Abstract
The nature of early interactions between Leishmania and macrophages which determine the outcome of infection can be related directly to parasite biological properties. Here we compared the capacity of L. major (Lm) strains, reported to be high (LmHV) and low virulent and (LmLV) in the mouse model and L. infantum (Li) strains, dermotropic (LiD) and viscerotropic (LiV), to infect and modulate cytokine production in human peripheral blood derived monocytes. Monocytes were infected with metacyclic promastigotes for 24, 48 and 72 h. Parasite burden was significantly higher in Lm- than in Li-infected monocytes. LmHV and LiD induced a significantly higher parasite burden than LmLV and LiV respectively. Cytokine production was evaluated in monocytes infected for 24 h. Contrary to interleukin (IL)-12p70, monocyte chemotactic protein-1 and transforming growth factor-β production was increased significantly in infected monocytes with no differences between strains. Lm isolates induced significantly higher quantities of tumour necrosis factor (TNF)-α than Li isolates. Low levels of IL-10 were induced by all Leishmania strains and, interestingly, co-stimulation with lipopolysaccharide (LPS) was accompanied by a dramatic increase in IL-10 production by infected monocytes. In conclusion, Lm isolates displaying different levels of virulence in mice exhibited significant differences in parasite burden but similar abilities to modulate cytokine production in human monocytes. Li strains showed weaker infectivity and TNF-α inducing-capacity compared with Lm strains. The dramatic increase of IL-10 production in infected monocytes co-stimulated by LPS may play a role in disease progression considering the presence of LPS during bacterial superinfections observed during human leishmaniasis.
Keywords: cytokines, Leishmania, monocyte, tropism, virulence
Introduction
Leishmania are intracellular protozoa that cause a wide spectrum of human diseases, ranging from self-healing cutaneous to lethal visceral leishmaniasis (VL) [1]. In Tunisia, L. major (Lm) causes cutaneous leishmaniasis (CL) which heals spontaneously [2], whereas L. infantum (Li) is mainly responsible for VL, fatal if untreated, [3] and occasionally for a sporadic form of CL with chronic and disfiguring lesions [4]. These various clinical outcomes are influenced by both differences in Leishmania species and nature of the host cellular immune response. In mice, resistance to Lm infection is based on an interleukin (IL)-12-driven T helper type 1 (Th1) response and interferon (IFN)-γ production resulting in macrophage activation and parasite killing. Development of Th2 cells with IL-10 and transforming growth factor (TGF)-β contribute to susceptibility by down-regulating Th1 cell differentiation and suppressing macrophage activity [5,6]. Information about the early interactions occurring between human monocytes or macrophages and Leishmania parasites is limited. These cells are the major site for parasite replication and, once a protective Th1 immune response is established, the principal effector cells responsible for their killing [7]. Among the mechanisms developed by Leishmania to promote their intracellular survival is the modulation of macrophage-associated cytokine production [8]. Leishmania promastigotes of different species are potent inhibitors of macrophage IL-12 production [9,10]. Tumour necrosis factor (TNF)-α was not detected in monocytes infected with L. donovani amastigotes [11] or with Lm or L. chagasi promastigotes [12,13], whereas it was produced by Lm amastigote-infected macrophages [14]. Leishmania can also induce production of macrophage deactivating cytokines such as IL-10 [15,16] or TGF-β[17–19]. Monocyte chemotactic protein-1 (MCP-1), a CC chemokine crucial in recruitment and activation of monocytes, is produced in Lm-infected human monocytes which stimulates the elimination of Leishmania by these cells directly [20]. Leishmania-mediated alterations of cytokine or chemokine production which influence the course of disease can vary depending on the Leishmania species or strain. Disease progression in BALB/c mice infected with different Lm strains isolated from human cutaneous lesions was heterogeneous, with differences observed in lesion size and some parameters of the immune response [21].
Here, we evaluated correlations between virulence or tropism of Leishmania strains and human monocyte response. We used two Lm strains showing different levels of virulence based on the severity of disease induced in BALB/c mice [21] and two Li strains, one viscerotropic and one dermotropic, to analyse infectivity and cytokine–chemokine production by human infected monocytes.
Materials and methods
Parasites
The Lm parasites isolated from human CL lesions [22] and Li parasites isolated from human sporadic CL lesions or from bone marrow aspirate of VL patients were used in this study. Lm isolates were selected on the basis of their experimental pathogenicity expressed in BALB/c mice: one high-virulence isolate (MHOM/TN/94/GLC94, identified as LmHV), inducing a severe disease, and one low-virulence isolate (MHOM/TN/94/GLC32, identified as LmLV) inducing a less severe disease [21]. Li were selected on the basis of their tropism: one viscerotropic isolate (MHOM/TN/98/LIPA843, identified as LiV) and one dermotropic isolate (MHOM/TN/99/LIPA922, identified as LiD). Parasites were cultivated on Novy–MacNeal–Nicolle medium at 26°C then adapted to RPMI-1640 (Sigma, St Louis, MO, USA) containing l-glutamine (2 mM), penicillin (100 U/ml), streptomycin (100 µg/ml) (RPMI PS/Glu) and 10% heat-inactivated fetal calf serum. Metacyclic promastigotes obtained by negative selection with peanut agglutinin were used for infection of human monocytes.
