Abstract
The biological cycle of Nosema spp. in honeybees depends on temperature. When expressed as total spore counts per day after infection, the biotic potentials of Nosema apis and N. ceranae at 33°C were similar, but a higher proportion of immature stages of N. ceranae than of N. apis were seen. At 25 and 37°C, the biotic potential of N. ceranae was higher than that of N. apis. The better adaptation of N. ceranae to complete its endogenous cycle at different temperatures clearly supports the observation of the different epidemiological patterns.
Biotic potential represents the maximum reproductive capacity of a population under optimum environmental conditions. Thus, a species fulfilling its biotic potential would exhibit maximal exponential population growth, thereby augmenting the possibilities of transmission of the species. A wide range of factors affects the biotic potential of each species, and among the external factors, temperature clearly influences the life cycles of most parasitic species (4).
Nosemosis is a common worldwide disease of adult honeybees (Apis mellifera) that is caused by microsporidia (19). Nosema apis was the only agent known to produce this disease in A. mellifera until N. ceranae was identified in this host in 2005, in Europe (11) and Taiwan (12). Both these microsporidia infect and multiply in the ventricular cells of A. mellifera, and they can be found under different environmental conditions in both the northern and southern hemispheres (17, 13). Significantly, N. ceranae seems to be more pathogenic than N. apis in caged worker bees (10, 18), and it has recently been related to significant losses of bees and colony collapses under field conditions (17, 8).
Due to the lack of comparative studies of the factors affecting parasite virulence, trials were designed to determine the influence of temperature on the biotic potentials of both microsporidia. Only the deleterious effect of high exogenous temperature on spores of N. apis has been checked previously (16).
In this work, purified N. apis and N. ceranae spores with a minimum viability of 99% (tested with 4% trypan blue) were obtained from experimentally infected honeybees always maintained at 33°C as described previously (9). The spores were counted using a hemocytometer chamber (19), while the Nosema species identification was confirmed by PCR (17).
The experimental infection of bees was carried out as described previously (10). Briefly, young Nosema-free honeybees were starved for 2 h and fed 2 μl of 50% sucrose solution containing 100,000 viable N. ceranae or N. apis spores. Honeybees were anesthetized with CO2, and later, a droplet of the spore solution was administered to each honeybee by touching a micropipette to its mouthparts until the entire droplet was consumed. The bees that did not consume the entire droplet were discarded. Uninfected control bees were fed 2 μl of 50% sucrose solution alone.
Three trials were carried out for 1 week each at three different temperatures (25, 33, and 37°C). Each trial included four replicate cages of 30 N. apis-infected honeybees, four replicate cages of 30 N. ceranae-infected honeybees, and four replicate cages of 30 uninfected honeybees. Three different refrigerated incubators (Memmert) were used for N. ceranae- or N. apis-infected bees and controls.
On days 1, 2, 3, 4, and 7 postinfection (p.i.), five living honeybees were removed from each of three cages (n = 15) in each incubator. Spore detection and counting were performed with each bee. To obtain the Nosema spore counts, the whole abdomen from each bee was placed into a sterile Eppendorf microtube in 200 μl of double-distilled water (PCR grade). After thorough grinding of the abdomen, the spore count for each bee was calculated with a hemocytometer. In addition, PCR was used to confirm the Nosema species identification. The biotic index was calculated as the total N. apis or N. ceranae spore count per day after infection.
Finally, on the same days p.i., a histopathological study was performed with two honeybees from the fourth cage in each incubator. The ventriculus of each bee, with the Malpighian tubules attached, was processed as described previously (10).
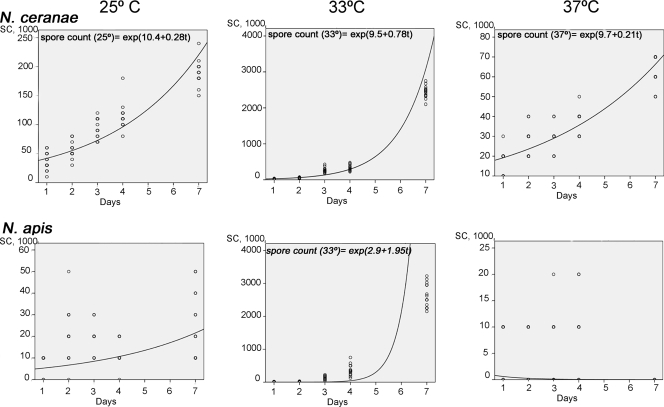
The daily spore count per bee and its relationship to the temperature were established with a nonparametric Mann-Whitney test for each Nosema species (level of significance, α = 0.05). Two curvilinear regression models for each temperature were established. The growth curves fitted predict the increases of the N. ceranae and N. apis spore counts over time (days p.i.), and the slopes and intercepts of the regression lines could be compared. SPSS (version 15.0) software was used to perform the tests and regression analysis.
