Abstract
Immunotherapeutic strategies under consideration for type 1 diabetes include modification of the autoimmune response through antigen-specific routes. Administration of short peptides representing T cell epitopes targeted by patients with the disease represents one approach. This study evaluated safety and mechanistic outcomes during first-in-man intradermal administration of a human leucocyte antigen-DR4 (HLA-DR4)-restricted peptide epitope of proinsulin (C19-A3). This randomized, open-label study assessed two major theoretical risks of peptide immunotherapy, namely induction of allergic hypersensitivity and exacerbation of the proinflammatory autoimmune response, using clinical assessment and mechanistic assays in vitro. Patients with long-standing type 1 diabetes and HLA-DRB1*0401 genotype received 30 µg (n = 18) or 300 µg (n = 18) of peptide in three equal doses at 0, 1 and 2 months or no intervention (n = 12). Proinsulin peptide immunotherapy in the dosing regimen used is well tolerated and free from risk of systemic hypersensitivity and induction/reactivation of proinsulin-specific, proinflammatory T cells. Peptide-specific T cells secreting the immune suppressive cytokine interleukin (IL)-10 were observed at month 3 in four of 18 patients in the low-dose group (versus one of 12 in the control group; P = not significant). Mean IL-10 response to peptide in the low-dose group increased between 0 and 3 months (P = 0·05 after stimulation with 5 µM peptide in vitro) and then declined to baseline levels between 3 and 6 months (P = 0·01 at 10 µM peptide in vitro). These studies pave the way for future investigations in new-onset patients designed to examine whether proinsulin peptide immunotherapy has beneficial effects on markers of T cell autoimmunity and preservation of β cell mass.
Keywords: immunotherapy, peptide, proinsulin, regulatory T cells, type 1 diabetes
Introduction
Intervention and prevention strategies currently under consideration for type 1 diabetes include agents designed to modify the autoimmune response through antigen-specific and non-antigen-specific routes [1]. Among the latter, studies of monoclonal anti-CD3 antibody therapy have established clearly that suppression of T cell immunity is effective in halting β cell damage when initiated at disease diagnosis, as indicated by preservation of residual C-peptide for 1–2 years of follow-up [2–4]. Future trials will need to address the question of whether repeated administration or maintenance of such immune modulation can extend this effect. However, the long-term sequelae of both acute and chronic immunosuppression in this study population remain unclear, as does the ability of such approaches to restore tolerance to β cells.
Antigen-specific modulation of the immune response is viewed as being more likely to restore immunological tolerance to β cells [1]. A variety of antigen-specific therapeutic strategies are effective in murine models of autoimmune diabetes [5], including administration of islet autoantigen (whole protein or peptide), via parenteral, oral and nasal routes. Recent translation of these approaches into man include therapeutic studies on oral and parenteral insulin [6,7] and safety studies of subcutaneous injection of glutamic acid decarboxylase-65 (GAD65) in alum [8] and an altered peptide ligand of insulin B9-23 [9]. The predominant focus upon using whole antigens as tolerogens ignores many of the potential advantages of using short peptides representing T cell epitopes targeted by patients with type 1 diabetes as part of the β cell-specific autoimmune response. These include the relatively modest costs of synthesis and the fact that on a weight-for-weight basis, peptide delivers up to 50 times more of the effective agent (i.e. presented epitope) than can be achieved with whole antigen [10].
There has been considerable recent interest in promoting peptide immunotherapy as an approach to restore immunological tolerance in autoimmune disease and allergy [10–12]. Theoretical risks include induction of an inflammatory response to the peptide, leading either to an allergic response and anaphylaxis or exacerbation of the autoimmune pathology. Indeed, a recent review suggested that the risks of the former were sufficiently high from animal studies for this approach to be relatively contraindicated in type 1 diabetes [13].
In order to take such an approach further towards full clinical evaluation in type 1 diabetes, we initiated a programme of studies to assess the preclinical safety of proinsulin peptide immunotherapy. The selected peptide is an epitope from the C–A chain junction present in proinsulin but not insulin. It is naturally processed and presented by human leucocyte antigen-DR4 (HLA-DR4) (B1*0401) and a prominent target of CD4 T cell responses [13–15]. In the present study we focused upon two major theoretical risks of peptide immunotherapy, namely induction of anaphylaxis and exacerbation of the proinflammatory autoimmune response. Our study shows that proinsulin peptide immunotherapy in the dosing regimen used is well tolerated and free from risk of systemic anaphylaxis or induction/reactivation of a proinflammatory autoimmune response. Future studies in new-onset patients will be required to examine whether it has beneficial effects on markers of T cell autoimmunity and preservation of C-peptide.
Research design and methods
Subjects
Subjects were recruited from the Bristol area. Inclusion criteria were: > 5 years’ duration of type 1 diabetes; HLA-DRB1*0401 genotype; C-peptide ≤ 0·2 nmol/l 6 min post-intravenous challenge with 1 mg glucagon; HbA1c < 10% at the time of recruitment. Exclusion criteria were: history of asthma, atopy or documented allergy; use of steroids or immunosuppressive drugs; other autoimmune disease (except thyroiditis); women not using effective contraception and pregnancy or breast feeding; retinopathy beyond background change; proteinuria; and raised creatinine. A total of 134 individuals were screened, of whom 57 were eligible and 48 were recruited sequentially into two groups of 24. Within each group subjects were randomized to receive either three intradermal injections of proinsulin peptide on a monthly basis (n = 18) or to receive no treatment (n = 6), resulting in a total of 18 subjects in the two treatment groups and 12 subjects in the control group. Treated subjects in the first group received 10 µg peptide and in the second group 100 µg, administered in 0·05 ml 0·9% NaCl. The study design is shown in Fig. 1. Dose and dosing interval were selected on the basis of studies in clinical allergy [16] and the DiaPep277 study in type 1 diabetes [17].
