The autoimmune disease, type 1 diabetes, presents a unique set of challenges to the clinical scientist trying to understand its aetiology. The β cells of the islets of Langerhans, which represent the immune system's target, are located within the pancreas, one of the more inaccessible organs in the body. The islets are too small to be imaged reliably with current technologies. It is estimated that approximately 1 million islet clusters, comprised of 1–2000 β cells each, are scattered throughout the pancreas, surrounded by exocrine cells and ducts filled with proteolytic enzymes. These enzymes are responsible for both the tendency of the pancreas to undergo rapid autolysis after death and for the inherent danger of pancreatic biopsy, which is considered widely as too hazardous a procedure for mere research purposes, even if inclusion of islets in the tissue sample could be guaranteed. To add to the problems of obtaining tissue for research, major advances in protocols for the management of disease at first clinical presentation and a greater public and physician awareness of the symptoms and signs of hyperglycaemia over recent decades have reduced the incidence of acutely fatal type 1 diabetes to a negligible level and hence all but ended the supply of diseased organs for study. Small wonder that there are so many unknowns in this disease.
Against this backdrop, a paper in the current issue of Clinical & Experimental Immunology [1] offers a unique insight into many hotly debated issues in this field, while inevitably raising several more. Willcox et al. [1] obtained what is probably the largest and most complete collection of post-mortem pancreatic organs from patients with type 1 diabetes, including many cases close to onset. These were assembled by one of the co-authors on the current paper, Alan Foulis, during the 1980s, through painstaking collection of paraffin blocks from post-mortem analyses conducted during the 25-year period from 1959 to 1984 in the United Kingdom [2]. The outcome is a definitive study of the lymphocytic infiltrates in the immediate microenvironment of the β cells as they are killed during the evolution of type 1 diabetes. Has the murderer been caught red-handed?
The cellular composition of insulitis
Chief among the insights is the identification of CD8 T cells as the major constituents of the insulitis that accompanies β cell destruction. Although this has been hinted at before [3–5], Willcox et al. provide evidence that CD8 T cell infiltrates peak and wane according to the number of functional β cells present, implying a direct relationship between the intensity of the attack and the death of the target. Macrophages are also present but at lower and more constant numbers throughout the destructive process. These findings have important implications for the current debate as to whether β cell death is due to the selective toxic effects of monokines, such as interleukin-1β and tumour necrosis factor-α [6], or whether it results from direct cytolytic action by CD8+ cytotoxic T lymphocytes (CTLs), recognizing β cell-specific autoantigens [7,8]. In our view, the close linkage of CD8 T cell count to β cell numbers that Willcox et al. have identified tips the balance in favour of CTLs as the smoking gun at the scene of β cell death. Furthermore, combined with our recent identification of prepoinsulin-specific CTL clones that kill β cells [7], there is now an imperative to move to more refined analysis of islet infiltrates in order to study T cell receptor CDR3 usage and compare, for example, putative pathogenic CTLs with insulitic CD8 T cells. Overall, the presence of a CD8-dominated insulitis reinforces our earlier suggestion that autoreactive CTLs are potentially important targets for immune-based interventions in type 1 diabetes [7,9].
The other striking finding in relation to the cellular composition of the insulitis is the subsequent influx of B lymphocytes into islets with the most advanced β cell destruction. The role of B lymphocytes in type 1 diabetes pathogenesis is also debated hotly, and these studies highlight its importance once more. Studies in the animal model, the non-obese diabetic mouse, provide good evidence that B lymphocytes contribute to disease, and that their removal at the latter stages of diabetes development has therapeutic benefit [10]. There is even an intriguing link between B lymphocytes and CTL survival in inflamed islets [11], which derives indirect support in the Willcox et al. study. In man, the situation is less certain: neither antibodies nor B lymphocytes are an absolute requirement for disease [12]. The outcome of the Diabetes TrialNet study of administration of Rituximab (monoclonal anti-CD20 antibody; http://www.diabetestrialnet.org) to patients with new-onset disease is awaited eagerly for its ability to settle this argument. In relation to this, an important further question is prompted by the Willcox et al. study: if Rituximab works in the TrialNet study conducted at diabetes onset, when the disease process is advanced and B lymphocytes are highly represented, does it necessarily follow that it will work earlier in disease, during the asymptomatic phase when perhaps fewer islets have such florid B lymphocyte infiltrates?
