Abstract
Autoimmune thyroid diseases are characterized by intrathyroidal infiltration of CD4+ and CD8+ T lymphocytes reactive to self-thyroid antigens. Early studies analysing T cell receptor (TCR) Vα gene usage have shown oligoclonal expansion of intrathyroidal T lymphocytes but not peripheral blood T cells. However, TCR Vβ diversity of the isolated CD4+ and CD8+ T cell compartments in the peripheral blood has not been characterized fully in these patients. We performed complementarity-determining region 3 (CDR3) spectratyping as well as flow cytometric analysis for the TCR Vβ repertoire in peripheral CD4+ and CD8+ T cells from 13 patients with Graves’ disease and 17 patients with Hashimoto's thyroiditis. Polyclonal TCR Vβ repertoire was demonstrated by flow cytometry in both diseases. In contrast, CDR3 spectratyping showed significantly higher skewing of TCR Vβ in peripheral CD8+ T cells but not CD4+ T cells among patients with Hashimoto's thyroiditis compared with healthy adults. We found trends towards a more skewed CDR3 size distribution in those patients having disease longer than 5 years and requiring thyroid hormone replacement. Patients with Graves’ disease exhibited no skewing both in CD4+ and CD8+ T cells. These findings indicate that clonal expansion of CD8+ T cells in Hashimoto's thyroiditis can be detected in peripheral blood and may support the role of CD8+ T cells in cell-mediated autoimmune attacks on the thyroid gland in Hashimoto's thyroiditis.
Keywords: autoimmune, Graves’ disease, Hashimoto's thyroiditis, TCR Vβ repertoire, spectratyping
Introduction
Autoimmune thyroid diseases, including Graves’ disease and Hashimoto's thyroiditis, are prototypes of human organ-specific autoimmune diseases. Hyperthyroidism in Graves’ disease is caused by thyroid-stimulating immunoglobulins that bind to and stimulate the thyroid-stimulating hormone receptor, whereas hypothyroidism in Hashimoto's thyroiditis is associated with autoantibodies to thyroperoxidase and thyroglobulins [1]. Variable numbers of locally infiltrating T and B lymphocytes may be involved in the production of such autoantibodies [2,3]. Indeed, approximately 10% of activated T cells infiltrating the thyroid gland in those patients has been shown to proliferate in response to thyroid cell antigens [4]. Analysis of the T cell receptor (TCR) repertoire is one of the most reliable tools to detect clonally expanded antigen-driven T cell populations. Although there is some controversy, studies of TCR Vα usage in patients with autoimmune thyroid diseases have demonstrated that clonally expanded T cells existed in the thyroid gland, and that TCR V gene expressions by intrathyroidal T cells were different from those found in peripheral T cells from the same patients [5–7].
A sensitive method to study TCR repertoire is to determine size distribution of cDNA for the complementarity-determining region 3 (CDR3) of TCR Vβ; that is, the hypervariable region generated by genetic rearrangements during T cell maturation in the thymus [8]. This CDR3 spectratyping allows us to determine clonal dominance or restriction within T cell populations. Using this method, in combination with flow cytometric analysis for TCR Vβ, we analysed the entire TCR Vβ repertoire both in peripheral CD4+ and CD8+ T cells among patients with autoimmune thyroid diseases and found significant skewing of TCR Vβ usage in circulating CD8+ T cells from Hashimoto's thyroiditis. We also discuss the correlation between the TCR Vβ restriction and clinical features in those patients.
Materials and methods
Patients
We studied 30 Japanese patients affected with autoimmune thyroid diseases: 13 with Graves’ disease and 17 with Hashimoto's thyroiditis (Table 1). All but three patients with Hashimoto's thyroiditis showed disease onset before 18 years of age. The diagnosis was based on clinical data and thyroid ultrasound imaging, in which all patients showed moderately heterogeneous, reduced echogenicity. Two patients had other autoimmune diseases: glomerulonephritis associated with anti-neutrophil cytoplasmic antibodies existed in patient 11 of Graves’ disease, and type 1 diabetes in patient 3 of Hashimoto's thyroiditis. No patients underwent fine-needle biopsy. All patients with Graves’ disease were treated with anti-thyroid drugs (methimazole or propylthiouracil) because of hyperthyroidism, and euthyroidism was achieved in six patients at the time of sample collection. Because of hypothyroidism, nine of 17 patients with Hashimoto's thyroiditis received levothyroxine, and one patient remained hypothyroid at the time of sampling (patient 3). Disease duration of Hashimoto's thyroiditis patients with and without levothyroxine requirement was 8·3 ± 7·0 and 7·5 ± 5·4 respectively. No patient was under treatment with immunomodulant therapies. Approval for the study was obtained from the Human Research Committee of Kanazawa University Graduate School of Medical Science, and informed consent was provided according to the Declaration of Helsinki.