Preparation and culture of human monocytes
Blood donations were collected from healthy volunteers (who provided informed consent) at the Blood Transfusion Service of Tunis. Plasma was saved following centrifugation, and peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) gradient centrifugation, and resuspended to 2 × 106/ml in RPMI PS/Glu supplemented with 10% fresh autologous plasma (AP). This suspension was added to gelatin/plasma-coated flasks and allowed to adhere for 2 h at 37°C, 5% CO2, as described previously [23]. Non-adherent cells were removed and adherent cells were collected by scraping with a plastic cell scraper and washed. Purity of monocytes was > 80% and contained less than 3% T cells, as determined by flow cytometry using a fluorescence activated cell sorter (FACScan) flow cytometer (Becton Dickinson, San Jose, CA, USA) and staining with anti-CD14, anti-CD3 and anti-CD19 monoclonal antibodies (BD Biosciences Pharmingen, San Diego, CA, USA). Isotype-matched control antibodies were used for detecting non-specific binding to cells. Data were analysed using Lysis II software.
Parasite infection of human monocytes
Freshly purified monocytes were adjusted to 106/ml in RPMI PS/Glu supplemented with 3% AP and distributed into plastic tissue culture dishes (eight-well Permanox chamber slides, Nunc, Naperville, IL, USA). Monocytes were incubated with metacyclic promastigotes, at a ratio of two parasites per cell, for 24 h at 37°C, 5% CO2. Extracellular parasites were washed with warm phosphate-buffered saline (at 37°C), and cultures were incubated for a further 24 and 48 h. Monocytes were then stained with Giemsa and May–Grunwald solutions and analysed using light microscopy. Results were expressed as the percentage of infected monocytes and the mean number of intracellular parasites per infected monocyte. Enumeration was performed on 50 cells.
Cytokine assay
Monocytes were resuspended at 106/ml in RPMI PS/Glu supplemented with 3% AP and distributed in 24-well plates. Monocytes were infected with metacyclic promastigotes, at a ratio of two parasites per cell, in the presence or absence of lipopolysaccharide (LPS) (1 µg/ml) or incubated overnight with recombinant human IFN-γ (100 ng/ml) (BD Biosciences Pharmingen) and then co-incubated with promastigotes, at a ratio of two parasites per cell, and LPS (1µg/ml). Supernatants were taken 24 h later and stored at –80°C. Levels of IL-10, TGF-β, IL-12p40 and p70, TNF-α and MCP-1 were measured using the enzyme-linked immunosorbent assay sandwich method [24]. Purified and biotinylated antibodies specific for these cytokines and human recombinant cytokines used as standards were purchased from BD Biosciences Pharmingen. The thresholds of cytokines detected were 13·6 pg/ml for IL-12p70 and p40; 4·5 pg/ml for TNF-α and IL-10; 3·9 pg/ml for MCP-1; and 41 pg/ml for TGF-β.
Statistical analysis
Results are expressed as mean ± standard error of the mean of three, four or five independent experiments. Statistical significance between treated and control cultures was analysed by Mann–Whitney U-test using Statview statistical software (version 5·0, 1998; SAS Institute Inc., San Diego, CA, USA). P values of P < 0·05 were considered significant.
Results
Human monocyte infection with L. major and L. infantum metacyclic promastigotes
Infection rates obtained with the two Lm isolates (100%) were similar and higher than those of Li isolates. The rate of infection was slightly higher for the dermotropic isolate LiD (average of 69%) compared with the viscerotropic isolate, LiV (average of 57%). Over the 3-day period, the number of intracellular parasites doubled for all isolates (Fig. 1). At 48 and 72 h post-infection, the mean number of intracellular parasites was significantly higher in monocytes infected with LmHV than with LmLV and was twice as high with LiD isolate compared with LiV isolate (P < 0·05). Parasite burden was twice as high in Lm- than in LiD-infected monocytes and threefold higher than in LiV-infected monocytes, and these differences were statistically significant (P < 0·05) (Fig. 1).
Fig. 1.
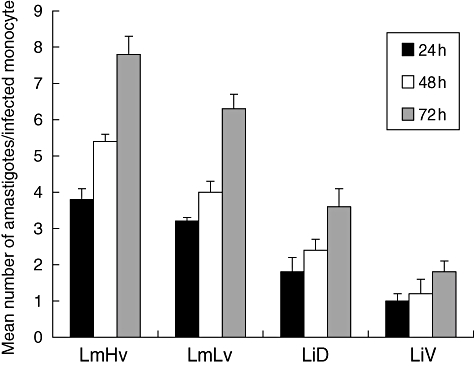
Intracellular multiplication of Leishmania major and L. infantum amastigotes in human monocytes. Results are mean ± standard deviation determined in three independent experiments.
