Abstract
Oxygen has a potent influence on the expression of genes and the activity of physiological and biochemical pathways in bacteria. We have found that oxygen significantly altered virulence-related phenotypic properties of Streptococcus mutans, the primary etiological agent of human dental caries. Transport of glucose, fructose, or mannose by the sugar:phosphotransferase system was significantly enhanced by growth under aerobic conditions, whereas aeration caused an extended lag phase and slower growth of S. mutans in medium containing glucose, fructose, or mannose as the carbohydrate source. Aeration resulted in a decrease in the glycolytic rate and enhanced the production of intracellular storage polysaccharides. Although aeration decreased the acid tolerance of S. mutans, aerobically grown cells had higher F-ATPase activity. Aeration altered biofilm architecture but did not change the ability of S. mutans to interact with salivary agglutinin. Growth in air resulted in enhanced cell-associated glucosyltransferase (Gtf) activity at the expense of cell-free Gtf activity. These results demonstrate that S. mutans can dramatically alter its pathogenic potential in response to exposure to oxygen, suggesting that the phenotype of the organism may be highly variable in the human oral cavity depending on the maturity of the dental plaque biofilm.
Streptococcus mutans is an important component of the biofilms on human teeth that are associated with dental caries. The ability of this organism to cause dental caries is dependent on adherence to the tooth surface, formation of biofilms, conversion of a wide variety of carbohydrates to organic acids through glycolysis, and tolerance of low pH (8, 9, 15). Oral biofilms are subjected to considerable fluxes in environmental conditions, including pH, nutrient availability, carbohydrate source, and oxygen tension, all of which appear to influence the composition and biological activities of the microbial populations (8, 15). The ability of oral bacteria to survive and persist in oral biofilms depends on their capacity to respond to environmental changes at genetic, physiological, and biochemical levels. Consistent with this idea, S. mutans has evolved a remarkable capacity to coordinate the expression of a variety of genes in response to environmental factors (8, 9, 15, 20).
It is well known that oxygen is one of the most important environmental factors regulating microbial gene expression and metabolism. The oral cavity is an overtly aerobic environment, and oxygen is utilized by many oral bacteria for respiration and energy generation. Oxygen flows into the mouth from the environment and circulatory system and rapidly diffuses into the biofilms on teeth, which are generally 100 μm deep or less (20). The oxygen concentration in oral biofilms can vary substantially, and thus, the mouth can support the growth of a wide variety of aerobic, facultative, and obligately anaerobic bacteria (13). In particular, cells that initially colonize an oral surface are exposed to higher levels of oxygen than those in mature biofilms, where diffusion of oxygen appears limited.
Recently our laboratory reported that exposure of S. mutans to oxygen inhibits biofilm formation on polystyrene surfaces and induces changes in cell surface protein profiles (2). Subsequent microarray analysis revealed that oxygen modulates the expression of a variety of genes in S. mutans, many of which participate in biofilm formation or contribute to virulence in some other way (3). Importantly, both transcriptional and posttranscriptional events appear to regulate the responses of S. mutans to oxygen (3). The purpose of this study was to evaluate the phenotypic consequences of growth of S. mutans in air, particularly in relation to known virulence attributes of the organism, to begin to establish a more complete understanding of how this pathogen may behave during the development of pathogenic biofilms.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
S. mutans UA159, a serotype c strain, was maintained in brain heart infusion (BHI) medium. To create a deletion of the fructosyltransferase (ftf) gene, 5′ and 3′ flanking regions of the gene were amplified from chromosomal DNA from S. mutans UA159, ligated using BamHI sites designed in the primer set, and cloned into the pGEM-T Easy vector (Promega, Madison, WI). The primers used were ftf-A (GCGGTGAGTTGACGGAAAAG) and ftf-BamHI-B (CCCAAAATTGGATCCTCTTATACAT) for the 5′ flanking region and ftf-BamHI-C (TCATCATGTGGATCCAGAAAAGAAA) and ftf-D (GGGAAATTGGCAAAACAAAGC) for 3′ flanking. The plasmid was digested with BamHI, and a kanamycin cassette (NPKm) released from pALH124 (4) with the same enzyme was inserted for the generation of a nonpolar mutation. The desired mutagenic plasmid was identified by PCR, isolated, and used to transform S. mutans UA159. Transformants were selected on BHI agar containing kanamycin (10 μg/ml), and the double-crossover ftf mutant was confirmed by PCR and sequencing.
For aerobic growth, an overnight culture of S. mutans UA159 was diluted 1:50 into a 250-ml conical flask containing 50 ml of BHI, and cultures were grown on a rotary shaker (150 rpm) at 37°C until late exponential growth (optical density at 600 nm [OD600] = 0.5). For anaerobic growth, cultures were similarly diluted and incubated, but the medium was overlaid with mineral oil (2). For biofilm formation, S. mutans was grown in a semidefined biofilm medium (BM) with either 20 mM glucose (BM-glucose) or 20 mM sucrose (BM-sucrose) as the carbohydrate source (19).
Bacterial adhesion.
Bacterial adhesion to polystyrene surfaces was measured as described previously (23). Briefly, cells from exponential-phase cultures (OD600 = 0.5) were washed two times with Tris-buffered saline (TBS) (pH 7.2) and resuspended to an OD600 of 0.5 (approximately 6 × 107 CFU ml−1). Bacterial cells were incubated with 10 μM SYTO 13 dye (Invitrogen, Carlsbad, CA) for 20 min in the dark. One hundred fifty microliters of stained cells was added to the plate (Microfluor 2 black; Thermo, Milford, MA) and incubated for 3 h in an aerobic atmosphere, with or without an overlay of mineral oil. Wells were then washed three times with TBS, and adherent bacteria were measured using a BioTek microplate scanning spectrophotometer (BioTek Instruments Inc., Winooski, VT) with excitation at 485 nm and emission at 528 nm. All experiments were performed in triplicate, and each experiment was repeated a minimum of four times.
Growth and biofilm assays.
For growth rate comparisons, tryptone-vitamin (TV) medium (3.5% tryptone, 0.04 μg ml−1 p-aminobenzoic acid, 0.2 μg ml−1 thiamine HCl, 1 μg ml−1 nicotinamide, 0.2 μg ml−1 riboflavin) supplemented with 0.5% glucose, fructose, or mannose was inoculated with 1:100 dilutions of overnight cultures of S. mutans UA159 and incubated at 37°C. The OD600 was manually recorded at routine time intervals or monitored using a Bioscreen C lab system (Helsinki, Finland). The Bioscreen C was set to continuously shake for aeration, and for anaerobic conditions, sterile mineral oil (50 μl per well) was placed on top of the cultures.
