), it was calculated that the strain was responsible for 26.2 mM of the acetate produced. The remaining 15.8 mM of acetate, as well as the traces of formate, ethanol, and lactate recovered, could be attributed to growth of B. breve Yakult. Hence, only 13.5% of the added carbon source was recovered in the end products of bifidobacterial metabolism. The estimations made above, including theoretical CO2 uptake and production by B. thetaiotaomicron LMG 11262 (24, 29, 37), allowed the calculation of the CR in the coculture as 101.7% (6, 10).
Coculture of Bacteroides thetaiotaomicron LMG 11262 and Bifidobacterium adolescentis LMG 10734 in MCB supplemented with inulin.
In a coculture of B. thetaiotaomicron LMG 11262 and B. adolescentis LMG 10734, inulin was depleted within 48 h of fermentation (Fig. 2C). The main metabolites produced were acetate, succinate, formate, and ethanol; traces of lactate (1.1 ± 0.1 mM) were found. Gas production was observed.
The qualitative inulin degradation profile (Fig. 2D) revealed simultaneous degradation of inulin fractions of all chain lengths. Oligofructose and short fractions of inulin accumulated in the fermentation medium during breakdown. A maximal free fructose concentration of 7.2 ± 0.1 mM was measured after 30 h of fermentation.
Considering the coculture conditions estimated above, it was calculated that 53.1% of the added carbon source ended up as metabolites of B. adolescentis LMG 10734 fermentation. The CR was calculated as 108.6%.
Coculture of Bacteroides thetaiotaomicron LMG 11262 and Bifidobacterium angulatum LMG 11039T in MCB supplemented with inulin.
After 48 h of fermentation, no residual inulin could be recovered in the fermentation medium after cocultivation of B. thetaiotaomicron LMG 11262 and B. angulatum LMG 11039T (Fig. 2E). Besides gases, acetate, succinate, formate, and ethanol were the main metabolites produced. Traces of lactate (3.3 ± 0.4 mM) were found.
Qualitative analysis of inulin degradation showed fast depletion of the short fractions of inulin, followed by simultaneous breakdown of the long ones (Fig. 2F). During the entire inulin degradation process, no accumulation of oligofructose or short fractions of inulin was observed. After 24 h of fermentation, a maximum free fructose concentration of 6.6 ± 0.2 mM was found.
Taking into account the coculture estimations made above, 54.1% of the added carbon source could be recovered as end products of bifidobacterial metabolism. The calculated CR was 106.6%.
Coculture of Bacteroides thetaiotaomicron LMG 11262 and Bifidobacterium longum LMG 11047 in MCB supplemented with inulin.
In a coculture of B. thetaiotaomicron LMG 11262 and B. longum LMG 11047, carbohydrate depletion occurred within 30 h of fermentation (Fig. 2G). Besides gases, acetate, formate, succinate, and ethanol were the main metabolites produced. Traces of lactate (4.3 ± 0.1 mM) were found.
Qualitative analysis of inulin degradation revealed fast substrate degradation without accumulation of oligofructose and short fractions of inulin (Fig. 2H). Free fructose concentrations peaked after 12 h of fermentation (5.1 ± 0.1 mM).
Considering the coculture conditions estimated above, it was calculated that 75.3% of the added carbon source was consumed by B. longum LMG 11047. The CR was calculated as 104.3%.
DISCUSSION
When referred to as target organisms for prebiotic stimulation by inulin-type fructans, the large-intestinal Bifidobacterium population is generally considered a homogeneous group of potentially beneficial microorganisms (15, 16). However, it has been shown that this population differs from one individual to another, not only in numbers but also in species composition (26, 27). Since the ability to metabolize oligofructose and inulin is a strain-specific feature among bifidobacteria, the outcome of a prebiotic dietary intervention is bound to be influenced by the host's specific situation (5, 19, 36, 45). The original bifidobacterial species composition not only will affect individual susceptibility toward prebiotic stimulation but will also determine which species will become dominant after the intake of oligofructose and inulin (23, 45). Taking into account the strain specificity of the health-promoting properties attributed to some bifidobacteria (12)—a major issue when the genus is cited in a probiotic context—these considerations might deserve more attention than currently granted. The ability of bifidobacteria to increase in numbers when a prebiotic becomes available as a fermentation substrate in the complex environment of the human colon ecosystem is speculated to be dependent on their fitness to compete with other large-intestinal fructan degraders (36). The latter include at least some Bacteroides species (17, 44). Since B. thetaiotaomicron LMG 11262 has been reported to be able to grow on oligofructose (44), it was selected as model test strain for the present in vitro competitiveness study.