Lymphocyte trafficking to islets of Langerhans
The spatial distribution of leucocytes in the insulitis associated with β cell destruction as defined here by Willcox et al. is intriguing. Some leucocytes are located within the islet, but most are concentrated around the periphery, extending into the tissue spaces between the exocrine glands. This is highly reminiscent of the peri-insulitis so often described in non-obese diabetic mice but not reported previously in man. Where are these cells coming from? The islets of Langerhans have two frontiers that lymphocytes do not normally cross: the endothelium lining the independent vasculature of the endocrine glands and the loose connective tissue that forms the barrier between the endocrine and exocrine components of the pancreas (Fig. 1). This continuum of lymphocytic infiltration within and around the islet and between the acini in type 1 diabetes suggests that the normal barriers to leucocyte migration between the islets and exocrine tissues are breached during disease pathogenesis. In a study of non-diseased human pancreas, we have noted that the major lymphocytic components of the normal exocrine organ are CD8+ cells that are situated both between the epithelial cells that form the acini and in the connective tissue between the glands (Fig. 2). As in the observations of Willcox et al. in diseased organs, CD4 T cells are relatively rare in the normal pancreas. This raises the question as to the relevance of these CD8+ T cells that are resident in the exocrine pancreas to the pathogenesis of type 1 diabetes. One speculation is that these cells may be early participants in the initiation of the pathological process that ends as diabetes, and this will need to be addressed in detailed studies that compare their characteristics with those of CD8 T cells present in the mature chronic insulitis.
Fig. 1.
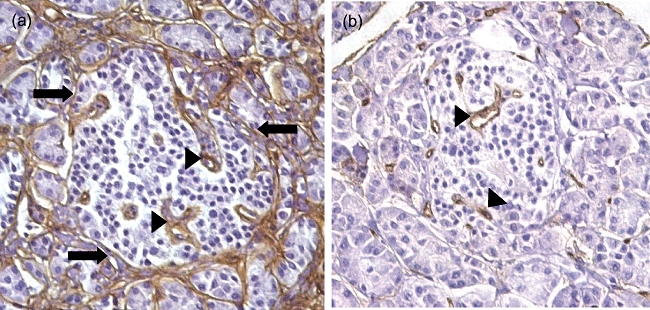
Paraffin sections of normal human pancreas stained using antibodies to (a) collagen IV and (b) endothelial antigen CD34 to highlight the peripheral boundary between the islets and the exocrine tissues (arrows) and blood vessels of the islet (arrow heads), neither of which are crossed by lymphocytes in healthy pancreas.
Fig. 2.
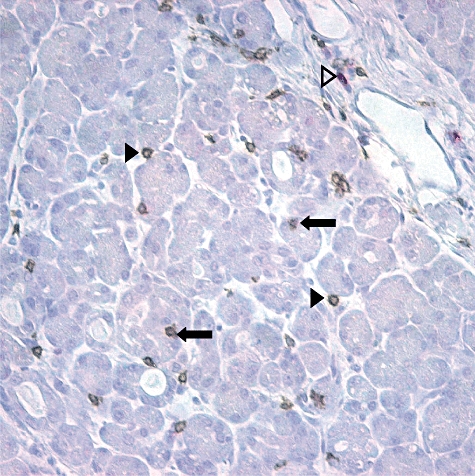
Paraffin section of normal human pancreas double-stained with a subtractive method to identify CD8+ cells (brown) and CD3+, CD8− cells (red). The most abundant T cell population in normal exocrine pancreas expresses CD8. CD8-expressing T cells are present between the epithelial cells of the acini (closed arrows) and in the connective tissue between the glands (closed arrow heads). CD8− T cells are evident in the connective tissue between lobules (open arrowheads). Additional experiments showed that the CD8+ cells in pancreas also express CD3.
In summary, it is striking how post-mortem samples collected between 35 and 60 years ago, in many cases before the autoimmune basis for diabetes was unravelled, should now provide such a novel set of insights into the complex processes at the heart of this common and debilitating condition. In practice, only the successful deployment of therapies that target specifically autoreactive CTLs, B lymphocytes or macrophages in the islet infiltrate will allow us to make a certain judgement about their culpability as agents of β cell death. In their defence, it remains plausible that some of the cells identified in the insulitis could be innocent bystanders, or even part of the host's attempt to restore order; but in our view they remain prime suspects. In a striking parallel with the modern forensic approach to unsolved crime, they may yet be caught as new ‘DNA evidence’ comes to light.