Species-specific cytokine induction by Leishmania-infected monocytes
Monocytes from five donors were exposed to metacyclic Lm and Li promastigotes. After 24-h culture, supernatants were collected and levels of IL-12p40, IL-12p70, IL-10, TNF-α, TGF-β cytokines and MCP-1 chemokine were analysed (Fig. 2). Both Lm isolates were able to induce significantly higher levels of TNF-α (897·5 pg/ml ± 161·8 for LmHV and 940 pg/ml ± 211·2 for LmLV), compared with Li isolates (285 pg/ml ± 111·5 for LiD and 307·5 pg/ml ± 98·1 for LiV, P < 0·05). No IL-12p70 was detected. However, unlike Li isolates, both Lm isolates induced low levels of IL-12p40. Significantly high levels of MCP-1, TGF-β and IL-10 were detected after stimulation with Lm or Li promastigotes, in comparison with uninfected cells. However, no significant difference in levels of these cytokines was observed between LmHV and LmLV or LiV and LiD.
Fig. 2.
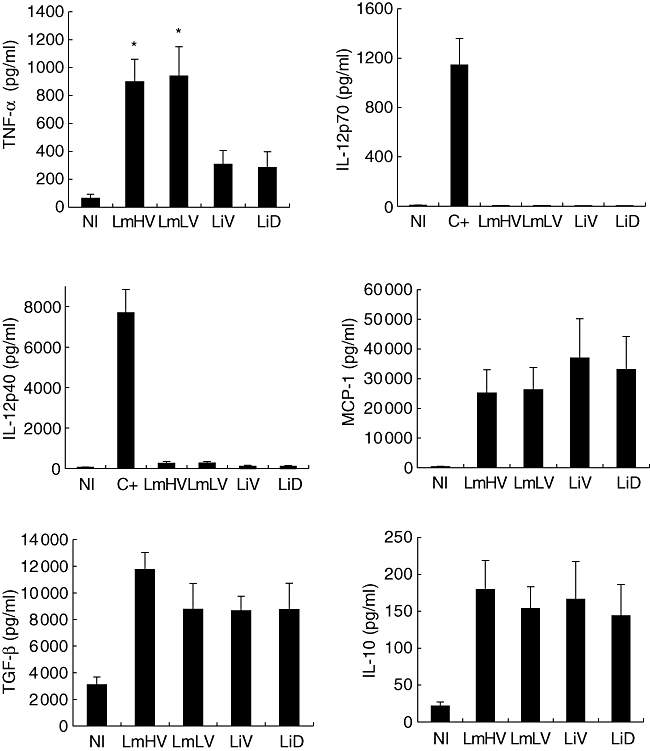
Comparative analysis of cytokine or chemokine patterns induced by Leishmania major and L. infantum isolates in human monocytes. Monocytes were infected with metacyclic promastigotes derived from each of the four isolates, at a ratio of two parasites per cell. Culture supernatants were collected after 24 h and cytokines were quantitated by enzyme-linked immunosorbent assay (ELISA). Control cultures were stimulated with interferon-γ and lipopolysaccharide (C+). Results are mean ± standard error of the mean of four or five experiments. P < 0·05 in comparison with non-infected (NI) cultures, except for interleukin (IL)-12p70 induced by the four isolates and for IL-12p40 induced by Leishmania viscerotropic (LiV) and Leishmania dermotropic (LiD) isolates. *P < 0·05 compared with LiV and LiD.
Effect of IFN-γ priming on cytokine-inducing capacity of the Leishmania isolates in infected monocytes
To determine whether IFN-γ priming could influence cytokine-inducing capacity of the Leishmania isolates, used as described previously [10], and whether this property could vary in accordance to tropism or virulence, monocytes were first primed with IFN-γ for 12 h and then infected with parasites (Fig. 3). A significant increase of TNF-α production was observed as a consequence of IFN-γ priming. This increase was fivefold greater for Li-infected monocytes compared with Lm-infected monocytes (twofold increase). However, no differences were observed in accordance to virulence or tropism. IFN-γ priming induced a weak increase in IL-12p40 production in infected monocytes (Fig. 3), but was inefficient in inducing IL-12p70 production (data not shown). Similar results were observed for all Leishmania isolates tested. Finally, preincubation with IFN-γ inhibited IL-10 and TGF-β production drastically in monocytes infected with all Leishmania isolates tested. The inhibitory rate varied from 70 to 90%.
Fig. 3.