Qualitative analysis of inulin breakdown by B. thetaiotaomicron LMG 11262 revealed simultaneous degradation of all different chain length fractions of the fructose polymer with accumulation of oligofructose and short fractions of inulin in the medium. The pattern observed showed striking similarities to the previously described nondiscriminative oligofructose breakdown mechanism of this strain, acting on fractions of all different chain lengths simultaneously and leading to an extracellular increase of free fructose (44). Such nonpreferential degradation profiles appear symptomatic for extracellular or periplasmic fructan degradation (10, 25, 37). When growing on less readily metabolizable substrates, such as oligofructose and inulin, B. thetaiotaomicron LMG 11262 produced more succinate at the expense of acetate, although the production of the latter yields more ATP (24, 29). However, succinate production allows regeneration of NAD+, which appears to be of higher value to the strain under circumstances of a reduced sugar consumption rate (24, 29, 37). A similar shift was previously reported for Bacteroides fragilis LMG 10263 (44).
The kinetics of carbohydrate consumption and metabolite production during coculture fermentations of selected bifidobacteria with B. thetaiotaomicron LMG 11262 reflected bifidobacterial variation in the inulin-type fructan degradation capacity corresponding with four phenotypically distinct clusters (9). B. breve Yakult (cluster A) hardly influenced bacteroidal growth and metabolite production; only around 13% of the added energy source could be recovered as end products of the strain's metabolism. The strains belonging to other clusters did manage to conquer at least part of the substrate at different time points during the inulin breakdown process. However, care needs to be taken when interpreting the percentages mentioned here; considering the assumptions made to calculate the numbers presented, they should be regarded as indicative and by no means absolute.
Considering formate and ethanol production as markers for in vitro bifidobacterial metabolic activity (10, 41), it was demonstrated that B. adolescentis LMG 10734 (cluster B) became more competitive toward the middle of a coculture fermentation with B. thetaiotaomicron LMG 11262. Qualitative analysis of inulin degradation revealed that at this point, oligofructose and short fractions of inulin started to accumulate in the fermentation medium. Metabolite production by B. angulatum LMG 11039T (cluster C) took place during the entire course of the coculture fermentation. Apparently, this strain was already competitive in an earlier stage of inulin degradation, which could be linked with its inulin-type-fructan breakdown capacities (9). The inulin degradation profile of this coculture showed accumulation neither of oligofructose nor of short fractions of inulin. Notwithstanding the kinetic differences discussed above, the overall competitiveness of B. adolescentis LMG 10734 and B. angulatum LMG 11039T was similar: slightly more than 50% of the added energy source was recovered as end products of their metabolism.
B. longum LMG 11047 was the only strain tested that was able to dominate in a coculture with B. thetaiotaomicron LMG 11262. Formate and acetate production were observed from the beginning of the fermentation, whereas succinate production remained limited. About 75% of the added energy source was recovered as metabolites resulting from bifidobacterial carbohydrate fermentation. This struggle for dominance stresses the importance of the choice of a Bifidobacterium strain to be applied as a probiotic (11, 12).
In none of the coculture fermentations was substantial bifidobacterial lactate production observed, which is in contradiction with the observations made during monoculture fermentation studies (9). This confirms an earlier reported shift in the Bifidobacterium metabolism toward more acetate, formate, and ethanol production at the expense of lactate when strains are grown under conditions of limited access to an energy source (10, 41-43).
In the present work, we set out to investigate whether the limited variation among bifidobacteria regarding their ability to degrade oligofructose and inulin is translated into a difference in their fitness to compete with other fructan-degrading colon bacteria (17, 36). Coculture fermentations of B. breve Yakult with a fructan-degrading, nonbifidobacterial human colon isolate revealed that the capacity to degrade oligofructose is a conditio sine qua non for bifidobacteria to be competitive when growing on inulin as the sole added energy source (19). Preferential oligofructose degradation, usually linked with cell-associated or intracellular carbohydrate breakdown (10, 44), provides strains with the weapons necessary to compete for the short fructan fractions. The ability to partially degrade inulin offers an additional competitive advantage but appears more effective when the bifidobacterial strain displays highly efficient fructose and oligofructose consumption. Although further in vivo confirmation is needed, the results presented in this study indicate that different groups of inulin-type-fructan prebiotics might stimulate distinct subgroups of the large-intestinal Bifidobacterium population, a variation that could be reflected in differences concerning their health-promoting effects.
