Abstract
Lactococcus lactis phage mutants that are insensitive to the recently characterized abortive infection mechanism AbiV were isolated and analyzed in an effort to elucidate factors involved in the sensitivity to AbiV. Whole-genome sequencing of the phage mutants p2.1 and p2.2 revealed mutations in an orf that is transcribed early, indicating that this orf was responsible for AbiV sensitivity. Sequencing of the homologous regions in the genomes of other AbiV-insensitive mutants derived from p2 and six other lactococcal wild-type phages revealed point mutations in the homologous orf sequences. The orf was named sav (for sensitivity to AbiV), and the encoded polypeptide was named SaV. The purification of a His-tagged SaV polypeptide by gel filtration suggested that the polypeptide formed a dimer in its native form. The overexpression of SaV in L. lactis and Escherichia coli led to a rapid toxic effect. Conserved, evolutionarily related regions in SaV polypeptides of different phage groups are likely to be responsible for the AbiV-sensitive phenotype and the toxicity.
Milk fermentation failure due to bacteriophage (phage) attack of bacterial starter cultures remains a significant risk in the dairy industry (1, 15). Several industrial cheese-making processes, which rely heavily on mesophilic cultures containing Lactococcus lactis strains to drive the fermentation, are particularly at risk for phage attacks (6, 53). Despite the diversity of the lactococcal phage population (24), members of only three genetically distinct phage groups (936, c2, and P335) are responsible for the majority of unsuccessful fermentations (51). As a defense against these phages, lactococci possess a wide variety of natural antiphage barriers, and more than 50 phage resistance systems in L. lactis have already been characterized (55). These antiphage hurdles are grouped into four general mechanisms of action, among which abortive infection (Abi) systems (15, 75) are considered to be the most efficient (51). To date, 23 Abi mechanisms in L. lactis have been characterized (15, 35). The Abi phenotype is often conferred by a single gene, but in a few cases, multiple genes are needed (8, 21, 60, 80). Overall, Abi systems constitute a heterogeneous group of proteins that share limited amino acid identity (38) but act after phage DNA entry and before the release of progeny phages. The consequence of the Abi activity is not only the inhibition of the phage infection but also the death of the infected cell, presumably due to phage-induced deleterious effects on critical cellular functions (76).
The extensive industrial use of lactococci containing phage resistance systems has led to the emergence of phage mutants that are insensitive to the applied antiphage barriers. This evolutionary process generates a need to constantly find new and/or improve existing phage resistance systems in order to keep phage populations under control in these man-made ecological niches (18, 51). To improve the efficiency of an Abi system, it is crucial to know the molecular details by which the system interacts with its target phages. For most of the characterized lactococcal Abi systems, only general effects on the phage life cycle have been revealed. For example, AbiA, AbiF, AbiK, AbiP, and AbiT were shown previously to interfere with DNA replication (8, 27, 31, 34, 36), while AbiB, AbiG, and AbiU affect RNA transcription (17, 21, 61). AbiC limits major capsid protein production (28), whereas AbiE, AbiI, and AbiQ affect phage packaging (30). Recently, it was demonstrated that the presence of AbiZ causes premature lysis of the infected cells (29). Based on the genetic diversity of the lactococcal Abi systems, their modes of action are likely to be diverse. Moreover, it has been inferred that their activities may be modulated depending on the infecting lactococcal phage species (5, 11, 15).
In order to reveal the individual mechanistic model responsible for the resistance phenotype, it is necessary to determine the molecular basis of the mode of action of an Abi system against various phages. From a practical standpoint, the analysis of Abi-insensitive phage mutants offers a good opportunity to identify the targeted phage component(s). By this approach, genes involved in the Abi sensitivity of lactococcal P335-like phages have been identified either by specific point mutations in the genomes of phage escape mutants or by the exchange of genetic material with a prophage (8-10, 25, 26, 43, 52). In the case of virulent 936 phages, Abi sensitivity genes were identified by point mutations in the phage escape mutants (4, 5, 8, 25, 32) or by recombination between two virulent phages (27).
Recently, we isolated and characterized the lactococcal abortive infection system AbiV, which inhibits the lytic cycles of 936 phages after DNA replication (35). Here, we report the identification of a specific gene in the genomes of phages of the 936 group and the c2 group that is involved in sensitivity to AbiV. Moreover, the phage gene product has a strong antimicrobial effect when expressed in L. lactis and Escherichia coli. The homologues of this gene in a wide variety of phages have an evolutionary relationship.
MATERIALS AND METHODS
Bacterial strains, phages, plasmids, and growth conditions.
Bacterial strains, phages, and plasmids used in this study are listed in Table 1. E. coli was grown at 37°C in Luria-Bertani medium (70). L. lactis was grown in M17 medium (77) supplemented with 0.5% glucose. L. lactis strains were grown at 30°C, except for strain JH-32, which was grown at 37°C to maintain the pGhost9::ISS1 insertion (35). In phage infection experiments, 10 mM CaCl2 was added to the medium. When appropriate, antibiotics were added as follows: for E. coli, 100 μg ml−1 of ampicillin, 10 μg ml−1 of chloramphenicol, 25 μg ml−1 of kanamycin, and 10 μg ml−1 of tetracycline; for L. lactis, 5 μg ml−1 of chloramphenicol, 3 μg ml−1 of erythromycin, and 5 μg ml−1 of tetracycline. Phage mutants insensitive to AbiV were isolated from single plaques obtained from a host expressing AbiV and then propagated in liquid medium on the same host. The latter procedure was repeated at least one more time for all isolated phage mutants to ensure a pure isolate.
TABLE 1.