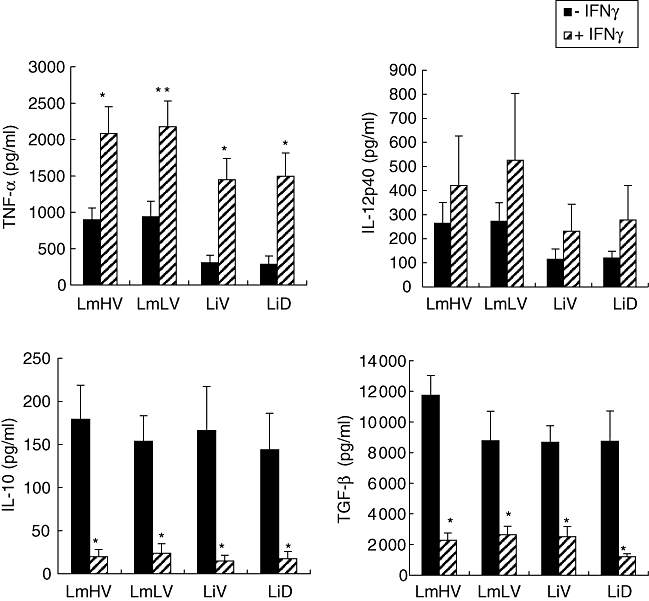
Comparative effect of interferon (IFN)-γ priming on cytokine production, in human monocytes infected by Leishmania parasites of different virulence and tropism. Monocytes were primed with IFN-γ for 12 h then infected with metacyclic Leishmania parasites for 24 h. Cytokines were quantified by enzyme-linked immunosorbent assay in culture supernatants. Results are expressed as mean cytokine production ± standard error of the mean determined from five experiments for interleukin (IL)-12p40 and IL-10, four experiments for tumour necrosis factor-α and three experiments for transforming growth factor-β. *P < 0·05; **P = 0·05 in comparison with unprimed monocytes.
Enhancing effect of LPS on Leishmania-induced IL-10 production by human monocytes
Leishmania parasites can modulate the ability of macrophages to produce cytokines in response to strong proinflammatory stimuli. The presence of LPS is required for the induction of IL-10 by Leishmania in murine macrophages [15]. Leishmania inhibit IL-12 production induced by IFN-γ/LPS stimulation in murine macrophages and human PBMC [9,10,25]. To investigate whether the capacity to modulate macrophage-associated cytokine production in response to LPS varies depending on virulence or tropism of Leishmania strains, monocytes were incubated overnight with or without IFN-γ then co-incubated with parasites and LPS for 2 h to analyse IL-12 and IL-10 production. Interestingly, in accordance with previous data obtained in mice, LPS had a powerful enhancing effect on Leishmania-induced IL-10 production by human monocytes (Fig. 4). IL-10 production in monocytes co-stimulated with LPS and Leishmania was 39–45-fold greater than in Leishmania-infected monocytes. No significant differences were observed, however, in accordance with virulence or tropism. Furthermore, and as shown previously [9,10,25], Leishmania inhibit IFN-γ/LPS induced-IL-12 p40 and p70 production (Fig. 5), but no significant difference was observed between studied isolates.
Fig. 4.
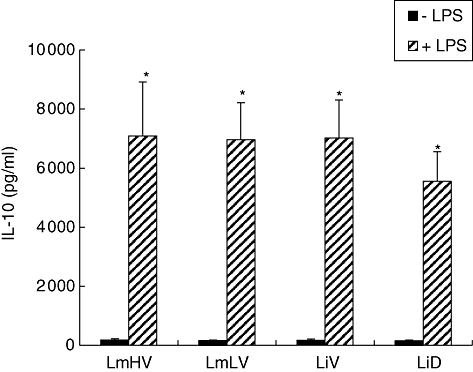
Comparative analysis of interkeukin (IL)-10 production induced by Leishmania isolates differing by virulence or tropism, in lipopolysaccharide (LPS)-activated monocytes. Monocytes were treated with LPS (1 µg/ml) and infected simultaneously with Leishmania parasites for 24 h. IL-10 was quantified in culture supernatants by enzyme-linked immunosorbent assay. Results are expressed as mean cytokine production ± standard error of the mean determined from five experiments. *P ≤ 0·01 in comparison with Leishmania-activated monocytes. Control cultures were monocytes stimulated with LPS (mean of IL-10 production 1965 pg/ml ± 318·6).
Fig. 5.
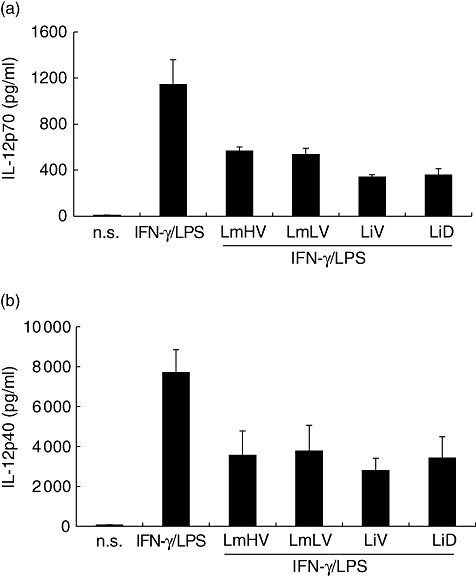
Effect of virulence or tropism of Leishmania major and L. infantum isolates on interleukin (IFN)-γ/lipopolysaccharide (LPS)-induced production of interleukin (IL)-12 after infection of human monocytes. Monocytes were primed for 12 h with IFN-γ then co-stimulated for 24h with Leishmania parasites and LPS. IL-12p70 (a) and IL-12p40 (b) were then quantified in culture supernatants. Results are mean ± standard error of the mean of three experiments; n.s.: non-stimulated and non-infected cultures.