Fig. 1.
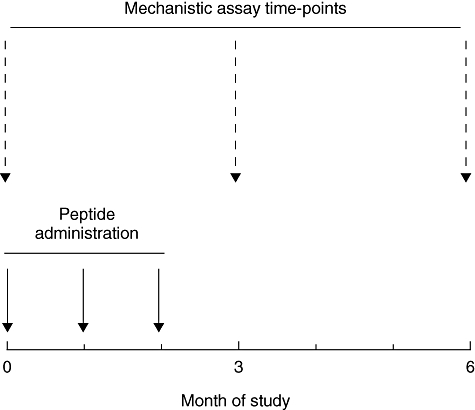
Graphical representation of study design, indicating timing of proinsulin peptide injections and major mechanistic studies. This template was followed for the 10 mg and 100 mg doses.
At baseline, prior to the second and third injections, and at study months 3 and 6, subjects underwent physical examination and measurement of antibodies to GAD65, the islet tyrosine phosphatase IA-2 (IA-2) and insulin, full blood count, liver enzymes, urea, creatinine and electrolytes, immunoglobulin (Ig) levels, thyroid stimulating hormone level, HbA1c and a lipid profile. Control subjects underwent identical clinical and biochemical assessments but did not receive any intervention. Because the primary outcome of this first-in-man study was safety of proinsulin peptide injection, clinical investigators were not blinded to a subject's treatment group.
The study was conducted in accordance with International Conference on Harmonization/World Health Organization Good Clinical Practice standards, with independent Data Safety Monitoring Board (DSMB) meetings on completion of each treatment group. The study was approved by the North Somerset and South Bristol Research Ethics Committee and the UK Medicines and Healthcare products Regulatory Agency. All subjects provided written informed consent prior to enrolment in the study.
The study protocol defined two primary outcome measures: (i) safety profile of proinsulin peptide administration [allergy, changes in routine biochemistry and metabolic control (HbA1c)]; and (ii) changes in cytokine responsiveness to proinsulin peptide detected by enzyme-linked immunospot (ELISPOT) 3 months after the first injection compared with baseline. Secondary outcome measures were: (i) changes in cytokine responsiveness to proinsulin peptide detected by ELISPOT 6 months after the first injection versus baseline; and (ii) changes in anti-GAD65, anti-IA-2, anti-insulin and anti-proinsulin peptide antibody levels at any time-point.
Peptide
Proinsulin peptide C19-A3 (GSLQPLALEGSLQKRGIV) was prepared to Good Manufacturing Practice standards by Calbiochem-Novabiochem AG Clinalfa, affiliate of Merck Pharmaceuticals (Läufelfingen, Switzerland). The peptide was > 95% pure, endotoxin-free and showed no toxicity in formal testing in two species (guinea-pigs and mice).
Mechanistic studies
Heparinized blood was obtained at baseline, 3 and 6 months and coded to blind the laboratory as to sample status (drug or non-drug; dose). Peripheral blood mononuclear cells (PBMCs) were purified within 6 h for cytokine ELISPOT assays for interferon (IFN)-γ, interleukin (IL)-4, IL-13, IL-5 and IL-10, as described previously [16,17]. For each cytokine, three separate aliquots of 106 PBMCs were stimulated with 10 µl study drug (proinsulin peptide) diluted in 0·9% saline to final concentrations of 1, 5 and 10 µM. Identical cultures were established for the negative and positive control conditions using 10 µl 0·9% saline or 6 µl/ml and 0·6 µl/ml of Pediacel (pertussis, diphtheria, Haemophilus influenza B, polio and tetanus toxoid vaccines; Sanofi Pasteur MSD, Maidenhead, UK). ELISPOTs were analysed as described [14] as the sum of triplicate wells and provided as the number of responder cells/106 PBMCs using a stimulation index (SI, derived as number of spots in test wells/number in saline control wells) of ≥ 3·0 to indicate a response to peptide. Inter- and intracoefficients of variation are 10·7% and 12·3% respectively. ELISPOT plate reading and the assignment of response/non-response was carried out while the samples remained coded. In the design of this study, we predetermined that a T cell response to proinsulin peptide C19-A3 would be recorded when an SI of ≥ 3·0 was seen at two or more of the peptide doses present in the cultures (1, 5 and 10 µM).
Predetermined criteria were used to assign cytokine ELISPOT results as indicating a ‘favourable’ T cell response to proinsulin peptide therapy. Designation of ‘favourable’ was based on the hypothesis that peptide immunotherapy operates through the induction of C19-A3-specific CD4 T cells secreting IL-10 [18] and/or deletion [10] or suppression [19] of proinflammatory (IFN-γ+) C19-A3-specific CD4 T cells. ‘Favourable responders’ had either (i) an IL-10 response to peptide induced at 3 months or (ii) lost a baseline IFN-γ response to peptide at 3 months.
IgG antibodies against C19-A3 were measured by enzyme-linked immunosorbent assay [20]. Rabbit polyclonal anti-serum against C19-A3 was used to derive a standard curve, against which patient sera (diluted 1:30, 1:100 and 1:1000) were measured. Further modification allowed measurement of IgE anti-peptide antibodies in undiluted test serum. Radioaimmunoassays were used to measure antibodies against IA-2, GAD65 [21] and insulin (RSR Ltd, Cardiff, UK).
Statistical analysis
Student's t-tests for paired samples were used to analyse changes in within-group cytokine ELISPOT responses at baseline, 3 and 6 months. Mean change in HbA1c levels from baseline for each treatment/control group was compared between groups using a Mann–Whitney U-test. The Wilcoxon matched-pairs test was used to analyse changes in HbA1c levels within each treatment group over time, the null hypothesis being that there would be no overall change. Mean change in anti-GAD65, anti-IA-2, anti-insulin and anti-proinsulin peptide antibody levels from baseline for each treatment/control group was compared between groups using a Mann–Whitney U-test. P-values < 0·05 were considered significant.