Table 1.
Patient characteristics.
| Graves’ disease | Hashimoto's thyroiditis | Normal range | |
|---|---|---|---|
| No. of cases | 13 | 17 | |
| Sex (no. male/female) | 4/9 | 2/15 | |
| Age* (years, mean ± s.d.) | 15·6 ± 4·2 | 21·0 ± 6·8 | |
| (no. < 15 years/≥ 15 years) | 4/9 | 4/13 | |
| Disease duration (years, mean ± s.d.) | 3·9 ± 5·0 | 7·9 ± 6·1 | |
| (no. < 5 years/≥ 5 years) | 8/5 | 6/11 | |
| Serum FT3* (pg/ml) | 6·14 ± 3·60 | 2·86 ± 0·38 | 2·20–4·30 |
| Serum FT4* (ng/dl) | 2·03 ± 0·9 | 1·17 ± 0·27 | 0·80–1·80 |
| Serum TSH* (µU/ml) | 0·27 ± 0·79 | 6·89 ± 16·30 | 0·27–4·65 |
| AbTg* (IU/ml) | 749·3 ± 1037·3 | 499·3 ± 739·1 | ≤ 44·0 |
| AbTPO* (IU/ml) | 315·6 ± 220·5 | 238·4 ± 251·8 | ≤ 7·2 |
Data at sample collection. FT3, free triiodothyronine; FT4, free thyroxine; TSH, thyroid-stimulating hormone; AbTg, anti-thyroglobulin antibody; AbTPO, anti-thyroid peroxidase antibody; s.d., standard deviation.
Cell preparations
Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque gradient centrifugation from the patients and controls. CD4+ and CD8+ T cells were purified by positive selection from PBMCs using monoclonal antibody (mAb)-coated magnetic beads according to the manufacturer's instructions (Becton Dickinson, San Diego, CA, USA) [9]. The purity of each isolated cell population exceeded 90% routinely, as assessed by flow cytometric analysis.
The CDR3 spectratyping
Total RNA was extracted from CD4+ and CD8+ T cells with Trizol reagent (Invitrogen, Carlsbad, CA, USA), and first-strand cDNA was generated from 2 µg total RNA with random hexamers and RAV-2 reverse transcriptase (Takara Bio Inc., Shiga, Japan). CDR3 size distribution assay was performed as described [10,11]. Briefly, each TCR Vβ fragment was amplified with one of the 25 Vβ-specific primers and a 6-fluorescein phosphoramidite-labelled Cβ primer recognizing both Cβ1 and Cβ2. Thirty-five cycles of amplification (94°C for 1 min, 55°C for 1 min, and 72°C for 1 min) were used. One µl of 1:10 diluted polymerase chain reaction (PCR) products was mixed with 12 µl of deionized formamide and 0·5 µl of size standard and heated at 95°C for 3 min. The size distribution of each fluorescent PCR product was determined by electrophoresis in performance optimized polymer 4 on the Applied Biosystems 310 automated sequencer, and data were analysed using GeneScan software (Applied Biosystems, Foster City, CA, USA). To assess each individual profile as normal or skewed, we used a previously reported complexity scoring system [12,13]; that is, a complexity score = (sum of all the peak heights/sum of the major peak heights) × (number of the major peaks). Major peaks were defined as those on the spectratype histogram whose amplitude was at least 10% of the sum of all the peak heights. The mean complexity scores of healthy adult controls was 5·06 (range: 4·89–5·19), as reported previously [13]. The number of TCR Vβ subfamilies with skewed CDR size pattern, as shown by a complex score lower than 4, was determined for each subject [13,14]. Analysis of differences among data groups was performed using the Student's t-test for unpaired samples. Values of P less than 0·05 were considered significant.