Throughout the study, Nosema infection was not evident in any of the control bees (Table 1), while N. ceranae was detected in all the bees (100%) exposed to this microsporidium. The proportion of the bees exposed to N. apis in which N. apis infection was detected varied during the period studied (0 to 100%).
TABLE 1.
Percentages of infected honey bees in the different test groupsa
| Group | Temp (°C) | % of infected bees on p.i. day:
|
||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 7 | ||
| N. apis-exposed bees | 25 | 33 | 53 | 100 | 100 | 100 |
| 33 | 66 | 93 | 100 | 100 | 100 | |
| 37 | 53 | 60 | 60 | 60 | 0 | |
| N. ceranae-exposed bees | 25 | 100 | 100 | 100 | 100 | 100 |
| 33 | 100 | 100 | 100 | 100 | 100 | |
| 37 | 100 | 100 | 100 | 100 | 100 | |
| Controls | 25 | 0 | 0 | 0 | 0 | 0 |
| 33 | 0 | 0 | 0 | 0 | 0 | |
| 37 | 0 | 0 | 0 | 0 | 0 | |
A bee is considered to be infected when at least one spore is observed in the hemocytometer's entire central square millimeter grid (19).
The biotic indices for the two microsporidia at 33°C were similar (P > 0.05; Mann-Whitney U test), and the lowest biotic index was recorded for microsporidia kept at 37°C. The indices for N. ceranae were higher than those for N. apis at 25 and 37°C (P < 0.05; Mann-Whitney U test), and even the N. ceranae spore counts were much higher than the N. apis spore counts at 33°C during the first 3 days after infection (P < 0.05; Mann-Whitney U test). However, this difference between the microsporidia incubated at 33°C was no longer evident by the end of the study period (P > 0.05; Mann-Whitney U test). Nevertheless, N. ceranae adapted to the temperature better than N. apis when the cages were incubated at 25°C, with a spore count more than double that for N. apis on each day of sampling (P < 0.05; Mann-Whitney U test). The spore counts for both microsporidia at 37°C were always lowest, and the spore count for N. ceranae was always higher than that for N. apis at this temperature (P < 0.05; Mann-Whitney U test).
The increase in the biotic index of N. ceranae at different temperatures fits well (R2 > 0.7) with a predictable model (Fig. 1). The biotic index at 33°C was predictably higher than that at any other temperature studied, as the slope (0.78) reflects a higher number of spores at this temperature than at any of the others. In contrast, the biotic index of N. apis does not fit with any model at any temperature (R2, 0.554 for 33°C). The values obtained for N. apis spores were always lower than those predicted.
FIG. 1.
Growth curves. Dependent variable: increasing of N. ceranae and N. apis spore counts (SC). Independent variable: days p.i.
Cells parasitized by N. apis and N. ceranae were observed only in the ventricular epithelium and never in Malpighian tubules or muscular layers.
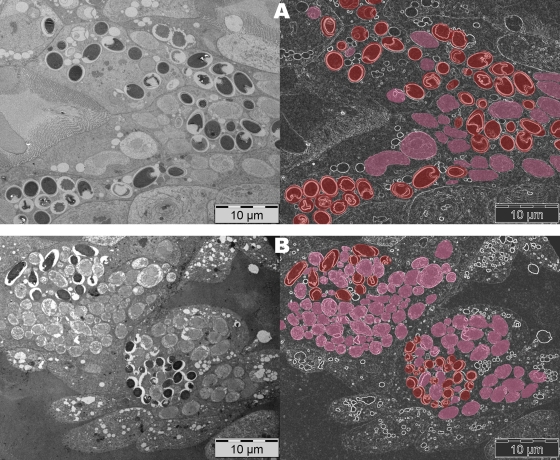
The two microsporidia produced similar morphological changes in the epithelial ventricular cells, as described previously (10), although there was variability in the number of cells infected and in the development of the infection, depending on the temperature. At 33°C, both microsporidia developed very well and parasitized cells could be seen from day 2 p.i. Indeed, empty spores were seen in cells parasitized by either microsporidium, confirming the completion of the endogenous cycle in less than 2 days. During the initial days, no differences in the calculated parasitic infection ratios were detected, although N. ceranae infection affected more cells than N. apis infection on day 4 p.i. (mean ± standard deviation, 76.14% ± 15.5% versus 54.6% ± 14.6%) and day 7 p.i. (83.14% ± 10.7% versus 80.3% ± 12.8%) and there was evidence of a higher proportion of immature stages of N. ceranae (70%) than of mature stages (Fig. 2B). In contrast, N. apis-infected cells displayed similar quantities of immature and mature stages (50% each) at this time (Fig. 2A). This finding may explain why the pathological consequences of infections with the two parasites are not the same, even when similar spore counts are detected. N. ceranae infected more epithelial cells than N. apis, but due to the impossibility of counting immature forms in a hemocytometer, the real number of affected cells in the bees cannot be accurately evaluated by using total spore counts. This approach is unreliable as a tool to evaluate the health status of infected bees, as also shown recently in field trials (8). Thus, a more reliable procedure must be used to include immature stages in these measurements. Since transmission electron microscopy evaluation is expensive and time-consuming and cannot be considered for routine analysis, quantitative real-time PCR may be a useful tool in this sense.