Results
Study enrolment and completion
Of the 48 subjects recruited, 45 (94%) completed the study. One subject (10 µg dose group) withdrew following the second treatment because of work commitments. Two subjects died during the course of the study. One subject, in the control group, died unexpectedly overnight (‘dead in bed’) before the 6-month study visit. Such deaths are recognized in diabetes [22], and no alternative diagnosis was made following a coroner's investigation. One subject (100 µg dose group) completed peptide administration but committed suicide 3 months after the last dose of peptide. Both events were carefully reviewed by the DSMB and the deaths were not considered to be related to participation in the study. The mean age of participants was 40·6 years (range 21–53 years) and mean duration of type 1 diabetes 23·8 years (range 7–45 years).
Tolerability and safety of intradermal proinsulin peptide administration
Proinsulin peptide administration was well tolerated by all subjects. There were no systemic allergic reactions, or other treatment-related serious adverse events, in any of the 36 treated subjects. Routine biochemical and haematological measurements did not change significantly during treatment.
Of the 18 subjects receiving the 10 µg dose, two (11%) had transient (maximum duration 2 h), localized erythematous skin reactions at the injection site with a maximum diameter of 20 mm. One subject experienced these after the first and third peptide injections, and the other after all three injections (Fig. 2a). These were not accompanied by weal, itching or pain at the injection site or systemic symptoms, and although present in the same subjects in their subsequent injections did not increase in speed of appearance, magnitude or duration with subsequent injections. Similar reactions were seen in significantly more of the subjects receiving the 100 µg dose (10 of 18; 56%, P = 0·012 versus 10 µg dose) on at least one occasion with a maximum diameter of 50 mm (Fig. 2b). Although skin reaction size increased in two subjects in this group, there did not appear to be any consistent pattern of escalation of size or frequency of the reaction with repeat administration. Reactions such as these, appearing within 30 s of injection, are suggestive of a local vasodilatatory response, and have been observed by others during peptide immunotherapy and attributed to minor chemical contaminants from the peptide synthesis (Mark Larché, personal communication). The lack of evidence of sensitization or amplification with further doses in the majority of subjects, along with the absence of induction of IgG or IgE anti-peptide antibody or T helper type 2 (Th2) cellular immune responses (see below), militate against a diagnosis of allergic hypersensitivity but are consistent with the possibility of an erythematous drug reaction, because of local vasoactive properties of either the peptide or a minor contaminant from the synthesis process. This conclusion is supported further by studies in which we have injected mice transgenic for HLA-DRB1*0401 with 10, 100 and 1000 µg of peptide using an identical regimen, and observed no induction of IL-4 producing T cells (data not shown).
Fig. 2.
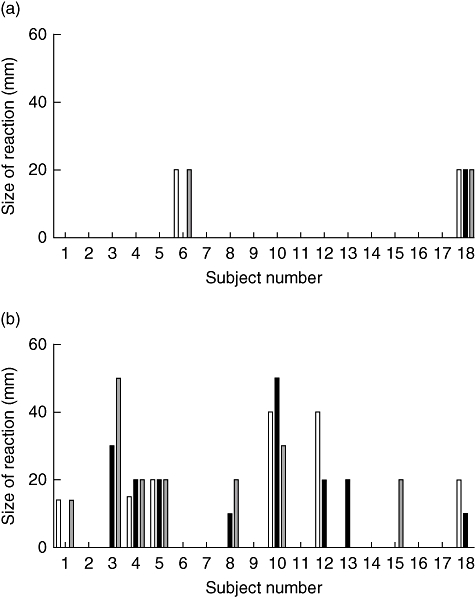
Prevalence and size of erythematous skin reactions among (a) 18 subjects receiving the 10 mg dose and (b) 18 subjects receiving the 100 mg dose. Bars represent reaction size in millimetre, and open, shaded and diagonal stripe bars represent first, second and third injections respectively.
Th2 cytokine responses to proinsulin peptide C19-A3
In order to address the risk of inducing anti-peptide allergic responses, we examined Th2-type responses using cytokine ELISPOTs to detect IL-4, IL-5 and IL-13. These are the prototypic type 2 cytokines associated typically with allergic responses and represent a surrogate measure of hypersensitivity. As shown in Fig. 3, baseline Th2 cytokine responses to proinsulin peptide were largely absent, consistent with our previous reports that Type 2 CD4 T cell autoreactivity in type 1 diabetes is rare [14,23]. Notably, considering the treatment groups as a whole, there was no evidence of induction of Th2 cytokines at 3 or 6 months after first peptide administration (Fig. 3). No subject showed any significant (SI ≥ 3·0) Th2 response to the peptide at any dose at any stage. In contrast, recall Th2 responses to the pentavalent vaccine Pediacel were detected frequently (data not shown).
Fig. 3.