List of bacteria, phages, and plasmids used in the study
| Bacterial strain, phage, or plasmid | Relevant characteristic(s)a | Source or reference |
|---|---|---|
| L. lactis strains | ||
| IL1403 | L. lactis subsp. lactis IL1403; host for phages P008, bIL170, and bIL67 | 7 |
| MB112 | L. lactis subsp. cremoris MG1363 Δupp; host for phages p2, sk1, jj50, and c2 | 50 |
| NZ9000 | L. lactis subsp. cremoris MG1363 pepN::nisRK | 40 |
| JH-20 | MB112(pJH2) Camr Abi+ | 35 |
| JH-22 | IL1403(pJH2) Camr Abi+ | 35 |
| JH-32 | MB112 containing pGhost9::ISS1 insert upstream of abiV on chromosome; Ermr Abi+ | 35 |
| JH-74 | NZ9000(pJH14) Camr | This study |
| JH-76 | NZ9000(pNZ8010) Camr | This study |
| JH-86 | MB112(pJH13, pAJ80) Camr Tetr | This study |
| JH-87 | MB112(pMAP84, pAJ80) Camr Tetr | This study |
| E. coli strains | ||
| MC1000 | Laboratory strain; cloning host | 64 |
| DH5α | Laboratory strain; cloning host | 70 |
| AJ177 | DH5α(pAJ80) Camr | DTUb strain collection |
| M15 | (pREP4) Kmr; used for cloning in pQE-70 His tag vector | Qiagen |
| JH-60 | AJ177(pJH13) Camr Tetr | This study |
| JH-65 | M15(pJH12) Ampr Kmr | This study |
| JH-77 | M15(pQE-70) Ampr Kmr | This study |
| JH-84 | DH5α(pJH15) Tetr | This study |
| JH-88 | AJ177(pJH15) Tetr | This study |
| JH-89 | MC1000(pJH14) Camr | This study |
| Phages | ||
| p2 | Small isometric headed; 936 species | 56 |
| sk1 | Small isometric headed; 936 species | 14 |
| jj50 | Small isometric headed; 936 species | 48 |
| P008 | Small isometric headed; 936 species | 48 |
| bIL170 | Small isometric headed; 936 species | 20 |
| c2 | Prolate headed; c2 species | 45 |
| bIL67 | Prolate headed; c2 species | 73 |
| p2.1 | Small isometric headed; 936 species; carries deletion and point mutation in orf26 | This study |
| p2.2, p2.5, p2.7, p2.8 | Small isometric headed; 936 species; carry nonsense mutation in orf26 | This study |
| p2.4 | Small isometric headed; 936 species; carries point mutation in orf26 start codon | This study |
| p2.3, p2.9, p2.10, p2.11 | Small isometric headed; 936 species; carry point mutation in RBS of orf26 | This study |
| p2.6 | Small isometric headed; 936 species; carries nonsense mutations in orf26 and orf28 | This study |
| sk1.1 | Small isometric headed; 936 species; carries mutation in orf26 start codon | This study |
| jj50.1 | Small isometric headed; 936 species; carries nonsense mutation in orf25 (homologue of p2 orf26) | This study |
| P008.1 | Small isometric headed; 936 species; carries nonsense mutation in orf33 (homologue of p2 orf26) | This study |
| bIL170.1 | Small isometric headed; 936 species; carries nonsense mutation in e24 (homologue of p2 orf26) | This study |
| c2.1 | Prolate headed; c2 species; carries mutation in e11 (homologue of p2 orf26) | This study |
| bIL67.1 | Prolate headed; c2 species; carries mutation in orf8 (homologue of p2 orf26) | This study |
| Plasmids | ||
| pAB223 | Vector containing genetic switch of phage TP901-1 | 12 |
| pAJ80 | pCI372 with cloned CI repressor gene from phage TP901-1 | 46 |
| pJH2 | Shuttle expression vector pLC5 with cloned abiV gene; Camr | 35 |
| pJH12 | pQE-70 with cloned fragment comprising nt 1005 to 1388; Ampr | This study |
| pJH13 | pMAP84 with cloned fragment comprising nt 872 to 1446; Tetr | This study |
| pJH14 | pNZ8010 with cloned fragment comprising nt 872 to 1446; Camr | This study |
| pJH15 | pMAP84 with cloned fragment from p2.1 corresponding to nt 872 to 1446; Camr | This study |
| pMAP84 | pPTPL with cloned PL promoter of phage TP901-1 (Fig. 1); Tetr | This study |
| pNZ8010 | PnisA-gusA cat Camr | 23 |
| pPTPL | Low-copy-number E. coli-L. lactis shuttle and promoter-cloning vector; Tetr | 63 |
| pQE-70 | Cloning vector for C-terminal His tagging of proteins; Ampr | Qiagen |
The fragments referred to are fragments of the sequence determined in this study. Abi+, phage resistance phenotype; Ampr, ampicillin resistance; Camr, chloramphenicol resistance; Ermr, erythromycin resistance; Kmr, kanamycin resistance; Tetr, tetracycline resistance.
DTU, Technical University of Denmark.
Phage assays.
The propagation of phages was performed as described by Emond et al. (31). The determination of titers of phage lysates (42) and efficiencies of plaque formation (EOP) (71) and cross-streaking assays (69) were performed as described previously. One-step growth curve experiments and center-of-infection assays (54) were performed using multiplicities of infection (MOIs) of 0.2 and 0.5, respectively. The efficiency of the center of infection (ECOI) was calculated as the number of phages (expressed as PFU per milliliter) propagated on an infected resistant strain divided by the number of phages (expressed as PFU per milliliter) propagated on an infected sensitive host. The burst size was calculated as reported elsewhere (54).
DNA isolation and manipulations.
Phage DNA was isolated from phage lysates (with minimum titers of 109 PFU ml−1) by using the lambda maxikit (Qiagen) with the following modifications to the manufacturer's recommendations: proteinase K (20 mg ml−1) was added to the cells resuspended in buffer L3, and the suspension was incubated (65°C for 30 min) before the addition of buffer L4. Plasmid DNA from E. coli cells and from L. lactis cells was isolated using the QIAprep Spin miniprep kit according to the recommendations of the manufacturer (Qiagen), with the exceptions that for L. lactis cells, lysozyme (15 mg ml−1) was added to buffer P1 and the lysis solution with the resuspended cells was incubated at 37°C for 30 min before the protocol proceeded. Restriction enzymes and T4 DNA ligase were used according to the instructions of the manufacturer (Roche). L. lactis was subjected to electroporation as described previously (37, 52).
Sequencing.
Phage genome sequencing was performed with an ABI Prism 3700 apparatus from the genomic platform at the research center of the Centre Hospitalier de l'Université Laval. Oligonucleotides previously used for the sequencing of other phage genomes (48) were used to sequence the major part of the phage p2.1 and p2.2 genomes. Other oligonucleotides were designed to complete the genome sequencing of the phage mutants. Sequence data were assembled using the Staden Pregap4 version 1.5 and Vector NTI version 10.3.0 software (Invitrogen). PCR amplicons of the sav genes and the flanking regions in other phage p2 mutants, as well as in other lactococcal phages, were sequenced.
DNA and protein analyses.
Sequence similarity searches were performed using BLAST (2) and PSI-BLAST (3) (http://www.ncbi.nlm.nih.gov/blast/Blast.cgi). Searches for helix, strand, and sheet elements of protein composition, as well as helix-turn-helix motifs, were done using the NPS@ website (http://npsa-pbil.ibcp.fr/). RNA secondary structure determination and the calculation of free energy were performed using the mfold website (http://frontend.bioinfo.rpi.edu/applications/mfold/cgi-bin/rna-form1.cgi). Molecular masses of the investigated proteins were estimated using Protein Calculator (http://www.scripps.edu/∼cdputnam/protcalc.html). Sequence alignment was performed using Multalin (19) (http://npsa-pbil.ibcp.fr/) and ClustalX (78) software. A phylogenetic tree was constructed using TREEVIEW software (67).
Protein purification and gel filtration.
The sav gene of phage p2 (nucleotides [nt] 1005 to 1388 in the sequence determined in this study) was cloned into the His tag vector pQE-70 (Qiagen) to create plasmid pJH12 in E. coli JH-65. The cloning was performed according to the recommendations in The QIAexpressionist (68) by using the promoter and ribosome binding site (RBS) of the vector. All cloned DNA fragments were verified by sequencing. The C-terminally His-tagged SaV was purified and subsequently run on a gel filtration column using the same conditions recently described (35).
Cloning strategies.
A 0.6-kb fragment from phage p2, corresponding to sav and the upstream gene orf27 (nt 872 to 1446 in the sequence determined in this study), was amplified by PCR and successfully cloned into the newly constructed expression vector pMAP84 (generating pJH13) by using the E. coli strain AJ177, in which expression is repressed (see below). The same 0.6-kb fragment was also successfully cloned into the nisin-inducible vector pNZ8010 (23) (generating pJH14). Finally, the mutated orf27-sav region of phage mutant p2.1 was also PCR amplified and successfully cloned into pMAP84 (yielding pJH15).
Construction of the expression vector pMAP84.
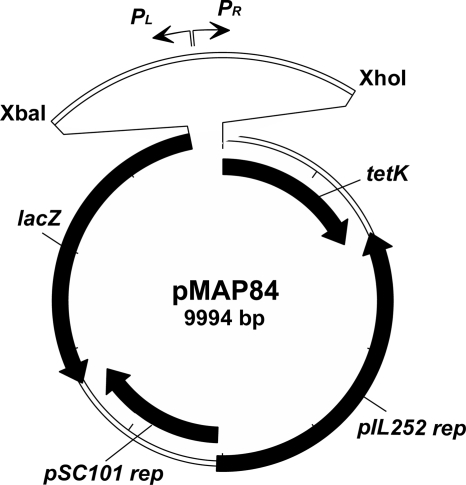
The low-copy-number promoterless shuttle vector pPTPL (63) was used to construct the novel repressible expression vector pMAP84. A DNA fragment encompassing nt 3142 to 3458 in the lactococcal phage TP901-1 (GenBank accession no. NC002747) was amplified by PCR using vector pAB223 as the template (12). This DNA fragment containing the PL promoter region from the genetic switch in TP901-1 (47) was inserted into the multiple-cloning site of pPTPL by using the restriction sites XhoI and XbaI so that the PL promoter points toward the lacZ reporter gene in the vector (Fig. 1). Expression from pMAP84 is repressed when the TP901-1 repressor protein CI is expressed from another vector (pAJ80).