Discussion
To evaluate the impact of virulence and tropism of Leishmania isolates on infectivity and cytokine/chemokine production by human monocytes, we selected two L. major strains showing different levels of virulence in BALB/c mice [21] and two L. infantum strains, one viscerotropic isolated from a VL patient and one dermotropic isolated from a patient with sporadic form of CL. Monocyte infection rates were similar for Lm strains. However, a significantly higher parasite burden was observed in monocytes infected with LmHV. These results suggest that although LmHV and LmLV are ingested similarly by monocytes, LmHV replicates more rapidly inside the phagolysosome. While correlation between virulence of Leishmania strains and ability to survive in murine macrophages has already been reported [21,26–28], much less is known about the human macrophage response. It was shown that unlike the wild-type parasite, an avirulent Lm mutant deleted for an essential metabolic gene (DHFR-TS) was phagocytized but unable to replicate in human macrophages [29]. For Li, the parasite burden in monocytes infected with LiD was significantly higher than in those infected with LiV. Moreover, infection rates were lower compared with those observed for Lm strains. Recently, Campos-Ponce et al. found no association between Li tropism (cutaneous versus visceral) and murine macrophage infection rates [30], whereas others showed higher infectivity for a visceral form compared with a cutaneous form [31]. Li tropism and hence virulence is certainly influenced largely by the host immune status as well as by strain genotype [32], and a variability within dermotropic or viscerotropic Li strains used could explain discrepancies observed.
To survive the hostile environment of macrophages, Leishmania developed subversion mechanisms including the modulation of cytokine production. Here, TNF-α levels were significantly higher for Lm- compared with Li-infected monocytes. We cannot exclude that differences in TNF-α production could be due to different rates of infection observed between Lm and Li strains. No correlations were found with virulence or tropism of Leishmania isolates, although data in murine models indicate that implication of TNF-α in the control of Leishmania infection seems to depend on parasite strain [33]. Variability in TNF-α-inducing capacity among Leishmania species has been shown previously in murine models [34,35]. In human, some studies demonstrated that Leishmania was able to infect monocytes without inducing TNF-α production [11–13], whereas others report the opposite [14]. In agreement with previous studies [11,14], we observed a significant increase of TNF-α production as a consequence of IFN-γ priming. This increase was greater in Lm than in Li-infected monocytes. These data, adding to the role of TNF-α in enhancing leishmanicidal activity in IFN-γ-activated macrophages [36], argue that TNF-α modulation by Leishmania during the early inflammatory events may be associated directly with the outcome of disease.
We showed that Leishmania strains did not induce IL-12 production, and inhibited the IFN-γ/LPS-induced IL-12 production. Furthermore, IFN-γ priming was inefficient to overcome the inhibition of IL-12 production by Leishmania isolates. However, no correlation was found between these response profiles and virulence or tropism. Similarly, the absence of IL-12 in supernatants of virulent or avirulent Lm-infected human macrophages was reported [29]. It was also shown that Lm and L. amazonensis were unable to induce IL-12 production in murine peritoneal macrophages in vitro and IFN-γ priming had no effect on IL-12 levels [37]. Adding to our results, all these data suggest that virulence of Leishmania does not influence the inhibition of IL-12 production by monocytes or macrophages.
We showed a pronounced induction of MCP-1 expression by Lm, as shown previously [12,20]. Similar results were obtained for Li-infected monocytes. MCP-1 was implicated in the recruitment of monocytes and the stimulation of macrophage leishmanicidal functions and its pattern of expression was correlated with the type of cutaneous disease [20,38]. In murine macrophages, MCP-1 expression seems to depend on the virulence of Leishmania strains [26]. These discrepancies could be explained by the different systems used (human versus mouse) or by comparison of virulent versus avirulent strains [26]versus Lm isolates which exhibit variable levels of virulence (our study).