Biofilm assays were carried out using polystyrene 96-well (flat-bottom) cell culture clusters (Costar 3595; Corning Inc., Corning, NY), as described elsewhere (30). Overnight cultures of S. mutans UA159 and its derivatives were transferred to prewarmed BHI and grown at 37°C in a 5%-CO2 atmosphere to an OD600 of 0.5. The cultures were then diluted 1:100 in prewarmed BM. Biofilms were allowed to form by incubating the plates on a rotary shaker at 150 rpm in a 37°C warm room, with or without an overlay of mineral oil. The culture medium was then decanted, and the plates were washed twice with 200 μl of sterile distilled water to remove planktonic and loosely bound cells. The adherent bacteria were stained with 50 μl of 0.1% crystal violet for 15 min. After rinsing twice with 200 μl of water, the bound dye was extracted from the stained cells using 200 μl of 99% ethanol. Biofilm formation was then quantified by measuring the absorbance of the solution at 575 nm. All biofilm assays were performed in quadruplicate and repeated five times.
Confocal microscopy.
S. mutans UA159 biofilms for use in confocal microscopy were generated in eight-well Lab-Tek Chamber Permanox slides (Nagle Nunc International, Rochester, NY). Overnight cultures were transferred to fresh BHI broth and allowed to grow to an OD600 of 0.5. The cultures were then diluted 1:100 in BM-glucose or BM-sucrose and incubated at 37°C for 24 h in air or in an anaerobe jar containing an anaerobic gas pack (GasPak EZ, Becton, Sparks, MD). Following 24 h of growth, each well was washed twice with 400 μl of TBS (pH 7.0), stained for 20 min in the dark with 300 μl of TBS containing 10 μM of SYTO 13 fluorescent dye, and washed once with 400 μl of TBS. The chamber walls were gently removed, 120 μl of TBS was deposited on each biofilm, and the chamber slides were covered with a coverslip that was secured with Superglue. Biofilms were examined by a Leica inverted fluorescence microscope with a Yokagowa spinning-disk confocal system (Yakogawa Corporation, Newnan, GA). Images were obtained using a ×60 oil objective. Simulated xyz three-dimensional images were generated using the ImageJ software package (National Institutes of Health, Bethesda, MD). Images were further colorized for display by using Adobe Photoshop.
Enzymatic assays.
To measure sugar transport by the phosphoenolpyruvate:sugar phosphotransferase system (PTS), cells were grown to an OD600 of 0.5 in TV medium supplemented with 0.5% (wt/vol) glucose, fructose, or mannose and assayed, as described previously (17). Briefly, cells were washed twice with 0.1 M sodium-potassium phosphate buffer (pH 7.2) and suspended in 10% of the original volume with the same buffer. The cell suspension was then permeabilized with 50 μl of toluene-acetone (1:9) per ml of cells. Permeabilized cells (10 to 50 μl) were assayed by the method of LeBlanc et al. (17). For F-ATPase assays, cells (OD600 = 0.5) were permeabilized with toluene and incubated with 5 mM ATP in ATPase buffer (6). Samples were removed at various intervals and assayed for inorganic phosphate released from ATP with the Fiske-Subbarow reagent (Sigma, St Louis, MO). Enzyme activities were normalized to total protein content as determined by a bicinchoninic acid assay (Sigma) with bovine serum albumin as the standard.
Oxidative and acid stress assays.
To assess the ability of cells to grow at low pH, overnight cultures of S. mutans UA159 were transferred to prewarmed BHI and grown at 37°C in a 5%-CO2 atmosphere to an OD600 of 0.5. The cells were then diluted in fresh BHI containing 0.001% hydrogen peroxide or 25 mM paraquat (methyl viologen; Sigma), and the impact of the agents on bacterial growth was monitored in a Bioscreen C lab system at 37°C under aerobic or anaerobic conditions, as detailed above. For oxidative killing assays, cells were grown to an OD600 of 0.3, harvested by centrifugation, and resuspended in 5 ml of 0.1 M glycine buffer, pH 7.0. Hydrogen peroxide (Fisher Scientific, Fair Lawn, NJ) was then added to the cell suspension to give a final concentration of 0.2% (vol/vol). A 100-μl sample was obtained immediately before or 30, 60, or 90 min after the addition of hydrogen peroxide. To inactivate hydrogen peroxide, 10 μl of catalase (5 mg/ml; Sigma) was added to samples immediately after collection. The survival rate was determined by plating in triplicate on BHI plates.
To assess the ability of cells to grow at low pH, overnight cultures of S. mutans UA159 were transferred to prewarmed BHI and grown at 37°C in a 5%-CO2 atmosphere to an OD600 of 0.5. Fresh BHI medium that had been acidified to pH 6.0 was inoculated with 1:50 dilutions of cell cultures. Growth was monitored in a Bioscreen C lab system at 37°C under aerobic or anaerobic conditions, as described above. For acid killing experiments, cells from mid-exponential-phase cultures (OD600 = 0.3) were washed once with 0.1 M glycine (pH 7.0) and resuspended in 0.1 M glycine (pH 2.8). Samples were stirred continuously at room temperature, and aliquots of cells were removed at 30, 60, and 90 min. Cells were serially diluted, plated on BHI agar, and incubated at 37°C for 2 days before colonies were counted. Cell viability at each time point was expressed as the percentage of viable cells at time zero.
pH drop experiments.
The glycolytic profile of cells was monitored by a pH drop assay (6). Briefly, cells from exponentially growing cultures (OD600 = 0.5) were washed with 1 culture volume of cold distilled water and resuspended in a solution of 50 mM KCl and 1 mM MgCl2 in 1/10 of the original culture volume. The suspension was titrated with 0.1 M KOH to a pH of 7.2, pH drops were initiated by addition of 55.6 mM glucose, and the pH was recorded over a 90-min period. To assess the capacity of each strain to lower the pH in the absence of exogenous carbohydrates, cells were grown as described above, washed twice with ice-cold water, and resuspended in 5 ml of 50 mM KCl-5 mM MgCl2, and the pH was recorded for 60 min.