FIG. 1.
Construction of the pMAP84 vector by cloning of the promoter region of the TP901-1 genetic switch into the multiple-cloning site of pPTPL.
Induction experiments.
L. lactis cultures containing either pJH14 or pNZ8010 were grown exponentially to an optical density at 600 nm (OD600) of 0.1, at which point each culture was split into two subcultures. At time zero, nisin (0.25 ng ml−1) was added to one of the subcultures, and samples for the determination of the OD600 and the quantity of surviving cells (in CFU per milliliter) were taken after 5, 35, and 135 min. A similar experiment was performed with E. coli JH-65, which contained sav cloned into the IPTG (isopropyl-β-d-thiogalactopyranoside)-inducible vector pQE-70, and with E. coli JH-55, which had only the pQE-70 vector, as a control. At an OD600 of 0.2 (2 min before time zero), IPTG (0.5 mM) was added, and samples for OD600 measurements and cell counts were taken after 2 min (at time zero) as well as after 30, 90, and 180 min.
Nucleotide sequence accession number.
The sequence determined in this study was submitted to the GenBank database under the accession number FJ010786.
RESULTS
Isolation of phage mutants insensitive to AbiV.
Phage p2 is a small-isometric-headed phage of the 936-like group that propagates on L. lactis subsp. cremoris MG1363. This phage was recently shown to be sensitive to AbiV (35). When propagated on AbiV+ L. lactis cells (strain JH-20), phage p2 forms small plaques (pinpoint size to 0.7 mm, compared to ca 1.6 mm when the phage is propagated on AbiV− cells), with an EOP of 10−4, where 1.0 represents the level of plaque formation by phage p2 on L. lactis subsp. cremoris MG1363 (or MB112) (35). One of these small plaques was picked at random and chosen for further characterization. The phage mutant (named p2.1) was purified on L. lactis JH-20, and its insensitivity to AbiV was confirmed by EOP assays. The above-described isolation procedure was similarly applied to obtain insensitive mutants of the following AbiV-sensitive wild-type phages: sk1 and jj50 (936 species; host is L. lactis MG1363), P008 and bIL170 (936 species; host is L. lactis IL1403), c2 (host is L. lactis MG1363), and bIL67 (c2 species; host is L. lactis IL1403). The AbiV-insensitive phage mutants were named sk1.1, jj50.1, P008.1, bIL170.1, c2.1, and bIL67.1, respectively. Finally, a set of 10 additional insensitive phage p2 mutants (named p2.2 to p2.11) were also isolated using the same procedure.
Microbiological analyses of phage mutant p2.1.
Phage mutant p2.1 was characterized by analyzing EOP, ECOI values, burst sizes, latency times, and plaque sizes on AbiV+ and AbiV− cells. EOP, ECOI values, burst sizes, and latency times on the two hosts were similar (Table 2). Moreover, they were also similar to those previously obtained with the wild-type phage p2 on the sensitive host L. lactis MB112 (35). Nonetheless, the plaque size of phage p2.1 was slightly smaller on AbiV+ cells (0.7 to 1 mm) than on AbiV− cells (1 mm). By comparison, the plaque size of the wild-type phage p2 on AbiV− cells is 1.5 mm. Taken together, these data indicated that the mutation in phage mutant p2.1 rendered the phage insensitive to AbiV with only minor fitness costs.
TABLE 2.
Effects of AbiV on lactococcal phage p2.1
| Host strain (AbiV phenotype) | EOPa | ECOI (%)b | Mean burst size ± SD (PFU/cell)c | Latency time (min)c | Plaque size (mm) |
|---|---|---|---|---|---|
| L. lactis MB112 (AbiV−) | 1.0 | 100.0 | 36.8 ± 4.6 | 20-30 | 1 |
| L. lactis JH-20 (AbiV+) | 1.1 | 94.0 ± 25.3 | 41.1 ± 4.6 | 20-30 | 0.7-1 |
EOP values are relative to the level of phage p2 plaque formation on L. lactis subsp. cremoris MG1363 (or MB112), which is set at 1.0.
MOI = 0.5; n = 3. The value for L. lactis JH-20 is the mean ± the standard deviation. The ECOI for phage p2 on L. lactis subsp. cremoris MG1363 (or MB112) is 100.0%.
MOI = 0.2; n = 3.
A gene that is transcribed early is involved in sensitivity to AbiV.
Genomic DNA was isolated from the phage mutant p2.1 and digested with EcoRV, and the restriction profile was compared to a restriction map of the wild-type phage p2. No difference was observed, indicating that no large deletion or non-reciprocally sized genomic rearrangement was responsible for the AbiV-insensitive phenotype (data not shown). The entire genome was then sequenced and compared to that of the wild-type phage p2. A comparative genome analysis revealed a total of three point mutations as well as a deletion of 56 nt in the p2.1 genome (Fig. 2). All the point mutations and the deletion were located within a 255-bp region of the genome that was previously shown to be transcribed early in the closely related phage sk1 (13, 14). Two point mutations were located in orf26, leading to amino acid changes (A45T and A60D), and one point mutation was located upstream of orf26 (Fig. 2). The deletion covered the 3′ end of orf27, as well as the RBS and the start codon of orf26. The deletion occurred between two GGTA sequences and led to a translational in-frame fusion of orf27 and the downstream orf26 in the phage mutant p2.1 (see below).
FIG. 2.
Localization of mutations in the genome of phage mutant p2.1. Mutations are marked with an asterisk and include three point mutations and a 56-nt deletion which encompasses the RBS and the sav start codon. The RBS and start codon in the wild-type sequence are underlined, and the orf27 stop codon and the sav start codon are in boldface. The deduced SaV amino acid sequence is shown below the nucleotide sequence, while the deduced sequence of Orf27 is shown above.
A second phage p2 mutant (p2.2) was selected, and its entire genome was also sequenced. The sequencing revealed only a point mutation, which was located in orf26 and caused a nonsense mutation in the first position of a glycine codon, resulting in a stop codon (G65→stop). orf26 and its flanking regions in the nine other phage p2 mutants (p2.3 to p2.11) were sequenced. Eight of the mutants had only a single point mutation in either the orf26 gene or its RBS (Table 3). The phage p2.6 mutant had two mutations, one in orf26 and one in orf28.
TABLE 3.
sav homologues in phage mutants insensitive to AbiV
| Phage | Mean EOP ± SDa | Mutant gene(s) | No. of amino acids in gene product | Nucleotide mutation(s)b | Amino acid mutation(s) or effect(s) |
|---|---|---|---|---|---|
| p2.1 | 1.1 ± 0.4 | orf26 | 128 | Deletion of 56 nt, GCT→ACT, GCT→GAT | A45T, A60D |
| p2.2 | 0.9 ± 0.3 | orf26 | 128 | GGA→TGA | G65→stop |
| p2.3 | 0.7 ± 0.4 | orf26 | 128 | RBS mutation: GGATTTGGGGT | |
| p2.4 | 0.9 ± 0.1 | orf26 | 128 | ATG→ATA | M1I |
| p2.5 | 0.9 ± 0.1 | orf26 | 128 | GAA→TAA | E19→stop |
| p2.6 | 1.4 ± 0.4 | orf26, orf28 | 128, 61 | GGA→TGA, GAA→TAA | G65→stop, E30→stop |
| p2.7 | 1.1 ± 0.3 | orf26 | 128 | CAG→TAG | Q37→stop |
| p2.8 | 0.9 ± 0.2 | orf26 | 128 | GAA→TAA | E16→stop |
| p2.9 | 0.8 ± 0.1 | orf26 | 128 | RBS mutation: GGATTGGTGGT | |
| p2.10 | 0.9 ± 0.1 | orf26 | 128 | RBS mutation: GGATTGCGGGT | |
| p2.11 | 0.6 ± 0.3 | orf26 | 128 | RBS mutation: GGATTTGGGGT | |
| sk1.1 | 0.8 ± 0.2 | orf26 | 128 | ATG→ATA | M1I |
| jj50.1 | 0.8 ± 0.2 | orf25 | 128 | GGA→TGA | G65→stop |
| P008.1 | 1.2 ± 0.4 | orf33 | 119 | TGG→TAG | W33→stop |
| bIL170.1 | 1.1 ± 0.2 | e24 | 130 | GAA→TAA | E58→stop |
| c2.1 | 1.0 ± 0.3 | e11 | 122 | ACT→CCT | T48P |
| bIL67.1 | 0.8 ± 0.2 | orf8 | 116 | TAC→TAA | Y10→stop |
EOP values are relative to the level of phage p2 plaque formation on L. lactis subsp. cremoris MG1363 (or MB112), which is set at 1.0.