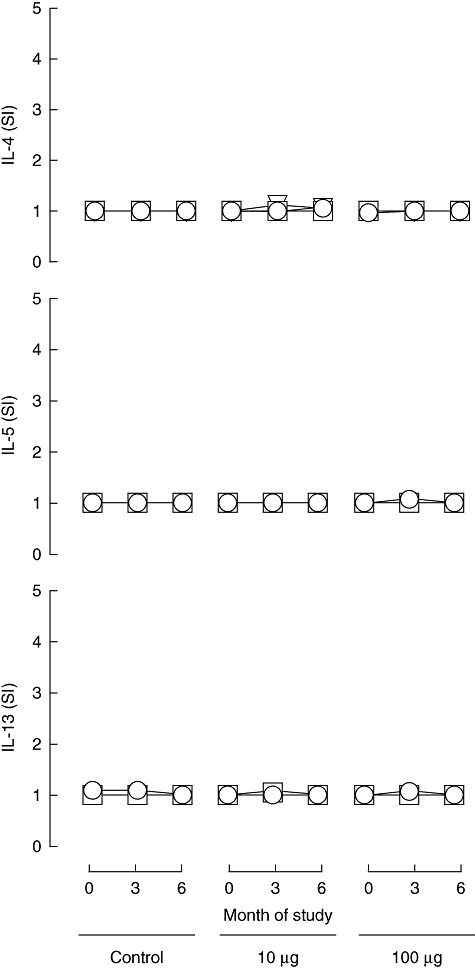
Th2 responses to proinsulin peptide. Mean (s.e.m.) stimulation indices [(number of spots in test)/(number in control)] for cytokine ELISPOT reactivity to proinsulin peptide for IL-4, IL-5 and IL-13 at 0-, 3- and 6-month duration of the study in the different treatment groups (control subjects, n = 12; subjects in 10 mg dose group, n = 18; subjects in 100 mg dose group, n = 18; these represent the same subjects as shown in Figs 2, 4 and 5). Circle, triangle and square symbols represent 1, 5 and 10 mM concentrations of peptide used in the ELISPOT assays in vitro.
The IFN-γ response to proinsulin peptide C19-A3
Our second safety concern was the possible induction of proinflammatory Th1-type responses to the administered peptide, as we have shown previously that this type of CD4 T cell autoreactivity to islet autoantigens is a hallmark of new-onset type 1 diabetes [14,23] and it is considered widely to play a role in β cell damage. We therefore examined anti-peptide responses using cytokine ELISPOTs to detect IFN-γ, the prototypic type 1 cytokine. As shown in Fig. 4, baseline IFN-γ cytokine responses to proinsulin peptide were largely absent in these subjects with long-standing disease. This is consistent with previous reports that islet autoreactive proinflammatory responses tend to wane after diagnosis. One subject in each of the treatment groups had a significant response to proinsulin peptide at baseline (SI ≥ 3·0 at two peptide concentrations) but not at subsequent analyses.
Fig. 4.
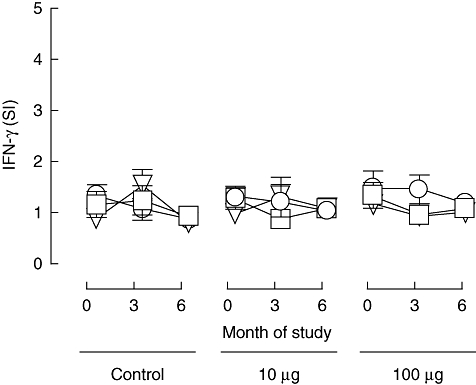
Proinflammatory responses to proinsulin peptide. Mean (s.e.m.) stimulation indices [(number of spots in test)/(number in control)] for cytokine ELISPOT reactivity to proinsulin peptide for IFN-γ at 0-, 3- and 6-month duration of the study in the different treatment groups (control subjects, n = 12; subjects in 10 mg dose group, n = 18; subjects in 100 mg dose group, n = 18; these represent the same subjects as shown in Figs 2, 4 and 5). Circle, triangle and square symbols represent 1, 5 and 10 mM concentrations of peptide used in the ELISPOT assays in vitro.
Considering the treatment groups as a whole, there was no evidence of induction of IFN-γ response to proinsulin peptide at 3 or 6 months after first peptide administration (Fig. 4). In one subject (10 µg group) a significant (SI ≥ 3·0 at two peptide concentrations) IFN-γ response to proinsulin peptide was observed at 3 months after enrolment and in another subject in the same group a significant response at 6 months after enrolment. In one subject (100 µg group) a significant response to peptide was observed at baseline and in one subject (control group) at the 3-month time-point. In contrast, recall IFN-γ responses to the pentavalent vaccine Pediacel were detected frequently (data not shown). Taken together, these data suggest that IFN-γ responses to the proinsulin C19-A3 epitope, which are known to be highly prevalent among DR4 subjects at diagnosis, have largely waned after diagnosis to become only sporadically detectable. There was no consistent indication of induction of a peptide-specific IFN-γ response following peptide administration and no significant changes in the group means.
The IL-10 response to proinsulin peptide C19-A3
In order to address the possibility that peptide immunotherapy is able to influence immune regulation, we examined peptide-specific IL-10 response by cytokine ELISPOT, as production of this cytokine is associated with subsets of CD4 T cells that have a regulatory phenotype. As shown in Fig. 5, mean baseline IL-10 responses to proinsulin peptide were low or absent in all study groups. In the 10 µg dosing group, there was a rise in mean IL-10 responses at all in vitro peptide concentrations at 3 months after enrolment, which was significant at the 5 µM peptide concentration in vitro (P = 0·05) but not at 10 µM (P = 0·15). This change was due largely to the effect on the group mean of four of 18 subjects who showed a significant (SI ≥ 3·0 at two peptide concentrations) response to peptide at this time-point, although this frequency of responders was not significant when compared with the untreated control group. Mean IL-10 responses to peptide (SI) declined between 3 and 6 months in the 10 µg dosing group (P = 0·08 and P = 0·01, respectively, at the 5 µM and 10 µM in vitro peptide concentrations). In the untreated control group, one subject responded significantly (SI ≥ 3·0 at two peptide concentrations) at 3 months to proinsulin peptide. In the 100 µg dosing group a similar IL-10 response was observed in one subject at baseline and one at 6 months. In neither of these groups was any significant change in mean IL-10 responses seen.
Fig. 5.