Cell surface hydrophobicity of S. mutans strains.
Bacterial cells from exponentially growing cultures (OD600 = 0.5) were washed twice and suspended in PUM buffer (17.4 g of K2HPO4, 7.26 g of KH2PO4, 1.8 g of urea, 0.2 g of MgSO4·7H2O, and distilled water to 1,000 ml; pH 7.1) to an OD600 of 0.8, as described previously (24). Then, 1.8 ml of hexadecane (Sigma) was added to 3.6 ml of the cell suspensions. The mixtures were vortexed for 60 s, and the aqueous phase was allowed to settle for 15 min. The cell density of the aqueous phase was then measured at 600 nm. The percentage of cells partitioned to hexadecane was calculated as [(OD600 before adsorption − OD600 after adsorption)/OD600 before adsorption] × 100.
Interaction of S. mutans with salivary components.
Unstimulated whole saliva (UWS) was collected from healthy volunteers with good oral health, as described previously (1). Salivary agglutinin (SAG) was prepared from S. mutans NG8 by affinity purification (7). To analyze the interactions of S. mutans with salivary preparations, bacterial aggregation and bacterial adhesion assays were performed. For bacterial aggregation assays, cells were grown aerobically or anaerobically to an OD600 of 0.5 in BHI broth, harvested by centrifugation at 4,000 × g for 10 min, washed twice with phosphate-buffered saline (PBS), and resuspended in PBS at an OD600 of 0.6. Nine hundred microliters of bacterial suspension, 5 μl of 0.1 M CaCl2, and 100 μl of SAG, UWS, or PBS were mixed in a test tube, vortexed, and transferred to cuvettes. After the cuvettes were equilibrated for 5 min at room temperature, the OD600 of the samples was recorded at 10-min intervals at 37°C for 120 min in a spectrophotometer equipped with a temperature-controlled multicuvette positioner. The percentage of aggregation (percent decrease in OD600) was calculated as follows: [(OD600 at 0 min − OD600 at 120 min)/(OD600 at 0 min)] × 100.
For bacterial adhesion assays, aerobically or anaerobically grown cells were harvested at an OD600 of 0.5, washed, and stained with SYTO 13, as described above. Adhesion assays were performed in two different ways: salivary preparations were added to each well with the cell suspensions (fluid-phase salivary preparations), or wells were first coated with salivary preparations before inoculation with cell suspensions (surface-bound salivary preparations). For the experiments with surface-bound salivary preparations, each well was conditioned with 100 μl of SAG, UWS, or PBS by incubating the plates with the salivary preparation at 37°C for 2 h with gentle shaking, followed by three washes with PBS. After air drying for 30 min, 150 μl of the cell suspension was inoculated into the wells. For experiments with fluid-phase salivary preparations, 150 μl of the cell suspension was inoculated into the wells concurrently with 15 μl of SAG, UWS, or PBS. Plates were incubated for 3 h in an aerobic or anaerobic environment and then washed three times with TBS. Adherent bacteria were quantified using a BioTek microplate scanning spectrophotometer (BioTek Instruments Inc.).
Measurement of cell-associated and cell-free Gtf activities.
Glucosyltransferase (Gtf) preparations were obtained from a strain of S. mutans UA159 lacking the fructosyltransferase gene, constructed as detailed above. The rationale for using this mutant strain was that the only remaining enzymatic activities in FTF-deficient strains that can polymerize constituents of sucrose are the Gtf enzymes. The FTF-deficient strain was grown to late exponential phase (OD600 = 0.5), and the culture was centrifuged at 4,000 × g for 10 min. The pH of the supernatant fluid was adjusted to 6.8 by addition of KOH, and phenylmethylsulfonyl fluoride and NaN3 were added to final concentrations of 1.0 and 3.1 mM, respectively. The supernatant fluid was concentrated 50-fold using a Centriprep (Millipore, Billerica, MA) with a 50-kDa-molecular-weight-cutoff membrane. Then, 50 volumes of potassium phosphate buffer (pH 6.8) containing phenylmethylsulfonyl fluoride and NaN3 as above were added, and the mixture was reconcentrated 50-fold. For measuring of cell-associated Gtf activity, cells from exponentially growing cultures (OD600 = 0.5) were washed twice and resuspended in potassium phosphate buffer (pH 6.8). Cells were then homogenized in a bead beater in the presence of glass beads. After removal of cell debris, the supernatant was used for measuring Gtf activity. The ability of cells to synthesize glucans from radiolabeled sucrose was measured, as described elsewhere (26). Briefly, an appropriate amount of the Gtf preparations was incubated in a reaction mixture containing 0.6 μCi per ml of [14C]sucrose (NEC100X; Perkin Elmer, Waltham, MA) for 2 or 4 h at 37°C. The amounts of water-insoluble glucan and methanol-insoluble glucan were determined after capture on glass fiber filters in a vacuum manifold. The amount of radiolabeled glucan was measured by scintillation counting of the material on the filters. The radioactive counts were divided by the total counts per minute of the original [14C]sucrose solution to calculate the percentage of glucose incorporated into polymer. Enzyme activity was normalized to the total protein content as determined by a bicinchoninic acid assay (Sigma) with bovine serum albumin as the standard or to the percent cpm per ml culture fluid to allow for calculation of the proportion of cell-associated or cell-free enzyme activity, respectively.
RESULTS
Oxygen alters growth and biofilm formation by S. mutans.
Compared to the doubling times and growth rates of anaerobically grown cells, aerobically grown cells showed a long lag phase and the growth rate was significantly decreased, regardless of the primary carbohydrate source (Fig. 1). In addition, the final yield of the cultures, as determined by optical density, was significantly lower when cells were cultured under aerobic conditions.
FIG. 1.
Growth curves of S. mutans UA159 grown under aerobic or anaerobic conditions. Growth in TV medium supplemented with glucose (A), fructose (B), or mannose (C) was monitored in a Bioscreen C system that was set to continuously shake (aerobic conditions). For anaerobic conditions, sterile mineral oil was placed on top of the broth culture.