Mutations in the putative RBS of sav, GGATTGGGGGT, are underlined and shown in bold.
A BLAST analysis of orf26 of phage p2 revealed sequence similarity to the lactococcal phage genes orf25 in jj50 (GenBank accession no. NC008371), orf26 in sk1 (GenBank accession no. NC001835), orf33 in P008 (GenBank accession no. NC008363), and e24 in bIL170 (GenBank accession no. NC001909), as well as e11 in phage c2 (GenBank accession no. NC001706) and orf8 in bIL67 (GenBank accession no. L33769). All these phages containing an orf26 homologue are sensitive to AbiV (35). Interestingly, an orf26 homologue is absent in the genome of the phage 712 (936 species), which is insensitive to AbiV. Similarly, lactococcal phages of the P335 species, which are also insensitive to AbiV, do not contain an orf26 homologue (35).
Since mutations were found in orf26 genes of p2 phage mutants, the corresponding regions in the other isolated phage mutants (jj50.1, sk1.1, P008.1, bIL170.1, c2.1, and bIL67.1) were sequenced and the results were compared to the wild-type sequences. Comparative analyses revealed point mutations in the orf26 homologues of all the phage mutants (Fig. 3). Taking these data together, we concluded that orf26 of phage p2 is involved in sensitivity to AbiV, and the gene was renamed sav (for sensitivity to AbiV).
FIG. 3.
Alignment of amino acid sequences of SaV proteins from phage mutants insensitive to AbiV. Mutations are underlined and shown in boldface; lines through letters indicate deletions, and X indicates a stop codon. Numbering refers to the start of the SaV sequence of p2.
Mutations in SaV.
The point mutations in the sav homologues of all the phage mutants were analyzed for effects on the translated SaV proteins (Fig. 3 and Table 3). Interestingly, almost all the mutations in the phage mutants led to either a stop codon (in p2.2, p2.5, p2.6, p2.7, p2.8, jj50.1, P008.1, bIL170.1, and bIL67.1), a modified RBS site (in p2.3, p2.9, p2.10, and p2.11), or a deleted start codon (in p2.4 and sk1.1). Mutations were mostly in the N-terminal part of SaV, indicating that this region serves an important function for the sensitivity to AbiV. The mutation in phage mutant c2.1 caused a single amino acid change (T48P), most likely leading to a conformational change in the protein. This point mutation is located in a region of the protein that has a high degree of conservation despite the otherwise large differences among the homologues of the c2- and 936-like phages (Fig. 3). The nature of the point mutations suggests that the gene product SaV is involved in AbiV sensitivity.
Analyses of the sav gene.
The sav gene in wild-type phage p2 consists of 384 bp, and its product contains 128 amino acids (aa) (molecular mass of 15.3 kDa). The sav gene marks the end of a putative five-gene operon consisting of orf30 to orf26 (sav). The region shares 97% nucleotide identity with a region (nt 17543 to 18862) found in the genome of lactococcal phage sk1 (GenBank accession no. NC001835) for which transcription analyses (13) are available. Preceding orf30 is a lactococcal consensus promoter, and there are overlaps between orf28, orf27, and sav. Indeed, the start codon of sav overlaps with the stop codon of the upstream gene orf27 (Fig. 2), and the start and stop codons of orf27 and orf28 also overlap, which suggests translational coupling of the gene products. The start codons of all the other sav homologues were overlapping the stop codon of a small upstream gene, except for the orf26 homologue in phage c2, which was separated (by 6 nt) from the upstream gene. The start codon of sav was preceded by a putative RBS (GGATTGGGGGT, where the underlined sequences match the Lactococcus consensus sequence). The RBS of orf27 (ACTTAGGAGGA) and that of orf28 (ACTAAGGAGAA) appeared to be closer to the consensus sequence and may therefore be stronger than the RBS of sav, supporting the possibility of translational coupling. This possibility was further supported by estimated secondary structures of mRNAs covering the RBS regions of the three genes. These structures in orf28, orf27, and sav mRNAs had calculated ΔG values of −6, −9, and −19 kcal/mol, respectively, indicating that sav likely needs the resolution of upstream RNA by ribosomes terminating the translation of upstream orf27.
In lactococcal phage mutant p2.1, a 56-nt deletion (Fig. 2) covered the 3′ end of orf27 (a 117-bp gene encoding a product of 39 aa with a molecular mass of 4.2 kDa), as well as the RBS and the 5′ end of sav. This deletion created an in-frame fusion between orf27 and sav, generating a fused protein of 17.6 kDa.
Analyses and overexpression of the SaV protein.
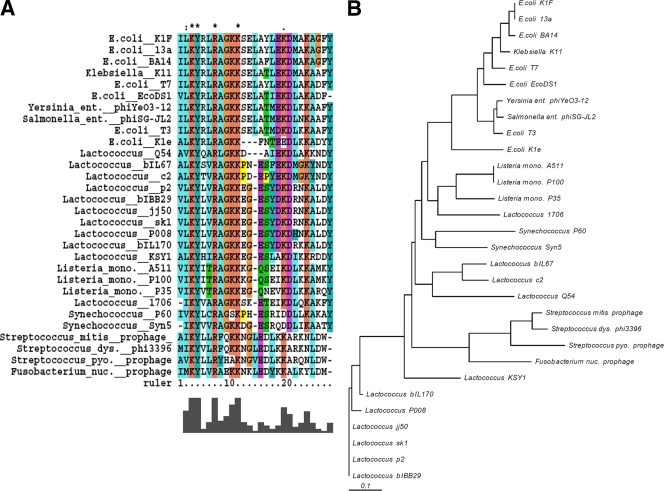
A His-tagged SaV protein (15.3 kDa) from phage p2 was purified after IPTG induction of exponentially growing E. coli cells. The molecular mass of the purified native SaV protein was estimated by gel filtration to be 33 ± 0.7 kDa (data not shown), suggesting that SaV forms a dimer in its native form. No similarity to proteins of known functions and no conserved domains were found in SaV. However, PSI-BLAST searches (five iterations) revealed that the conserved region observed in the SaV homologues in 936-like and c2-like phages (positions 38 to 63) (Fig. 3) is also present in proteins from phages infecting other hosts, such as Klebsiella pneumoniae, E. coli, Yersinia enterocolitica, Salmonella enterica, Listeria monocytogenes, Fusobacterium nucleatum, Streptococcus sp., and Synechococcus sp. (Fig. 4).
FIG. 4.