Acknowledgments
This research was funded by the Research Council of the Vrije Universiteit Brussel, the Fund for Scientific Research—Flanders (FWO-AL418), the Institute for the Promotion of Innovation through Science and Technology in Flanders (IWT-Vlaanderen) (GBOU Project IWT-010054), Yakult Belgium, and Institute Danone. Gwen Falony was a recipient of a Ph.D. grant from the IWT-Vlaanderen. Frédéric Leroy was supported by a postdoctoral fellowship from the FWO.
We thank the analytical laboratory team of BENEO-Orafti NV for their support.
Footnotes
Published ahead of print on 27 February 2009.
REFERENCES
- 1.Bäckhed, F., R. E. Ley, J. L. Sonnenburg, D. A. Peterson, and J. I. Gordon. 2005. Host-bacterial mutualism in the human intestine. Science 307:1915-1920. [DOI] [PubMed] [Google Scholar]
- 2.Belenguer, A., S. H. Duncan, G. Calder, G. Holtrop, P. Louis, G. E. Lobley, and H. J. Flint. 2006. Two routes of metabolic cross-feeding between Bifidobacterium adolescentis and butyrate-producing anaerobes from the human gut. Appl. Environ. Microbiol. 72:3593-3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bjursell, M. K., E. C. Martens, and J. I. Gordon. 2006. Functional genomic and metabolic studies of the adaptations of a prominent adult human gut symbiont, Bacteroides thetaiotaomicron, to the suckling period. J. Biol. Chem. 281:36269-36279. [DOI] [PubMed] [Google Scholar]
- 4.Comstock, L. E., and M. J. Coyne. 2003. Bacteroides thetaiotaomicron: a dynamic, niche-adapted human symbiont. Bioessays 25:926-929. [DOI] [PubMed] [Google Scholar]
- 5.de Preter, V., T. Vanhoutte, G. Huys, J. Swings, P. Rutgeerts, and K. Verbeke. 2008. Baseline microbiota activity and initial bifidobacteria counts influence responses to prebiotic dosing in healthy subjects. Aliment. Pharmacol. Ther. 27:504-513. [DOI] [PubMed] [Google Scholar]
- 6.Duncan, S. H., A. Barcenilla, C. S. Stewart, S. E. Pryde, and H. J. Flint. 2002. Acetate utilization and butyryl-coenzyme A (CoA): acetate-CoA transferase in butyrate-producing bacteria from the human large intestine. Appl. Environ. Microbiol. 68:5186-5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duncan, S. H., P. Louis, and H. J. Flint. 2007. Cultivable bacterial diversity from the human colon. Lett. Appl. Microbiol. 44:343-350. [DOI] [PubMed] [Google Scholar]
- 8.Eckburg, P. B., E. M. Bik, C. N. Bernstein, E. Purdom, L. Dethlefsen, M. Sargent, S. R. Gill, K. E. Nelson, and D. A. Relman. 2005. Diversity of the human intestinal microbial flora. Science 308:1635-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falony, G., K. Lazidou, A. Verschaeren, S. Weckx, D. Maes, and L. De Vuyst. 2009. In vitro kinetic analysis of fermentation of prebiotic inulin-type fructans by Bifidobacterium species reveals four different phenotypes. Appl. Environ. Microbiol. 75:454-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Falony, G., A. Vlachou, K. Verbrugghe, and L. De Vuyst. 2006. Cross-feeding between Bifidobacterium longum BB536 and acetate-converting, butyrate-producing colon bacteria during growth on oligofructose. Appl. Environ. Microbiol. 72:7835-7841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.FAO/WHO. 2002. Guidelines for the evaluation of probiotics in food. Report of a joint FAO/WHO expert consultation. http://www.who.int/foodsafety/publications/fs_management/probiotics2/en/index.html.
- 12.FAO/WHO. 2001. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Report of a joint FAO/WHO expert consultation. http://www.who.int/foodsafety/publications/fs_management/en/probiotics.pdf.