Growth in oxygen impairs biofilm formation on polystyrene surfaces (2). When the architectures of biofilms formed under anaerobic and aerobic conditions was examined using confocal microscopy, cells grown under aerobic conditions did not accumulate and form mature biofilms on polystyrene surfaces compared to those grown in anaerobic conditions, irrespective of the carbohydrate source (Fig. 2A and B). Biofilms formed under anaerobic conditions had an average thickness of 11.7 μm (± 3.1 μm) in BM-glucose and 14.5 μm (± 5.7 μm) in BM-sucrose and were uniformly distributed across the surface. In contrast, biofilms formed under aerobic conditions were thin and sparsely scattered about the surface.
FIG. 2.
Confocal microscopic images of S. mutans UA159 biofilms grown under aerobic or anaerobic conditions. For biofilm formation, S. mutans was grown in BM with either 20 mM glucose (A) or sucrose (B) for 24 h. Cells were stained with SYTO 13 for 20 min. Data presented here are representative of at least three independent experiments.
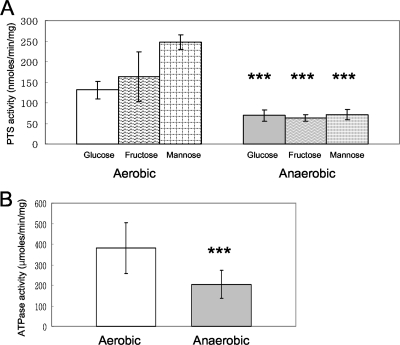
Aeration increases PTS and ATPase activities.
To determine whether sugar transport by PTS permeases was influenced by growth of cells with aeration, glucose-, fructose-, and mannose-specific PTS activities were measured in late-exponential cells grown in glucose, fructose, or mannose. Interestingly, cells cultured with aeration had significantly higher PTS activities, ranging from 2.1- to 4.7-fold higher for all assayed sugars, with the largest differences observed in cells grown in the presence of mannose (Fig. 3A).
FIG. 3.
Sugar transport activity by the PTS (A) or ATPase activity (B) in S. mutans UA159 grown under aerobic or anaerobic conditions. PTS activity is expressed as nanomoles of NADH oxidized in a phosphoenolpyruvate-dependent manner min−1 (mg protein)−1. ATPase activity is expressed as μmoles of released phosphate min−1 (mg protein)−1. Data are expressed as means for at least three independent experiments. Error bars represent standard deviations. ***, P < 0.001, Mann-Whitney U test.
The activities of the H+-translocating ATPase were also compared in aerobically and anaerobically growing cells. Aerobically grown cells had nearly twice the ATPase activity as those grown without aeration (Fig. 3B).
Oxygen exposure alters stress tolerance of S. mutans.
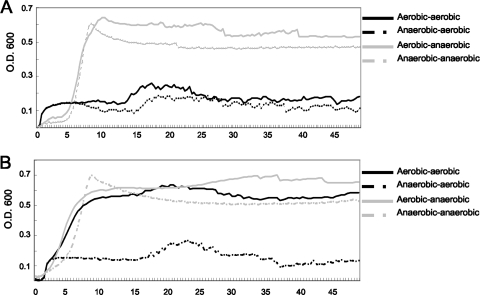
Aeration did not significantly influence oxidative stress tolerance of S. mutans when mid-exponential-phase cells were exposed to H2O2 in killing experiments (Fig. 4A). However, aerated cells had a substantially decreased ability to grow in the presence of hydrogen peroxide or paraquat (Fig. 4B and C). Notably, we did observe that S. mutans showed an enhanced ability to grow in the presence of paraquat when the cells were precultured in an aerobic environment, compared with cells that were precultured anaerobically (Fig. 5).
FIG. 4.
Oxidative stress assays with S. mutans UA159 grown under aerobic or anaerobic conditions. For hydrogen peroxide killing (A), aerobically or anaerobically grown cells were harvested and suspended in BHI containing 0.2% hydrogen peroxide for 30, 60, or 90 min. The growth rate of S. mutans under 0.001% hydrogen peroxide (B) or 25 mM paraquat (C) was monitored using a Bioscreen C system to analyze the ability of cells to withstand oxidative stress under aerobic or anaerobic conditions. Data presented here are representative of at least three independent experiments.
FIG. 5.
Changes in oxidative tolerance in S. mutans UA159 when cells are reexposed to oxygen. Cells were grown under aerobic or anaerobic conditions, diluted into fresh BHI broth containing 0.001% hydrogen peroxide (A) or 25 mM paraquat (B), and regrown under anaerobic (overlaid with mineral oil) or aerobic (without mineral oil) conditions. The growth rate of S. mutans was monitored using a Bioscreen C system (solid black line, aerobically grown cells were diluted and regrown under aerobic conditions; dotted black line, anaerobically grown cells were diluted and regrown under aerobic conditions; solid gray line, aerobically grown cells were diluted and regrown under anaerobic conditions; dotted gray line, anaerobically grown cells were diluted and regrown under anaerobic conditions). Data presented here are representative of at least three independent experiments.
As shown in Fig. 6, aeration significantly increased the susceptibility of S. mutans to acid stress. Acid killing experiments revealed that the survival rate of cells after incubation in buffer at pH 2.8 for 90 min was up to 1 log greater for anaerobically cultured cells than for cells that had been grown with aeration (Fig. 6A). Reduced acid tolerance in aerated cells was also observable as a decrease in the doubling time of the cells at pH 6.0 and by a decrease in the yield of cells, as assessed by comparisons of the final optical density attained by cultures (Fig. 6B).
FIG. 6.
Acid tolerance assays with S. mutans UA159 grown under aerobic or anaerobic conditions. For killing assays (A), aerobically or anaerobically grown cells were exposed to pH 2.8 for 30, 60, or 90 min. The growth rate of S. mutans at pH 6.0 (B) was monitored using a Bioscreen C system under aerobic or anaerobic conditions. Data presented here are representative of at least three independent experiments.
Aeration affects carbohydrate catabolism.
Experiments using pH drop protocols showed that cells grown under anaerobic conditions were able to lower the pH through glycolysis slightly faster than cells grown under aerobic conditions (Fig. 7A). However, the pH achieved after 90 min by the cell suspensions did not differ as a function of the atmosphere in which cells were grown.
FIG. 7.
Glycolytic acidification by S. mutans UA159 grown under aerobic or anaerobic conditions in the presence of added glucose (A) or from endogenous stores (B). Experiments were performed as detailed in Materials and Methods, and the data shown are representative of at least three independent experiments.