Alignment of sequences of the conserved region in SaV homologues of different phages by using ClustalX. (A) The bar diagram shows the degrees of conservation. Asterisks indicate fully conserved residues; colons indicate conservation of either M, I, L, or V; and dots indicate conservation of either S, N, D, E, Q, or K. Color coding reflects a consensus of amino acids belonging to the same group. Default settings of ClustalX were used. Yersinia ent., Yersinia enterocolitica; Salmonella ent., Salmonella enterica; Listeria mono., Listeria monocytogenes; Streptococcus dys., Streptococcus dysgalactiae; Streptococcus pyo., Streptococcus pyogenes; Fusobacterium nuc., Fusobacterium nucleatum. (B) Bootstrapped phylogenetic tree based on the conserved region in SaV.
SaV is toxic to L. lactis and E. coli cells.
We investigated whether AbiV efficiency could be decreased or increased by expressing sav in trans. A new vector with regulated gene expression was constructed and named pMAP84 (Fig. 1). In this vector, transcription from the PL promoter can be repressed by the lactococcal phage TP901-1 repressor protein CI, expressed by a separate vector (pAJ80) (47). A PCR fragment encompassing sav, orf27, and the upstream region with the RBS of wild-type phage p2 was ligated into pMAP84, and E. coli DH5α and E. coli AJ177 (containing pAJ80 and expressing the CI repressor) were transformed with the resulting plasmid (named pJH13). No transformants of DH5α were obtained, whereas cloning into E. coli AJ177 (yielding strain JH-60) was successful. To test if pJH13 could exist within the cell without repression by CI (expressed by pAJ80), total plasmid (pJH13 and pAJ80) was extracted from E. coli JH-60 and used to transform E. coli MC1000 and L. lactis MB112, after which the strains were subjected to tetracycline selection (selection for pJH13 only). Fifty E. coli and 100 L. lactis tetracycline-resistant transformants were tested for tetracycline and chloramphenicol resistance. All transformants were resistant to both antibiotics, indicating that cotransformation with pJH13 and pAJ80 had occurred in all cases. We therefore concluded that pJH13 alone is toxic to the cells, probably due to the expression of unrepressed sav.
A cell growth experiment was conducted using lactococcal cells expressing the CI repressor and either pJH13 or the cloning vector pMAP84 alone. The cells containing pJH13 and pAJ80 had a 50% reduced growth rate and a 15% reduced final yield compared to cells with pMAP84 and pAJ80. The same experiment was conducted with E. coli cells harboring a repressed sav gene (strain JH-60) or expressing mutated sav (strain JH-84). Similarly, cells of E. coli JH-60 (SaV+) had a 50% reduced growth rate and a 15% reduced final yield compared to cells of E. coli JH-84 (data not shown). The above-described results strongly indicate that SaV is toxic in both E. coli and L. lactis, even when expressed at low levels.
Further, we wanted to determine if the coexistence of AbiV and repressed SaV in the same cell was possible. Cells expressing AbiV at low (L. lactis JH-32) and high (L. lactis JH-20) levels (35) were transformed with the plasmids pJH13 and pAJ80. PCR analysis of 40 colonies from each transformation showed intact sav but deleted abiV in all transformants. These observations were supported by sequencing and phage resistance tests, which showed that all the transformants had become sensitive to phage p2 (data not shown).
Finally, a cloning experiment was performed in which a DNA fragment encompassing orf27-sav was PCR amplified from phage mutant p2.1 by using the same primer set used for the wild-type cloning. This fragment was successfully cloned into the vector pMAP84 in the E. coli strains AJ177 (carrying pAJ80 with the CI repressor) and DH5α (carrying no repressor), yielding strains JH-88 and JH-84, respectively. The finding that it was possible to clone a mutated orf27-sav fragment in an unrepressed state suggested that the mutations conferring resistance to AbiV also reduce or eliminate SaV toxicity.
Induction of sav causes rapid cell death.
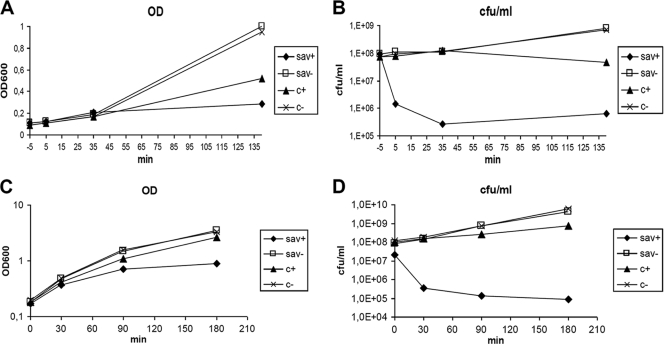
A nisin-inducible expression system was used to time the expression of sav in L. lactis. A PCR-amplified fragment containing orf27-sav and the RBS of wild-type phage p2 was ligated into pNZ8010 (23) (generating pJH14) and cloned into E. coli MC1000 (yielding strain JH-89). The construct was then transferred into L. lactis NZ9000 (resulting in strain JH-74) and used for the nisin induction experiments (22). Due to the toxic properties of nisin, the nisin-induced control reached only 50% of the cell density obtained in the uninduced cultures (Fig. 5A). In the induced sav-expressing cultures, the OD600 rose from 0.1 to 0.2 in the first 30 min, after which no further increase of OD600 was observed. Five minutes after the induction, the number of cells able to form colonies on plates of M17 medium supplemented with 0.5% glucose was reduced by 100-fold. It was reduced another 10-fold after 35 min and ended at 0.5% of the original cell count after 135 min (Fig. 5B).
FIG. 5.
Growth (A and C) and number of surviving cells (B and D) of L. lactis (A and B) or E. coli (C and D) after the induction of sav with nisin or IPTG, respectively. Sav indicates vector pNZ8010 (A and B) or pQE70 (C and D) with cloned sav, while c indicates an empty vector. + and − indicate induction and no induction, respectively, with nisin at time zero and IPTG at 2 min before time zero.
A similar experiment was performed with the IPTG-inducible E. coli clone JH-65 overexpressing C-terminally His-tagged SaV (see above). The IPTG-induced control (Fig. 5C and D) was only marginally inhibited during the experiment. However, the number of surviving SaV+ cells was severely reduced following the induction (Fig. 5D). After 30 min, the cell count was reduced 500-fold, and the cell count ended at a 1,000-fold reduction after 180 min. The above-described results indicate that the expression of sav has a rapid antimicrobial effect.
DISCUSSION
Lactococcal phage mutants that are insensitive to the abortive infection mechanism AbiV can be obtained at a frequency of 10−4. The analysis of these phage mutants was used in the present study to further investigate the mode of action of AbiV. The phage mutants p2.1 and p2.2 were chosen for whole-genome sequencing. Two mutations in orf26 (sav), as well as a 56-nt deletion in a flanking region, were found in the genome of the phage p2.1 mutant, while only a point mutation was found in orf26 of the phage p2.2 mutant. orf26 is presumably the last gene of a five-gene cluster that is transcribed early and shares 97% nucleotide identity with a confirmed five-gene operon (orf30 to orf26) found in phage sk1 (13). This cluster is transcribed from a consensus promoter (81) upstream of orf30 (14), which is likely recognized by host RNA polymerase. Such host-recognized consensus promoters are typically found in the early-transcription regions of lactococcal phages (13).
Analyses of the sav regions in other AbiV-insensitive phage mutants from both the 936 and c2 groups revealed amino acid changes in the central region of the SaV protein, which is highly conserved among SaV homologues (Fig. 3). The point mutation in phage c2.1 (T48P) most likely induces a conformational change in the conserved region of the polypeptide. Furthermore, two point mutations in phage p2.1 led to radical amino acid changes (A45T and A60D), which may also alter the property of the polypeptide due to the changes from neutral alanine (A) to polar (T and D) and charged (D) residues. Although the phage mutant p2.1 also contains a 56-nt deletion which prevents the expression of native SaV, this deletion creates an in-frame translational fusion of N-terminal Orf27 (corresponding to codons 1 to 25) with an almost complete SaV polypeptide (residues 4 to 128), which may not affect the function of SaV. Other mutations likely led to the production of a significantly shorter SaV protein (stop codon mutations) or a protein produced at lower levels (RBS mutations) which conferred insensitivity to AbiV while still allowing the assembly of functional phages.