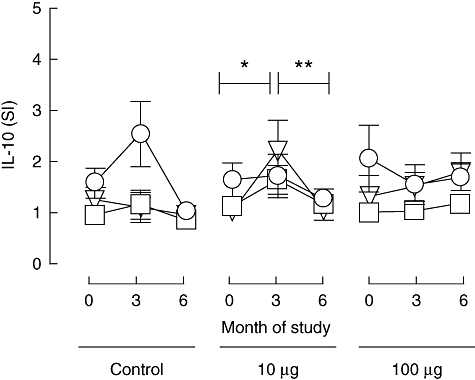
IL-10 responses to proinsulin peptide. Mean (s.e.m.) stimulation indices [(number of spots in test)/(number in control)] for cytokine ELISPOT reactivity to proinsulin peptide at 0-, 3- and 6-month duration of the study in the different treatment groups (control subjects, n = 12; subjects in 10 mg dose group, n = 18; subjects in 100 mg dose group, n = 18; these represent the same subjects as shown in Figs 2, 3 and 5). Circle, triangle and square symbols represent 1, 5 and 10 mM concentrations of peptide used in the ELISPOT assays in vitro. *Signifies that among the 10 mg dosing group the mean stimulation index rose between 0 and 3 months (P = 0·05 after in vitro stimulation with 5 mM peptide and P = 0·15 after in vitro stimulation with 10 mM); **signifies that it fell between 3 and 6 months (P = 0·08 after in vitro stimulation with 5 mM and P = 0·01 after in vitro stimulation with 10 mM peptide).
Designation of favourable response to proinsulin peptide administration
Using our predetermined criteria (see Methods), one subject in each of the peptide treatment groups was designated a ‘favourable responder’ through having a significant IFN-γ response to proinsulin peptide at baseline (SI ≥ 3·0 at two peptide concentrations), which was absent at all subsequent analyses. Similarly, four subjects in the 10 µg and one control subject were designated as ‘favourable responders’ through having induction of significant IL-10 responses (SI ≥ 3·0 at two peptide concentrations) to proinsulin peptide at 3 months. Examples of these responses are shown in Fig. 6.
Fig. 6.
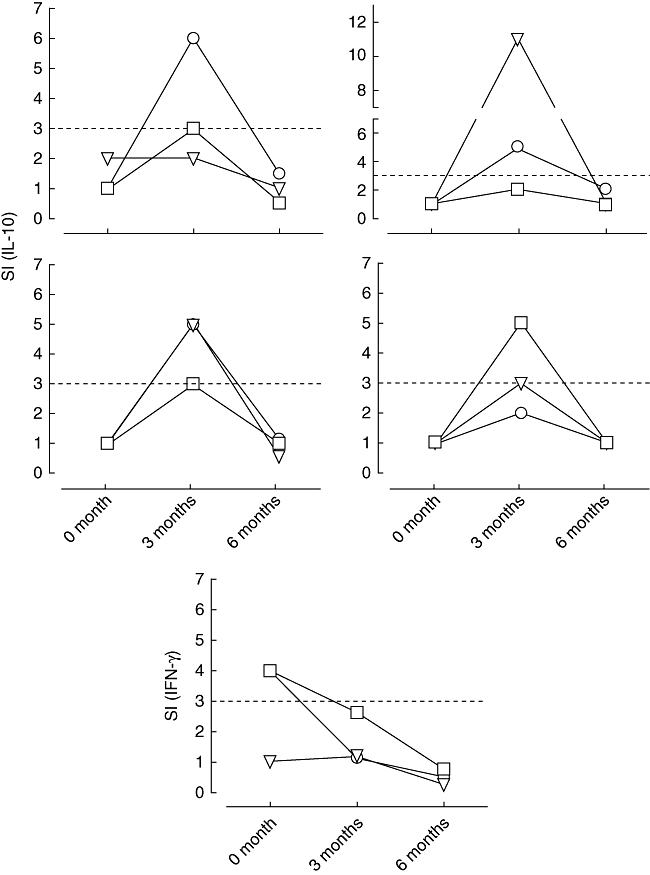
Representative examples of the five patients who received proinsulin peptide immunotherapy at the 10 mg dose and showed a ‘favourable’ T-cell response, either designated as induction of IL-10 secreting cells or designated as loss of IFN-γ secreting cells at 3 months [signified by stimulation index (SI) 33 at 32 peptide concentrations; dotted line indicates the cut-off]. Circle, triangle and square symbols represent 1, 5 and 10 mM concentrations of peptide used in the ELISPOT assay in vitro.
Glycaemic control
At baseline, there was no significant difference in HbA1c level between any of the study groups. Mean [standard deviation (s.d.)] value in the 10 µg dosing group was 7·70% (0·99); in the 100 µg group 8·41% (0·91) and in the control group 7·9% (1·03). No significant change in HbA1c values was observed at 3 months in any of the study groups (Fig. 7a). However, at 6 months, HbA1c levels in the 10 µg dosing group fell significantly compared with baseline [mean change −0·23%, 95% confidence interval (CI) −0·42 to −0·03, P = 0·02] and compared with the control subjects (mean change in controls subjects %HbA1c 0·35, 95% CI −0·01 to 0·71, P = 0·02) (Fig. 7b). Of interest, HbA1c levels fell in all five subjects in the 10 µg dosing group who were considered to have a favourable T cell response to peptide therapy at 3 months, compared with only four of the 12 subjects who did not show a T cell response at this time (P = 0·03) (Fig. 7). In this dosing group, mean change in HbA1c was higher among T cell responders than non-responders, although this difference did not reach statistical significance (P = 0·06). In the 100 µg dosing group, there was no significant change in HbA1c level at any time-point. Of the two subjects in this group who had a favourable T cell response to treatment, one showed a fall and the other showed a rise in HbA1c level.
Fig. 7.
