Abstract
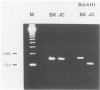
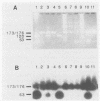
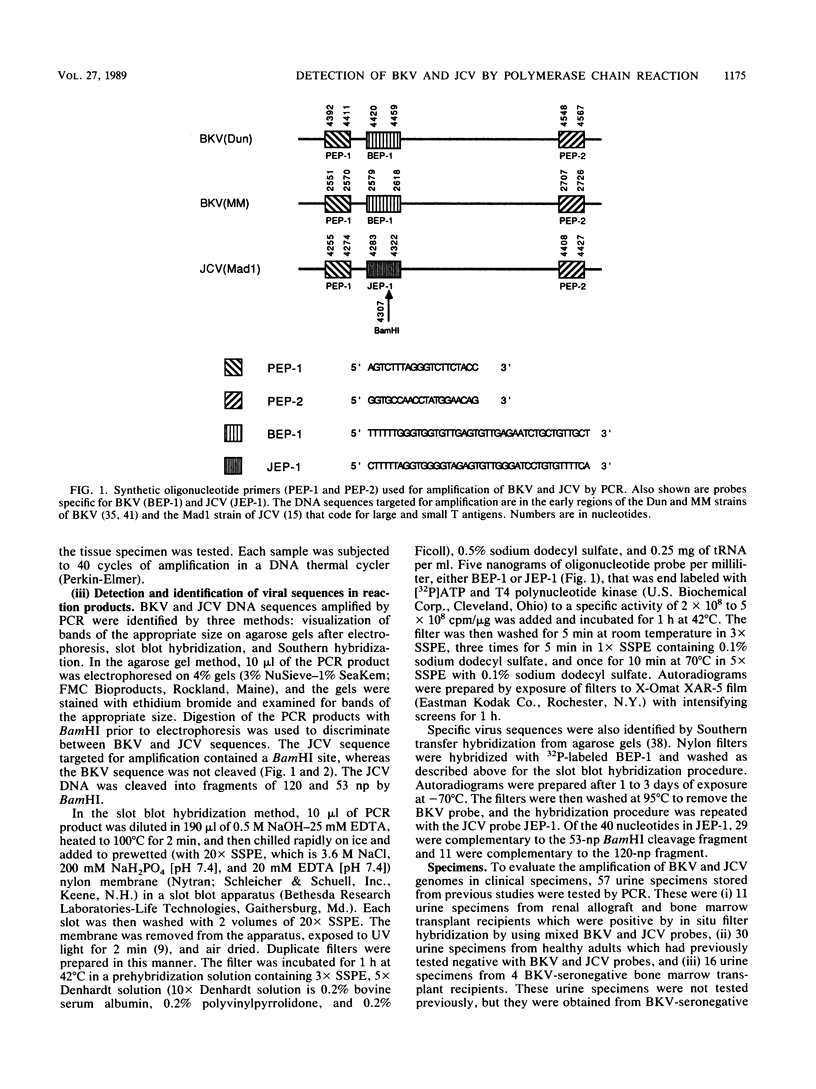
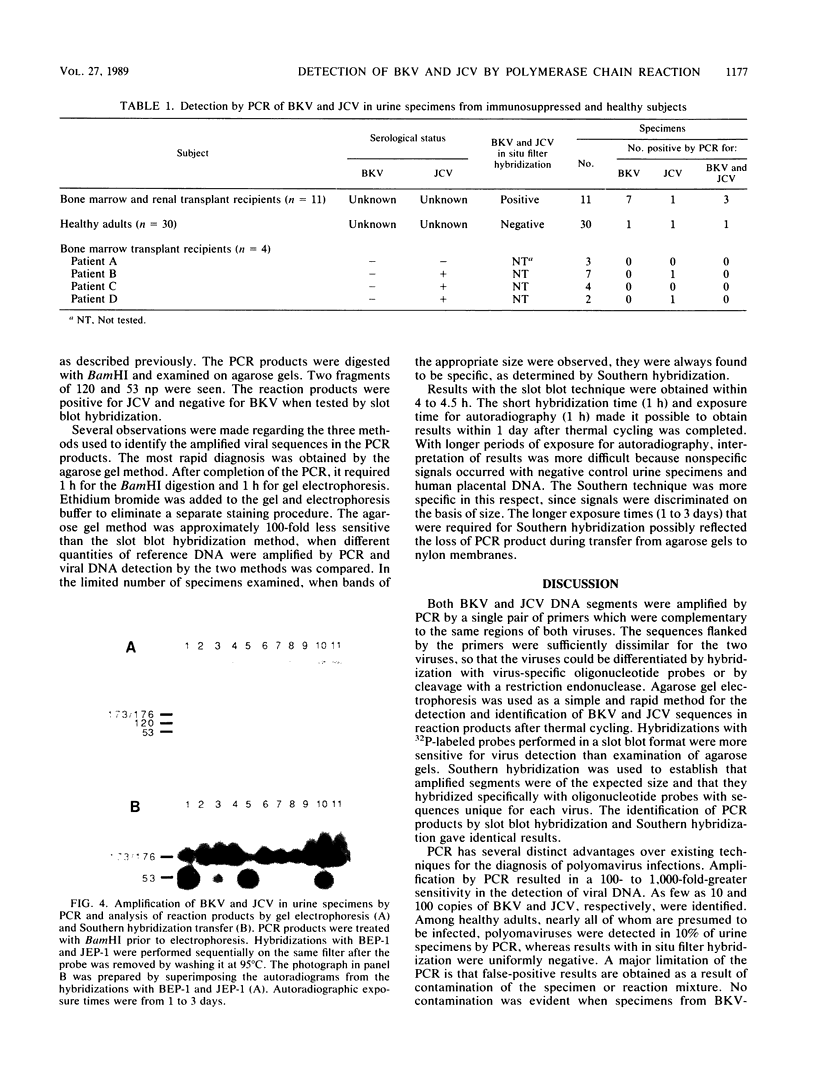
DNAs of the human polyomaviruses BK virus (BKV) and JC virus (JCV) were amplified by the polymerase chain reaction (PCR) by using a single pair of 20-base oligonucleotide primers that were complementary to the same regions of both viruses. The sequences flanked by the primers were unique for each virus and could be differentiated by hybridization with 40-base, 32P-labeled oligonucleotide probes or by cleavage with BamHI. The DNA fragments resulting from amplification of BKV and JCV were 176 and 173 nucleotide pairs, respectively. The sensitivities of PCR for amplification of cloned BKV and JCV DNAs were 10 and 100 copies, respectively. Hybridization with the oligonucleotide probes was specific for each virus. A total of 57 urine samples from three groups of subjects were processed by DNA extraction or boiling and were tested by PCR. Urine samples collected from immunosuppressed patients (n = 11) and previously documented to be positive for BKV, JCV, or both were positive by PCR. Ten percent of urine samples from healthy adults (n = 30) that were previously negative for BKV and JCV were positive for one or both viruses by PCR. Urine samples (n = 16) from four seronegative bone marrow transplant recipients were uniformly negative for BKV. JCV was detected in deparaffinized brain tissue from a patient with progressive multifocal leukoencephalopathy. Specific diagnosis of virus in clinical specimens could be made within 1 day of receipt of the specimens. The PCR method is attractive for use in diagnosing polyomavirus infections because of its sensitivity, specificity, and rapid turnaround time.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apperley J. F., Rice S. J., Bishop J. A., Chia Y. C., Krausz T., Gardner S. D., Goldman J. M. Late-onset hemorrhagic cystitis associated with urinary excretion of polyomaviruses after bone marrow transplantation. Transplantation. 1987 Jan;43(1):108–112. doi: 10.1097/00007890-198701000-00024. [DOI] [PubMed] [Google Scholar]
- Arthur R. R., Beckmann A. M., Li C. C., Saral R., Shah K. V. Direct detection of the human papovavirus BK in urine of bone marrow transplant recipients: comparison of DNA hybridization with ELISA. J Med Virol. 1985 May;16(1):29–36. doi: 10.1002/jmv.1890160105. [DOI] [PubMed] [Google Scholar]
- Arthur R. R., Shah K. V., Baust S. J., Santos G. W., Saral R. Association of BK viruria with hemorrhagic cystitis in recipients of bone marrow transplants. N Engl J Med. 1986 Jul 24;315(4):230–234. doi: 10.1056/NEJM198607243150405. [DOI] [PubMed] [Google Scholar]