The pH drop protocol calls for the cell suspensions to be titrated with 0.1 M KOH to achieve a relatively stable pH of around 7.2 prior to initiating the pH drop by addition of glucose. It was observed that cells grown under aerobic conditions routinely required a larger volume of KOH to achieve a stable neutral pH. Microarray analysis has shown that the genes for the production of intracellular polysaccharide (IPS), a glycogen-like storage polymer, were increased in cells exposed to aeration (3). When pH drops were performed without titration with KOH or addition of glucose, cells grown under aerobic conditions were capable of lowering the pH by more than one full pH unit compared to cells grown under anaerobic conditions (Fig. 7B). Since S. mutans does not produce a capsule, these results demonstrate that the aerobically grown cells have accumulated greater quantities of IPS than cells growing anaerobically. When the amounts of IPS were directly compared between aerobically and anaerobically grown cells by spectrophotometrically measuring polysaccharide-iodine complexes, aerobically grown cells had significantly larger amounts of IPS than those grown anaerobically (see Fig. S1 in the supplemental material).
Effects of oxygen exposure on surface hydrophobicity and adhesion pattern of S. mutans.
We analyzed changes in surface hydrophobicity between aerobically and anaerobically grown cells, because growth in oxygen was reported to induce changes in cell surface biogenesis by S. mutans (2). Aerobically grown cells were more hydrophobic than anaerobically grown cells as assessed in a hexadecane partitioning assay (see Fig. S2 in the supplemental material). Changes in surface hydrophobicity can influence adherence of S. mutans, so the abilities of aerobically and anaerobically grown cells to adhere to polystyrene surfaces were compared. The results demonstrated that aerobically grown cells showed significantly greater adhesion to polystyrene surfaces than anaerobically grown cells (see Fig. S2 in the supplemental material).
Oxygen exposure does not influence interactions of S. mutans with salivary agglutinin.
An important contributor to colonization of the oral cavity by S. mutans is the ability to interact with salivary macromolecules. In particular, the binding of salivary agglutinin is a major mechanism for S. mutans adherence to the tooth surface in the absence of sucrose (7, 10). The potential for interaction of S. mutans with salivary preparations may be altered under aerobic conditions, since it was shown that growth in oxygen alters the surface composition of S. mutans (2). Our results demonstrated that growth in oxygen does not significantly affect the interaction of S. mutans with salivary preparations (Fig. 8). Specifically, aggregation of S. mutans in the presence of UWS and SAG did not differ between aerobically and anaerobically grown cells (Fig. 8A). In terms of the ability of the cells to adhere to polystyrene, surface-adsorbed salivary preparations inhibited cell adhesion to plastic surfaces more than fluid-phase preparations, but there were no significant differences in bacterial adhesion between aerobically and anaerobically grown cells. The amount of biofilm formed was at the low end of detection under aerobic conditions when fluid-phase and adsorbed salivary preparations were utilized (data not shown), so it was not possible to compare the difference in biofilm formation by the salivary preparations between aerobic and anaerobic conditions.
FIG. 8.
Bacterial aggregation (A) or adhesion (B) assay to analyze the interaction of S. mutans with salivary preparations. In bacterial aggregation assays, cell suspensions were mixed with SAG, UWS, or PBS and transferred to cuvettes. The OD600 of the samples was recorded at 10-min intervals at 37°C for 120 min in a spectrophotometer equipped with a temperature-controlled multicuvette positioner. Percentage aggregation (percent decrease in OD600) was calculated as follows: [(OD600 at 0 min − OD600 at 120 min)/(OD600 at 0 min)] × 100. For bacterial adhesion, aerobically or anaerobically grown cells were harvested at an OD600 of 0.5 and stained with SYTO 13. Adhesion assays were performed in two different ways: salivary preparations were added to each well with the cell suspensions (fluid-phase salivary preparations), or wells were first coated with salivary preparations before inoculation with cell suspensions (surface-adsorbed salivary preparations). For the experiments with surface-adsorbed salivary preparations, each well was conditioned with 100 μl of SAG, UWS, or PBS for 2 h, washed, and air dried. For the experiments with fluid-phase salivary preparations, 150 μl of the cell suspension was inoculated into the wells concurrently with 15 μl of SAG, UWS, or PBS. Plates were incubated for 3 h in an aerobic or anaerobic environment and washed, and adherent bacteria were measured using a BioTek microplate scanning spectrophotometer. The data represented herein were from incubation under aerobic conditions, because there was no significant difference in bacterial adhesion to polystyrene wells between aerobic and anaerobic incubation. The error bars represent standard deviations. Data presented here are representative of at least three independent experiments.
Aeration alters distribution of Gtf activity.
Sucrose-dependent adherence is mediated by water-insoluble glucans produced from sucrose by Gtf enzymes (14), which contribute in major ways to adherence and accumulation on tooth surfaces, particularly smooth surfaces (18). When Gtf activities were compared between cell cultures grown under anaerobic or aerobic conditions, aerobically grown cells showed greater cell-associated Gtf activities, but less cell-free Gtf activity was detected in the culture supernatant fluid than was the case for anaerobically grown cells (Fig. 9). No significant difference in protein concentrations of cells or culture supernatants between the two cell populations was noted (data not shown).
FIG. 9.
Results of glucan synthetic activities by an FTF-deficient mutant of strain UA159 grown under aerobic or anaerobic conditions. Cell-associated glucan synthetic activity (A) was measured using [14C]sucrose after homogenization of aerobically or anaerobically grown cells in the presence of glass beads, while glucan synthetic activity of cell culture supernatant fluids (B) was measured after 50-fold concentration. Enzyme activities from cells and supernatants were normalized to the total protein content as determined by a bicinchoninic acid assay with bovine serum albumin as the standard or to percentage cpm per ml culture fluid, respectively. Data presented here are representative of at least three independent experiments.