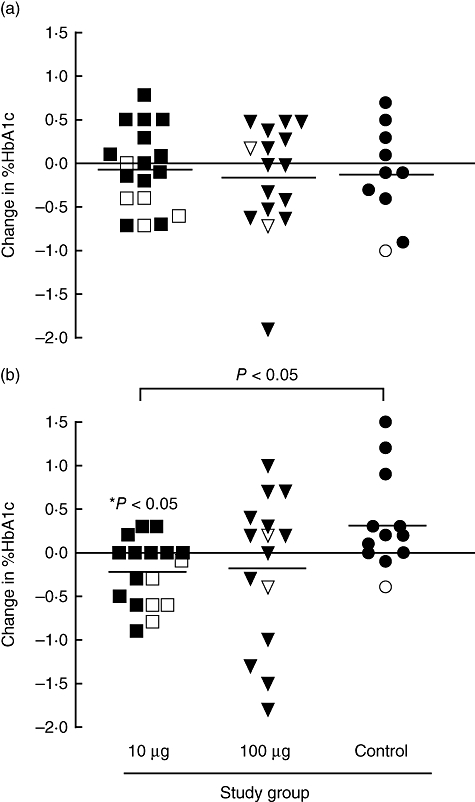
Change in HbA1c. Graph shows change in HbA1c level (%) between baseline and (a) 3 months (data available on 18, 14 and 10 subjects in the 10 mg, 100 mg and control groups respectively) and (b) between baseline and 6 months for each subject in each treatment group (data available on 17, 15 and 12 subjects in the 10 mg, 100 mg and control groups respectively). Subjects classified as showing favourable T-cell responses (designated as induction of IL-10 secreting cells or loss of IFN-γ secreting cells at 3 months; see Fig. 6) are shown with open symbols. Panel (b) shows that mean change in HbA1c is significantly greater in subjects in the 10-mg dosing group compared with control subjects (P < 0·05) at 6 months. *Signifies that the mean change in HbA1c (reduction from baseline) is significant in the 10 mg group (P < 0·05) but not the other groups and that in the 10 mg dosing group the IL-10 responders had a significantly higher frequency of reduction in HbA1c than the non-responder group (P < 0·05).
Anti-proinsulin peptide and islet cell antibodies
None of the subjects had detectable IgG class anti-proinsulin peptide antibodies at baseline or during the course of the study. In six subjects in whom skin reactions to peptide injection were observed, IgE-class anti-peptide antibodies were also measured, but were not detectable. There were no significant changes in levels of anti-GAD65, anti-IA-2 or anti-insulin antibodies in any of the treatment groups.
Discussion
This is the first study in which a natural peptide sequence representing a CD4 T cell epitope of an islet autoantigen has been administered to patients with type 1 diabetes. The study shows that intradermal injection of proinsulin C19-A3 is both well tolerated and safe. In particular, our study shows no evidence that proinsulin peptide immunotherapy is associated with risk of systemic allergic hypersensitivity or induction/reactivation of potentially damaging proinflammatory T cell responses. Given that these represent the major theoretical hazards of this approach, our study signifies an important milestone in the clinical evaluation of peptide immunotherapy for type 1 diabetes.
Peptide immunotherapy has been used in the successful prevention and treatment of numerous animal models of human inflammatory and autoimmune diseases, including type 1 diabetes [10]. In terms of clinical translation, the most experience thus far has been gained in the setting of type I (allergic) hypersensitivity. A recent review highlighted the fact that 418 patients with cat and bee venom allergies have been treated in 11 studies, 10 of which reported positive findings, including improved symptom scores and changes in T cell responses to allergen, including IL-10 production and induction of allergen-specific regulatory T cells [11]. Given the success of peptide immunotherapy in this clinical setting, it may seem paradoxical that one of the main safety concerns addressed in the present study was induction of allergy and anaphylaxis. This concern was based on studies in the non-obese diabetic mouse model involving administration of the dominant CD4 T cell autoantigenic epitope insulin B9-23 [24,25]. However, in these studies relatively large quantities (total > 1 mg) and repeated (seven times) dosing of peptide were used. Our own study shows that within the range of a total dose of 300 µg given over three injections in man, there was neither clinical nor immunological evidence of a systemic hypersensitivity response. It remains a possibility that the skin reactions we observed were due to local hypersensitivity reactions that were not associated with systemic changes in T cell or antibody responses.
Our second safety concern related to induction/reactivation of proinflammatory, proinsulin-specific autoreactivity. In the absence of any observed induction of anti-peptide antibodies or emergence of proinsulin peptide-specific CD4 T cells secreting the proinflammatory cytokine IFN-γ (a hallmark of type 1 diabetes diagnosis; [14]) it seems reasonable to conclude that disease exacerbation may be of limited concern. This is consistent with studies in animal models, in which induction or acceleration of disease through antigen administration is exceedingly rare [10]. However, it is noteworthy that proinflammatory responses to proinsulin peptide C19-A3 were not detectable at baseline in the long-standing patients enrolled in this study. This signifies a difference between patients with long-standing disease and those with new-onset type 1 diabetes, as we and others have shown that near to diagnosis T cell reactivity against the C19-A3 region is a prominent feature of disease [10,14,15,26,27]. Thus, there remains a theoretical possibility that peptide administration under the latter circumstances could promote activation of primed autoreactive T cells and accelerate β loss. Studies on patients near to diagnosis will be required to evaluate this potential risk.