Flow cytometric analysis for TCR Vβ repertoire
Three-colour immunofluorescence analysis was used to study TCR Vβ repertoire distribution, as described previously [11]. Briefly, after washing twice in phosphate-buffered saline, peripheral blood samples were incubated with appropriate phycoerythrin (PE)-conjugated mAbs with specificity for TCR Vβ 1-23 (Immunotech, Marseille, France), fluorescein isothiocyanate-conjugated anti-CD8 (Becton Dickinson) and R-PE-Cy5-conjugated anti-CD4 (Dako, Glostrup, Denmark) mAbs. After lysis of erythrocytes and washing, stained cells were analysed with a fluorescence activated cell sorter Calibur flow cytometer using CellQuest software (BD Bioscience, Tokyo, Japan). TCR Vβ expression was represented as a percentage of CD4+ or CD8+ cells for each family.
Three-dimensional graphic display of TCR diversity
Qualitative alterations of TCR Vβ repertoire obtained by CDR3 spectratyping were combined with the quantity of specific Vβ+ CD4+ and CD8+ T cells for each Vβ subfamily and plotted as landscape columns, as described previously [11,15].
Results
Skewed CDR3 size pattern in Hashimoto's thyroiditis but not Graves’ disease
In healthy controls, the majority of Vβ subfamilies exhibited a Gaussian curve with six peaks or more, reflecting a diverse TCR repertoire. Consistent with previous reports [16], CD8+ T cells exhibited a more skewed CDR3 profile than CD4+ T cells regardless of the presence of disease, probably because of age-related CD8+ T cell clonal expansion (Fig. 1a–c). All 25 different Vβ segments were amplified from CD4+ and CD8+ T cells obtained from each patient's sample. As shown in Fig. 1b and 1c, patients with Graves’ disease showed a diverse distribution in the majority of their Vβ subfamilies both in CD4+ and CD8+ T cells. In contrast, the frequency of skewed TCR Vβ subfamilies of patients with Hashimoto's thyroiditis was significantly higher than that of Graves’ disease and healthy controls in CD8+ T cells but not CD4+ T cells. No evidence was found for preferential skewing of particular Vβ subfamilies in patients with Hashimoto's thyroiditis. Because none of the patients had clinical and laboratory evidence of acute infection at the time of sample collection, we conclude that these alterations reflect a stable state of the TCR repertoire in these patients.
Fig. 1.
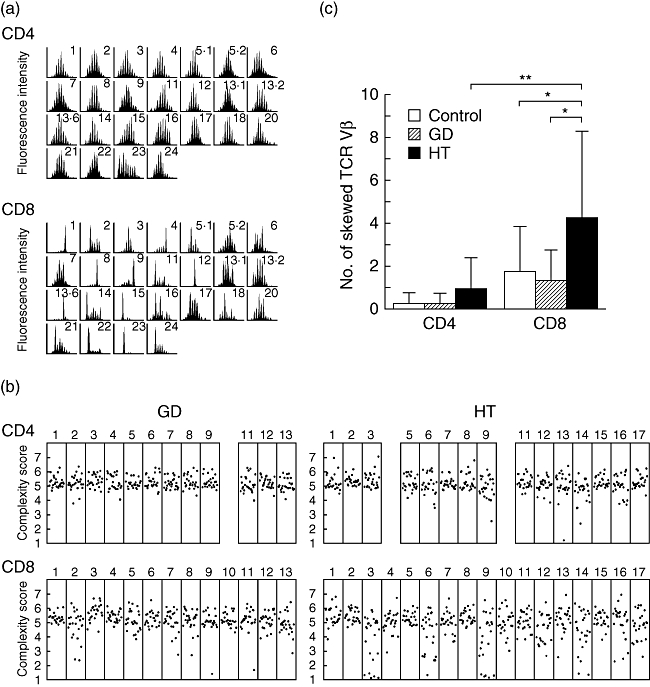
CDR3 spectratyping of T cell receptor (TCR) Vβ. (a) CDR3 size distribution. Each TCR Vβ fragment was amplified from cDNA with one of 25 Vβ-specific primers. The size distribution of polymerase chain reaction (PCR) products was determined by an automated sequencer and GeneScan software. Shown are the results of CDR3 size distribution in CD4+ and CD8+ T cells from patient 16 of Hashimoto's thyroiditis. (b) CDR3 complexity scores. Complexity scores for each TCR Vβ were shown. (c) Frequency of skewed TCR Vβ repertoire. Shown are the mean (±standard deviation) numbers of skewed TCR Vβ obtained from CD4+ and CD8+ T cells of the control and patients with Graves’ disease and with Hashimoto's thyroiditis. GD, Graves’ disease; HT, Hashimoto's thyroiditis. *P < 0·05; **P < 0·01.