- 13.Flint, H. J. 2006. The significance of prokaryote diversity in the human gastrointestinal tract, p. 65-90. In N. A. Logan, H. M. Lappin-Scott, and P. C. F. Oyston (ed.), Prokaryotic diversity: mechanisms and significance. SGM Symposium, vol. 66. Cambridge University Press, Cambridge, United Kingdom. [Google Scholar]
- 14.Frank, D. N., A. L. S. Amand, R. A. Feldman, E. C. Boedeker, N. Harpaz, and N. R. Pace. 2007. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. USA 104:13780-13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gibson, G. R., H. M. Probert, J. A. E. Van Loo, R. A. Rastall, and M. B. Roberfroid. 2004. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr. Res. Rev. 17:259-275. [DOI] [PubMed] [Google Scholar]
- 16.Gibson, G. R., and M. B. Roberfroid. 1995. Dietary modulation of the human colonic microbiota—introducing the concept of prebiotics. J. Nutr. 125:1401-1412. [DOI] [PubMed] [Google Scholar]
- 17.Gibson, G. R., and X. Wang. 1994. Bifidogenic properties of different types of fructo-oligosaccharides. Food Microbiol. 11:491-498. [Google Scholar]
- 18.Gill, S. R., M. Pop, R. T. DeBoy, P. B. Eckburg, P. J. Turnbaugh, B. S. Samuel, J. I. Gordon, D. A. Relman, C. M. Fraser-Liggett, and K. E. Nelson. 2006. Metagenomic analysis of the human distal gut microbiome. Science 312:1355-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huebner, J., R. L. Wehling, and R. W. Hutkins. 2007. Functional activity of commercial prebiotics. Int. Dairy J. 17:770-775. [Google Scholar]
- 20.Joye, D., and H. Hoebregs. 2000. Determination of oligofructose, a soluble dietary fiber, by high-temperature capillary gas chromatography. J. AOAC Int. 83:1020-1025. [PubMed] [Google Scholar]
- 21.Ley, R. E., D. A. Peterson, and J. I. Gordon. 2006. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 124:837-848. [DOI] [PubMed] [Google Scholar]
- 22.Macfarlane, G. T., and J. H. Cummings. 1991. The colonic flora, fermentation and large bowel digestive function, p. 51-92. In S. F. Phillips, J. H. Pemberton, and R. G. Shorter (ed.), The large intestine: physiology, pathophysiology and disease. Raven Press Ltd., New York, NY.
- 23.Macfarlane, S., G. T. Macfarlane, and J. H. Cummings. 2006. Prebiotics in the gastrointestinal tract. Aliment. Pharmacol. Ther. 24:701-714. [DOI] [PubMed] [Google Scholar]
- 24.Macy, J. M., L. G. Ljungdahl, and G. Gottschalk. 1978. Pathway of succinate and propionate formation in Bacteroides fragilis. J. Bacteriol. 134:84-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Makras, L., G. Van Acker, and L. De Vuyst. 2005. Lactobacillus paracasei subsp. paracasei 8700:2 degrades inulin-type fructans exhibiting different degrees of polymerization. Appl. Environ. Microbiol. 71:6531-6537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsuki, T., K. Watanabe, J. Fujimoto, Y. Kado, T. Takada, K. Matsumoto, and R. Tanaka. 2004. Quantitative PCR with 16S rRNA-gene-targeted species-specific primers for analysis of human intestinal bifidobacteria. Appl. Environ. Microbiol. 70:167-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCartney, A. L., W. Z. Wang, and G. W. Tannock. 1996. Molecular analysis of the composition of the bifidobacterial and Lactobacillus microflora of humans. Appl. Environ. Microbiol. 62:4608-4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McWilliam Leitch, E. C., A. W. Walker, S. H. Duncan, G. Holtrop, and H. J. Flint. 2007. Selective colonization of insoluble substrates by human faecal bacteria. Environ. Microbiol. 9:667-679. [DOI] [PubMed] [Google Scholar]
- 29.Miller, T. L. 1978. Pathway of formation of acetate and succinate from pyruvate by Bacteroides succinogenes. Arch. Microbiol. 117:145-152. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen, T. V., P. L. Van, C. L. Huy, K. N. Gia, and A. Weintraub. 2006. Etiology and epidemiology of diarrhea in children in Hanoi, Vietnam. Int. J. Infect. Dis. 10:298-308. [DOI] [PubMed] [Google Scholar]
- 31.Picard, C., J. Fioramonti, A. Francois, T. Robinson, F. Neant, and C. Matuchansky. 2005. Bifidobacteria as probiotic agents—physiological effects and clinical benefits. Aliment. Pharmacol. Ther. 22:495-512. [DOI] [PubMed] [Google Scholar]
- 32.Rigottier-Gois, L., V. Rochet, N. Garrec, A. Suau, and J. Doré. 2003. Enumeration of Bacteroides species in human faeces by fluorescent in situ hybridisation combined with flow cytometry using 16S rRNA probes. Syst. Appl. Microbiol. 26:110-118. [DOI] [PubMed] [Google Scholar]
- 33.Roberfroid, M. B. 2005. The gastrointestinal system: a major target for functional foods, p. 17-36. In M. B. Roberfroid and I. Wolinsky (ed.), Inulin-type fructans: functional food ingredients. CRC Press LCC, Boca Raton, FL.