FIG. 2.
Detailed views of ventricular epithelial cells parasitized at 7 days p.i. at 33°C. N. apis-infected cells (A) displayed similar quantities of immature and mature stages (red), while N. ceranae-infected cells (B) exhibited a higher proportion of immature stages (pink) at this time.
N. ceranae infection affects more cells than N. apis infection when honeybees are maintained at the same temperature, and such differences may explain the higher mortality observed when N. ceranae infects A. mellifera than when N. apis is the infecting agent (9, 18).
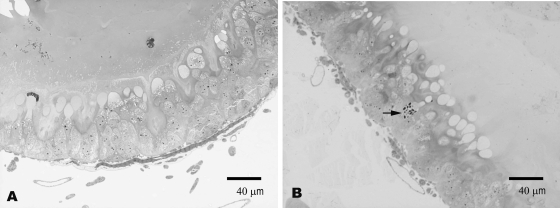
N. ceranae clearly infected bees kept at 25°C by day 2, when infected cells were easily seen in all the visualized fields, while N. apis-infected cells were not seen until day 7, when at least one parasitized cell could be detected in almost all areas (Fig. 3).
FIG. 3.
Ventricular epithelial cells 2 days p.i. at 25°C. In honeybees infected by N. apis, no parasitic forms were detected (A), but different parasitic stages (arrow) in cells from N. ceranae-infected honeybees were seen (B).
The temperature of 37°C was the least favorable for either species, as very few parasitized cells were detected in N. ceranae-infected bees and none were detected in N. apis-infected bees at this temperature.
Nevertheless, the fact that a few infected cells could be found in all the areas studied from day 2 onwards indicated that N. ceranae was at least capable of infecting cells at this temperature. Neither spores nor infected cells in N. apis-infected bees kept at 37°C were detected on day 7 p.i., confirming the data from previous studies with this microsporidium species (3). Indeed, a previous study reported the temperature of 37°C to be the maximal growth temperature for N. apis (15) and also stated that Nosema-infected bees recover when kept at this temperature. Nosema spores counted at 37°C in the previous days may be remanent spores in transit.
The better adaptation of N. ceranae than of N. apis to temperature, enabling it to complete its endogenous cycle with a higher biotic index, is clearly in agreement with the epidemiological differences between these microsporidia. Indeed, while N. ceranae-infected honeybees can be detected in all four seasons (17, 8), N. apis infection is more prevalent in milder seasons such as the spring and autumn (1, 6, 7, 19).
It is generally accepted that the earth's temperature is progressively increasing, and the consequences of this effect on the endogenous and external life cycles of parasites are of concern (2). As described previously for Aethina tumida (14), increasing temperatures due to climate change will promote the extension of the distribution of honeybee pathogens or pests. Changes in climate may affect the distribution, seasonality, and severity of infectious diseases (5), such as nosemosis in honeybees, and the plasticities of species to adapt to new triggering situations will increase the probabilities not only of colonizing but also of consolidating the occupancy of new ecosystems under different environmental conditions.
Acknowledgments
We thank M. L. García and A. Fernández for image processing.
R. Martín-Hernández was cofinanced by the Junta de Comunidades de Castilla-La Mancha (JCCM) and the INIA-European Social Fund. The work reported here was supported by JCCM and Ministerio de Medio Ambiente y Medio Rural y Marino (API/FEGA-FOUNDS).
Footnotes
Published ahead of print on 20 February 2009.
REFERENCES
- 1.Bailey, L. 1955. The epidemiology and control of Nosema disease of the honeybee. Ann. Appl. Biol. 43:379-389. [Google Scholar]
- 2.Brooks, D. R., and E. P. Hoberg. 2007. How will global climate change affect parasite-host assemblages? Trends Parasitol. 23:571-574. [DOI] [PubMed] [Google Scholar]
- 3.Burnside, C. F., and L. Revell. 1948. Observation on Nosema disease of honey bees. J. Econ. Entomol. 41:603-607. [Google Scholar]
- 4.Bush, A. O., J. C. Fernández, G. W. Esch, and J. R. Seed. 2001. Parasitism: the diversity and ecology of animal parasites. Cambridge University Press, Cambridge, United Kingdom.