DISCUSSION
The results presented herein show that exposure to oxygen has a profound effect on phenotypic properties of S. mutans that directly impact the virulence of this pathogen. Clearly, higher concentrations of oxygen do not provide the preferred environment for growth of S. mutans. Growth in air is slower than that under anaerobic conditions, which may arise from the substantial amount of energy needed to maintain proper NAD+/NADH balances in the presence of oxygen. In particular, oxygen removal by S. mutans mainly involves NADH oxidases and NADH peroxidases, which convert oxygen and some of its metabolites to water or hydrogen peroxide using NADH (11, 12). A major mechanism for regeneration of NADH by S. mutans growing under aerated conditions involves the partial tricarboxylic acid (TCA) cycle, identified through genome sequencing (5). Under aerobic conditions, the genes encoding the pyruvate dehydrogenase complex and components of the TCA cycle are transcriptionally activated (3), so carbohydrate that would normally be directed to organic acid production can be shunted into the TCA cycle to regenerate NADH. Consistent with this observation is the finding that acid production from glucose is slower than for cells grown anaerobically (Fig. 7A). Likewise, one would expect the decrease in yield noted above for aerobically cultured cells if carbohydrate was directed away from ATP generation.
Consistent with the increased demands on energy generation in aerated cells, the primary pathways for transport and catabolism of carbohydrates are substantially more active in aerated cells. Our observations of increases in PTS activity in the presence of air are consistent with transcriptional profiling data showing that a variety of genes for carbohydrate uptake systems, including sugar-specific PTS permeases, were substantially upregulated in cells exposed to oxygen (3). Such upregulation could allow the cells to more efficiently scavenge and internalize carbohydrates. Notably, this enhancement in carbohydrate uptake does not translate into more-efficient glycolysis as measured by glycolytic acidification of the environment (Fig. 7A). While the reduced glycolytic rate of aerobically grown S. mutans in part reflects movement of carbon into the TCA cycle, we also observed that the production of glycogen-like intracellular polysaccharide was increased in cells grown in air. These results are consistent with microarray experiments showing upregulation of the glg genes, encoding enzymes for glycogen production, in aerated cells (3). In most bacteria, catabolism of intracellular polysaccharides is highly regulated by transcriptional and allosteric mechanisms, and excess carbohydrate generally stimulates production of IPS and represses catabolism. A more detailed analysis of various mutants lacking glycogen biosynthetic or catabolic enzymes would help to clarify the impact of aeration on observed glycolytic rates. Nevertheless, it is clear that growth in air stimulates IPS accumulation and adversely affects the rate at which cells can produce acid from glucose. In terms of oral biofilm ecology, these results support the idea that in relatively immature biofilms where O2 tension would be highest, S. mutans preferentially moves available glucose into storage polymers. Upregulation of the PTS and glycogen production capacities under these conditions may allow the organism to capture a greater amount of transiently present dietary carbohydrates for catabolism after exogenous sources are depleted. As dental plaque matures, the need to store carbohydrates intracellularly may be diminished because more dietary carbohydrate may be retained in the biofilms.
Aeration significantly decreased the ability of S. mutans grown in the presence of low concentrations of hydrogen peroxide or paraquat (Fig. 4B and C). These findings likely reflect the finite capacity of the organism to cope with oxidative stress; i.e., the challenge of growth with aeration and the presence of oxidative stress agents simply overwhelm the cells. However, the data do point out that the compensation the organisms make to grow well in air does not enhance their ability to grow or cope with hydrogen peroxide or superoxide. Again, this observation may be relevant to the behavior of S. mutans in early biofilms, which competes with other species that are better adapted to growth in air or that produce substantial amounts of hydrogen peroxide (28). Although our microarray data showed that expression of the NADH oxidase and superoxide dismutase (SOD) genes was upregulated under aerobic conditions (3), we found no significant difference in SOD activity in aerated and anaerobic cells when we directly measured the enzyme (data not shown).
In contrast to the results obtained when monitoring growth in the presence of paraquat or hydrogen peroxide, we did note that aerobic growth in the absence of stressors allowed the cells to undergo an adaptation that let them cope with a subsequent challenge with paraquat but not with hydrogen peroxide (Fig. 5). These results indicate that aeration can induce changes that enhance resistance to superoxide ions but do not offer protection from hydrogen peroxide. Given that measurements of SOD did not reveal changes in the levels of the enzymes in the two populations (aerated versus anaerobic), S. mutans must alter other metabolic or structural constituents to cope with the damage done by superoxide. The fact that S. mutans cannot adapt well to the presence of hydrogen peroxide emphasizes the conclusions of previous studies (16, 28), which showed that production of H2O2 by commensal bacteria can be a deterrent to establishment of S. mutans in oral biofilms.
The ability of S. mutans to tolerate acid was significantly reduced by exposure to oxygen (Fig. 6). One possible explanation for these findings is that alterations in cell envelope composition induced by aeration (2) result in diminished tolerance to low pH, perhaps through increased proton permeability. Still, our results indicate that S. mutans cells physiologically adapt to an aerobic environment by enhancing their ability to maintain pH homeostasis. Specifically, previous studies have shown that adaptation to low pH by mutans streptococci can involve upregulation of the membrane-associated F ATPase (6) and/or increased glucose PTS activity (21). Thus, the increased PTS permease and ATPase activities (Fig. 3A and B) seen in aerated cells, coupled with a movement of carbohydrate into the TCA cycle, may signal that anaerobically grown cells are compensating for a diminished capacity to maintain ΔpH. Also consistent with the notion that an important adaptation by S. mutans to growth in air is an enhanced capacity to generate energy, Sheng and Marquis have shown that this organism can use the F ATPase not only to pump protons but also as an ATP synthase, typical of organisms with a complete respiratory chain (27). Others have reported that lactococci and certain streptococci can also use the ATPase as an ATP synthase if provided with constituents of the cytochrome system (29, 31). Whether ATP synthesis via the F1FO ATPase of S. mutans occurs in human dental plaque remains to be determined.
A previous study (22) noted that adaptation to low pH concomitantly conferred cross-protection of S. mutans against oxidative stresses. In contrast, the data presented herein show that aeration of cells does not confer a phenotype similar to what is typically noted for acid adaptation by S. mutans, i.e., increased resistance to acid killing and an increased ability to lower the pH faster and to a greater degree than is the case for unadapted cells. While the microarray data (3) and the data shown in Fig. 5 reveal a genetic and phenotypic adaptation to growth in air, the regulon(s) controlling this adaptation apparently does not overlap completely with that regulating acid adaptation. Since a variety of laboratories are working on molecular mechanisms of acid and oxidative stress tolerance, with a particular focus on DNA binding proteins governing the responses, progress should be made relatively quickly on the underlying basis for the phenotypic observations on stress adaptation.