To elucidate further the characteristics of skewing of TCR Vβ usage in Hashimoto's thyroiditis, those patients were divided into different subgroups based on age, disease duration and levothyroxine requirement (Fig. 2a–c). Although clonal T cell expansion can be seen in healthy individuals with age [16], no difference was observed between patients 14 years of age or younger and those older. In contrast, there were trends towards more skewing of CDR3 size distribution in CD8+ T cells from patients having disease longer than 5 years and requiring replacement therapy of levothyroxine. Of note, six of nine patients with Hashimoto's thyroiditis who showed a more skewed pattern in CD8+ T cells (no. of skewed TCR Vβ, 6·5 ± 3·8) and having disease longer than 5 years required replacement therapy. Numbers of skewed TCR Vβ did not correlate with the thyroid hormones levels or autoantibody titres in patients with Hashimoto's thyroiditis (data not shown).
Fig. 2.
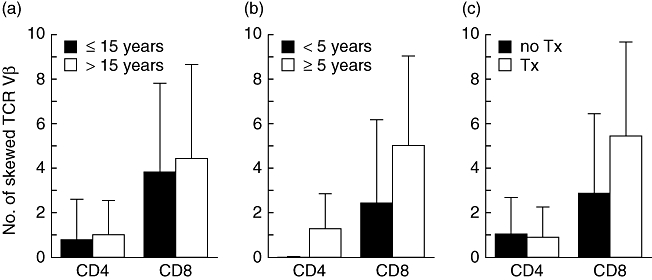
Frequency of skewed T cell receptor (TCR) Vβ in subgroups of Hashimoto's thyroiditis. Shown are the mean (±standard deviation) numbers of skewed TCR Vβ obtained from CD4+ and CD8+ T cells in each subgroup divided based on (a) age, (b) disease duration and (c) levothyroxine requirement of Hashimoto's thyroiditis. Tx, treatment.
Polyclonal TCR Vβ repertoire by mAbs in both diseases
The relative TCR Vβ usage of CD4+ and CD8+ T cells was also investigated using a panel of mAbs specific for 20 different Vβ regions (Fig. 3a,b). None of the patients with Graves’ disease and Hashimoto's thyroiditis exhibited a high percentage (> 30%) of T cells expressing a certain Vβ. Although relative expansion of TCR Vβ subfamilies (i.e. above the mean ± 2 standard deviations) was seen in both diseases, the frequency was not high either in CD4+ or CD8+ T cells, and predominance of expansions in CD8+ T cells was not seen, unlike in CDR3 spectratyping (Fig. 3b). In addition, flow cytometric analysis demonstrated that the Vβ subfamily having a skewed CDR3 size pattern in CD4+ and CD8+ T cells in a particular patient did not necessarily show relative expansion compared with controls. No significant differences in the percentage of peripheral CD3+, CD4+ and CD8+ T cells, the ratio of CD4+ to CD8+ T cells and the percentage expression of CD45RO+ memory marker in CD4+ and CD8+ T cells were found among Graves’ disease, Hashimoto's thyroiditis and control subjects (data not shown).
Fig. 3.