- 5.De la Rocque, S., J. A. Rioux, and J. Slingenbergh. 2008. Climate change: effects on animal disease systems and implications for surveillance and control. Rev. Sci. Tech. Off. Int. Epizoot. 27:339-354. [PubMed] [Google Scholar]
- 6.Doull, K. M., and J. E. Eckert. 1962. A survey of the incidence of Nosema disease in California. J. Econ. Entomol. 3:313-317. [Google Scholar]
- 7.Dyes, E. G., and C. A. Wilson. 1978. A study of the seasonal variations of Nosema apis Zander of honey bees in Mississippi. Am. Bee J. 118:33-35. [Google Scholar]
- 8.Higes, M., R. Martín-Hernández, C. Botías, E. Garrido-Bailón, A. V. González-Porto, L. Barrios, M. J. del Nozal, J. L. Bernal, J. J. Jiménez, P. García-Palencia, and A. Meana. 2008. How natural infection by Nosema ceranae causes honeybee colony collapse. Environ. Microbiol. 10:2659-2669. [DOI] [PubMed] [Google Scholar]
- 9.Higes, M., R. Martín-Hernández, E. Garrido-Bailón, C. Botías, P. García-Palencia, and A. Meana. 2008. Regurgitated pellets of Merops apiaster as fomites of infective Nosema ceranae (Microsporidia) spores. Environ. Microbiol. 10:1374-1379. [DOI] [PubMed] [Google Scholar]
- 10.Higes, M., P. García-Palencia, R. Martín-Hernández, and A. Meana. 2007. Experimental infection of Apis mellifera honeybees with Nosema ceranae (Microsporidia). J. Invertebr. Pathol. 94:211-217. [DOI] [PubMed] [Google Scholar]
- 11.Higes, M., R. Martín, and A. Meana. 2006. Nosema ceranae, a new microsporidian parasite in honeybees in Europe. J. Invertebr. Pathol. 92:93-95. [DOI] [PubMed] [Google Scholar]
- 12.Huang, W. F., J. H. Jiang, Y. W. Chen, and C. H. Wang. 2007. A Nosema ceranae isolate from the honeybee Apis mellifera. Apidologie 38:30-37. [Google Scholar]
- 13.Klee, J., A. M. Besana, E. Genersch, S. Gisder, A. Nanetti, D. Q. Tam, T. X. Chinh, F. Puerta, J. M. Ruz, P. Kryger, D. Message, F. Hatjina, S. Korpela, I. Fries, and R. J. Paxton. 2007. Widespread dispersal of the microsporidian Nosema ceranae, an emergent pathogen of the western honey bee, Apis mellifera. J. Invertebr. Pathol. 96:1-10. [DOI] [PubMed] [Google Scholar]
- 14.Le Compte, Y., and M. Navajas. 2008. Climate change: impact on honey bee populations and diseases. Rev. Sci. Tech. Off. Int. Epizoot. 27:499-510. [PubMed] [Google Scholar]
- 15.Lotmar, R. 1943. Über den Einfluss der Temperatur auf den Parasiten Nosema apis. Beih. Schweiz. Bienen-Ztg. 1:261-284. [Google Scholar]
- 16.Malone, L. A., H. S. Gatehouse, and E. L. Tregidga. 2001. Effects of time, temperature, and honey on Nosema apis (Microsporidia: Nosematidae), a parasite of the honeybee, Apis mellifera (Hymenoptera: Apidae). J. Invertebr. Pathol. 77:258-268. [DOI] [PubMed] [Google Scholar]
- 17.Martín-Hernández, R., A. Meana, L. Prieto, A. Martínez Salvador, E. Garrido-Bailón, and M. Higes. 2007. Outcome of colonization of Apis mellifera by Nosema ceranae. Appl. Environ. Microbiol. 73:6331-6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paxton, R., J. Klee, S. Korpela, and I. Fries. 2007. Nosema ceranae has infected Apis mellifera in Europe since at least 1998 and may be more virulent than Nosema apis. Apidologie 38:558-565. [Google Scholar]
- 19.World Organisation for Animal Health. January 2008, posting date. Manual of diagnostic tests and vaccines for terrestrial animals (terrestrial manual), 6th ed., p. 410-414. World Organisation for Animal Health, Paris, France. http://www.oie.int/eng/normes/mmanual/2008/pdf/2.02.04_NOSEMOSIS.pdf.