Analyses of the secondary structures of SaV proteins revealed high (69 to 84%) contents of α-helixes in the investigated 936 and c2 phages. The distributions of α-helixes (interspersed with random coils) in the investigated phages were remarkably similar, despite the lack of sequence similarity (data not shown). Interestingly, when PSI-BLAST was used to search for more distant SaV homologues, similarities to deduced protein sequences from phages infecting diverse bacterial hosts (gram-positive and gram-negative bacteria and cyanobacteria) were found. The similarities were observed primarily in the conserved region, and an alignment of the sequences of this region revealed a high degree of conservation of specific residues (Fig. 4A). A phylogenetic analysis suggested that this SaV-like region has evolved from a common ancestor (Fig. 4B). No putative function could be assigned to SaV, though the conservation of the middle region of SaV suggests a common function in a wide range of phages.
The start codon of sav overlaps with the stop codon of the upstream gene orf27 (Fig. 2). Such out-of-phase overlapping genes are often observed in phages, which are known to have compact genomes (59, 72). Terminal overlap may cause translational coupling (65), a mechanism that ensures the tightly coupled translation of the two overlapping genes (72). The terminal overlap of sav with an upstream gene of about half the size was observed in all the investigated AbiV-sensitive lactococcal phages, except for phage c2, in which the two genes were separated by 6 bp. Similarly, 77% of the homologous sav genes (Fig. 4) overlap with an upstream gene. It is therefore tempting to speculate that orf27 and sav are translationally coupled. On the other hand, the mutations in the RBS of sav indicate that a functional RBS is important for the translation of sav. Translational coupling ensures an equimolar ratio of two polypeptides and may not entail a direct functional relationship between them. However, in the case of a terminal overlap, it is generally observed that the two genes almost always encode structurally or functionally related polypeptides (72). Translational coupling has been demonstrated previously for phage structural genes (16, 33, 74) but also for polypeptides in the DNA polymerase holoenzymes of coliphage T4 (79) and lactococcal phage sk1 (14).
To study the interaction of SaV with AbiV, we tried to complement the AbiV-insensitive phage mutant p2.1 with SaV from the wild-type phage p2 by providing the sav gene in trans during infection with phage mutant p2.1 (4, 25). The sav cloning experiments, together with subsequent SaV induction experiments, showed that the expression of the protein causes cell death (Fig. 5). It has been demonstrated in a number of cases that the products of phage genes that are transcribed early can have antimicrobial activity when expressed in bacterial cells (58, 49, 62, 66). These phage proteins produced soon after the beginning of the infection quickly inactivate or redirect critical processes of the bacterial cell machinery in order to shut down host metabolic activities and facilitate the production of phage components (39, 41, 44, 57). For example, early phage proteins have been demonstrated to inhibit transcription in E. coli by interaction with RNA polymerase (58) or transcription factors (66) or to redirect DnaB helicase to favor phage DNA replication (49, 62, 63). The quick toxic effects in both L. lactis and E. coli indicate that SaV may be such an early regulatory protein that targets a critical cellular mechanism common to the two distantly related bacterial species. At this time, the role and the antimicrobial mode of action of SaV are unknown. However, the expression of the mutated Orf27-SaV fusion from phage p2.1 was not toxic in E. coli, which indicates that the mutations in the conserved region of SaV rendering the phage insensitive to AbiV are also involved in the antimicrobial activity of the protein. It is therefore likely that it is the translated SaV polypeptide that is somehow involved in both the AbiV phenotype and toxicity toward the host cell.
In conclusion, the early-transcription phage protein SaV was demonstrated to be involved in the sensitivity of 936- and c2-like phages to the recently described abortive infection mechanism AbiV. The middle regions of SaV proteins in distantly related phages are conserved and evolutionarily related. Due to the fast-working toxic effect of SaV in both L. lactis and E. coli, we suggest that the function of SaV during the phage infection is to shut down or redirect essential host functions.
Acknowledgments
We thank Margit Pedersen and Cengiz Binici for constructing the pMAP84 vector, as well as D. van Sinderen and O. Kuipers for the gifts of pPTPL and pNZ8010, respectively.
This work was funded in part by a graduate scholarship from the Technical University of Denmark to J.H. and a strategic grant from the Natural Sciences and Engineering Research Council (NSERC) of Canada to S.M.
Footnotes
Published ahead of print on 6 March 2009.
REFERENCES
- 1.Allison, G. E., and T. R. Klaenhammer. 1998. Phage resistance mechanisms in lactic acid bacteria. Int. Dairy J. 8:207-226. [Google Scholar]
- 2.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altschul, S. F., J. C. Wootton, E. M. Gertz, R. Agarwala, A. Morgulis, A. A. Schaffer, and Y. K. Yu. 2005. Protein database searches using compositionally adjusted substitution matrices. FEBS J. 272:5101-5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bidnenko, E., M. C. Chopin, S. D. Ehrlich, and J. Anba. 2002. Lactococcus lactis AbiD1 abortive infection efficiency is drastically increased by a phage protein. FEMS Microbiol. Lett. 214:283-287. [DOI] [PubMed] [Google Scholar]
- 5.Bidnenko, E., D. Ehrlich, and M. C. Chopin. 1995. Phage operon involved in sensitivity to the Lactococcus lactis abortive infection mechanism AbiD1. J. Bacteriol. 177:3824-3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bissonnette, F., S. Labrie, H. Deveau, M. Lamoureux, and S. Moineau. 2000. Characterization of mesophilic mixed starter cultures used for the manufacture of aged cheddar cheese. J. Dairy Sci. 83:620-627. [DOI] [PubMed] [Google Scholar]
- 7.Bolotin, A., P. Wincker, S. Mauger, O. Jaillon, K. Malarme, J. Weissenbach, S. D. Ehrlich, and A. Sorokin. 2001. The complete genome sequence of the lactic acid bacterium Lactococcus lactis ssp. lactis IL1403. Genome Res. 11:731-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouchard, J. D., E. Dion, F. Bissonnette, and S. Moineau. 2002. Characterization of the two-component abortive phage infection mechanism AbiT from Lactococcus lactis. J. Bacteriol. 184:6325-6332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouchard, J. D., and S. Moineau. 2004. Lactococcal phage genes involved in sensitivity to AbiK and their relation to single-strand annealing proteins. J. Bacteriol. 186:3649-3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bouchard, J. D., and S. Moineau. 2000. Homologous recombination between a lactococcal bacteriophage and the chromosome of its host strain. Virology 270:65-75. [DOI] [PubMed] [Google Scholar]
- 11.Boucher, I., E. Emond, E. Dion, D. Montpetit, and S. Moineau. 2000. Microbiological and molecular impacts of AbiK on the lytic cycle of Lactococcus lactis phages of the 936 and P335 species. Microbiology 146:445-453. [DOI] [PubMed] [Google Scholar]