- 34.Roberfroid, M. B. 2005. Inulin-type fructans and the modulation of the intestinal microflora, p. 151-181. In M. B. Roberfroid and I. Wolinsky (ed.), Inulin-type fructans: functional food ingredients. CRC Press LCC, Boca Raton, FL.
- 35.Robizadeh, S., K. J. Rhee, S. G. Wu, D. Huso, C. M. Gan, J. E. Golub, X. Q. Wu, M. Zhang, and C. L. Sears. 2007. Enterotoxigenic Bacteroides fragilis: a potential instigator of colitis. Inflamm. Bowel Dis. 13:1475-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rossi, M., C. Corradini, A. Amaretti, M. Nicolini, A. Pompei, S. Zanoni, and D. Matteuzzi. 2005. Fermentation of fructooligosaccharides and inulin by bifidobacteria: a comparative study of pure and fecal cultures. Appl. Environ. Microbiol. 71:6150-6158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salyers, A. A. 1984. Bacteroides of the human lower intestinal-tract. Annu. Rev. Microbiol. 38:293-313. [DOI] [PubMed] [Google Scholar]
- 38.Savage, D. C. 1977. Microbial ecology of the gastrointestinal tract. Annu. Rev. Microbiol. 31:107-133. [DOI] [PubMed] [Google Scholar]
- 39.Schell, M. A., M. Karmirantzou, B. Snel, D. Vilanova, B. Berger, G. Pessi, M. C. Zwahlen, F. Desiere, P. Bork, M. Delley, R. D. Pridmore, and F. Arigoni. 2002. The genome sequence of Bifidobacterium longum reflects its adaptation to the human gastrointestinal tract. Proc. Natl. Acad. Sci. USA 99:14422-14427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sonnenburg, J. L., J. Xu, D. D. Leip, C. H. Chen, B. P. Westover, J. Weatherford, J. D. Buhler, and J. I. Gordon. 2005. Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science 307:1955-1959. [DOI] [PubMed] [Google Scholar]
- 41.Van der Meulen, R., T. Adriany, K. Verbrugghe, and L. De Vuyst. 2006. Kinetic analysis of bifidobacterial metabolism reveals a minor role for succinic acid in the regeneration of NAD+ through its growth-associated production. Appl. Environ. Microbiol. 72:5204-5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van der Meulen, R., L. Avonts, and L. De Vuyst. 2004. Short fractions of oligofructose are preferentially metabolized by Bifidobacterium animalis DN-173 010. Appl. Environ. Microbiol. 70:1923-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van der Meulen, R., N. Camu, T. Van Vooren, C. Heymans, and L. De Vuyst. 2008. In vitro kinetic analysis of carbohydrate and aromatic amino acid metabolism of different members of the human colon. Int. J. Food Microbiol. 124:27-33. [DOI] [PubMed] [Google Scholar]
- 44.Van der Meulen, R., L. Makras, K. Verbrugghe, T. Adriany, and L. De Vuyst. 2006. In vitro kinetic analysis of oligofructose consumption by Bacteroides and Bifidobacterium spp. indicates different degradation mechanisms. Appl. Environ. Microbiol. 72:1006-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vanhoutte, T., V. De Preter, E. De Brandt, K. Verbeke, J. Swings, and G. Huys. 2006. Molecular monitoring of the fecal microbiota of healthy human subjects during administration of lactulose and Saccharomyces boulardii. Appl. Environ. Microbiol. 72:5990-5997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zocco, M. A., M. E. Ainora, G. Gasbarrini, and A. Gasbarrini. 2007. Bacteroides thetaiotaomicron in the gut: molecular aspects of their interaction. Dig. Liver Dis. 39:707-712. [DOI] [PubMed] [Google Scholar]
- 47.Zoetendal, E. G., A. von Wright, T. Vilpponen-Salmela, K. Ben-Amor, A. D. L. Akkermans, and W. M. de Vos. 2002. Mucosa-associated bacteria in the human gastrointestinal tract are uniformly distributed along the colon and differ from the community recovered from feces. Appl. Environ. Microbiol. 68:3401-3407. [DOI] [PMC free article] [PubMed] [Google Scholar]