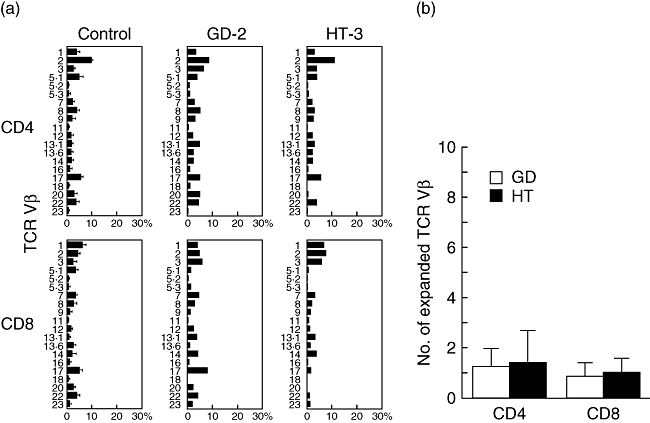
Flow cytometric analysis of T cell receptor (TCR) Vβ. (a) Expression profiles of TCR Vβ subfamilies. Peripheral blood samples were stained with monoclonal antibodies (mAbs) for individual TCR Vβ together with anti-CD4 and anti-CD8 mAbs. The percentage of each TCR Vβ expression within CD4+ and CD8+ T cells was analysed by flow cytometry. (b) Frequency of expanded TCR Vβ repertoire. Shown are the mean (±standard deviation) numbers of expanded TCR Vβ obtained from CD4+ and CD8+ T cells of patients with Graves’ disease and Hashimoto's thyroiditis.
Combined qualitative and quantitative analysis
To assess the pattern of clonal CD8+ T cell dominance in Hashimoto's thyroiditis in more detail, we used a combined analysis of TCR Vβ repertoire distribution and CDR3 size distribution. Although such analysis provided a better visual representation, again there was no evidence for preferential expansion of particular Vβ subfamilies (Fig. 4).
Fig. 4.
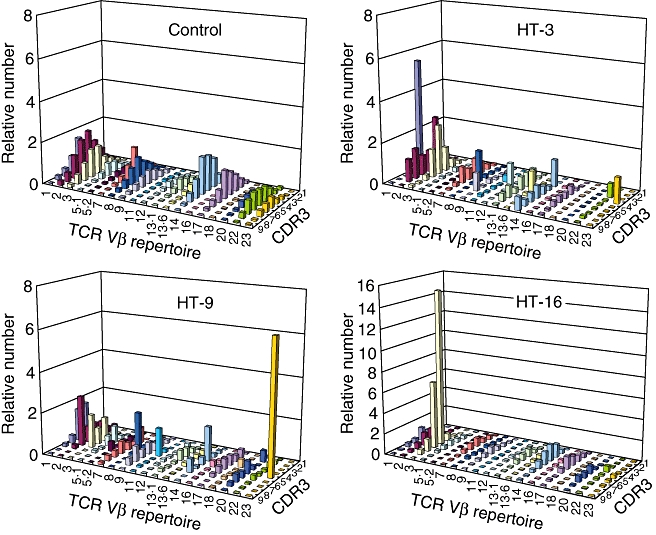
Combined display of flow cytometric analysis of T cell receptor (TCR) Vβ and CDR3 spectratyping. Data from qualitative (CDR3 spectratyping) and quantitative (flow cytometric analysis for TCR Vβ) analysis are displayed visually as graphic landscape columns for CD4+ and CD8+ T cells.
Discussion
Early studies of intrathyroidal T lymphocytes from patients with Graves’ disease and Hashimoto's thyroiditis demonstrated significant restriction in TCR Vα but not in Vβ gene usage [5,6]. Restricted usage of TCR Vα as well as TCR Vβ was also shown in T cells infiltrating extrathyroidal sites in addition to thyroid from Graves’ disease [17]. On the contrary, other reports demonstrated no restriction of intrathyroidal TCR Vα subfamilies in Graves’ disease [18–20]. In Hashimoto's thyroiditis, a marked restriction of Vα usage in intrathyroidal CD8+ T cell but not in CD4+ T cell has been shown in a small-scale study [7]. These controversies over the presence of restricted TCR Vα and Vβ usage in T cells from patients with autoimmune thyroid diseases may be due to the different sensitivity of each method used for assessing the TCR repertoire. In addition, in most studies whole T lymphocytes but not isolated CD4+ and CD8+ T cells were utilized, resulting in possible underestimation of TCR restriction. On the other hand, no restriction has been demonstrated in peripheral blood T cells from patients with autoimmune thyroid diseases, even though intrathyroidal T cells showed restricted TCR Vα usage [5–7,17]. Because organ-specific autoimmune disorders may result from molecular mimicry between environmental antigens and self-antigens of target organs, thyroid-infiltrating lymphocytes are assumed to play a pathogenic role and are the most suitable source to assess TCR restriction in autoimmune thyroid diseases. However, the availability of such samples may be limited because thyroid fine-needle aspiration and biopsies are performed rarely in those patients. Recent advances in molecular and cellular techniques analysing the TCR Vβ repertoire may allow us to obtain a reliable assessment of autoimmune conditions in peripheral blood [21,22]. We therefore set out to study in more detail the possible presence of TCR Vβ restriction among circulating CD4+ and CD8+ T cells from patients with autoimmune thyroid diseases.