Lm and Li isolates were able to induce significantly elevated levels of IL-10 in human monocytes regardless of their virulence or tropism. On the contrary, using Lm, L. chagasi or L. amazonensis, others found that parasite infection does not induce IL-10 release in human PBMC [10] or monocytes [13]. Despite the fact that we did not detect any significant association between IL-10-inducing capacity and virulence of Lm or Li isolates, discrepancies observed between our results and literature data can be attributed to variability in Leishmania strains or species. In addition, these different results may be due to the use of different cell populations, PBMC or macrophages or monocytes. It was suggested that the absence of IL-10 induction could be explained by the fact that optimal IL-10 production by macrophages probably depends on FcγR ligation by surface IgG [15]. However, our results and others, showing that in vitro infection of murine macrophages with non-opsonized L. donovani promastigotes caused significant induction of IL-10 release, suggest that other mechanisms may contribute to IL-10 induction in macrophages [39]. Interestingly, we also observed that infection had a powerful enhancing effect on LPS-induced IL-10 production by human monocytes. On the contrary, it was shown that IL-10 production by LPS-stimulated L. chagasi-infected monocytes was similar to uninfected controls [13]. However, IL-10 induction in mice by Leishmania amastigotes required the presence of LPS [15]. Considering the presence of LPS during bacterial superinfections that can be observed during visceral and CL [40,41], it is tempting to speculate that the simultaneous presence of LPS and parasites could induce a robust production of IL-10 that may play a role in progression of disease.
Finally, significantly elevated levels of TGF-β were detected following stimulation with all Leishmania strains, but no correlation with virulence or tropism was observed. A direct correlation between the amounts of TGF-β produced following in vitro infection of murine macrophages and virulence of two L. braziliensis isolates was demonstrated [17]. It was also reported that Li strains with the higher infectivity induce higher levels of TGF-β in murine macrophages [28]. These discrepancies can be attributed to the use of human versus mouse models and point out once again the difficulties of relating results observed in experimental murine leishmaniasis to human leishmaniasis.
In conclusion, we showed in this study that Lm strains displaying different levels of virulence in BALB/c mice and viscerotropic and dermotropic Li strains displayed similar MCP-1-, IL-10- and TGF-β-inducing capacities as well as similar abilities to inhibit IL-12 production in human monocytes, despite significant differences in infectivity. Such results obtained in vitro, where several factors playing an important role in in vivo Leishmania–macrophage infection are absent, do not exclude an implication of parasite virulence in the innate immune response of the human host, as shown in the mouse model. Nevertheless, our results reveal that Li are weaker TNF-α inducers than Lm in human monocytes, indicating that TNF-α modulation by Leishmania, during the early inflammatory events, may be associated directly with the type of species-specific pathogenesis that will develop later.
Acknowledgments
We thank Dr Yosser Ben Achour-Chenik and Dr Melika Ben Ahmed for manuscript reading, Dr Belhassen Kaabi for help in statistical analysis, the Blood Transfusion Service of Tunis for blood samples and especially blood donors for the generous donation of their cells. This work was supported by the Tunisian Ministry for Research and Technology (IMM23).
References
- 1.World Health Organization. 2008. Leishmaniasis, background information. Available at: http://www.who.int/leishmaniasis/en/
- 2.Ben Salah A, Kamarianakis Y, Chlif S, Alaya NB, Prastacos P. Zoonotic cutaneous Leishmaniasis in central Tunisia: spatio temporal dynamics. Int J Epidemiol. 2007;37:991–1000. doi: 10.1093/ije/dym125. [DOI] [PubMed] [Google Scholar]
- 3.Ben Salah A, Ben Ismail R, Amri F, et al. Investigation of the spread of human visceral leishmaniasis in central Tunisia. Trans R Soc Trop Med Hyg. 2000;94:382–6. doi: 10.1016/s0035-9203(00)90112-3. [DOI] [PubMed] [Google Scholar]
- 4.BenSaid M, Guerbouj S, Saghrouni F, Fathallah-Mili A, Guizani I. Occurence of Leishmania infantum cutaneous leishmaniasis in central Tunisia. Trans Roy Soc Trop Med Hyg. 2006;100:521–6. doi: 10.1016/j.trstmh.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 5.Sacks D, Noben-Trauth N. The immunology of susceptibility and resistance to Leishmania major in mice. Nat Rev Immunol. 2002;2:845–58. doi: 10.1038/nri933. [DOI] [PubMed] [Google Scholar]