- Arthur R. R., Shah K. V., Charache P., Saral R. BK and JC virus infections in recipients of bone marrow transplants. J Infect Dis. 1988 Sep;158(3):563–569. doi: 10.1093/infdis/158.3.563. [DOI] [PubMed] [Google Scholar]
- Arthur R. R., Shah K. V., Yolken R. H., Charache P. Detection of human papovaviruses BKV and JCV in urines by ELISA. Prog Clin Biol Res. 1983;105:169–176. [PubMed] [Google Scholar]
- Bauer W. R., Turel A. P., Jr, Johnson K. P. Progressive multifocal leukoencephalopathy and cytarabine. Remission with treatment. JAMA. 1973 Oct 8;226(2):174–176. [PubMed] [Google Scholar]
- Brown P., Tsai T., Gajdusek D. C. Seroepidemiology of human papovaviruses. Discovery of virgin populations and some unusual patterns of antibody prevalence among remote peoples of the world. Am J Epidemiol. 1975 Oct;102(4):331–340. doi: 10.1093/oxfordjournals.aje.a112169. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman D. V., Gardner S. D., Field A. M. Human polyomavirus infection in renal allograft recipients. Br Med J. 1973 Aug 18;3(5876):371–375. doi: 10.1136/bmj.3.5876.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman D. V., Mackenzie E. F., Gardner S. D., Poulding J. M., Amer B., Russell W. J. Human polyomavirus (BK) infection and ureteric stenosis in renal allograft recipients. J Clin Pathol. 1978 Apr;31(4):338–347. doi: 10.1136/jcp.31.4.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman D. V., Wolfendale M. R., Daniel R. A., Dhanjal N. K., Gardner S. D., Gibson P. E., Field A. M. A prospective study of human polyomavirus infection in pregnancy. J Infect Dis. 1980 Jul;142(1):1–8. doi: 10.1093/infdis/142.1.1. [DOI] [PubMed] [Google Scholar]
- Corallini A., Pagnani M., Viadana P., Silini E., Mottes M., Milanesi G., Gerna G., Vettor R., Trapella G., Silvani V. Association of BK virus with human brain tumors and tumors of pancreatic islets. Int J Cancer. 1987 Jan 15;39(1):60–67. doi: 10.1002/ijc.2910390111. [DOI] [PubMed] [Google Scholar]
- Dörries K., Loeber G., Meixensberger J. Association of polyomaviruses JC, SV40, and BK with human brain tumors. Virology. 1987 Sep;160(1):268–270. doi: 10.1016/0042-6822(87)90071-7. [DOI] [PubMed] [Google Scholar]
- Frisque R. J., Bream G. L., Cannella M. T. Human polyomavirus JC virus genome. J Virol. 1984 Aug;51(2):458–469. doi: 10.1128/jvi.51.2.458-469.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner S. D., Field A. M., Coleman D. V., Hulme B. New human papovavirus (B.K.) isolated from urine after renal transplantation. Lancet. 1971 Jun 19;1(7712):1253–1257. doi: 10.1016/s0140-6736(71)91776-4. [DOI] [PubMed] [Google Scholar]