Acknowledgments
Research data included in this article from our own laboratory was supported by a grant from the Juvenile Diabetes Research Foundation (award number 25-2007-834) and conducted by Adelaide Annan using post-mortem samples obtained through the JDRF Network for Pancreatic Organ Donors with Diabetes (nPOD).
References
- 1.Willcox A, Richardson SJ, Bone AJ, Foulis AK, Morgan NC. Analysis of islet inflammation in human type 1 diabetes. Clin Exp Immunol. 2009 doi: 10.1111/j.1365-2249.2008.03860.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foulis AK, Liddle CN, Farquharson MA, Richmond JA, Weir RS. The histopathology of the pancreas in type 1 (insulin-dependent) diabetes mellitus: a 25-year review of deaths in patients under 20 years of age in the United Kingdom. Diabetologia. 1986;29:267–74. doi: 10.1007/BF00452061. [DOI] [PubMed] [Google Scholar]
- 3.Bottazzo GF, Dean BM, McNally JM, MacKay EH, Swift PG, Gamble DR. In situ characterization of autoimmune phenomena and expression of HLA molecules in the pancreas in diabetic insulitis. N Engl J Med. 1985;313:353–60. doi: 10.1056/NEJM198508083130604. [DOI] [PubMed] [Google Scholar]
- 4.Hanninen A, Jalkanen S, Salmi M, Toikkanen S, Nikolakaros G, Simell O. Macrophages, T cell receptor usage, and endothelial cell activation in the pancreas at the onset of insulin-dependent diabetes mellitus. J Clin Invest. 1992;90:1901–10. doi: 10.1172/JCI116067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Somoza N, Vargas F, Roura-Mir C, et al. Pancreas in recent onset insulin-dependent diabetes mellitus. Changes in HLA, adhesion molecules and autoantigens, restricted T cell receptor V beta usage, and cytokine profile. J Immunol. 1994;153:1360–77. [PubMed] [Google Scholar]
- 6.Cnop M, Welsh N, Jonas JC, Jorns A, Lenzen S, Eizirik DL. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes. 2005;54(Suppl. 2):S97–107. doi: 10.2337/diabetes.54.suppl_2.s97. [DOI] [PubMed] [Google Scholar]
- 7.Skowera A, Ellis RJ, Varela-Calvino R, et al. CTLs are targeted to kill beta cells in patients with type 1 diabetes through recognition of a glucose-regulated preproinsulin epitope. J Clin Invest. 2008;118:3390–402. doi: 10.1172/JCI35449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Lorenzo TP, Peakman M, Roep BO. Translational mini-review series on type 1 diabetes: systematic analysis of T cell epitopes in autoimmune diabetes. Clin Exp Immunol. 2007;148:1–16. doi: 10.1111/j.1365-2249.2006.03244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Staeva-Vieira T, Peakman M, von Herrath M. Translational mini-review series on type 1 diabetes: immune-based therapeutic approaches for type 1 diabetes. Clin Exp Immunol. 2007;148:17–31. doi: 10.1111/j.1365-2249.2007.03328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu CY, Rodriguez-Pinto D, Du W, et al. Treatment with CD20-specific antibody prevents and reverses autoimmune diabetes in mice. J Clin Invest. 2007;117:3857–67. doi: 10.1172/JCI32405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brodie GM, Wallberg M, Santamaria P, Wong FS, Green EA. B-cells promote intra-islet CD8+ cytotoxic T-cell survival to enhance type 1 diabetes. Diabetes. 2008;57:909–17. doi: 10.2337/db07-1256. [DOI] [PubMed] [Google Scholar]
- 12.Martin S, Wolf-Eichbaum D, Duinkerken G, et al. Development of type 1 diabetes despite severe hereditary B-lymphocyte deficiency. N Engl J Med. 2001;345:1036–40. doi: 10.1056/NEJMoa010465. [DOI] [PubMed] [Google Scholar]