- 6.Mansueto P, Vitale G, Di Lorenzo G, Rini GB, Mansuelo S, Cillari E. Immunopathology of leishmaniasis: an update. Int J Immunopathol Pharmacol. 2007;20:435–45. doi: 10.1177/039463200702000302. [DOI] [PubMed] [Google Scholar]
- 7.Basu MK, Ray M. Macrophage and Leishmania: an unacceptable coexistence. Crit Rev Microbiol. 2005;31:145–54. doi: 10.1080/10408410591005101. [DOI] [PubMed] [Google Scholar]
- 8.Sacks D, Sher A. Evasion of immunity by parasitic protozoa. Nature. 2002;3:1041–7. doi: 10.1038/ni1102-1041. [DOI] [PubMed] [Google Scholar]
- 9.Carrera L, Gazzinelli RT, Badolato R, et al. Leishmania promastigotes selectively inhibit interleukin-12 induction in bone marrow-derived macrophages from susceptible and resistant mice. J Exp Med. 1996;183:515–26. doi: 10.1084/jem.183.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sartori A, Oliveira MAP, Scott P, Trinchieri G. Metacyclogenesis modulates the ability of Leishmania promastigotes to induce IL-12 production in human mononuclear cells. J Immunol. 1997;159:2849–57. [PubMed] [Google Scholar]
- 11.Reiner NE, Ng W, Wilson CB, McMaster WR, Burchett SK. Modulation of in vitro monocyte cytokine response to Leishmania donovani. Interferon-gamma prevents parasite-induced inhibition of interleukin-1 production and primes monocytes to respond to Leishmania by producing both tumor necrosis factor-alpha and interleukin 1. J Clin Invest. 1990;85:1914–24. doi: 10.1172/JCI114654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Badolato R, Sacks DL, Savoia D, Musso T. Leishmania major: infection of human monocytes induces expression of IL-8 and MCAF. Exp Parasitol. 1996;82:21–6. doi: 10.1006/expr.1996.0003. [DOI] [PubMed] [Google Scholar]
- 13.De Almeida MC, Cardoso SA, Barral-Netto M. Leishmania chagasi infection alters the expression of cell adhesion and costimulatory molecules on human monocytes and macrophages. Int J Parasitol. 2003;3:153–62. doi: 10.1016/s0020-7519(02)00266-7. [DOI] [PubMed] [Google Scholar]
- 14.Green SJ, Crawford RM, Hockmeyer JT, Meltzer MS, Nacy CA. Leishmania major amastigotes initiates the L-arginine-dependent killing mechanism in IFN-gamma stimulated macrophages by induction of tumor necrosis factor-alpha. J Immunol. 1990;145:4290–7. [PubMed] [Google Scholar]
- 15.Kane MM, Mosser DM. The role of IL-10 in promoting disease progression in leishmaniasis. J Immunol. 2001;16:1141–7. doi: 10.4049/jimmunol.166.2.1141. [DOI] [PubMed] [Google Scholar]
- 16.Guizani-Tabbane L, Ben Aissa K, Belghith M, Sassi A, Dellagi K. Leishmania major amastigotes induce p50/c-Rel NF-kappa B transcription factor in human macrophages: involvement in cytokine synthesis. Infect Immun. 2004;72:2582–9. doi: 10.1128/IAI.72.5.2582-2589.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barral A, Barral-Netto M, Yong EC, Brownell CE, Twardzik DR, Reed SG. Transforming growth factor β as a virulence mechanism for Leishmania braziliensis. Proc Natl Acad Sci USA. 1993;90:3442–6. doi: 10.1073/pnas.90.8.3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barral A, Teixeira M, Reis P, et al. Transforming growth factor β in human cutaneous leishmaniasis. Am J Pathol. 1995;147:947–654. [PMC free article] [PubMed] [Google Scholar]
- 19.Gantt KR, Schultz-Cherry S, Rodriguez N, et al. Activation of TGF-β by Leishmania chagasi. importance for parasite survival in macrophages. J Immunol. 2003;170:2613–20. doi: 10.4049/jimmunol.170.5.2613. [DOI] [PubMed] [Google Scholar]
- 20.Ritter U, Moll H. Monocyte chemotactic protein-1 stimulates the killing of Leishmania major by human monocytes, acts synergistically with IFN-γ and is antagonized by IL-4. Eur J Immunol. 2000;30:3111–20. doi: 10.1002/1521-4141(200011)30:11<3111::AID-IMMU3111>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 21.Kebaier C, Louzir H, Chenik M, Ben Salah A, Dellagi K. Heterogeneity of wild Leishmania major isolates in experimental murine pathogenicity and specific immune response. Infect Immun. 2001;69:4906–15. doi: 10.1128/IAI.69.8.4906-4915.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Louzir H, Melby PC, Ben Salah A, et al. Immunologic determinants of disease evolution in localized cutaneous leishmaniasis due to Leishmania major. J. Infect Dis. 1998;177:1687–95. doi: 10.1086/515297. [DOI] [PubMed] [Google Scholar]
- 23.Jones BM, Nicholson JK, Colman RC, Hubbard M. Comparison of monocyte separation methods using flow cytometric analysis. J Immunol Methods. 1989;125:41–7. doi: 10.1016/0022-1759(89)90076-8. [DOI] [PubMed] [Google Scholar]