- Gardner S. D., MacKenzie E. F., Smith C., Porter A. A. Prospective study of the human polyomaviruses BK and JC and cytomegalovirus in renal transplant recipients. J Clin Pathol. 1984 May;37(5):578–586. doi: 10.1136/jcp.37.5.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber M. A., Shah K. V., Thung S. N., Zu Rhein G. M. Immunohistochemical demonstration of common antigen of polyomaviruses in routine histologic tissue sections of animals and man. Am J Clin Pathol. 1980 Jun;73(6):795–797. [PubMed] [Google Scholar]
- Gibson P. E., Gardner S. D., Porter A. A. Detection of human polyomavirus DNA in urine specimens by hybridot assay. Arch Virol. 1985;84(3-4):233–240. doi: 10.1007/BF01378975. [DOI] [PubMed] [Google Scholar]
- Goudsmit J., Wertheim-van Dillen P., van Strien A., van der Noordaa J. The role of BK virus in acute respiratory tract disease and the presence of BKV DNA in tonsils. J Med Virol. 1982;10(2):91–99. doi: 10.1002/jmv.1890100203. [DOI] [PubMed] [Google Scholar]
- Grinnell B. W., Padgett B. L., Walker D. L. Distribution of nonintegrated DNA from JC papovavirus in organs of patients with progressive multifocal leukoencephalopathy. J Infect Dis. 1983 Apr;147(4):669–675. doi: 10.1093/infdis/147.4.669. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hogan T. F., Borden E. C., McBain J. A., Padgett B. L., Walker D. L. Human polyomavirus infections with JC virus and BK virus in renal transplant patients. Ann Intern Med. 1980 Mar;92(3):373–378. doi: 10.7326/0003-4819-92-3-373. [DOI] [PubMed] [Google Scholar]
- Hogan T. F., Padgett B. L., Walker D. L., Borden E. C., McBain J. A. Rapid detection and identification of JC virus and BK virus in human urine by using immunofluorescence microscopy. J Clin Microbiol. 1980 Feb;11(2):178–183. doi: 10.1128/jcm.11.2.178-183.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecatsas G., Prozesky O. W., van Wyk J., Els H. J. Papova virus in urine after renal transplantation. Nature. 1973 Feb 2;241(5388):343–344. doi: 10.1038/241343a0. [DOI] [PubMed] [Google Scholar]
- Miller J. R., Barrett R. E., Britton C. B., Tapper M. L., Bahr G. S., Bruno P. J., Marquardt M. D., Hays A. P., McMurtry J. G., 3rd, Weissman J. B. Progressive multifocal leukoencephalopathy in a male homosexual with T-cell immune deficiency. N Engl J Med. 1982 Dec 2;307(23):1436–1438. doi: 10.1056/NEJM198212023072307. [DOI] [PubMed] [Google Scholar]