Enhanced abilities to adhere to the tooth surface should offer S. mutans an advantage in becoming established in dental plaque. Exposure to aeration clearly increased surface hydrophobicity, which might be predicted to enhance the ability of the organisms to adhere to the tooth (25). Aerobically grown cells could adhere to polystyrene surfaces better than anaerobically grown cells (see Fig. S2 in the supplemental material), but aeration did not significantly influence interaction of S. mutans with salivary preparations (Fig. 8). Although marked surface remodeling due to cultivation in the presence of oxygen was noted in previous work, there were no apparent changes in the expression of spaP, which encodes the P1 adhesin that binds to salivary agglutinin, and changes in P1 levels or localization were not seen in aerated cells (2, 3). Consistent with our previous findings, the ability of S. mutans to form biofilms was severely impaired by oxygen exposure. As noted previously, the lack of efficient biofilm formation in air may be due to alterations in cell surface biogenesis and composition caused by aeration (2), and these changes may be mediated, in large part, by the AtlA autolysin. Also, as previously noted (2), the presence of salivary preparations did not allow S. mutans to overcome the inhibitory effect of oxygen on biofilm formation (data not shown). One might predict that a strategy for S. mutans to enhance colonization might be to upregulate its ability to bind to a saliva-coated surface, but that does not appear to be the case, at least under the conditions used here. However, further analysis of interactions of S. mutans with salivary components under aerobic conditions is needed to determine if other adhesins may be affected by growth in air.
In contrast to the adherence results, the data presented here do support that a strategy used by S. mutans to become established in early biofilms involves enhancing the capacity to produce cell-associated, water-insoluble glucans. Localization of the Gtf enzymes of S. mutans is different in cells exposed to air and cells grown anaerobically (Fig. 9). Consistent with a previous report from our laboratory that showed aerobically grown cells had a greater amount of GtfC associated with the cell surface than anaerobically grown cells (3), total Gtf activity was enhanced in the cell-associated fractions of aerobically cultivated cells, at the expense of cell-free Gtf activity. Whether this change in localization reflects a nonspecific increase in affinity of the Gtfs for the cell surface induced by growth in air, a specific interaction mediated by glucan binding proteins, or a mechanism involving as yet unknown proteins that specifically recruit Gtf to the cell, the finding supports that cells that are in immature biofilms may have an enhanced capacity to produce cell-associated glucans. Such a strategy could strengthen the association of the organisms with enamel pellicle and aid in the recruitment of S. mutans via glucan-mediated attachment (26). Gtfs have also been found in early enamel pellicle, so enhanced binding of Gtfs at the cell surface, such that the cells would use Gtf as a receptor in pellicle, may be yet another strategy to colonize the tooth. Given the overall inhibitory effect of oxygen on biofilm formation, one could interpret these findings collectively to argue that the major route of the organisms in immature plaque to establish biofilms is to produce an adhesive, glucan-rich matrix as quickly as possible so as to compete effectively with other organisms that are more adept at formation of tooth biofilms.
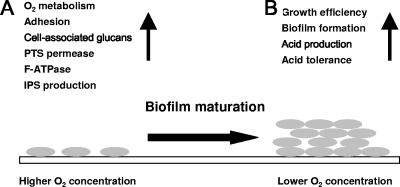
Summary.
The present study indicates that S. mutans in immature biofilms may display behaviors much different from those in mature biofilms, where oxygen concentrations are lower. At elevated oxygen concentrations, adhesion capacity and cell-associated glucan production may be enhanced, which would promote initial colonization on teeth and recruit additional mutans streptococci to early biofilms by glucan-mediated accumulation, respectively (Fig. 10A). During this early phase, increases in PTS and ATPase activities could optimize energy production by the cells as they are required to devote more energy to maintenance. Concurrently, IPS stores would be increased to assist in optimal retention of carbohydrates from the environment. Then, as biofilms mature, oxygen tension and redox potential are reduced, allowing S. mutans to transition into a more efficient growth state that improves biofilm formation and enhances acidogenesis and acid tolerance (Fig. 10B). Collectively, the results support that strategies to perturb acquisition of S. mutans may need to be considerably different from those designed to eradicate S. mutans from established biofilms.
FIG. 10.
Changes in phenotypic and biochemical properties of S. mutans during biofilm maturation. S. mutans cells dramatically change core physiologic properties according to oxygen levels. Cells in immature biofilms (A) may show greater oxygen metabolism, adhesion capacity, PTS permease activity, F-ATPase activity, and intracellular polysaccharide storage to survive and persist in a higher oxygen concentration. As biofilms mature (B), redox potential and oxygen levels fall, and cells display more-efficient growth, improved biofilm formation, and enhanced acidogenic and acid tolerance properties.
Supplementary Material
Acknowledgments
This study was supported by grant no. DE13239 from the National Institute of Dental and Craniofacial Research.
Footnotes
Published ahead of print on 27 February 2009.