In the present study we demonstrated significantly higher skewing of TCR Vβ in peripheral CD8+ T cells by using CDR3 spectratyping among patients with Hashimoto's thyroiditis compared with healthy adults. Interestingly, the patients requiring thyroid hormone replacement who may have suffered from more severe autoimmune attacks on the thyroid gland tended to have more skewing of CDR3 size distribution than those without replacement therapy, which supports the idea that these TCR alterations in peripheral CD8+ T cells may be reflected in the local events and may be involved in the development and maintenance of chronic thyroiditis. Similar results were obtained from the study of the TCR Vβ repertoire by using CDR3 spectratyping of patients affected with myasthenia gravis, another organ-specific autoimmune disorder, where persistent TCR expansions in peripheral blood T cells correlated with clinical severity [21]. Moreover, patients with disease longer than 5 years also tended towards more skewing of TCR Vβ compared with those with shorter disease duration in our cross-sectional study. These findings contrast with the hypothesis of Davies et al.[23], that TCR restrictions seen in the thyroid aspirates from patients with autoimmune thyroid diseases represent an early disease marker. However, autoantigens may be sustained in the thyroid gland and cause chronic antigenic stimulation, probably resulting in the accumulation of antigen-specific T cell clones. A longitudinal examination will be needed to explain the persistence and progression of TCR restriction in Hashimoto's thyroiditis. In contrast, flow cytometric analysis for TCR Vβ repertoire failed to show expansion or deletion of particular TCR Vβ either in CD4+ or CD8+ T cells. Because the circulating pathogenic T cells in a large pool of peripheral T cells may be very small, it is reasonable to assume that they seldom reach the detection threshold of flow cytometric analysis.
It has been demonstrated recently that interferon-γ-inducible chemokines such as CXC chemokine ligand 10 (CXCL10) play an important role in the initial stage of autoimmune disorders involving endocrine gland [24]. Indeed, elevated levels of circulating CXCL10 have been reported in autoimmune thyroid diseases [25]. Both lymphocytes and thyroid follicular cells may account for production of CXCL10 [24,26]. Although we had no data concerning chemokines, it is tempting to speculate that CD8+ T cells with skewed TCR Vβ might be associated with secretion of CXCL10. Further studies are necessary in order to address these important issues.
In Graves’ disease, CDR3 spectratyping and flow cytometric analysis of TCR Vβ repertoire demonstrated no restricted TCR Vβ usage either in peripheral CD4+ or CD8+ T cells regardless of the severity, duration of the disease or levels of the thyroid hormones. Although our methods were sensitive enough to detect clonal expansion in peripheral T cells in Hashimoto's thyroiditis, the possibility that specific antigen-driven T cells were mildly expanded in the thyroid gland but undetectable in the circulation of patients with Graves’ disease because of sensitivity problems cannot be ruled out. Alternatively, the absence of restricted TCR Vβ usage could be ascribed to anti-thyroid drugs themselves, as the drugs have immunomodulant actions [27]. Further studies assessing TCR Vβ repertoire in intrathyroidal and peripheral CD4+ and CD8+ T cells simultaneously will be useful to define the presence of clonal dominance in Graves’ disease.
In summary, our studies provide evidence for clonal expansion of peripheral CD8+ T cells from patients with Hashimoto's thyroiditis. Characterization of the clonal composition of such CD8+ T cells that may be associated with cell-mediated autoimmune attacks on the thyroid gland will provide important insights into the pathogenesis of the disease and deserve close investigation.
Acknowledgments
We thank Ms Harumi Matsukawa and Ms Shizu Kouraba for excellent technical assistance, Dr Akira Asai for clinical samples and Dr Yoshihito Kasahara for insightful discussion. This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan and a grant from the Ministry of Health, Labour and Welfare of Japan, Tokyo.