The mechanisms through which peptide immunotherapy mediates its beneficial effects are not known with certainty, but include induction of anergy and deletion of pathogenic T cells that target the administered sequence [10]. There is also evidence for induction of CD4 T cells with regulatory properties [19]. Peptide immunotherapy promotes dominant presentation by immature dendritic cells through direct binding to surface major histocompatibility complex (MHC) class II molecules, conditions known to favour the induction of CD4 T cells secreting IL-10 [18,28], and such cells have been observed following peptide immunotherapy for clinical allergy [28]. Our study offers preliminary evidence that proinsulin peptide immunotherapy is associated with the appearance of C19-A3-specific IL-10+ CD4 T cells. It was notable that the appearance of such cells was relatively short-lived, with the number detected declining significantly between months 3 and 6 in the 10 µg group. It should be emphasized that the study had limited power to examine the induction of immune regulation, especially in subjects with long-standing disease in whom there was no pre-existing proinflammatory response. The IL-10 phenotype has been associated with later age of onset of type 1 diabetes [14] and improved glycaemic control [29,30], and therefore represents an important potential surrogate of immune regulation. Future studies in new-onset patients with type 1 diabetes will be required to explore whether induction of these cells is reproducible and enhanced by repeated or extended dosing.
Finally, the observation that metabolic control at 6 months was improved significantly in the low-dose peptide group compared with control subjects is intriguing. In our study design, control subjects did not receive any form of injection, and it is possible that the improved metabolic control we observed in the 10 µg-treated group is a placebo effect among subjects undergoing the true intervention. However, it is worth noting that the improved metabolic control was observed at month 6, following a period of 3 months during which neither the treatment nor control groups had any interaction with the clinical team. Furthermore, there is the additional observation that all the subjects receiving low-dose peptide in whom a favourable T cell response to peptide was noted had improved HbA1c levels, a significant difference in frequency compared with non-responders. Beneficial effects on HbA1c levels were not seen in the 100 µg-treated group, in whom favourable T cell responses were not seen. These results should be interpreted cautiously, and as we did not collect information on the insulin requirements of subjects during the study there is no additional corroborative evidence of an effect of peptide therapy on metabolic control. Whether proinsulin peptide injections induced a regulated immunological environment that allowed insulin-secreting cells, known to exist in the pancreata of patients with long-standing type 1 diabetes in close proximity to T cells and macrophages [31], to exert functional effects, remains a speculation that will be best resolved in Phase II studies.
Acknowledgments
This work was supported by grants from the Diabetes Vaccine Development Centre, Australia working in concert with the Juvenile Diabetes Research Foundation and the National Health and Medical Research Council of Australia. The authors also acknowledge financial support from the Department of Health via the National Institute for Health Research (NIHR) comprehensive Biomedical Research Centre award to Guy's and St Thomas’ NHS Foundation Trust in partnership with King's College London. We are grateful to Professor Mark Larché for helpful discussions and to the patients who participated in this study. We would like to acknowledge the contribution to this work of William Hall, who died in tragic circumstances shortly after completion of his undergraduate studies at King's College London (Biomedical Sciences, 2003–06).
Note in proof
After the data in this report were obtained, the investigators were notified that the filter used to sterilise the peptide by Clinalfa had not been tested correctly. Sterility and pyrogen testing of aliquots of the peptide were, however, consistently negative throughout the study. By agreement with the UK Medicines and Healthcare Regulatory Agency, no further doses of the peptide were given after February 2008.
References
- 1.Staeva-vieira T, Peakman M, von Herrath M. Translational mini-review series on type 1 diabetes: immune-based therapeutic approaches for type 1 diabetes. Clin Exp Immunol. 2007;148:17–31. doi: 10.1111/j.1365-2249.2007.03328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bougneres PF, Landais P, Boisson C, et al. Limited duration of remission of insulin dependency in children with recent overt type I diabetes treated with low-dose cyclosporin. Diabetes. 1990;39:1264–72. doi: 10.2337/diab.39.10.1264. [DOI] [PubMed] [Google Scholar]
- 3.Herold KC, Hagopian W, Auger JA, et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med. 2002;346:1692–8. doi: 10.1056/NEJMoa012864. [DOI] [PubMed] [Google Scholar]
- 4.Keymeulen B, Vandemeulebroucke E, Ziegler AG, et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med. 2005;352:2598–608. doi: 10.1056/NEJMoa043980. [DOI] [PubMed] [Google Scholar]
- 5.Shoda LK, Young DL, Ramanujan S, et al. A comprehensive review of interventions in the NOD mouse and implications for translation. Immun. 2005;23:115–26. doi: 10.1016/j.immuni.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Diabetes Prevention Trial-T1D Study Group. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med. 2002;346:1685–91. doi: 10.1056/NEJMoa012350. [DOI] [PubMed] [Google Scholar]
- 7.Skyler JS, Krischer JP, Wolfsdorf J, et al. Effects of oral insulin in relatives of patients with type 1 diabetes: the diabetes prevention trial – type 1. Diabetes Care. 2005;28:1068–76. doi: 10.2337/diacare.28.5.1068. [DOI] [PubMed] [Google Scholar]