- Mäntyjärvi R. A., Meurman O. H., Vihma L., Berglund B. A human papovavirus (B.K.), biological properties and seroepidemiology. Ann Clin Res. 1973 Oct;5(5):283–287. [PubMed] [Google Scholar]
- O'Reilly R. J., Lee F. K., Grossbard E., Kapoor N., Kirkpatrick D., Dinsmore R., Stutzer C., Shah K. V., Nahmias A. J. Papovavirus excretion following marrow transplantation: incidence and association with hepatic dysfunction. Transplant Proc. 1981 Mar;13(1 Pt 1):262–266. [PubMed] [Google Scholar]
- Padgett B. L., Walker D. L. Prevalence of antibodies in human sera against JC virus, an isolate from a case of progressive multifocal leukoencephalopathy. J Infect Dis. 1973 Apr;127(4):467–470. doi: 10.1093/infdis/127.4.467. [DOI] [PubMed] [Google Scholar]
- Padgett B. L., Walker D. L. Virologic and serologic studies of progressive multifocal leukoencephalopathy. Prog Clin Biol Res. 1983;105:107–117. [PubMed] [Google Scholar]
- Padgett B. L., Walker D. L., ZuRhein G. M., Eckroade R. J., Dessel B. H. Cultivation of papova-like virus from human brain with progressive multifocal leucoencephalopathy. Lancet. 1971 Jun 19;1(7712):1257–1260. doi: 10.1016/s0140-6736(71)91777-6. [DOI] [PubMed] [Google Scholar]
- Rand K. H., Johnson K. P., Rubinstein L. J., Wolinsky J. S., Penney J. B., Walker D. L., Padgett B. L., Merigan T. C. Adenine arabinoside in the treatment of progressive multifocal leukoencephalopathy: use of virus-containing cells in the urine to assess response to therapy. Ann Neurol. 1977 May;1(5):458–462. doi: 10.1002/ana.410010509. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Seif I., Khoury G., Dhar R. The genome of human papovavirus BKV. Cell. 1979 Dec;18(4):963–977. doi: 10.1016/0092-8674(79)90209-5. [DOI] [PubMed] [Google Scholar]
- Shah K. V., Daniel R. W., Warszawski R. M. High prevalence of antibodies to BK virus, an SV40-related papovavirus, in residents of Maryland. J Infect Dis. 1973 Dec;128(6):784–787. doi: 10.1093/infdis/128.6.784. [DOI] [PubMed] [Google Scholar]
- Shibata D. K., Arnheim N., Martin W. J. Detection of human papilloma virus in paraffin-embedded tissue using the polymerase chain reaction. J Exp Med. 1988 Jan 1;167(1):225–230. doi: 10.1084/jem.167.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Traystman M. D., Gupta P. K., Shah K. V., Reissig M., Cowles L. T., Hillis W. D., Frost J. K. Identification of viruses in the urine of renal transplant recipients by cytomorphology. Acta Cytol. 1980 Nov-Dec;24(6):501–510. [PubMed] [Google Scholar]
- Yang R. C., Wu R. BK virus DNA: complete nucleotide sequence of a human tumor virus. Science. 1979 Oct 26;206(4417):456–462. doi: 10.1126/science.228391. [DOI] [PubMed] [Google Scholar]