- 12.Breüner, A., L. Brøndsted, and K. Hammer. 1999. Novel organization of genes involved in prophage excision identified in the temperate lactococcal bacteriophage TP901-1. J. Bacteriol. 181:7291-7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chandry, P. S., B. E. Davidson, and A. J. Hillier. 1994. Temporal transcription map of the Lactococcus lactis bacteriophage sk1. Microbiology 140:2251-2261. [DOI] [PubMed] [Google Scholar]
- 14.Chandry, P. S., S. C. Moore, J. D. Boyce, B. E. Davidson, and A. J. Hillier. 1997. Analysis of the DNA sequence, gene expression, origin of replication and modular structure of the Lactococcus lactis lytic bacteriophage sk1. Mol. Microbiol. 26:49-64. [DOI] [PubMed] [Google Scholar]
- 15.Chopin, M. C., A. Chopin, and E. Bidnenko. 2005. Phage abortive infection in lactococci: variations on a theme. Curr. Opin. Microbiol. 8:473-479. [DOI] [PubMed] [Google Scholar]
- 16.Christie, G. E., L. M. Temple, B. A. Bartlett, and T. S. Goodwin. 2002. Programmed translational frameshift in the bacteriophage P2 FETUD tail gene operon. J. Bacteriol. 184:6522-6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cluzel, P. J., A. Chopin, S. D. Ehrlich, and M. C. Chopin. 1991. Phage abortive infection mechanism from Lactococcus lactis subsp. lactis, expression of which is mediated by an Iso-ISS1 element. Appl. Environ. Microbiol. 57:3547-3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coffey, A., and R. P. Ross. 2002. Bacteriophage-resistance systems in dairy starter strains: molecular analysis to application. Antonie van Leeuwenhoek 82:303-321. [PubMed] [Google Scholar]
- 19.Combet, C., C. Blanchet, C. Geourjon, and G. Deleage. 2000. NPS@: network protein sequence analysis. Trends Biochem. Sci. 25:147-150. [DOI] [PubMed] [Google Scholar]
- 20.Crutz-Le Coq, A. M., B. Cesselin, J. Commissaire, and J. Anba. 2002. Sequence analysis of the lactococcal bacteriophage bIL170: insights into structural proteins and HNH endonucleases in dairy phages. Microbiology 148:985-1001. [DOI] [PubMed] [Google Scholar]
- 21.Dai, G., P. Su, G. E. Allison, B. L. Geller, P. Zhu, W. S. Kim, and N. W. Dunn. 2001. Molecular characterization of a new abortive infection system (AbiU) from Lactococcus lactis LL51-1. Appl. Environ. Microbiol. 67:5225-5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Ruyter, P. G., O. P. Kuipers, M. M. Beerthuyzen, I. van Alen-Boerrigter, and W. M. de Vos. 1996. Functional analysis of promoters in the nisin gene cluster of Lactococcus lactis. J. Bacteriol. 178:3434-3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Ruyter, P. G., O. P. Kuipers, and W. M. de Vos. 1996. Controlled gene expression systems for Lactococcus lactis with the food-grade inducer nisin. Appl. Environ. Microbiol. 62:3662-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deveau, H., S. J. Labrie, M. C. Chopin, and S. Moineau. 2006. Biodiversity and classification of lactococcal phages. Appl. Environ. Microbiol. 72:4338-4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dinsmore, P. K., and T. R. Klaenhammer. 1997. Molecular characterization of a genomic region in a Lactococcus bacteriophage that is involved in its sensitivity to the phage defense mechanism AbiA. J. Bacteriol. 179:2949-2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dinsmore, P. K., and T. R. Klaenhammer. 1994. Phenotypic consequences of altering the copy number of abiA, a gene responsible for aborting bacteriophage infections in Lactococcus lactis. Appl. Environ. Microbiol. 60:1129-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Domingues, S., A. Chopin, S. D. Ehrlich, and M. C. Chopin. 2004. The lactococcal abortive phage infection system AbiP prevents both phage DNA replication and temporal transcription switch. J. Bacteriol. 186:713-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Durmaz, E., D. L. Higgins, and T. R. Klaenhammer. 1992. Molecular characterization of a second abortive phage resistance gene present in Lactococcus lactis subsp. lactis ME2. J. Bacteriol. 174:7463-7469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Durmaz, E., and T. R. Klaenhammer. 2006. Abortive phage resistance mechanism AbiZ speeds the lysis clock to cause premature lysis of phage-infected Lactococcus lactis. J. Bacteriol. 189:1417-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Emond, E., E. Dion, S. A. Walker, E. R. Vedamuthu, J. K. Kondo, and S. Moineau. 1998. AbiQ, an abortive infection mechanism from Lactococcus lactis. Appl. Environ. Microbiol. 64:4748-4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Emond, E., B. J. Holler, I. Boucher, P. A. Vandenbergh, E. R. Vedamuthu, J. K. Kondo, and S. Moineau. 1997. Phenotypic and genetic characterization of the bacteriophage abortive infection mechanism AbiK from Lactococcus lactis. Appl. Environ. Microbiol. 63:1274-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Forde, A., and G. F. Fitzgerald. 1999. Bacteriophage defence systems in lactic acid bacteria. Antonie van Leeuwenhoek 76:89-113. [PubMed] [Google Scholar]
- 33.Fortier, L. C., A. Bransi, and S. Moineau. 2006. Genome sequence and global gene expression of Q54, a new phage species linking the 936 and c2 phage species of Lactococcus lactis. J. Bacteriol. 188:6101-6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garvey, P., G. F. Fitzgerald, and C. Hill. 1995. Cloning and DNA sequence analysis of two abortive infection phage resistance determinants from the lactococcal plasmid pNP40. Appl. Environ. Microbiol. 61:4321-4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haaber, J., S. Moineau, L. C. Fortier, and K. Hammer. 2008. AbiV, a novel antiphage abortive infection mechanism on the chromosome of Lactococcus lactis subsp. cremoris MG1363. Appl. Environ. Microbiol. 74:6528-6537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hill, C., L. A. Miller, and T. R. Klaenhammer. 1990. Nucleotide sequence and distribution of the pTR2030 resistance determinant (hsp) which aborts bacteriophage infection in lactococci. Appl. Environ. Microbiol. 56:2255-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holo, H., and I. F. Nes. 1989. High-frequency transformation, by electroporation, of Lactococcus lactis subsp. cremoris grown with glycine in osmotically stabilized media. Appl. Environ. Microbiol. 55:3119-3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Josephsen, J., and H. Neve. 2004. Bacteriophage and antiphage mechanisms of lactic acid bacteria, p. 295-350. In S. Salminen, A. von Wright, and A. Ouwehand (ed.), Lactic acid bacteria: microbiological and functional aspects. CRC Press, London, United Kingdom.
- 39.Karam, J. 1994. Molecular biology of bacteriophage T4. ASM Press, Washington, DC.
- 40.Kuipers, O., P. G. de Ruyter, M. Kleerebezem, and W. de Vos. 1998. Quorum-sensing-controlled gene expression in lactic acid bacteria. J. Biotechnol. 64:15-21. [Google Scholar]
- 41.Kutter, E., R. Raya, and K. Carlson. 2005. Molecular mechanisms of phage infection, p. 165-222. In E. Kutter and A. Sulakvelidze (ed.), Bacteriophages, biology and applications. CRC Press, London, United Kingdom.
- 42.Kutter, E., and A. Sulakvelidze. 2005. Bacteriophages, biology and applications. CRC Press, London, United Kingdom.