References
- 1.Caturegli P, Kimura H, Rocchi R, Rose NR. Autoimmune thyroid diseases. Curr Opin Rheumatol. 2007;19:44–8. doi: 10.1097/BOR.0b013e3280113d1a. [DOI] [PubMed] [Google Scholar]
- 2.Canonica GW, Bagnasco M, Cosulich ME, Torre G, McLachlan SM, Smith BR. Why thyroid is major site of thyroid autoantibody synthesis in autoimmune thyroid disease. Lancet. 1983;1:1163. doi: 10.1016/s0140-6736(83)92891-x. [DOI] [PubMed] [Google Scholar]
- 3.Utiger RD. The pathogenesis of autoimmune thyroid disease. N Engl J Med. 1991;325:278–9. doi: 10.1056/NEJM199107253250410. [DOI] [PubMed] [Google Scholar]
- 4.MacKenzie WA, Schwartz AE, Friedman EW, Davies TF. Intrathyroidal T cell clones from patients with autoimmune thyroid disease. J Clin Endocrinol Metab. 1987;64:818–24. doi: 10.1210/jcem-64-4-818. [DOI] [PubMed] [Google Scholar]
- 5.Davies TF, Martin A, Concepcion ES, Graves P, Cohen L, Ben-Nun A. Evidence of limited variability of antigen receptors on intrathyroidal T cells in autoimmune thyroid disease. N Engl J Med. 1991;325:238–44. doi: 10.1056/NEJM199107253250404. [DOI] [PubMed] [Google Scholar]
- 6.Davies TF, Martin A, Concepcion ES, et al. Evidence for selective accumulation of intrathyroidal T lymphocytes in human autoimmune thyroid disease based on T cell receptor V gene usage. J Clin Invest. 1992;89:157–62. doi: 10.1172/JCI115556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McIntosh RS, Watson PF, Weetman AP. Analysis of the T cell receptor V alpha repertoire in Hashimoto's thyroiditis: evidence for the restricted accumulation of CD8+ T cells in the absence of CD4+ T cell restriction. J Clin Endocrinol Metab. 1997;82:1140–6. doi: 10.1210/jcem.82.4.3868. [DOI] [PubMed] [Google Scholar]
- 8.Nikolich-Zugich J, Slifka MK, Messaoudi I. The many important facets of T-cell repertoire diversity. Nat Rev Immunol. 2004;4:123–32. doi: 10.1038/nri1292. [DOI] [PubMed] [Google Scholar]
- 9.Shibata F, Toma T, Wada T, et al. Skin infiltration of CD56bright CD16− natural killer cells in a case of X-SCID with Omenn syndrome-like manifestations. Eur J Haematol. 2007;79:81–5. doi: 10.1111/j.1600-0609.2007.00874.x. [DOI] [PubMed] [Google Scholar]
- 10.Wada T, Schurman SH, Otsu M, et al. Somatic mosaicism in Wiskott–Aldrich syndrome suggests in vivo reversion by a DNA slippage mechanism. Proc Natl Acad Sci USA. 2001;98:8697–702. doi: 10.1073/pnas.151260498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okamoto H, Arii C, Shibata F, et al. Clonotypic analysis of T cell reconstitution after haematopoietic stem cell transplantation (HSCT) in patients with severe combined immunodeficiency. Clin Exp Immunol. 2007;148:450–60. doi: 10.1111/j.1365-2249.2007.03378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bomberger C, Singh-Jairam M, Rodey G, et al. Lymphoid reconstitution after autologous PBSC transplantation with FACS-sorted CD34+ hematopoietic progenitors. Blood. 1998;91:2588–600. [PubMed] [Google Scholar]
- 13.Wada T, Schurman SH, Garabedian EK, Yachie A, Candotti F. Analysis of T-cell repertoire diversity in Wiskott–Aldrich syndrome. Blood. 2005;106:3895–7. doi: 10.1182/blood-2005-06-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mizuno K, Yachie A, Nagaoki S, et al. Oligoclonal expansion of circulating and tissue-infiltrating CD8+ T cells with killer/effector phenotypes in juvenile dermatomyositis syndrome. Clin Exp Immunol. 2004;137:187–94. doi: 10.1111/j.1365-2249.2004.02500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yawalkar N, Ferenczi K, Jones DA, et al. Profound loss of T-cell receptor repertoire complexity in cutaneous T-cell lymphoma. Blood. 2003;102:4059–66. doi: 10.1182/blood-2003-04-1044. [DOI] [PubMed] [Google Scholar]