- 8.Agardh CD, Cilio CM, Lethagen A, et al. Clinical evidence for the safety of GAD65 immunomodulation in adult-onset autoimmune diabetes. J Diabetes Complications. 2005;19:238–46. doi: 10.1016/j.jdiacomp.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Alleva DG, Maki RA, Putnam AL, et al. Immunomodulation in type 1 diabetes by NBI-6024, an altered peptide ligand of the insulin B epitope. Scand J Immunol. 2006;63:59–69. doi: 10.1111/j.1365-3083.2005.01705.x. [DOI] [PubMed] [Google Scholar]
- 10.Peakman M, Dayan CM. Peptide immunotherapy: fighting fire with fire? Immunology. 2001;104:361–6. doi: 10.1046/j.1365-2567.2001.01335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larche M. Update on the current status of peptide immunotherapy. J Allergy Clin Immunol. 2007;119:906–9. doi: 10.1016/j.jaci.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 12.Larche M, Wraith DC. Peptide-based therapeutic vaccines for allergic and autoimmune diseases. Nat Med. 2005;11:S69–76. doi: 10.1038/nm1226. [DOI] [PubMed] [Google Scholar]
- 13.McDevitt H. Specific antigen vaccination to treat autoimmune disease. Proc Natl Acad Sci USA. 2004;101(Suppl.)(2):14627–30. doi: 10.1073/pnas.0405235101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arif S, Tree TI, Astill TP, et al. Autoreactive T cell responses show proinflammatory polarization in diabetes but a regulatory phenotype in health. J Clin Invest. 2004;113:451–63. doi: 10.1172/JCI19585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Lorenzo TP, Peakman M, Roep BO. Translational mini-review series on type 1 diabetes: systematic analysis of T cell epitopes in autoimmune diabetes. Clin Exp Immunol. 2007;148:1–16. doi: 10.1111/j.1365-2249.2006.03244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oldfield WL, Larche M, Kay AB. Effect of T-cell peptides derived from Fel d 1 on allergic reactions and cytokine production in patients sensitive to cats: a randomised controlled trial. Lancet. 2002;360:47–53. doi: 10.1016/s0140-6736(02)09332-7. [DOI] [PubMed] [Google Scholar]
- 17.Raz I, Elias D, Avron A, Tamir M, Metzger M, Cohen IR. Beta-cell function in new-onset type 1 diabetes and immunomodulation with a heat-shock protein peptide (DiaPep277): a randomised, double-blind, phase II trial. Lancet. 2001;358:1749–53. doi: 10.1016/S0140-6736(01)06801-5. [DOI] [PubMed] [Google Scholar]
- 18.Wraith DC. Role of interleukin-10 in the induction and function of natural and antigen-induced regulatory T cells. J Autoimmun. 2003;20:273–5. doi: 10.1016/s0896-8411(03)00046-5. [DOI] [PubMed] [Google Scholar]
- 19.Verhoef A, Alexander C, Kay AB, Larche M. T cell epitope immunotherapy induces a CD4+ T cell population with regulatory activity. PLoS Med. 2005;2:e78. doi: 10.1371/journal.pmed.0020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bogdanos DP, Baum H, Sharma UC, et al. Antibodies against homologous microbial caseinolytic proteases P characterise primary biliary cirrhosis. J Hepatol. 2002;36:14–21. doi: 10.1016/s0168-8278(01)00252-5. [DOI] [PubMed] [Google Scholar]
- 21.Achenbach P, Warncke K, Reiter J, et al. Stratification of type 1 diabetes risk on the basis of islet autoantibody characteristics. Diabetes. 2004;53:384–92. doi: 10.2337/diabetes.53.2.384. [DOI] [PubMed] [Google Scholar]
- 22.Start RD, Barber C, Kaschula RO, Robinson RT. The ‘dead in bed syndrome’– a cause of sudden death in Type 1 diabetes mellitus. Histopathology. 2007;51:843–5. doi: 10.1111/j.1365-2559.2007.02829.x. [DOI] [PubMed] [Google Scholar]
- 23.Peakman M, Stevens EJ, Lohmann T, et al. Naturally processed and presented epitopes of the islet cell autoantigen IA-2 eluted from HLA-DR4. J Clin Invest. 1999;104:1449–57. doi: 10.1172/JCI7936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu E, Moriyama H, Abiru N, et al. Anti-peptide autoantibodies and fatal anaphylaxis in NOD mice in response to insulin self-peptides B:9-23 and B:13-23. J Clin Invest. 2002;110:1021–7. doi: 10.1172/JCI15488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu E, Moriyama H, Abiru N, et al. Preventing peptide-induced anaphylaxis: addition of C-terminal amino acids to produce a neutral isoelectric point. J Allergy Clin Immunol. 2004;114:607–13. doi: 10.1016/j.jaci.2004.03.052. [DOI] [PubMed] [Google Scholar]
- 26.Durinovic-Bello I, Boehm BO, Ziegler AG. Predominantly recognized proinsulin T helper cell epitopes in individuals with and without islet cell autoimmunity. J Autoimmun. 2002;18:55–66. doi: 10.1006/jaut.2001.0566. [DOI] [PubMed] [Google Scholar]
- 27.Narendran P, Williams AJ, Elsegood K, Leech NJ, Dayan CM. Humoral and cellular immune responses to proinsulin in adults with newly diagnosed type 1 diabetes. Diabetes Metab Res Rev. 2003;19:52–9. doi: 10.1002/dmrr.332. [DOI] [PubMed] [Google Scholar]
- 28.Tarzi M, Klunker S, Texier C, et al. Induction of interleukin-10 and suppressor of cytokine signalling-3 gene expression following peptide immunotherapy. Clin Exp Allergy. 2006;36:465–74. doi: 10.1111/j.1365-2222.2006.02469.x. [DOI] [PubMed] [Google Scholar]
- 29.Alizadeh BZ, Hanifi-Moghaddam P, Eerligh P, et al. Association of interferon-gamma and interleukin 10 genotypes and serum levels with partial clinical remission in type 1 diabetes. Clin Exp Immunol. 2006;145:480–4. doi: 10.1111/j.1365-2249.2006.03172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanda S, Roep BO, von Herrath M. Islet antigen specific IL-10(+) immune responses but not CD4(+)CD25(+)FoxP3(+) cells at diagnosis predict glycemic control in type 1 diabetes. Clin Immunol. 2008;127:138–43. doi: 10.1016/j.clim.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 31.Meier JJ, Bhushan A, Butler AE, Rizza RA, Butler PC. Sustained beta cell apoptosis in patients with long-standing type 1 diabetes: indirect evidence for islet regeneration? Diabetologia. 2005;48:2221–8. doi: 10.1007/s00125-005-1949-2. [DOI] [PubMed] [Google Scholar]