- 43.Labrie, S. J., and S. Moineau. 2007. Abortive infection mechanisms and prophage sequences significantly influence the genetic makeup of emerging lytic lactococcal phages. J. Bacteriol. 189:1482-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu, J., M. Dehbi, G. Moeck, F. Arhin, P. Bauda, D. Bergeron, M. Callejo, V. Ferretti, N. Ha, T. Kwan, J. McCarty, R. Srikumar, D. Williams, J. J. Wu, P. Gros, J. Pelletier, and M. DuBow. 2004. Antimicrobial drug discovery through bacteriophage genomics. Nat. Biotechnol. 22:185-191. [DOI] [PubMed] [Google Scholar]
- 45.Lubbers, M. W., N. R. Waterfield, T. P. Beresford, R. W. Le Page, and A. W. Jarvis. 1995. Sequencing and analysis of the prolate-headed lactococcal bacteriophage c2 genome and identification of the structural genes. Appl. Environ. Microbiol. 61:4348-4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Madsen, P. L., A. H. Johansen, K. Hammer, and L. Brøndsted. 1999. The genetic switch regulating activity of early promoters of the temperate lactococcal bacteriophage TP901-1. J. Bacteriol. 181:7430-7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Madsen, S. M., D. Mills, G. Djordjevic, H. Israelsen, and T. R. Klaenhammer. 2001. Analysis of the genetic switch and replication region of a P335-type bacteriophage with an obligate lytic lifestyle on Lactococcus lactis. Appl. Environ. Microbiol. 67:1128-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mahony, J., H. Deveau, S. Mc Grath, M. Ventura, C. Canchaya, S. Moineau, G. F. Fitzgerald, and D. van Sinderen. 2006. Sequence and comparative genomic analysis of lactococcal bacteriophages jj50, 712 and P008: evolutionary insights into the 936 phage species. FEMS Microbiol. Lett. 261:253-261. [DOI] [PubMed] [Google Scholar]
- 49.Mallory, J. B., C. Alfano, and R. McMacken. 1990. Host virus interactions in the initiation of bacteriophage lambda DNA replication. Recruitment of Escherichia coli DnaB helicase by lambda P replication protein. J. Biol. Chem. 265:13297-13307. [PubMed] [Google Scholar]
- 50.Martinussen, J., and K. Hammer. 1994. Cloning and characterization of upp, a gene encoding uracil phosphoribosyltransferase from Lactococcus lactis. J. Bacteriol. 176:6457-6463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moineau, S. 1999. Applications of phage resistance in lactic acid bacteria. Antonie van Leeuwenhoek 76:377-382. [PubMed] [Google Scholar]
- 52.Moineau, S., S. Pandian, and T. Klaenhammer. 1994. Evolution of a lytic bacteriophage via DNA acquisition from the Lactococcus lactis chromosome. Appl. Environ. Microbiol. 60:1832-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moineau, S., D. Tremblay, and S. Labrie. 2002. Phages of lactic acid bacteria: from genomics to industrial applications. ASM News 68:388-393. [Google Scholar]
- 54.Moineau, S., E. Durmaz, S. Pandian, and T. R. Klaenhammer. 1993. Differentiation of two abortive mechanisms by using monoclonal antibodies directed toward lactococcal bacteriophage capsid proteins. Appl. Environ. Microbiol. 59:208-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moineau, S., and C. Levesque. 2005. Control of bacteriophages in industrial fermentations, p. 285-296. In E. Kutter and A. Sulakvelidze (ed.), Bacteriophages, biology and applications. CRC Press, London, United Kingdom.
- 56.Moineau, S., S. A. Walker, E. R. Vedamuthu, and P. A. Vandenbergh. 1995. Cloning and sequencing of LlaDCHI [corrected] restriction/modification genes from Lactococcus lactis and relatedness of this system to the Streptococcus pneumoniae DpnII system. Appl. Environ. Microbiol. 61:2193-2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mosig, G., and F. Eiserling. 2006. T4 and related phages: structure and development, p. 225-267. In R. Calendar (ed.), The bacteriophages. Oxford University Press, Oxford, United Kingdom.
- 58.Nechaev, S., and K. Severinov. 1999. Inhibition of Escherichia coli RNA polymerase by bacteriophage T7 gene 2 protein. J. Mol. Biol. 289:815-826. [DOI] [PubMed] [Google Scholar]
- 59.Normark, S., S. Bergstrom, T. Edlund, T. Grundstrom, B. Jaurin, F. P. Lindberg, and O. Olsson. 1983. Overlapping genes. Annu. Rev. Genet. 17:499-525. [DOI] [PubMed] [Google Scholar]
- 60.O'Connor, L., A. Coffey, C. Daly, and G. F. Fitzgerald. 1996. AbiG, a genotypically novel abortive infection mechanism encoded by plasmid pCI750 of Lactococcus lactis subsp. cremoris UC653. Appl. Environ. Microbiol. 62:3075-3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.O'Connor, L., M. Tangney, and G. F. Fitzgerald. 1999. Expression, regulation, and mode of action of the AbiG abortive infection system of Lactococcus lactis subsp. cremoris UC653. Appl. Environ. Microbiol. 65:330-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Odegrip, R., S. Schoen, E. Haggard-Ljungquist, K. Park, and D. K. Chattoraj. 2000. The interaction of bacteriophage P2 B protein with Escherichia coli DnaB helicase. J. Virol. 74:4057-4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.O'Driscoll, J., F. Glynn, O. Cahalane, M. O'Connell-Motherway, G. F. Fitzgerald, and D. van Sinderen. 2004. Lactococcal plasmid pNP40 encodes a novel, temperature-sensitive restriction-modification system. Appl. Environ. Microbiol. 70:5546-5556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oliver, D. B., and J. Beckwith. 1982. Regulation of a membrane component required for protein secretion in Escherichia coli. Cell 30:311-319. [DOI] [PubMed] [Google Scholar]
- 65.Oppenheim, D. S., and C. Yanofsky. 1980. Translational coupling during expression of the tryptophan operon of Escherichia coli. Genetics 95:785-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Orsini, G., M. Ouhammouch, J. P. Le Caer, and E. N. Brody. 1993. The asiA gene of bacteriophage T4 codes for the anti-sigma 70 protein. J. Bacteriol. 175:85-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Page, R. D. 1996. TreeView: an application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 12:357-358. [DOI] [PubMed] [Google Scholar]
- 68.Qiagen. June 2003, posting date. The QIAexpressionist: a handbook for high-level expression and purification of 6xHis-tagged proteins, 5th ed. Qiagen, Valencia, CA. http://www.biochem.wisc.edu/courses/biochem660/Reading/090808_reading1.pdf.
- 69.Ross, W., S. H. Shore, and M. M. Howe. 1986. Mutants of Escherichia coli defective for replicative transposition of bacteriophage Mu. J. Bacteriol. 167:905-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 71.Sanders, M. E., and T. R. Klaenhammer. 1980. Restriction and modification in group N streptococci: effect of heat on development of modified lytic bacteriophage. Appl. Environ. Microbiol. 40:500-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Scherbakov, D. V., and M. B. Garber. 2000. Overlapping genes in bacterial and bacteriophage genomes. Mol. Biol. 34:572-583. [PubMed] [Google Scholar]
- 73.Schouler, C., S. D. Ehrlich, and M. C. Chopin. 1994. Sequence and organization of the lactococcal prolate-headed bIL67 phage genome. Microbiology 140:3061-3069. [DOI] [PubMed] [Google Scholar]
- 74.Selianov, N. A., A. G. Prilipov, V. P. Efimov, E. I. Marusich, and V. V. Mesyanzhinov. 1990. Cascade of overlapping late genes in bacteriophage T4. Biomed. Sci. 1:55-62. [PubMed] [Google Scholar]
- 75.Sing, W. D., and T. Klaenhammer. 1990. Plasmid-induced abortive infection in lactococci: a review. J. Dairy Sci. 73:2239-2251. [Google Scholar]
- 76.Snyder, L. 1995. Phage-exclusion enzymes: a bonanza of biochemical and cell biology reagents? Mol. Microbiol. 15:415-420. [DOI] [PubMed] [Google Scholar]
- 77.Terzaghi, B. E., and W. E. Sandine. 1975. Improved medium for lactic streptococci and their bacteriophages. Appl. Microbiol. 29:807-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Torgov, M. Y., D. M. Janzen, and M. K. Reddy. 1998. Efficiency and frequency of translational coupling between the bacteriophage T4 clamp loader genes. J. Bacteriol. 180:4339-4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Twomey, D. P., P. J. De Urraza, L. L. McKay, and D. J. O'Sullivan. 2000. Characterization of AbiR, a novel multicomponent abortive infection mechanism encoded by plasmid pKR223 of Lactococcus lactis subsp. lactis KR2. Appl. Environ. Microbiol. 66:2647-2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.van de Guchte, M., J. Kok, and G. Venema. 1992. Gene expression in Lactococcus lactis. FEMS Microbiol. Rev. 8:73-92. [DOI] [PubMed] [Google Scholar]