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Ahn, S.-J., S.-J. Ahn, Z. T. Wen, L. J. Brady, and R. A. Burne. 2008. Characteristics of biofilm formation by Streptococcus mutans in the presence of saliva. Infect. Immun. 76:4259-4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahn, S.-J., and R. A. Burne. 2007. Effects of oxygen on biofilm formation and the AtlA autolysin of Streptococcus mutans. J. Bacteriol. 189:6293-6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahn, S.-J., Z. T. Wen, and R. A. Burne. 2007. Effects of oxygen on virulence traits of Streptococcus mutans. J. Bacteriol. 189:8519-8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahn, S.-J., Z. T. Wen, and R. A. Burne. 2006. Multilevel control of competence development and stress tolerance in Streptococcus mutans UA159. Infect. Immun. 74:1631-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ajdic, D., W. M. McShan, R. E. McLaughlin, G. Savic, J. Chang, M. B. Carson, C. Primeaux, R. Tian, S. Kenton, H. Jia, S. Lin, Y. Qian, S. Li, H. Zhu, F. Najar, H. Lai, J. White, B. A. Roe, and J. J. Ferretti. 2002. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc. Natl. Acad. Sci. USA 99:14434-14439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belli, W. A., and R. E. Marquis. 1991. Adaptation of Streptococcus mutans and Enterococcus hirae to acid stress in continuous culture. Appl. Environ. Microbiol. 57:1134-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brady, L. J., D. A. Piacentini, P. J. Crowley, P. C. Oyston, and A. S. Bleiweis. 1992. Differentiation of salivary agglutinin-mediated adherence and aggregation of mutans streptococci by use of monoclonal antibodies against the major surface adhesin P1. Infect. Immun. 60:1008-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burne, R. A. 1998. Oral streptococci. Products of their environment. J. Dent. Res. 77:445-452. [DOI] [PubMed] [Google Scholar]
- 9.Burne, R. A., R. G. Quivey, Jr., and R. E. Marquis. 1999. Physiologic homeostasis and stress responses in oral biofilms. Methods Enzymol. 310:441-460. [DOI] [PubMed] [Google Scholar]
- 10.Crowley, P. J., L. J. Brady, S. M. Michalek, and A. S. Bleiweis. 1999. Virulence of a spaP mutant of Streptococcus mutans in a gnotobiotic rat model. Infect. Immun. 67:1201-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higuchi, M., M. Shimada, Y. Yamamoto, T. Hayashi, T. Koga, and Y. Kamio. 1993. Identification of two distinct NADH oxidases corresponding to H2O2-forming oxidase and H2O-forming oxidase induced in Streptococcus mutans. J. Gen. Microbiol. 139:2343-2351. [DOI] [PubMed] [Google Scholar]
- 12.Higuchi, M., Y. Yamamoto, L. B. Poole, M. Shimada, Y. Sato, N. Takahashi, and Y. Kamio. 1999. Functions of two types of NADH oxidases in energy metabolism and oxidative stress of Streptococcus mutans. J. Bacteriol. 181:5940-5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kenney, E. B., and M. M. Ash, Jr. 1969. Oxidation reduction potential of developing plaque, periodontal pockets and gingival sulci. J. Periodontol. 40:630-633. [DOI] [PubMed] [Google Scholar]
- 14.Koga, T., H. Asakawa, N. Okahashi, and S. Hamada. 1986. Sucrose-dependent cell adherence and cariogenicity of serotype c Streptococcus mutans. J. Gen. Microbiol. 132:2873-2883. [DOI] [PubMed] [Google Scholar]
- 15.Kolenbrander, P. E. 2000. Oral microbial communities: biofilms, interactions, and genetic systems. Annu. Rev. Microbiol. 54:413-437. [DOI] [PubMed] [Google Scholar]
- 16.Kreth, J., J. Merritt, W. Shi, and F. Qi. 2005. Competition and coexistence between Streptococcus mutans and Streptococcus sanguinis in the dental biofilm. J. Bacteriol. 187:7193-7203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.LeBlanc, D. J., V. L. Crow, L. N. Lee, and C. F. Garon. 1979. Influence of the lactose plasmid on the metabolism of galactose by Streptococcus lactis. J. Bacteriol. 137:878-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loesche, W. J. 1986. Role of Streptococcus mutans in human dental decay. Microbiol. Rev. 50:353-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loo, C. Y., D. A. Corliss, and N. Ganeshkumar. 2000. Streptococcus gordonii biofilm formation: identification of genes that code for biofilm phenotypes. J. Bacteriol. 182:1374-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marquis, R. E. 1995. Oxygen metabolism, oxidative stress and acid-base physiology of dental plaque biofilms. J. Ind. Microbiol. 15:198-207. [DOI] [PubMed] [Google Scholar]
- 21.Nascimento, M. M., J. A. Lemos, J. Abranches, R. B. Goncalves, and R. A. Burne. 2004. Adaptive acid tolerance response of Streptococcus sobrinus. J. Bacteriol. 186:6383-6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quivey, R. G., Jr., R. C. Faustoferri, K. A. Clancy, and R. E. Marquis. 1995. Acid adaptation in Streptococcus mutans UA159 alleviates sensitization to environmental stress due to RecA deficiency. FEMS Microbiol. Lett. 126:257-261. [DOI] [PubMed] [Google Scholar]
- 23.Roche, F. M., R. Downer, F. Keane, P. Speziale, P. W. Park, and T. J. Foster. 2004. The N-terminal A domain of fibronectin-binding proteins A and B promotes adhesion of Staphylococcus aureus to elastin. J. Biol. Chem. 279:38433-38440. [DOI] [PubMed] [Google Scholar]
- 24.Rosenberg, M., A. Perry, E. A. Bayer, D. L. Gutnick, E. Rosenberg, and I. Ofek. 1981. Adherence of Acinetobacter calcoaceticus RAG-1 to human epithelial cells and to hexadecane. Infect. Immun. 33:29-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Satou, N., J. Satou, H. Shintani, and K. Okuda. 1988. Adherence of streptococci to surface-modified glass. J. Gen. Microbiol. 134:1299-1305. [DOI] [PubMed] [Google Scholar]
- 26.Schilling, K. M., and W. H. Bowen. 1992. Glucans synthesized in situ in experimental salivary pellicle function as specific binding sites for Streptococcus mutans. Infect. Immun. 60:284-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheng, J., and R. E. Marquis. 2006. Enhanced acid resistance of oral streptococci at lethal pH values associated with acid-tolerant catabolism and with ATP synthase activity. FEMS Microbiol. Lett. 262:93-98. [DOI] [PubMed] [Google Scholar]
- 28.Tong, H., W. Chen, J. Merritt, F. Qi, W. Shi, and X. Dong. 2007. Streptococcus oligofermentans inhibits Streptococcus mutans through conversion of lactic acid into inhibitory H2O2: a possible counteroffensive strategy for interspecies competition. Mol. Microbiol. 63:872-880. [DOI] [PubMed] [Google Scholar]
- 29.Vido, K., H. Diemer, A. Van Dorsselaer, E. Leize, V. Juillard, A. Gruss, and P. Gaudu. 2005. Roles of thioredoxin reductase during the aerobic life of Lactococcus lactis. J. Bacteriol. 187:601-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wen, Z. T., and R. A. Burne. 2002. Functional genomics approach to identifying genes required for biofilm development by Streptococcus mutans. Appl. Environ. Microbiol. 68:1196-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamamoto, Y., C. Poyart, P. Trieu-Cuot, G. Lamberet, A. Gruss, and P. Gaudu. 2005. Respiration metabolism of Group B Streptococcus is activated by environmental haem and quinone and contributes to virulence. Mol. Microbiol. 56:525-534. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.