- 24.Sassi A, Louzir H, Ben Salah A, Mokni M, Ben Osman A, Dellagi K. Leishmanin skin test lymphoproliferative responses and cytokine production after symptomatic or asymptomatic Leishmania major infection in Tunisia. Clin Exp Immunol. 1999;116:127–32. doi: 10.1046/j.1365-2249.1999.00844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Belkaid Y, Butcher B, Sacks DL. Analysis of cytokine production by inflammatory mouse macrophages at the single-cell level: selective impairment of IL-12 induction in Leishmania infected cells. Eur J Immunol. 1998;28:1369–400. doi: 10.1002/(SICI)1521-4141(199804)28:04<1389::AID-IMMU1389>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 26.Racoosin EL, Beverley SM. Leishmania major: promastigotes induce expression of a subset of chemokine genes in murine macrophages. Exp Parasitol. 1997;85:283–95. doi: 10.1006/expr.1996.4139. [DOI] [PubMed] [Google Scholar]
- 27.Chakraborty P, Ghosh D, Basu MK. Modulation of macrophage mannose receptor affects the uptake of virulent and avirulent Leishmania donovani promastigotes. J Parasitol. 2001;87:1023–7. doi: 10.1645/0022-3395(2001)087[1023:MOMMRA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 28.Baptista-Fernandes T, Marques C, Rodrigues OR, Santos-Gomes GM. Intra-specific variability of virulence in Leishmania infantum zymodeme MON-1 strains. Comp Immun Microbiol Infect Dis. 2007;30:41–53. doi: 10.1016/j.cimid.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Brodskyn C, Beverley SM, Titus RG. Virulent or avirulent (dhfr−ts-) Leishmania major elicit predominantly a type-1 cytokine response by human cells in vitro. Clin Exp Immunol. 2000;119:299–304. doi: 10.1046/j.1365-2249.2000.01122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campos-Ponce M, Ponce C, Ponce E, Maingon RDC. Leishmania chagasi/infantum: further investigations on Leishmania tropism in atypical cutaneous and visceral leishmaniasis foci in Central America. Exp Parasitol. 2005;109:209–19. doi: 10.1016/j.exppara.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 31.Louassini M, Adroher FJ, Foulquie MR, Benitez R. Investigations on the in vitro metacyclogenesis of a visceral and a cutaneous human strain of Leishmania infantum. Acta Trop. 1998;70:355–68. doi: 10.1016/s0001-706x(98)00041-2. [DOI] [PubMed] [Google Scholar]
- 32.Gradoni L, Gramiccia M. Leishmania infantum tropism: strain genotype or host immune status? Parasitol Today. 1994;10:264–7. doi: 10.1016/0169-4758(94)90142-2. [DOI] [PubMed] [Google Scholar]
- 33.Ritter U, Mattner J, Rocha JS, Bogdan C, Korner H. The control of Leishmania major by TNF in vivo is dependent on the parasite strain. Microbes Infect. 2004;6:559–65. doi: 10.1016/j.micinf.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 34.Matte C, Olivier M. Leishmania-induced cellular recruitment during the early inflammatory response: modulation of pro-inflammatory mediators. J Infect Dis. 2002;185:673–81. doi: 10.1086/339260. [DOI] [PubMed] [Google Scholar]
- 35.Gomes IN, de Carvalho Calabrich AF, da Silva Tavares R, Wietzerbin J, de Freitas LAR, Tavares Veras PS. Differential properties of CBA/J mononuclear phagocytes recovered from an inflammatory site and probed with two different species of Leishmania. Microbes Infect. 2003;5:251–60. doi: 10.1016/s1286-4579(03)00025-x. [DOI] [PubMed] [Google Scholar]
- 36.Liew FY, Li Y, Millot S. Tumor necrosis factor-α synergizes with IFN-γ in mediating killing of Leishmania major through the induction of nitric oxide. J Immunol. 1990;145:4306–10. [PubMed] [Google Scholar]
- 37.Oliveira MAP, Santiago HC, Lisboa CR, et al. Leishmania sp: comparative study with Toxoplasma gondii and Trypanosoma cruzi in their ability to initialize IL-12 and IFN-γ synthesis. Exp Parasitol. 2000;95:96–105. doi: 10.1006/expr.2000.4523. [DOI] [PubMed] [Google Scholar]
- 38.Ritter U, Moll H, Laskay T, et al. Differential expression of chemokines in patients with localized and diffuse cutaneous American leishmaniasis. J Infect Dis. 1996;173:699–709. doi: 10.1093/infdis/173.3.699. [DOI] [PubMed] [Google Scholar]
- 39.Bhattacharyya S, Ghosh S, Jhonson PL, Bhattacharyya SK, Majumdar S. Immunomodulatory role of interleukin-10 in visceral leishmaniasis: defective activation of protein kinase C-mediated signal transduction events. Infect Immun. 2001;69:1499–507. doi: 10.1128/IAI.69.3.1499-1507.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.El-On J, Sneier R, Elias E. Leishmania major: bacterial contamination of cutaneous lesions in experimental animals. Israel J Med Sci. 1992;28:847–51. [PubMed] [Google Scholar]
- 41.Kadivar MR, Kajbaf TZ, Karimi A, Alborzi A. Childhood visceral leishmaniasis complicated by bacterial infections. East Mediterr Health J. 2000;6:879–83. [PubMed] [Google Scholar]