- 16.Schwab R, Szabo P, Manavalan JS, et al. CD4+ and CD8+ T cell clones in elderly humans. J Immunol. 1997;158:4493–9. [PubMed] [Google Scholar]
- 17.Heufelder AE, Wenzel BE, Scriba PC. Antigen receptor variable region repertoires expressed by T cells infiltrating thyroid, retroorbital, and pretibial tissue in Graves’ disease. J Clin Endocrinol Metab. 1996;81:3733–9. doi: 10.1210/jcem.81.10.8855831. [DOI] [PubMed] [Google Scholar]
- 18.McIntosh RS, Tandon N, Pickerill AP, Davies R, Barnett D, Weetman AP. IL-2 receptor-positive intrathyroidal lymphocytes in Graves’ disease. Analysis of V alpha transcript microheterogeneity. J Immunol. 1993;151:3884–93. [PubMed] [Google Scholar]
- 19.McIntosh RS, Watson PF, Pickerill AP, Davies R, Weetman AP. No restriction of intrathyroidal T cell receptor V alpha families in the thyroid of Graves’ disease. Clin Exp Immunol. 1993;91:147–52. doi: 10.1111/j.1365-2249.1993.tb03370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caso-Pelaez E, McGregor AM, Banga JP. A polyclonal T cell repertoire of V-alpha and V-beta T cell receptor gene families in intrathyroidal T lymphocytes of Graves’ disease patients. Scand J Immunol. 1995;41:141–7. doi: 10.1111/j.1365-3083.1995.tb03546.x. [DOI] [PubMed] [Google Scholar]
- 21.Matsumoto Y, Matsuo H, Sakuma H, et al. CDR3 spectratyping analysis of the TCR repertoire in myasthenia gravis. J Immunol. 2006;176:5100–7. doi: 10.4049/jimmunol.176.8.5100. [DOI] [PubMed] [Google Scholar]
- 22.Mazziotti G, Sorvillo F, Naclerio C, et al. Type-1 response in peripheral CD4+ and CD8+ T cells from patients with Hashimoto's thyroiditis. Eur J Endocrinol. 2003;148:383–8. doi: 10.1530/eje.0.1480383. [DOI] [PubMed] [Google Scholar]
- 23.Davies TF, Concepcion ES, Ben-Nun A, Graves PN, Tarjan G. T-cell receptor V gene use in autoimmune thyroid disease: direct assessment by thyroid aspiration. J Clin Endocrinol Metab. 1993;76:660–6. doi: 10.1210/jcem.76.3.8445022. [DOI] [PubMed] [Google Scholar]
- 24.Rotondi M, Chiovato L, Romagnani S, Serio M, Romagnani P. Role of chemokines in endocrine autoimmune diseases. Endocr Rev. 2007;28:492–520. doi: 10.1210/er.2006-0044. [DOI] [PubMed] [Google Scholar]
- 25.Antonelli A, Rotondi M, Fallahi P, et al. High levels of circulating CXC chemokine ligand 10 are associated with chronic autoimmune thyroiditis and hypothyroidism. J Clin Endocrinol Metab. 2004;89:5496–9. doi: 10.1210/jc.2004-0977. [DOI] [PubMed] [Google Scholar]
- 26.Ejrnaes M, Videbaek N, Christen U, Cooke A, Michelsen BK, von Herrath M. Different diabetogenic potential of autoaggressive CD8+ clones associated with IFN-gamma-inducible protein 10 (CXC chemokine ligand 10) production but not cytokine expression, cytolytic activity, or homing characteristics. J Immunol. 2005;174:2746–55. doi: 10.4049/jimmunol.174.5.2746. [DOI] [PubMed] [Google Scholar]
- 27.Wilson R, McKillop JH, Chopra M, Thomson JA. The effect of antithyroid drugs on B and T cell activity in vitro. Clin Endocrinol (Oxf) 1988;28:389–97. doi: 10.1111/j.1365-2265.1988.tb03670.x. [DOI] [PubMed] [Google Scholar]


